Abstract
Background
Eribulin mesylate is a tubulin inhibitor with activity superior to paclitaxel in NIH:OVCAR-3 human epithelial ovarian cancer xenograft models. We sought to assess the efficacy of eribulin in platinum-resistant and platinum-sensitive recurrent ovarian cancer.
Methods
Patients with recurrent measurable epithelial ovarian cancer, ≤2 prior cytotoxic regimens, and adequate organ function were enrolled into two separate cohorts: 1) Platinum resistant (progression-free interval from last platinum-based therapy <6 months); and 2) Platinum sensitive (progression-free interval from last platinum-based therapy ≥6 months). Treatment: Eribulin 1.4 mg/m2 over 15 minutes by vein on days 1 and 8, every 21 days. Efficacy was determined by objective response by computed tomography.
Results
Platinum-resistant cohort: Thirty-seven patients enrolled. Thirty-six patients were evaluable for response and toxicity. Two patients achieved partial response (PR, 5.5%). Sixteen (44%) had a best response of stable disease. Median progression-free survival was 1.8 months (95% confidence interval, 1.4–2.8 months). Platinum-sensitive cohort: Thirty-seven patients enrolled, and all were evaluable for response. Seven patients achieved partial response (PR, 19%). Median progression-free survival was 4.1 months (95% confidence interval, 2.8–5.8 months). The major toxicity was grade 3 or 4 neutropenia (42% in platinum-resistant patients; 54% in platinum-sensitive patients).
Conclusions
Eribulin achieved objective response in 5.5% of women with platinum-resistant recurrent ovarian cancer and in 19% of women with platinum-sensitive disease. Median progression-free survival was 1.8 months in the platinum-resistant group and 4.1 months in the platinum-sensitive group.
Keywords: eribulin, ovarian cancer, phase II, recurrence
INTRODUCTION
In 2010, epithelial ovarian cancer was diagnosed in approximately 21,880 women in the U.S., and approximately 13,850 American women died from this disease.1 Nearly 75% of women with epithelial ovarian cancer have stage III or IV disease at the time of diagnosis. While greater than 80% of patients with advanced-stage ovarian cancer will demonstrate a clinical response to first-line platinum-based chemotherapy, the majority will ultimately succumb to their disease, with 5-year overall survival rates of 5–30%.2,3
For patients with disease that progresses on first-line therapy (primary refractory disease) and patients whose disease progresses within the first 6–12 months of completing first-line platinum-based chemotherapy, the efficacy of further chemotherapy is poor. While a number of agents with some evidence of activity have been identified in phase II trials, responses (generally partial responses at best) are observed in approximately 15–30% of women (depending on the platinum sensitivity of the population). Responses in platinum-resistant patients are seen in only approximately 15% of patients, and duration of response is only approximately 4 months.
Eribulin mesylate (E7389, halichondrin B) is a tubulin inhibitor whose mechanism of action differs from that of other anti-tubulin agents, effecting cell cycle block at G2/M, disruption of mitotic spindle formation, and initiation of apoptosis.4 Eribulin mesylate appears to have a tubulin interaction mechanism that differs from that of paclitaxel. In in vivo studies using NIH:OVCAR-3 human ovarian cancer xenograft models, treatment with eribulin mesylate increased survival and reduced the size and number of metastases, with activity superior to that of paclitaxel.4,5
There is a clinical need for new, effective agents for the treatment of ovarian cancer, particularly for patients who have had prior taxane-platinum-based chemotherapy. Because the response rates to second-line treatment are generally higher among patients with platinum-sensitive disease, we designed this study to determine whether eribulin mesylate could achieve objective responses among patients with recurrent ovarian cancer, studied in two separate cohorts: platinum-resistant patients and platinum-sensitive patients.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients were 18 years or older and had histologically or cytologically confirmed epithelial ovarian, fallopian tube, or primary peritoneal cancer, measurable disease (defined by RECIST Version 1.0), no more than two prior therapies for this cancer, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Prior surgery, chemotherapy, and/or radiation must have been completed at least 4 weeks prior to enrollment (6 weeks for nitrosoureas or mitomycin C), and patients must have recovered from any prior treatment-related adverse events. Patients were required to have adequate bone marrow function (white blood count [WBC] ≥3,000/μL, absolute neutrophil count [ANC] ≥1,500/μL, and platelets ≥100,000/μL), renal function (creatinine within normal institutional limits), and hepatic function (total bilirubin within normal institutional limits, serum glutamic oxaloacetic transaminase [SGOT] and serum glutamic pyruvic transaminase [SGPT] ≤2.5 × institutional upper limit of normal). Eligible patients had to have a life expectancy of > 2 months, and the ability to understand and willingness to sign a written informed consent. Patients were not permitted to take concomitant anti-tumor hormonal therapy and/or investigational agents, and had to have discontinued any prior hormonal therapy at least 14 days before starting study treatment. Patients with another invasive malignancy within the past 5 years were excluded, except for patients with a history of stage IA or IB endometrial cancer (who were considered disease free from endometrial cancer) and patients with a history of non-melanoma skin cancer. Other exclusions included: patients with known brain metastases, patients who were pregnant or breastfeeding, patients with known human immunodeficiency virus (HIV), and patients with uncontrolled intercurrent illness including, but not limited to, ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements. Patients previously treated with eribulin or patients with a history of allergic reaction to compounds of similar chemical or biological composition of eribulin were ineligible. An effort was made to switch patients who were taking enzyme-inducing anticonvulsant agents and any other medications/substances known to affect (or with the potential to affect) the pharmacokinetics of eribulin to other medications before starting study treatment. Patients were assigned to the platinum-resistant cohort if the progression-free interval from last platinum-based therapy was < 6 months, and to the platinum-sensitive cohort if the progression-free interval from last platinum-based therapy was ≥ 6 months.
All patients signed written informed consent. The protocol was reviewed annually by the Institutional Review Boards/Privacy Boards of the participating institutions.
Treatment
Eribulin mesylate 1.4 mg/m2 was administered intravenously over 15 minutes on days 1 and 8, every 21 days (to accommodate scheduling difficulties, all day 1 treatments could be given 1 day early or up to 2 days late, provided all treatment parameters were met). On day 1 of each cycle the ANC was required to be ≥1000/μL and platelets ≥100,000/μL. On Day 8 of each cycle, eribulin was held if the ANC was <500/μLor the platelets were <50,000/μL. If the Day 8 eribulin was held for myelosuppression, the dose was not “made up”. Day 1 treatment delays were permitted for up to 2 weeks for recovery of ANC to ≥1000/μLand platelets to ≥100,000/μL. The use of granulocyte colony stimulating factors (GCSFs) was not allowed during cycle 1 of treatment, and prophylactic or routine use during subsequent cycles was not recommended. In the case of neutropenia without fever or febrile neutropenia, administration of GCSFs, per current ASCO guidelines, was allowed. If GCSFs were used in subsequent cycles, a 24-hour window before and after treatment with eribulin was recommended. The use of GCSFs between days 1 and 8 of treatment was not recommended. Dose reduction of eribulin to 1 mg/m2 for all subsequent cycles was required in the event of: febrile neutropenia; platelet count <10,000/μL; grade 4 neutropenia lasting ≥7 days; grade 3 or 4 elevations in SGOT, SGPT, or alkaline phosphatase; grade 3 or 4 stomatitis; or grade 2 or worse renal toxicity. Eribulin-related toxicities had to resolve to within eligibility parameters, within 2 weeks, before the next cycle could be given.
Treatment with eribulin was continued for as long as there was no evidence of disease progression, unacceptable toxicity, an intercurrent illness that prevented further treatment, patient withdrawal of consent from study, or any change in the patient’s condition that in the opinion of the investigator rendered the patient unable to continue safely on study.
Monitoring for toxicity and assessment of response
All patients had the following evaluations: physical examination, vital signs, assessment of performance status, weekly complete blood count and serum chemistries, serum CA125 on day 1 of each cycle, and ongoing assessment of adverse events and concurrent medications. For evaluation of response, all patients had computed tomography (CT) of chest/abdomen/pelvis every other cycle. Radiographic response was determined by RECIST Version 1.0,6 with the following definitions: Complete Response (CR): disappearance of all target lesions; Partial Response (PR): at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum of the LD; Progressive Disease (PD): at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions; Stable Disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started. Patients were considered to be evaluable by CA125 if the CA125 was >35 units/mL prior to the start of study treatment. Patients were considered to have had a CA125 response if there had been a serial decrease in CA125 of at least 50% over four samples or a serial decrease of at least 75% over three samples.7,8
Statistical considerations
The primary endpoint of the study was to determine the frequency of objective response to treatment with eribulin in patients with recurrent ovarian, fallopian tube, or peritoneal cancer. The study was designed to assess the objective response rate (CR+PR) separately for the following two cohorts: 1) patients with platinum-resistant disease (progression-free interval from last platinum-based therapy <6 months); and 2) patients with potentially platinum-sensitive disease (progression-free interval from last platinum-based therapy ≥6 months). The two cohorts were accrued and analyzed separately. Within each cohort, an optimal two-stage Simon design was utilized in which a 20% response rate was considered promising, and a 5% response rate was considered unacceptable.9 With both Type I error and Type II error set at 10%, 12 patients were to be initially enrolled and treated with eribulin, with enrollment expanded to 37 patients if at least one patient had an objective response (CR or PR) in each cohort.
The treatment regimen was considered as having demonstrated a promising level of single-agent activity in the respective cohort if at least 4 of 37 patients were shown to have objective responses. Within each cohort, this design yields at least a 90% probability of a positive result if the true response rate is at least 20%, and it yields a 90% probability of a negative result if the true response rate is 5%. Platinum-sensitive patients and platinum-resistant patients were analyzed separately. Descriptive statistics were provided on patient characteristics, toxicities, and responses. Objective response rates were calculated for each cohort. Additionally, the response rate and its one-sided 90% confidence intervals were estimated using an exact inference method that appropriately accounts for the two-stage design feature.10 The Kaplan-Meier method11 was used to estimate the progression-free survival. Analyses were performed using R package “clinfun” and SAS software (version 9.2, SAS Institute Inc., Cary, NC).12 Although not designated as a study endpoint, in response to reviewers, the “disease control rate” defined as the percentage of patients achieving CR or PR or SD, and its 90% confidence interval, was calculated for each cohort.
RESULTS
Patient demographics and response rates for both cohorts are summarized in Table 1. Toxicity results are summarized in Table 2. Responses, toxicities, and survival outcomes are detailed below for each cohort.
Table 1.
Demographics and treatment responses for platinum-resistant and platinum-sensitive ovarian cancer patients treated with eribulin
| Platinum Resistant | Platinum Sensitive | |
|---|---|---|
| Patients enrolled/evaluable | 37/36 | 37/37 |
| Median age (range) | 61 years (38–80) | 60 years (45–77) |
| Median platinum-free interval (range) | 3 months (0.1–5.9) | 10 months (6.5–45) |
| Median # prior regimens (range) | 2 (1–2) | 1.5 (1–2) |
| Median PS (range) | 0 (0–1) | 0 (0–1) |
| Median # cycles delivered | 2 (1–10) | 6 (1–51) |
| Partial response | 2/36 (5.5%) | 7/37 (19%) |
| Stable disease | 16/36 (44%) | 21/37 (57%) |
| CA125 response | 3/31 (9.6%) | 11/31 (35%) |
PS: Eastern Cooperative Oncology Group (ECOG) performance status
Table 2.
At least possibly treatment-related grade 3 and 4 toxicities for platinum-resistant and platinum-sensitive ovarian cancer patients treated with eribulin, per patient
| Platinum Resistant (n=36) | Platinum Sensitive (n= 37) | |
|---|---|---|
| Neutropenia | 15 (42%) | 20 (54%) |
| Febrile neutropenia | 1 (2.7%) | 0 |
| Leucopenia | 12 (33%) | 11 (30%) |
| Thrombocytopenia | 0 | 0 |
| Anemia (grade 3) | 1 (2.7%) | 0 |
| Lymphopenia | 1 (2.7%) | 2 (5.4%) |
| Thrombosis | 2 (5.5%) | 1 (2.7%) |
| Infection (pneumonia, urinary tract infection) | 2 (5.5%) | 0 |
| Pain (grade 3) | 0 | 3 (8%) |
| Hyperglycemia (grade 3) | 1 (2.7%) | 1 (2.7%) |
| Hypokalemia (grade 3) | 2 (5.5%) | 0 |
| Hyponatremia (grade 3) | 0 | 1 (2.7%) |
| Hypophosphatemia (grade 3) | 0 | 1 (2.7%) |
| Hypomagnesemia (grade 3) | 0 | 1 (2.7%) |
| Generalized muscle weakness (grade 3) | 1 (2.7%) | 2 (5.4%) |
| Elevated SGOT/SGPT (grade 3) | 0 | 2 (5.4%) |
| Diarrhea (grade 3) | 0 | 1 (2.7%) |
| Ataxia (grade 3) | 0 | 1 (2.7%) |
SGOT: serum glutamic oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase
Platinum-resistant cohort
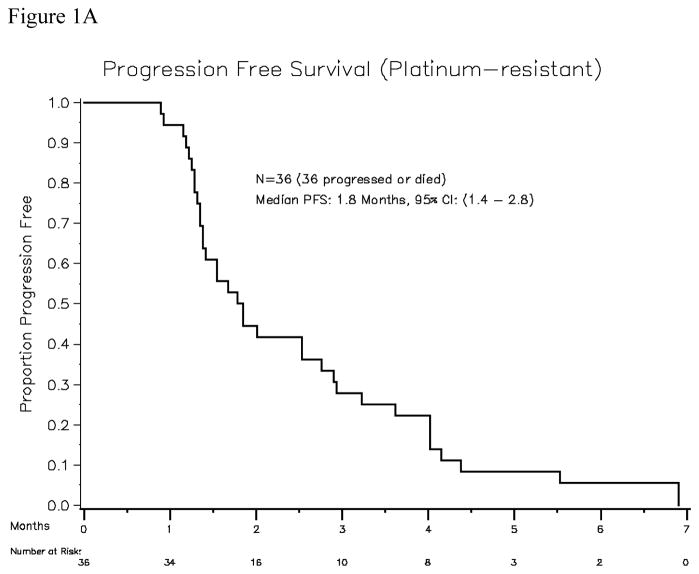
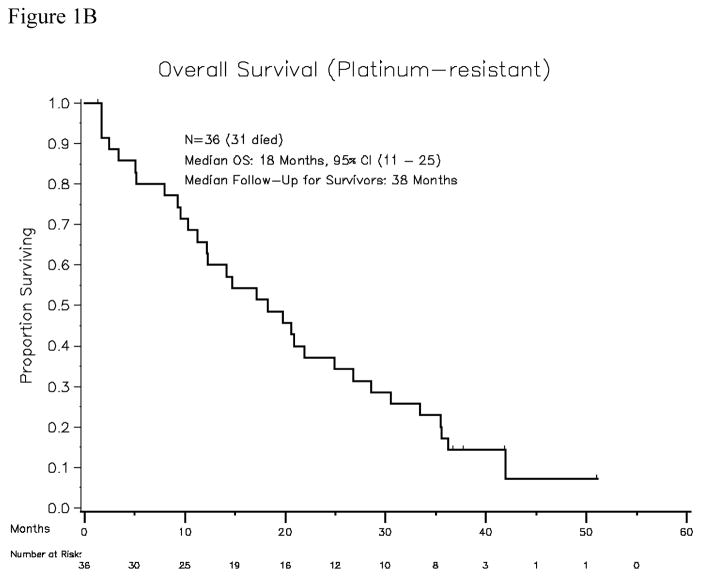
Thirty-seven patients were accrued, of which 36 were evaluable for response and toxicity (1 patient was removed from study prior to initiating study treatment due to declining performance status). All patients had received platinum-taxane first-line treatment (carboplatin or cisplatin plus paclitaxel in 34 patients, docetaxel in 2 patients), and had a median platinum-free interval of 3 months (range, 0.1–5.9). Thirty patients had received second-line treatment: five were re-treated with paclitaxel plus carboplatin, ten received gemcitabine plus carboplatin, ten received liposomal doxorubicin, two received carboplatin plus pemetrexed, one received carboplatin plus liposomal doxorubicin, one received single-agent carboplatin, and one received topotecan. In addition, two patients had received maintenance paclitaxel after achieving complete clinical remission with first-line treatment. The median age was 61 years (range, 38–80), and the median ECOG performance status was 0. Among the 36 evaluable patients, the median number of cycles delivered per patient was 2 (range, 1–10). Two patients achieved partial response (PR, 5.5%). Calculating the response rate using a bias-correcting estimate yields an objective response rate of 9.9%, with the lower bound of the one-sided 90% CI being 2%. The response durations of these two partial responses were 84 days and 128 days. The time to achieve objective PR was 2.8 months in both patients. Sixteen patients had best response of stable disease (SD, 44%). Thirty-one patients had serum CA125 values that were evaluable for response. Only 3 (9.6%) of 31 had a CA125 response. Using a definition of CR + PR + SD to define the “disease control rate”, 18 of 36 patients would be considered to have disease control (50%, with 90% confidence interval 38–100%). However, the median progression-free survival was only 1.8 months (95% CI, 1.4–2.8 months). Median overall survival among platinum-resistant patients was 18 months (95% CI, 11–25 months) (Figures 1A and 1B).
Figure 1.
Figure 1A. Progression-free survival for the platinum-resistant patient cohort (N=36)
Figure 1B. Overall survival for the platinum-resistant patient cohort (N=36)
Per patient, grade 3 or 4, at least possibly treatment-related adverse events included: neutropenia 42%, leucopenia 33%, anemia 2.7%, lymphopenia 2.7%, febrile neutropenia 2.7%, infection 5.5%, thrombosis 5.5%, hypokalemia 5.5%, hyperglycemia 2.7%, and generalized muscle weakness 2.7%.
Platinum-sensitive cohort
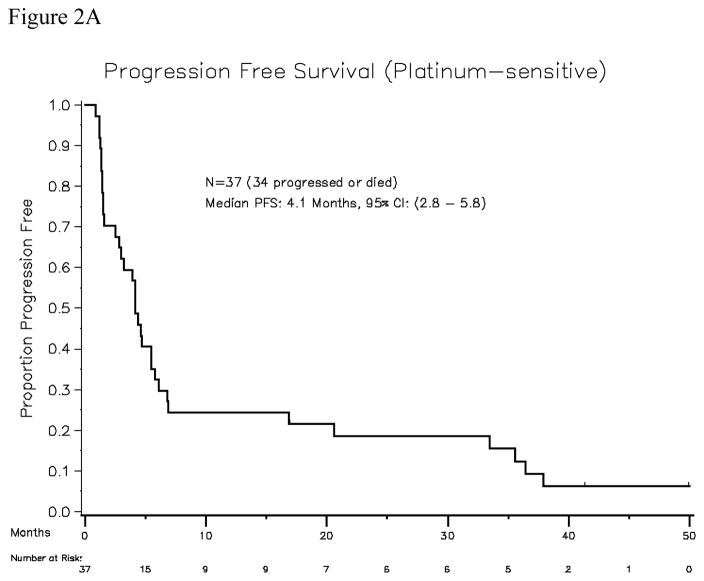
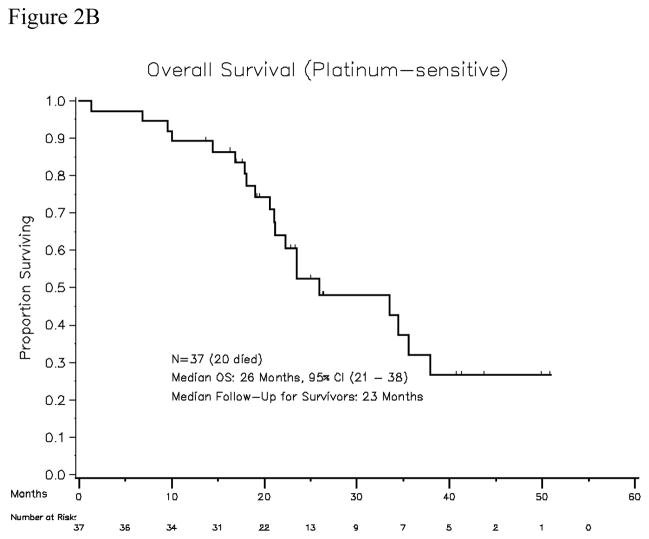
Thirty-seven patients were accrued, of which all were evaluable for response. The median platinum-free interval was 10 months (range, 6.5–45). All patients had received platinum (carboplatin or cisplatin) plus taxane-based first line treatment (36 received paclitaxel, 1 received docetaxel). Sixteen patients had received second-line therapy: six patients were re-treated with paclitaxel plus carboplatin; six patients were treated with gemcitabine plus carboplatin, one received oxaliplatin plus gemcitabine, one received carboplatin plus pemetrexed, one received liposomal doxorubicin, and one received bevacizumab. In addition, two patients in the platinum-sensitive cohort had been treated with maintenance paclitaxel after achieving first complete clinical remission. The median age of the patients in the platinum-sensitive cohort was 60 years (range, 45–77), and the median ECOG performance status was 0. The median number of cycles delivered was 6 per patient (range, 1–51). Seven of 37 evaluable patients achieved objective response (PR, 19%), with no patient achieving a complete response. Calculating the response rate using a bias-correcting estimate yields an objective response rate of 19.8%, with the lower bound of the one-sided 90% CI being 11%. Twenty-one patients had a best response of stable disease (SD, 57%). Thirty-one patients had serum CA125 values that were evaluable, and 11 patients (35%) had CA125 responses. The “disease control rate” (CR + PR+ SD) was 28 of 37 patients (76%, with 90% confidence interval 61–100% ). Response durations for the 7 patients who achieved objective response are shown in Table 3. Median progression-free survival was 4.1 months (95% CI, 2.8–5.8 months). Median overall survival among platinum-sensitive patients was 26 months (95% CI, 21–38 months) (Figures 2A and 2B).
Table 3.
Response durations for the 7 patients in platinum-sensitive cohort who achieved objective response
patient came off study due to excessive toxicity or withdrawal of consent, but had no evidence of progression of disease.
Figure 2.
Figure 2A. Progression-free survival for the platinum-sensitive patient cohort (N=37)
Figure 2B. Overall survival for platinum-sensitive patient cohort (N=37)
Per patient, grade 3 or 4, at least possibly treatment-related adverse events included: neutropenia 54%, leucopenia 30%, lymphopenia 5.4%, pain 8%, generalized muscle weakness 5.4%, elevated liver enzymes 5.4%, thrombosis 2.7%, hyperglycemia 2.7%, hyponatremia 2.7%, hypophosphatemia 2.7%, hypomagnesemia 2.7%, diarrhea 2.7%, and ataxia 2.7%.
DISCUSSION
This study was designed with two separate cohorts of patients with recurrent ovarian cancer in order to determine the activity of eribulin in platinum-resistant and platinum-sensitive patients. Objective response was observed in only 5.5% of platinum-resistant patients; in contrast, 19% of platinum-sensitive patients achieved objective response. A similar contrast in sensitivity to non-platinum chemotherapy agents has been reported with most other agents tested in ovarian cancer. In a large phase III trial, liposomal doxorubicin was compared to topotecan, with patients stratified as platinum resistant or platinum sensitive prior to randomization. Objective response rates did not differ substantially between the two drugs, but they were higher in the platinum-sensitive patients (liposomal doxorubicin, 28%; topotecan, 28%) than in the platinum-resistant patients (liposomal doxorubicin, 12.3%; topotecan, 6.5%).13 In a phase III trial comparing gemcitabine to liposomal doxorubicin in platinum-resistant ovarian cancer patients, objective responses were observed in 9.2% of patients assigned to gemcitabine and in 11.7% of patients assigned to liposomal doxorubicin.14 In a single-arm phase II trial of liposomal doxorubicin in platinum-resistant patients, 9.9% of patients had objective response15; however, an earlier trial reported an 18% response rate in a platinum- and taxane-resistant population.16 While the observed response rate of 19% among platinum-sensitive ovarian cancer patients makes eribulin a promising new agent for this population, effective treatment for platinum-resistant ovarian cancer remains a significant therapeutic challenge.
The treatment schedule of eribulin is convenient, with a short infusion time and no required steroid pre-medications. Neutropenia was the most common toxicity, although neutropenic fever events were rare (2.7% of patients in the platinum-resistant cohort and no events in the platinum-sensitive cohort). Patients in this study had received only one or two prior regimens. Subsequent to the design and conduct of this study for patients with ovarian cancer, eribulin was approved by the Food and Drug Administration for the treatment of metastatic breast cancer based on results from a randomized phase III trial.17 Prolonged neutropenia and neuropathy were both more common in the breast cancer studies18 than they were in this ovarian cancer study, perhaps because patients in the breast cancer studies were much more heavily pre-treated.
Interestingly, in the breast cancer studies, eribulin achieved high objective response rates despite the fact that most patients had received prior taxane therapy. We observed a similar result in our study: all patients enrolled had received prior platinum and all had received prior taxane, yet objective responses were observed despite prior taxane therapy.
There has been increasing focus on progression-free survival, rather than objective response rate, as a measure of drug efficacy, particularly for clinical trials involving targeted therapeutic agents.19 In this two-cohort phase II study, progression-free survival was a secondary objective. Among patients in the platinum-resistant cohort, progression-free survival did not appear to be prolonged by treatment with eribulin (median progression-free survival, 1.8 months). In the platinum-sensitive cohort, progression-free survival was 4.1 months, which is similar to what has been observed with other agents with activity in platinum-sensitive ovarian cancer. Some studies have used “disease control rate” as a measure of treatment efficacy. Although this study was not designed with disease control rate as an endpoint, in response to manuscript reviewers, we have reported these results. The disease-control rate in the platinum-sensitive cohort is high (76%) which mirrors the relatively high objective response rate. Although the observed disease control rate in the platinum-resistant cohort appears favorable (50%), the wide confidence interval and the very short progression-free survival of 1.8 months support the conclusion that eribulin is not active in the platinum-resistant population.
This study is the first to demonstrate the efficacy of eribulin in recurrent ovarian cancer. We show that eribulin is a novel and active agent for patients with platinum-sensitive recurrent disease; and that eribulin is inactive in platinum-resistant disease.
Acknowledgments
Funding for this study was provided by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute, Clinicaltrials.gov registration #NCT00334893.
Footnotes
Results of this study were presented, in part, at the American Society of Clinical Oncology meeting.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefoi H, A’Hern RP, Fisher C, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol. 1999;17:767–775. doi: 10.1200/JCO.1999.17.3.767. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.CTEP Rapid Communication. Solicitation for Letters of Intent: E7389, Halichondrin B Analog (NSC 707389) 2005. [Google Scholar]
- 5.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Rustin GJ, Nelstrop AE, McClean P, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996;5:1545–1551. doi: 10.1200/JCO.1996.14.5.1545. [DOI] [PubMed] [Google Scholar]
- 8.Rustin GJ, Bast RC, Jr, Kelloff GJ, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin Cancer Res. 2004;10:3919–3926. doi: 10.1158/1078-0432.CCR-03-0787. [DOI] [PubMed] [Google Scholar]
- 9.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 12.Seshan VE. clinfun: Clinical Trial Design and Data Analysis Functions. R package version 0.9.6. 2010 http://CRAN.R-project.org/package=clinfun.
- 13.Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 14.Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 15.Markman M, Kennedy A, Webster K, et al. Phase 2 trial of liposomal doxorubicin (40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000;78(3 Pt 1):369–372. doi: 10.1006/gyno.2000.5921. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 17.Twelves C, Loesch D, Blum JL, et al. on behalf of the Study 305 investigators. A phase III study (EMBRACE) of eribulin mesylate versus treatment of physician’s choice in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:18s. (suppl; abstr CRA1004) [Google Scholar]
- 18.Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 19.Sabbatini P, Spriggs D, Aghajanian C, et al. Consolidation strategies in ovarian cancer: observations for future clinical trials. Gynecol Oncol. 2010;116:66–71. doi: 10.1016/j.ygyno.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]