Abstract
Rationale
Noncontingent administration of amphetamine into the ventral striatum or systemic nicotine increases responses rewarded by inconsequential visual stimuli. When these drugs are contingently administered, rats learn to self-administer them. We recently found that rats self-administer the GABAB receptor agonist baclofen into the median (MR) or dorsal (DR) raphe nuclei.
Objectives
We examined whether noncontingent administration of baclofen into the MR or DR increases rats’ investigatory behavior rewarded by a flash of light.
Results
Contingent presentations of a flash of light slightly increased lever presses. Whereas noncontingent administration of baclofen into the MR or DR did not reliably increase lever presses in the absence of visual stimulus reward, the same manipulation markedly increased lever presses rewarded by the visual stimulus. Heightened locomotor activity induced by intraperitoneal injections of amphetamine (3 mg/kg) failed to concur with increased lever pressing for the visual stimulus. These results indicate that the observed enhancement of visual stimulus seeking is distinct from an enhancement of general locomotor activity. Visual stimulus seeking decreased when baclofen was co-administered with the GABAB receptor antagonist, SCH 50911, confirming the involvement of local GABAB receptors. Seeking for visual stimulus also abated when baclofen administration was preceded by intraperitoneal injections of the dopamine antagonist, SCH 23390 (0.025 mg/kg), suggesting enhanced visual stimulus seeking depends on intact dopamine signals.
Conclusions
Baclofen administration into the MR or DR increased investigatory behavior induced by visual stimuli. Stimulation of GABAB receptors in the MR and DR appears to disinhibit the motivational process involving stimulus–approach responses.
Keywords: Median and dorsal raphe nuclei, GABAB receptors, Baclofen, Serotonin, Dopamine, Visual stimuli, Incentive motivation, Sensation seeking
Introduction
Sensory stimulus presentations, such as a flash of light, can trigger approach responses in rats and other animals; in other words, rats will increase responses if the occurrence of a flash of light is contingent upon responding such as lever pressing (Kish 1966; Stewart 1960). This effect is also characterized as visual stimulus-induced investigation or visual sensation (VS) seeking. Seeking effects of a flash of light are weak: in our hands, VS seeking declines quickly over the first few minutes of the session. But, VS seeking recovers and shows a similar rapid decline in following sessions. Such a diminishing effect of inconsequential sensory stimuli seems to be adaptive because it allows animals to minimize unnecessary energy expenditure or engage in other possibly important pursuits. The lack of it may relate to phenomena clinically characterized as obsessive and compulsive.
We recently found that amphetamine administration into the ventral striatum, a primary region of the mesolimbic dopamine system, increases investigatory responses rewarded by visual stimuli and slowed behavioral inhibition (Shin et al. 2010). It is important to reiterate that the VS seeking we are describing does not depend on prior conditioning with classical primary rewards like food: experimentally naive rats markedly increase responding for unconditioned visual stimuli when they receive amphetamine in the ventral striatum. Such intracranial chemical manipulations are useful in elucidating the neural circuitry of VS seeking; these findings may shed new light on the nature and mechanisms of motivation and reward.
Neural mechanisms of VS seeking are virtually unknown; however, many studies examined neural mechanisms of motivated behavior controlled by conditioned stimuli. Amphetamine-enhanced VS seeking may have been predicted from the widely held view that the activation of the mesolimbic dopamine system markedly increases responses controlled by conditioned stimuli (Berridge and Robinson 1998; Ikemoto and Panksepp 1999). For example, injections of amphetamine into the ventral striatum increase seeking rewarded by conditioned stimuli (Kelley and Delfs 1991; Taylor and Robbins 1984), while dopamine depletion induced by 6-hydroxydopamine infusions into the ventral striatum decreases such responses (Taylor and Robbins 1986).
In addition to the mesolimbic dopamine system, seeking behaviors associated with conditioned sensory stimuli appear to be controlled by the median and dorsal raphe nuclei (MR and DR, respectively), regions that contain ascending serotonergic neurons. For example, selective lesions of MR–DR serotonergic neurons by 5,7-dihydroxytryptamine increase seeking rewarded by conditioned stimuli (Fletcher et al. 1999). Similarly, intra-MR infusions of the GABAA agonist muscimol facilitate reinstatement of stimulus-guided alcohol seeking (Le et al. 2008). These findings suggest that inhibition of the MR–DR system invigorates seeking rewarded by conditioned stimuli. Indeed, opposing functional relationships between the DR serotonin system and the midbrain dopamine system have been suggested (Daw et al. 2002; Deakin 1983; Kapur and Remington 1996). Because the MR is involved in similar behavioral functions as the DR and because non-serotonergic projection neurons are also involved in similar behavioral functions as serotonergic neurons, we suggest here a modified notion referred to as the tug-of-war hypothesis, which includes both serotonergic and non-serotonergic projection neurons in the MR and DR functionally opposing the mesolimbic dopamine system.
The present study aims to test the following hypothesis: the GABAB receptor agonist baclofen administered into the MR or DR will increase investigatory behavior rewarded by unconditioned visual stimuli. This hypothesis has arisen from two independent views. The first view hinges on the tug-of-war hypothesis. According to this hypothesis, since the activation of the mesolimbic dopamine system leads to an increase in VS seeking (Shin et al. 2010), the inhibition of the MR–DR system should lead to a similar behavioral consequence. Furthermore, the tug-of-war hypothesis predicts that effects of intra-MR/DR baclofen depend on intact dopamine system. We test this notion using systemic injections of a dopamine receptor antagonist.
The other view arises from the approach drive1 theory of drug reward (see Ikemoto 2010). Briefly, the theory consists of the following premises: there exists a brain process that energizes approach-type behaviors (e.g., exploratory, investigatory, and seeking behaviors) to obtain information and rewards by coordinating sensory–perceptual, visceral, and motor processes. Some drug manipulations, such as intra-ventral striatal amphetamine, activate the approach drive. Furthermore, mere activation of this drive leads to reinforcement (Ikemoto 2010). We recently found that administration of baclofen into the MR or DR is reinforcing in that the manipulation induces conditioned place preference, and baclofen was self-administered into these regions (Shin and Ikemoto 2010). The reinforcing effects of intra-MR or DR baclofen may result from its ability to induce the approach drive. Specifically, we hope to demonstrate a synergistic relationship between intra-MR/DR baclofen and positively salient stimuli: baclofen in the MR or DR should facilitate an animal’s interaction with the environment when salient stimuli are available. In contrast, in the absence of salient stimuli, environmental interaction should be minimal. Therefore, the present study examines whether the stimulation of GABAB receptors in the MR or DR facilitates VS seeking.
Materials and methods
Animals
We used 47 male Wistar rats (Harlan, Dublin, VA) weighing 250–350 g at the time of surgery. Testing sessions occurred during the dark cycle of a reverse 12-h dark 12-h light cycle (8:00 a.m. off). Food and water were given ad libitum except during 90-min testing sessions. All procedures were approved by the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Drug Abuse and were in accordance with the National Institutes of Health guidelines.
Surgery
Each rat unilaterally received a guide cannula (24 gauge) implant under sodium pentobarbital (31 mg/kg, intraperitoneal route (IP)) and chloral hydrate (142 mg/kg, IP) anesthesia. The guide cannula ended 1.0 mm above either the MR or DR. All cannulae were inserted into the left hemisphere at a lateral angle (10° or 20°) toward the midline, using the flat skull position (Paxinos and Watson 2005). Stereotaxic coordinates (in millimeters) were 7.4–8.0 posterior to the bregma (P), 1.6 lateral to the midline (L), and 7.9–8.0 ventral to the skull surface (V) angled at 10° for the MR and P7.2, L2.6, and V5.7 with a 20° angle for the DR. Following surgery, rats were housed individually and rested 5–7 days before the start of experiments described below.
Drugs
The GABAB receptor agonist (R) baclofen and the GABAB receptor antagonist SCH 50911 (Tocris Bioscience, MO) were dissolved in artificial cerebrospinal fluid consisting of (in millimolars): 148 NaCl, 2.7 KCl, 1.2 CaCl2, and 0.85 MgCl2, pH adjusted to 6.5–7.5. The D1 receptor antagonist SCH 23390 (Sigma–Aldrich, MO) was dissolved in 0.9% sterile saline prior to testing.
Experimental apparatuses and intracranial injection procedure
Experimental apparatuses were previously described (Shin et al. 2010). Briefly, each rat was placed individually in a standard test chamber equipped with two retractable levers (10 cm above the floor) and two cue light receptacles above the levers on a side wall. An injection cannula (31 gauge) was inserted and secured into the guide cannula, which was connected by polyethylene tubing to a micropump (Ikemoto and Sharpe 2001) that hung a few millimeters above the rat’s head. Activation of the micropump’s step motor turned its shaft in incremental steps (9° per step) over 5 s, driving its threaded shaft into the drug reservoir and, in turn, pushing a volume out of the reservoir into the brain. In experiment 1, infusions were delivered into either the MR or DR on a fixed 90-s interval schedule (unit volume of 100 nl every 90 s×60 infusions=6 μl over the course of a 90-min session), adapted from our recent study (Shin et al. 2010). In subsequent experiments 2–4, infusions were delivered into the MR on a fixed 250-s interval schedule (unit volume of 75 nl every 250 s×21=1.6 μl over the course of a 90-min session), to minimize drug diffusion. During experiments 2–3, locomotor activity of rats was detected by the Roto-Rat™ counter (Med Associates), which quantified turns of the electrical commutator used for the microinjection pump.
General procedure and visual stimulus presentation
Experimentally naive rats were used for each of the three experiments described below except experiment 3, which used rats going through experiments 2 and 4. Three rats were eliminated from analysis because one lost its guide cannula implant during experiment 4, and two became ill during experiment 3. One day before the start of testing, rats were habituated in testing chambers with levers retracted for 90 min. Rats were individually placed in the test chamber with the visual stimulus procedure described below. Rats were given a single session daily, and each session lasted 90 min.
Responding on the active lever illuminated the cue light just above the lever for 1 s and extinguished the house light for 7 s, during which lever pressing was counted but did not produce a programmed consequence. Responding on the inactive lever had no programmed consequence. To facilitate differential responding between the two levers, the number of lever presses required to produce a visual stimulus increased by one every ten visual stimulus presentations that the rat earned. The left–right locations of the active and inactive levers were counterbalanced among rats, and the assignment of active and inactive functions between the levers remained the same for each rat throughout the experiment.
Experiment 1: effects of baclofen concentration
In all sessions, active lever pressing resulted in presentation of a visual stimulus as described above. The concentration of baclofen present in the noncontingent infusions varied from session to session according to the following schedule: 0 mM (only vehicle) in sessions 1 and 8, 0.02 mM in sessions 2 and 3, 0.1 mM in sessions 4 and 5, and 0.5 mM in sessions 6 and 7. We focused on the MR and DR in this experiment because regions just outside the MR and DR do not support self-administration of baclofen in our previous study (Shin and Ikemoto 2010).
Experiment 2: interaction between visual stimuli and intra-MR baclofen
In sessions 1 through 4, responding on either lever had no programmed consequence (i.e., no resulting visual stimulus presentation). In sessions 5 through 8, responding on the active lever presented visual stimuli as described above. The rats received vehicle in sessions 1, 2, 5, and 6 and 0.5 mM baclofen into the MR in sessions 3, 4, 7, and 8.
Experiment 3: effects of amphetamine-induced hyperactivity on visual stimulus seeking
The rats received 0.9% saline (1 ml/kg, IP) just prior to session 1, amphetamine (3 mg/kg, IP) just prior to session 2, and intra-MR baclofen (0.5 mM) during session 3. During sessions 1 and 2 following an IP injection, rats were similarly fastened to the drug infusion micropump but without cannulae, to keep experimental conditions consistent with session 3.
Experiment 4: effects of the GABAB receptor antagonist SCH 50911 and the dopamine receptor antagonist SCH 23390
Rats received vehicle, 0.5 mM baclofen, 1 mM SCH50911, and a mixture of 0.5 mM baclofen and 1 mM SCH50911 into the MR over four sessions. The order of testing these injections was counterbalanced among rats.
These rats were tested in four additional sessions with the visual stimulus procedure described above. Immediately before each 90-min session, the rats received an injection of either 0.9% saline (1 ml/kg, IP) or SCH 23390 (0.025 mg/kg, IP). This dose was chosen on the basis of previous studies suggesting that this dose disrupts sensory–motor integrative rather than motor processes (Liu and Ikemoto 2007; Sanger 1987). During sessions, they received intra-MR infusions of either vehicle or 0.5 mM baclofen. The order of these four injection manipulations was counterbalanced among rats.
Histology
Upon completion of the experiments, the rats’ brains were removed after the rats were deceased under pentobarbital (31 mg/kg, IP) and chloral hydrate (142 mg/kg, IP) anesthesia. After a minimum of 2 days in a 10% formalin solution, the brains were sectioned on a cryostat. Coronal sections (40-μm thickness) were mounted on slides and stained with cresyl violet for microscopic examination.
Data and statistical analyses
Lever preference ratios (LPR) were obtained using the following formula: LPR=(active lever presses-inactive lever presses)/(active lever presses+inactive lever presses). The formula produces values ranging between 1 and −1; 0 indicates no preference, while 1 and −1 indicate absolute preference for the active and inactive levers, respectively. To meet the assumptions of normal distribution and homogeneous variance, lever press and locomotor data were square-root transformed; if a set of data contained 0, 1 was added to each number of the set before the transformation (McDonald 2009). Data were analyzed with repeated factors ANOVAs (Statistica, version 6.1, StatSoft, Inc., Tulsa, OK), which were followed by Newman–Keuls post hoc tests when appropriate. Data are presented as mean ± SEM.
Results
Experiment 1: effects of baclofen concentration
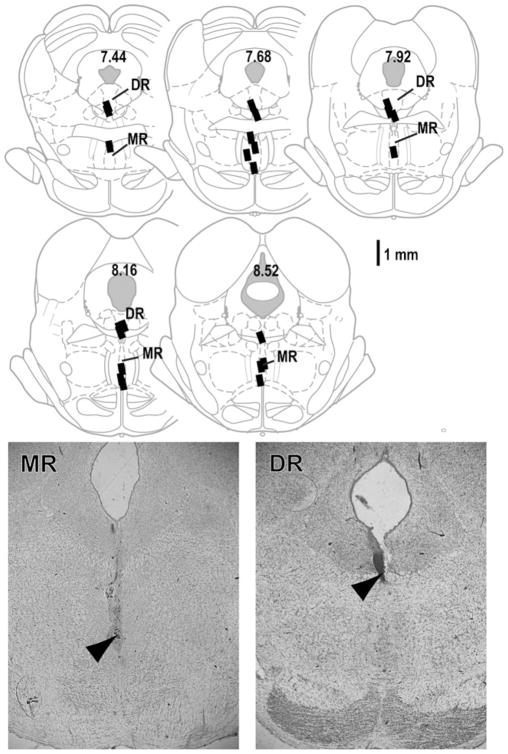
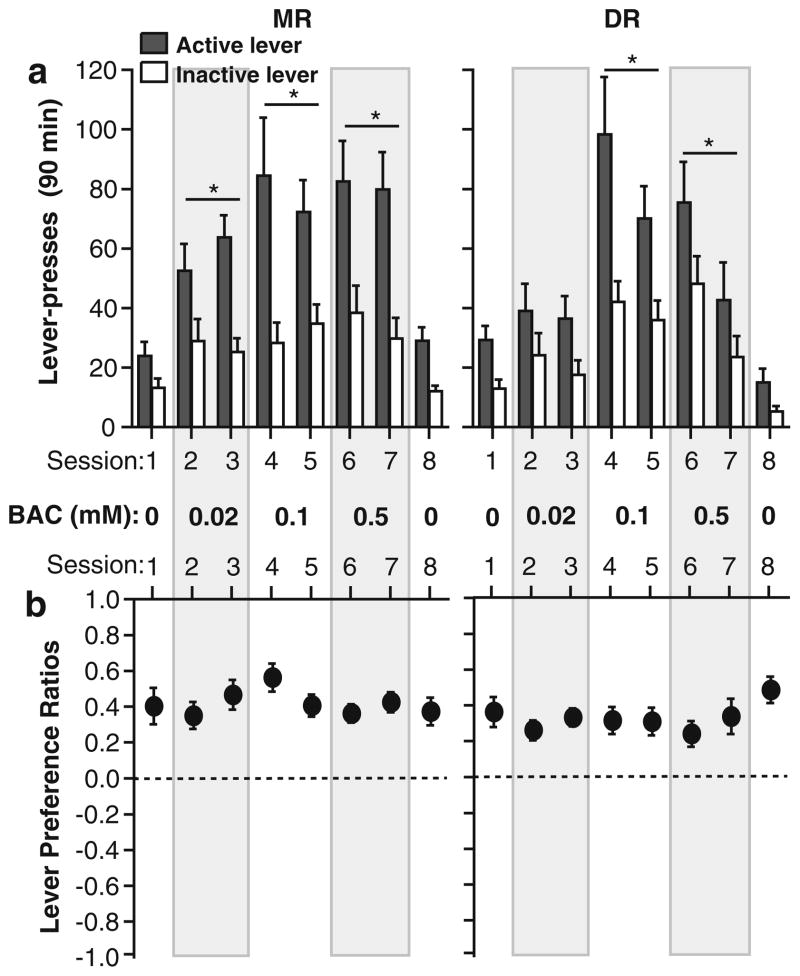
The diagrams in Fig. 1 depict each of the injection sites. When rats received vehicle injected into the MR or DR in session 1, rats moderately responded on the levers and tended to respond on the active lever more than the inactive lever (Fig. 2a). Noncontingent baclofen injections into the MR or DR in subsequent sessions increased lever pressing (Fig. 2a). All concentrations of baclofen injected into the MR reliably increased lever presses, while medium and high, but not low, concentrations injected into the DR increased lever presses, suggesting that the MR is more responsive to baclofen than the DR. These observations are confirmed by 4×2×2 (concentration×session×lever) ANOVAs on lever presses with significant main effects of concentration (MR: F3,33=12.29, P<0.0001, and DR: F3,33= 8.38, P=0.0003) and lever (MR: F3,11=63.10, P<0.0001, and DR: F3,11=36.89, P<0.0001).
Fig. 1.
Intracranial injection sites of experiment 1. Injection cannula tips (0.3 mm, N=24) were shown on drawings adopted from Paxinos and Watson (2005).
Numbers indicate posterior distances from bregma.
Arrowheads in the photomicrograms indicate representative tips of injection cannula
Fig. 2.
Noncontingent administration of baclofen into the MR or DR facilitates lever pressing when rewarded by visual stimuli. a Mean lever presses (±SEM) are shown for MR (N=12) and DR (N=12) injections. *P<0.05, the concentration significantly increased lever presses than the vehicle. b Lever preference ratios are shown. BAC baclofen
ANOVAs on lever preference ratio indicate that lever preference for the active lever did not significantly increase as a function of baclofen concentration for either MR or DR injections (Fig. 2b). Baclofen injections appear to have increased responding on both levers while keeping relative preference unchanged.
Experiment 2: interaction between visual stimuli and intra-MR baclofen
Experiment 1’s results led us to address two issues: one is to determine whether the increase in lever pressing during baclofen administration depends on the presentation of the visual stimulus. The other is to better understand the lack of selective active lever increase during baclofen administration, while keeping active lever preference induced by the visual stimulus. Experiment 2 examined the interaction between baclofen and visual stimulus presentation on active and inactive lever pressing and locomotor activity.
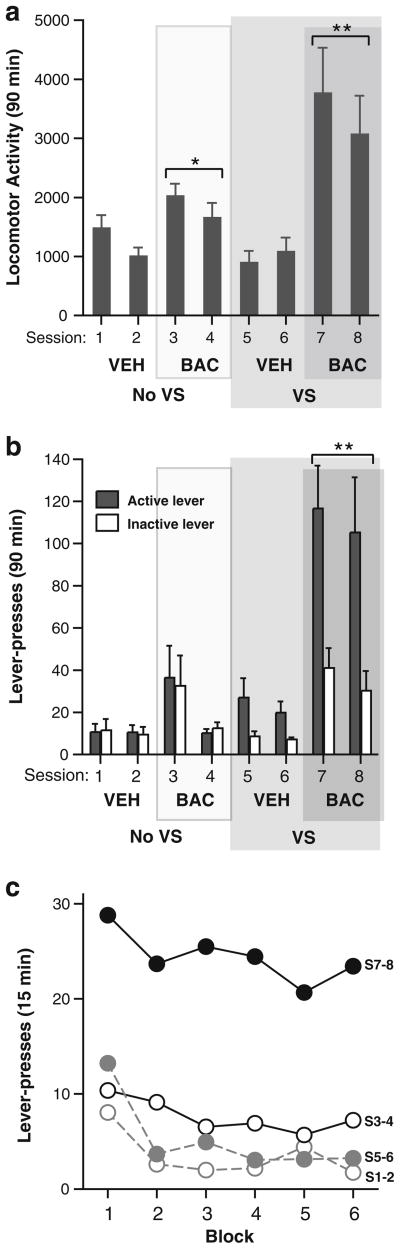
While baclofen injections alone reliably facilitated locomotor activity more than vehicle injections (sessions 3–4 vs. sessions 1–2 or 5–6), baclofen injections accompanied by the presentation of the visual stimulus (sessions 7–8) increased locomotion significantly more than all other conditions (Fig. 3a). These observations were confirmed by a 2×2×2 (visual stimulus × baclofen × repeated condition) ANOVA on locomotor activity that showed a significant interaction between visual stimulus and baclofen (F1,7= 34.33, P=0.0006).
Fig. 3.
Interaction between contingent visual stimuli and noncontingent intra-MR baclofen injections on active and inactive lever presses and locomotor activity. a A combination of visual stimuli and baclofen injections more effectively increased lever pressing than either manipulation alone (N=8). **P<0.01, the BAC + VS condition had significantly greater lever presses than other conditions. Both active and inactive lever presses in sessions 7–8 were significantly greater than any active or inactive lever responding in other conditions (P< 0.05). b An interaction between visual stimuli and baclofen injections was also present on locomotor activity. *P<0.01, significantly different from the values in sessions 1–2 or 5–6. **P<0.01, significantly different from the values in any other condition. c The distributions of lever presses over the course of the 90-min session are shown. S1–2, VEH + no VS; S3–4, BAC + no VS; S5–6, VEH + VS; S7–8: BAC + VS. BAC baclofen, No VS no visual stimuli, S session, VEH vehicle, VS visual sensation
A more pronounced interaction was found for lever pressing. Without visual stimulus, baclofen injections tended to increase lever pressing more than vehicle injections (Fig. 3b; sessions 1–2 vs. 3–4); this effect was not reliable and was largely due to two rats with markedly increased lever pressing in session 3. When active lever pressing was rewarded with visual stimuli, baclofen injections increased lever pressing markedly more than any other condition (sessions 7–8). Similar to what we found in experiment 1, baclofen injections increased both active and inactive lever presses while keeping active lever preference, which depended on contingent visual stimuli. It should be noted that inactive lever presses in sessions 7–8 were significantly greater than those in sessions 3–4, suggesting that increased inactive lever presses in session 7–8 also depended on the visual stimulus rewarding active lever pressing. These observations were confirmed by a 2× 2×2×2 (visual stimulus × baclofen × repeated condition × lever) ANOVA on lever responses with significant interactions between visual stimulus and baclofen (F1,7=10.95, P=0.0130) and between visual stimulus, baclofen, and lever (F1,7=11.08, P=0.0126).
We also examined the distribution of lever presses over the course of each session (Fig. 3c). In vehicle sessions (S1–2 and S5–6), rats tended to mostly lever press during the first 15 min, while in baclofen sessions (S3–4 and S7–8), lever presses were distributed over the full course of the session. These observations suggest that lever pressing habituated quickly in the absence of baclofen.
Experiment 3: effects of amphetamine-induced hyperactivity on visual stimulus seeking
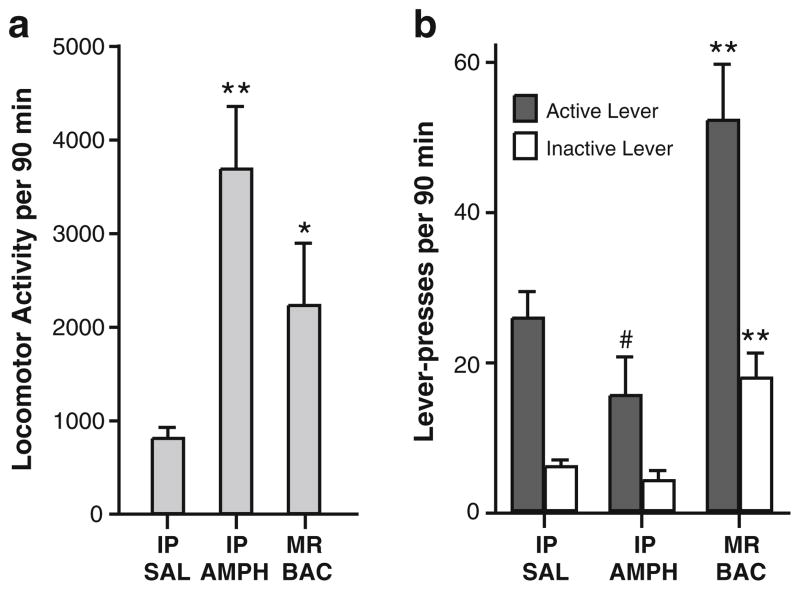
We sought to replicate our previous finding that systemic administration of d-amphetamine (3 mg/kg, IP) makes rats hyperactive, but not interactive with visual stimuli (Shin et al. 2010). This shows that hyperactivity is not sufficient for increasing lever pressing that we employed as the measure of investigatory motivation in the present study. We compared effects induced by systemic injections of d-amphetamine with those induced by intra-MR baclofen using a within subjects design.
As shown in Fig. 4a, systemic administration of amphetamine (3 mg/kg, IP) markedly increased locomotor activity greater than saline control or intra-MR baclofen (a significant main effect of manipulation, F2,34 =18.46, P<0.0001, obtained by a one-way ANOVA on locomotor activity). On the other hand, the systemic amphetamine decreased active lever presses lower than those of saline control or intra-MR baclofen (Fig. 4b; a significant main effect of manipulation, F2,34=27.10, P<0.0001, obtained by a 3×2 (manipulation x lever) ANOVA), while it did not reliably decrease inactive lever presses (F2,34=24.20, P<0.0001). These data confirm that hyperactivity is not sufficient for increasing active or inactive lever pressing and suggest that systemic administration of amphetamine facilitates hyperactivity/arousal while interfering with seeking.
Fig. 4.
Effects of hyperactivity induced by systemic amphetamine administration on lever pressing. a Effects on locomotor activity. *P< 0.01, significantly greater than IP saline (SAL) values. **P<0.01, significantly greater than IP saline or MR baclofen (BAC) values. b Effects on active and inactive lever presses. #P<0.01, significantly lower than IP SAL values. **P<0.01, significantly greater than IP SAL or IP amphetamine (AMPH)
Experiment 4: effects of the GABAB receptor antagonist SCH 50911 and the dopamine receptor antagonist SCH 23390
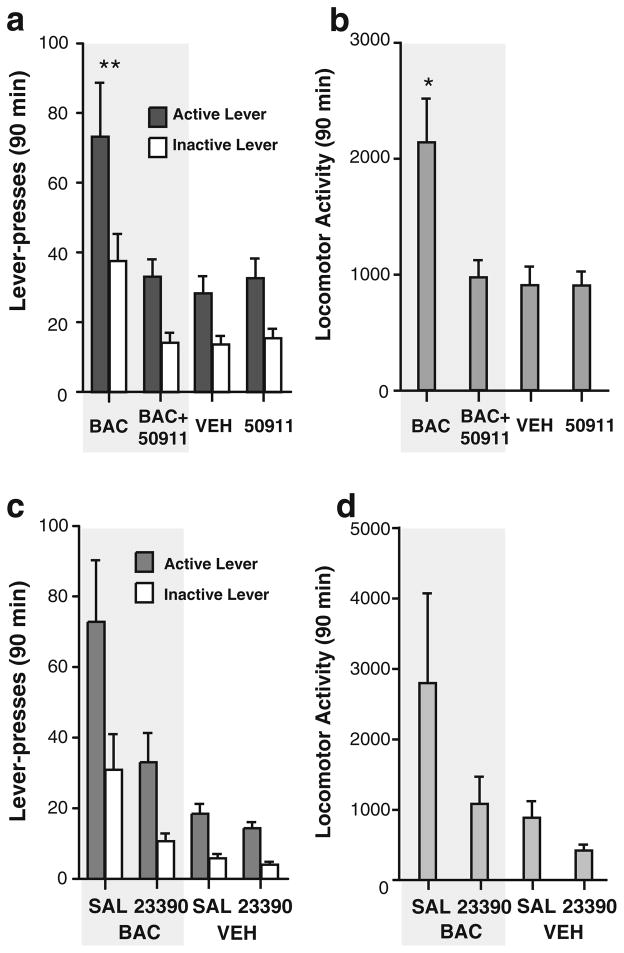
Co-administration of SCH 50911 and baclofen completely blocked facilitating effects of baclofen on visual stimulus seeking (Fig. 5a), while not altering preference between the two levers. Co-administration of SCH 50911 and baclofen also completely blocked locomotion facilitated by baclofen during seeking sessions (Fig. 5b), although SCH 50911 alone did not have any detectable effect on lever pressing or locomotion. These observations are confirmed by a 2×2×2 (SCH 50911 × baclofen × lever) ANOVA on lever pressing (a significant interaction between SCH 50911 and baclofen, F1,14=8.46, P=0.0115) and a 2×2 (SCH 50911 × baclofen) ANOVA on locomotion (a significant interaction between SCH 50911 and baclofen, F1,14=7.17, P=0.0180).
Fig. 5.
Effects of the GABAB receptor antagonist SCH 50911 and the dopamine receptor antagonist SCH 23390 on visual stimulus seeking facilitated by intra-MR baclofen. Data are means (±SEM) of lever presses (a and c) and locomotor activity (b and d). a and b Visual stimulus seeking or locomotor activity facilitated by intra-MR baclofen was diminished by co-administration of SCH 50911 (N= 15). **P<0.01, *P<0.05, baclofen alone had significantly greater lever presses or locomotor activity than any other manipulation. c and d Only main effects of baclofen and SCH 23390 were significant (see text, N=14). 50911 SCH 50911, 23390 SCH 23390, BAC baclofen, SAL saline, VEH vehicle
Systemic administration of SCH 23390 (0.025 mg/kg, IP) reduced lever pressing (a significant SCH 23390 main effect, F1,13 =14.74, P=0.0020, with a 2×2×2 (SCH 23390×baclofen×lever) ANOVA) with lever and baclofen factors collapsed together (Fig. 5c). The main effect of intra-MR baclofen was also significant (F1,13=16.06, P= 0.0015). There was a strong trend on the interaction between SCH 23390×baclofen (F1,13=4.02, P=0.0663) on lever presses. Similarly, main effects of SCH 23390 and baclofen on locomotor activity were significant (Fig. 5d; F1,13 =14.18, P=0.0023, and F1,13 =5.16, P=0.0408, respectively, following a 2×2 (SCH 23390×baclofen) ANOVA). No interaction between SCH 23390 and baclofen on locomotion was detected.
Discussion
Our findings on the effects of intra-MR baclofen support the hypothesis that intra-raphe baclofen can increase investigatory responses rewarded by inconsequential stimuli. Experiment 2 illustrated that in the absence of intra-raphe baclofen, lever pressing rewarded by visual stimuli habituated quickly after the first 15 min. Furthermore, in the absence of visual stimulus reward, intra-raphe baclofen had minimal effects on lever pressing and locomotor activity. However, an identical dose of intra-raphe baclofen paired with the availability of visual stimulus reward resulted in a robust increase in lever pressing. It may be intuitive to speculate that the observed increase in responding under the influence of intra-raphe baclofen is due to an increase in general arousal or mere motor activity; however, this speculation is not tenable, considering the evidence from experiment 2 revealing that intra-raphe baclofen alone only minimally increases measures of general locomotor activity and lever pressing. In addition, experiment 3 confirms that the two levers are not physically placed to pick up unintended presses due to mere hyperactivity because systemic amphetamine that increased general activity failed to increase either active or inactive lever presses (see below for additional discussion on effects of systemic amphetamine). The explanation that intra-MR baclofen increased sensory pleasure of the visual stimulus is not satisfying either, because the presentation of the visual stimulus did not selectively increase active lever pressing, but increased inactive lever pressing and locomotor activity.
The present study is consistent with predictions generated by the theory of approach drive (Ikemoto 2010) and indicates that the MR and DR are relevant components of approach drive processes. In brief, the approach drive theory describes the central processes that coordinate perceptual, visceral, and motor functions in order to energize approach-type behaviors. In addition to the MR and DR, approach drive is most likely mediated by extensive structures and neurotransmitter systems. The mesolimbic dopamine system has been suggested to play an integral role in general seeking function (Ikemoto 2007, 2010; Ikemoto and Panksepp 1999; Panksepp 1998). Consistently, amphetamine administration into the ventral striatum, but not dorsal striatum, facilitates seeking responses rewarded by inconsequential visual stimuli, and this enhancement in VS seeking depends on dopamine transmission at both D1 and D2 receptors (Shin et al. 2010). Evidence from experiment 4 of the present study is also consistent because systemic injections of a low dose (0.025 mg/kg, IP) of the D1 receptor antagonist SCH 23390 decreased lever presses rewarded by the visual stimulus with or without intra-MR baclofen. This dose of SCH 23390 is not sufficient to cause motor incapacity (Sanger 1987), and therefore, the decreased VS seeking can most likely be attributed to a decrease in motivational processes. It appears that systemic administration of nicotine also influences approach drive. Whereas response-contingent presentation of visual stimuli or intravenous administration of nicotine alone only supports low responding, the combination of visual stimulus presentation and nicotine administration synergistically increases responding (Chaudhri et al. 2006; Donny et al. 2003; Palmatier et al. 2006). Although these data have been primarily discussed from the point of view of nicotine’s role in tobacco addiction, they complement the notion that the approach drive is generated by extensive structures utilizing a number of neurotransmitter systems.
The approach drive perspective offers possible explanations for other aspects of the present findings as well. For example, the data show that during baclofen administration, an increase in responses on the active lever is accompanied by a proportional increase in responses on the inactive lever. In order to explain this, we must first emphasize that the approach drive energizes a flexible category of behaviors or investigatory tendencies rather than a fixed behavior. This behavior is guided by environmental stimuli with positive valence (i.e., having an attractive quality rather than being aversive or neutral). Different environmental stimuli have different strengths of positive (or negative) valence: the stronger the positive valence, the more vigorous the approach behavior. Therefore, the approach drive facilitates flexible, general investigatory tendencies, without terminal goals such as food intake. Intra-MR baclofen combined with the availability of visual stimuli may have increased general investigatory tendencies, thereby responses on the inactive lever as well as locomotor activity.
The same principle applies to the Wirtshafter et al. (1993) finding that intra-MR baclofen (63–500 ng) increases locomotor activity in a large hole-board chamber several fold greater than control injections. In the present study, however, intra-MR baclofen (169 ng) only slightly increased general activity in the absence of visual stimulus availability. According to the approach drive perspective, locomotor activity should be facilitated by intra-MR baclofen in a large chamber equipped with many investigatory objects to visit (e.g., nose-poke holes), whereas in a small environment lacking salient objects to explore, locomotion is not facilitated by intra-MR baclofen.
Although the activation of approach drive processes appears to increase measures of general locomotor activity, we have reason to believe that measures of general locomotor activity can be influenced by a multitude of behavioral processes; therefore, an increase in these measures does not necessarily indicate the presence of approach processes. Previous studies in rats have shown that the effects of systemic amphetamine on locomotor activity peak around 3 mg/kg and decrease with higher doses (Fray et al. 1980; Kelley et al. 1986). Using a rotation device identical to the one used in the present study to measure general locomotor activity, our previous studies have shown that doses (0.3–3 mg/kg) of amphetamine progressively increase locomotor activity (Shin et al. 2010). A 3-mg/kg amphetamine dose markedly increases locomotor activity while decreasing VS seeking, an effect replicated in the present study. A 1-mg/kg amphetamine dose increases both locomotion and VS seeking; however, the increase in VS seeking is modest. Injections of amphetamine directly into the ventral striatum markedly increase VS seeking but only moderately increase locomotor activity. These data seem to suggest that locomotor activity and VS seeking are dissociable. Although both the 3-mg/kg amphetamine dose and intra-MR baclofen increased locomotor activity, the brain processes resulting from intra-raphe baclofen appear to be qualitatively different from those following a high dose of systemic amphetamine.
Evidence suggests that approach behavior and consummatory behavior (feeding and drinking) are generated by overlapping, yet distinct, circuitries. Consider the following differences between baclofen administration into the MR or DR and amphetamine administration into the ventral striatum: baclofen administration into the MR or DR increases approach drive (present study) and increases feeding and drinking (Wirtshafter et al. 1993) at the same dose range. Although amphetamine injections into the ventral regions of the striatum can increase food intake (Evans and Vaccarino 1990), this effect of amphetamine may depend on the precise location of injections. Amphetamine administration into the ventral striatum most effectively increases approach behaviors than that into other striatal regions (Shin et al. 2010; Taylor and Robbins 1984), whereas amphetamine into the ventrolateral striatum more effectively increases food intake than the ventral striatum (Kelley et al. 1989). In addition, systemic nicotine positively influences approach drive (see paragraph 2 of this Discussion section), but suppresses appetite.2
Our results complement the tug-of-war hypothesis that is modified from similar notions emphasizing serotonin and the DR (Daw et al. 2002; Deakin 1983; Kapur and Remington 1996). The tug-of-war hypothesis suggests that the MR–DR system and the mesolimbic dopamine system play functionally opposing roles. Previous studies have found that behavioral responses energized by the activation of the mesolimbic dopamine system can be further energized or oppositely inhibited by respective inhibition or activation of the MR–DR system (Fletcher 1996, 1995; Fletcher and Korth 1999; Fletcher et al. 1999). However, the tug-of-war hypothesis fails to account for the lack of feeding and drinking effects upon the activation of the mesolimbic dopamine system, whereas intra-MR or DR baclofen readily increases such behaviors (Wirtshafter et al., 1993). Thus, this hypothesis appears to be limited to approach-type, but not consummatory, responses.
Our behavioral findings on the effects of intra-MR baclofen may be attributable to the inhibition of serotonergic neurons; however, available evidence is circumstantial and inconsistent. In the present study, we confirm that baclofen’s effects were mediated through GABAB receptors. Because GABAB receptors appear to be selectively localized on serotonergic neurons (Varga et al. 2002; Wirtshafter and Sheppard 2001), intra-MR injections of baclofen may have selectively inhibited serotonin neurons. However, intra-MR baclofen failed to decrease serotonin levels in the hippocampus, while intra-MR muscimol or 8-OHDPAT decreased them (Shim et al. 1997); moreover, locomotor activity induced by intra-MR baclofen was unaltered in rats treated with the serotonin synthesis inhibitor p-chlorophenylalanine (Wirtshafter et al. 1993). The action of baclofen in the DR is also complex. Baclofen injections into the DR could lead to activation, instead of inhibition, of serotonin neurons in the DR (Takahashi et al. 2010). This activation is presumably mediated by baclofen’s action on GABAergic afferents (Abellán et al. 2000) and appears to be concentration dependent; higher concentrations can inhibit serotonergic neurons (Abellán et al. 2000). Although the inhibition of serotonergic neurons appears to play a role in the enhancement of seeking rewarded by conditioned stimuli (Fletcher et al. 1999), it is unclear whether the inhibition of serotonergic neurons was involved in visual stimulus seeking facilitated by baclofen administration into the MR or DR.
In summary, our results confirm the hypothesis that administration of the GABAB receptor agonist baclofen into the MR or DR increases investigatory responses rewarded by presentations of unconditioned visual stimuli. The present study shows that it is not necessary for sensory stimuli to be conditioned with classic primary rewards like food, to strongly control behavior. Rather, the intrinsic salience of sensory stimuli is sufficient to strongly modulate behavior under some states induced by such pharmacological manipulations as intra-raphe baclofen. These results support the predictions generated by the tug-of-war hypothesis as well as the approach drive theory of drug reward.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The authors thank the NIH Fellows Editorial Board for editorial assistance.
Footnotes
Drive is shorthand for central coordinating process of perceptual, visceral, and motor functions for energizing voluntary behavior.
Nicotine’s appetite suppressant effect appears to be mediated by different mechanisms from those that are reward-related (Mineur et al. 2011).
We declare that we do not have any financial conflict of interest and that the experiments comply with the current laws of the USA in which they were performed.
Contributor Information
Fiori R. Vollrath-Smith, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services, Baltimore, MD 21224, USA
Rick Shin, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services, Baltimore, MD 21224, USA.
Satoshi Ikemoto, Email: sikemoto@intra.nida.nih.gov, Behavioral Neuroscience Branch, National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services, Baltimore, MD 21224, USA, National Institute on Drug Abuse, 251 Bayview Boulevard, Suite 200, Baltimore, MD 21224, USA.
References
- Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABA(B) receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning or incentive salience? Brian Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Deakin JFW. Roles of serotonergic systems in escape, avoidance and other behaviour. In: Cooper SJ, editor. Theory in psychopharmacology. Academic; London: 1983. pp. 149–193. [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev. 1990;14:9–22. doi: 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. Effects of d-fenfluramine and metergoline on responding for conditioned reward and the response potentiating effect of nucleus accumbens d-amphetamine. Psychopharmacology. 1995;118:155–163. doi: 10.1007/BF02245834. [DOI] [PubMed] [Google Scholar]
- Fletcher P. Injection of 5-HT into the nucleus accumbens reduces the effects of d-amphetamine on responding for conditioned reward. Psychopharmacology. 1996;126:62–69. doi: 10.1007/BF02246412. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM. RU-24969 disrupts d-amphetamine self-administration and responding for conditioned reward via stimulation of 5-HT1B receptors. Behav Pharmacol. 1999;10:183–193. doi: 10.1097/00008877-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Chambers JW. Selective destruction of brain serotonin neurons by 5,7-dihydroxytryptamine increases responding for a conditioned reward. Psychopharmacology. 1999;147:291–299. doi: 10.1007/s002130051170. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology. 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psych. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology. 1991;103:187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Winnock M, Stinus L. Amphetamine, apomorphine and investigatory behavior in the rat: analysis of the structure and pattern of responses. Psychopharmacology. 1986;88:66–74. doi: 10.1007/BF00310515. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine micro-injections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res. 1989;35:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kish GB. Studies of sensory reinforcement. In: Honing WK, editor. Operant behavior: areas of research and application. Appleton-Century-Crofts; New York: 1966. pp. 109–159. [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology. 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Ikemoto S. The midbrain raphe nuclei mediate primary reinforcement via GABA(A) receptors. Eur J Neurosci. 2007;25:735–743. doi: 10.1111/j.1460-9568.2007.05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JH. Handbook of biological statistics. 2. Sparky House; Baltimore: 2009. [Google Scholar]
- Mineur Y, Abizaid A, Rao Y, Salas R, DiLeone R, Gndisch D, Diano S, De Biasi M, Horvath T, Gao X-B, Picciotto M. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332:1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier M, Evans-Martin F, Hoffman A, Caggiula A, Chaudhri N, Donny E, Liu X, Booth S, Gharib M, Craven L, Sved A. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: the foundations of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Elsevier; Burlington: 2005. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. The actions of SCH 23390, a D1 receptor antagonist, on operant and avoidance behavior in rats. Pharmacol Biochem Behav. 1987;26:509–513. doi: 10.1016/0091-3057(87)90157-2. [DOI] [PubMed] [Google Scholar]
- Shim I, Javaid J, Wirtshafter D. Dissociation of hippocampal serotonin release and locomotor activity following pharmacological manipulations of the median raphe nucleus. Behav Brain Res. 1997;89:191–198. doi: 10.1016/s0166-4328(97)00060-0. [DOI] [PubMed] [Google Scholar]
- Shin R, Ikemoto S. The GABA(B) receptor agonist baclofen administered into the median and dorsal raphe nuclei is rewarding as shown by intracranial self-administration and conditioned place preference in rats. Psychopharmacology. 2010;208:545–554. doi: 10.1007/s00213-009-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PLoS One. 2010;5:e8741. doi: 10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioral control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Varga V, Sik A, Freund TF, Kocsis B. GABA(B) receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience. 2002;109:119–132. doi: 10.1016/s0306-4522(01)00448-1. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Sheppard AC. Localization of GABAB receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR, Pitzer MR. Studies on the behavioral activation produced by stimulation of GABAB receptors in the median raphe nucleus. Behav Brain Res. 1993;59:83–93. doi: 10.1016/0166-4328(93)90154-i. [DOI] [PubMed] [Google Scholar]