Abstract
Aims
Erythropoietin stimulating agents (ESAs) is an active area of clinical investigation in heart failure (HF) but can cause hypertension and higher hemoglobin concentrations (Hb) that have been associated with adverse outcomes. We evaluated a dosing algorithm and potential confounders’ effect on Hb and BP in a clinical trial.
Methods
In an ongoing randomized, placebo controlled, single blind clinical trial of ESA (Epoetin alfa) in anemic patients with heart failure and a preserved ejection fraction (HFPEF), Hb was measured weekly as was BP, weight and concomitant medical therapy. A repeated measure mixed model evaluated determinants of weekly changes in Hb and BP.
Results
Among 45 subjects (78±11 years, 67% women, EF=57±9%) with a total of 780 repeated weekly Hb measures, Hb significantly increased over time in those assigned to ESA (β= 0.933, p< 0.0001), compared to placebo. Dose (β = −0.108, p<0.0001), patient weight (β = −0.016, p=0.0037), diuretic use (β = −0.124, p=0.0389), and time (β = 0.003, p=0.0331), were all significantly associated with Hb change. Increased diuretic dose and weight change were significantly inversely associated with changes in Hb. ESA administration and dose were not significant determinants of absolute BP or changes in BP from baseline.
Discussion
In addition to ESA dose and duration of therapy, factors indicative of volume status including weight and diuretic use are determinants of hemoglobin levels in HF subjects.
Conclusion
The currently employed dosing algorithm which adjusts the administration of ESA based on the absolute hemoglobin and weekly change in hemoglobin increases Hb with relatively a low weekly dose of ESA without significant effects on blood pressure.
Introduction
Anemia is common in patients with heart failure irrespective of ejection fraction,(1) and associated with increased morbidity and mortality (2, 3). Erythropoietin stimulating agents (ESAs) are an active area of clinical investigation in heart failure (HF).(4–6) However, higher hemoglobin concentrations (Hb) have been associated with adverse outcomes in populations other than heart failure.(7, 8) A secondary analysis of one dataset suggests that the excess risk attributable to ESAs were not seen in patients with heart failure nor those who achieved a target hemoglobin(9), though significant concerns about such therapy are still raised.(5, 10)
Among the factors that could contribute to the excess risk associated with ESA include the dose of the ESA, the rapidity of the rise in and increases in blood pressure, among others.(10) Of note, the principle proposed uses of ESAs for management of anemia is for patients that also have conditions associated with alterations in plasma volume, including renal failure and heart failure. In addition to ESA dose and pharmacological effect, changes in volume status arising from routine medical therapy for HF can cause alterations in Hb and effect blood pressure.
As part of an ongoing controlled clinical trial, we evaluated a structured ESA dosing algorithm and it’s effects on surrogates of volume status including Hb, blood volume, and blood pressure, in order to monitor and evaluate safety and efficacy. Accordingly, the purposes of this study were to: (1) determine the efficacy (active vs. placebo) of the dosing algorithm for raising hemoglobin values and the effect on blood volume components, (2) characterize the dose of epoetin alfa employed and the time course of the response, (3) identify relevant confounders of hemoglobin changes in the heart failure population, and (4) evaluate the safety of the dosing algorithm specifically focusing on changes in blood pressure and hemoglobin.
Methods
Study Design
This is an analysis of an ongoing prospective, randomized, single blind, twenty-four week study (NCT00286182) among community dwelling, independently living, older adult patients with anemia and heart failure with a preserved ejection fraction. The diagnosis of heart failure was based on the NHANES CHF Criteria with a score ≥ 3(11) and study participants were considered to have a preserved ejection fraction if three-dimensional echocardiographically determined ejection fraction was >40%. Anemia was defined as hemoglobin < 12 g/dL(12). Informed consent was obtained in all subjects. The study was approved by the Columbia University Medical Center IRB.
Patients were excluded from the study if they had uncontrolled hypertension (SBP >160 mmHg and/or DBP > 90 mmHg, resting heart rate >120 bpm, baseline 6 minute walk > 450 meters, valvular heart disease greater than mild stenotic or greater than moderate regurgitant lesions by transthoracic echocardiography, infiltrative cardiac disease such as hemochromatosis and amyloidosis, hypertrophic cardiomyopathy, chronic pulmonary disease (FEV1 < 60% predicted), renal failure (GFR < 15 mL/min), hemoglobin < 8 g/dL, exercise limited by angina, claudication or neurological diseases, severe liver dysfunction, cardiac surgery less than 3 months prior, known iron deficiency anemia from chronic blood loss, significant alcohol or illicit drug use, known hypercoagulable state or an active hematologic disease. Patients were also excluded if they had a history of deep venous thrombosis or pulmonary embolus within 12 months before study entry, had a history of CVA or TIA within 6 months, or an acute coronary syndrome within 6 months of study entry, had an allergy or sensitivity to human serum albumin, or had a known hypersensitivity to mammalian cell-derived products.
The primary endpoint of this ongoing clinical trial is the change in end diastolic volume as measured by three dimensional echocardiography, while secondary endpoints include submaximal exercise capacity as assessed by six minute walk, maximal exercise capacity as assessed by cardiopulmonary exercise testing with cycle ergometry, NYHA class and quality of life as assessed by the KCCQ and Minnesota Living with Heart Failure Questionnaire.
Randomization and Blinding
Randomization was 1:1 to either epoetin alfa or placebo. To limit chance imbalances in a trial of this size and because the differences in hemoglobin levels between genders and the strong association of renal function with hemoglobin levels, randomization was stratified by gender and baseline renal function (dichotomized by cut point of an estimated Creatinine clearance of 40 ml/min). Study subjects were blinded to treatment assignment, which was maintained throughout the study, by providing weekly injections of epoetin alfa or placebo in unmarked syringes. While study subjects were unaware of treatment assignment, study personnel administering therapy were aware of treatment assignment but endpoints were performed by study personnel blinded to the result of randomization (e.g. prospective, randomized, single blinded endpoint assessment).
Study drug administration and dosing
Epogen (Epoetin alpha, Ortho Biotec, Inc) was administered weekly by subcutaneous injection using a pre-specified dosing algorithm.(13) The dosing algorithm (Table 1) was designed to make adjustments based on the rate of rise (ROR) of the hemoglobin over a one week period, as well as the absolute hemoglobin value. All subjects randomized to active treatment initially received 7,500 units of erythropoietin given weekly by subcutaneous injection, while placebo subjects received the same injection volume of normal saline. Placebo injection volumes were changed based on study algorithm during the study, to give the appearance that dose adjustments were occurring in the placebo group. Subjects were carefully monitored (e.g. every week) when beginning therapy to avoid rapid increases in hemoglobin, which was measured by a point of care system (Hemocue Inc. Sweeden) and/or increases in blood pressure, measured by a automated cuff sphygmomanometer (Omron, Kyoko City, Japan). No dose adjustments were made for the first three doses of erythropoietin (7,500 units/week) unless the hemoglobin rose too rapidly (greater than 0.4 g/dL) in any given weekly interval.
Table 1.
Dosing Algorithm
| Most Recent Hemoglobin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10.5 | 10.5 – 11.0 | 11.1 – 11.5 | 11.6 – 12.0 | 12.1 – 12.5 | 12.6 – 13.0 | 13.1 – 13.5 | 13.6 – 14.0 | > 14.0 | ||
| ROR/ week | −0.3 | +50% | +50% | +50% | +25% | +25% | +25% | NC | Hold° | Hold* |
| −0.2 | +50% | +50% | +50% | +25% | +25% | NC | NC | Hold° | Hold* | |
| −0.1 | +50% | +50% | +50% | +25% | +25% | NC | NC | Hold° | Hold* | |
| 0 | +50% | +50% | +50% | +25% | +25% | NC | −25% | Hold° | Hold* | |
| 0.1 | +50% | +50% | +25% | +25% | NC | NC | −50% | Hold° | Hold* | |
| 0.2 | +50% | +25% | +25% | +25% | NC | NC | −50% | Hold° | Hold* | |
| 0.3 | NC | NC | NC | NC | NC | −25% | Hold° | Hold° | Hold* | |
| 0.4 | NC | NC | NC | NC | −25% | Hold* | Hold* | Hold* | Hold* | |
| 0.5 | Hold+ | Hold+ | Hold+ | Hold+ | Hold+ | Hold+ | Hold+ | Hold* | Hold* | |
Hold dose and check weekly hemoglobins and reduce dose by 50% when ROR is 0 and Hgb is 12.6–13.5.
Hold dose and check weekly hemoglobins and reduce dose by 50% when ROR is <0.4 gm/dl
Hold dose and check weekly hemoglobins and reduce dose by 25% when ROR is 0 gm/dl and Hgb is 12.6–13.5
Blood volume analysis
Blood volume was determined at baseline and after 24 weeks of therapy in a subset of subjects by intravenous administration of iodine131–labeled albumin (Volumex, Daxor Corp., New York City, New York) as previously described(14). Plasma volume was determined as the zero-time volume of distribution of the radiolabeled albumin obtained by semilogarithmic extrapolation of values measured from at least 3 samples drawn twelve minutes after injection at 6-minute intervals. Plasma radioactivity of each sample was measured in a semi-automated counter (BVA-100 Blood Volume Analyzer, Daxor Corp). Blood volume and red blood cell volumes were calculated from the plasma volume measurement and then compared with normal values for age, gender, height, and weight based on the subject’s ideal weight(15).
Statistical analyses
SAS for Windows (Version 9.1.3, SAS Institute Inc., Cary, North Carolina) was used for all analyses. Results are expressed as mean ± standard deviation unless otherwise noted. We used a repeated measure mixed model (PROC MIXED) to evaluate factors associated with weekly changes in hemoglobin and blood pressure. Each subject’s baseline level (intercept) was entered as random effect in the model, which accounted for the variability of the baseline levels between subjects. In the first model evaluating absolute hemoglobin and hemoglobin change from baseline we included ESA administration, ESA dose and duration of therapy as well as surrogates of volume status including changes in weight recorded weekly and weekly increases in diuretic dose. All interactions tested were not significant and were excluded from the model. In the modeling of blood pressure, we examined separately the steady and pulsatile components, respectively, by modeling both mean arterial pressure (MAP) and pulse pressure (PP). In this model, we included ESA administration, ESA dose and duration of therapy along with increases in antihypertensive medications and the interaction of increases in antihypertensive therapy and time, in order to determine if ESA increased blood pressure and if this increase was in part masked by increases in antihypertensive therapy. To evaluate for changes in blood volume and its components including plasma volume and red cell volume, we employed a student’s t-test for paired comparisons between baseline and repeat testing at 6 months, stratified by study drug allocation. SAS for Windows (Version 8.0, SAS Institute Inc., Cary, North Carolina) was used for those analyses.
Results
Clinical and Demographic Characteristics
The clinical and demographic characteristics of the study population are shown in Table 2. Subjects were predominately Hispanic women who on average were older than 75 years of age and had multiple other co-morbid conditions in addition to hypertension including diabetes, obesity, coronary artery disease and chronic kidney disease. They were anemic, as required by the protocol and had elevated B-type natriuretic peptide consistent with the diagnosis of heart failure. Almost all were on chronic diuretics (94%) and a significant percentage were treated with ACE inhibitors or angiotensin receptor antagonists (69%), beta blockers (67%) and calcium channel blockers (52%)at baseline to manage their high blood pressureDosing Algorithm: As shown in Figure 1 (Upper left panel) subjects randomized to ESA as compared to placebo had a greater increase in hemoglobin throughout the study period. The increase in hemoglobin appeared as early as 10 days after randomization and was on average 1.4 gm/dl higher than baseline throughout the study period. This was achieved with an average weekly dose of erythropoietin of 4655 Units/week which tended to decline throughout the study period (see Figure 1, lower left panel). Notably the cohort randomized to placebo also had a significant increase in hemoglobin which averaged 0.4 gm/dl throughout the study. Despite this “placebo effect”, the hemoglobin was higher in those on active therapy and placebo throughout the study period (p<0.0001 for comparisons done every 10 days).
Table 2.
Demographic and Clinical Characteristics of Study Subjects
| Parameter | Values |
|---|---|
| Number of Subjects | 48 |
| Age | 78±11 |
| Gender (% Female) | 32 (67%) |
| Ethnicity (% Latino) | 29 (60%) |
| Race (White/Black/Asian) | 30/17/1 |
| Co Morbid Conditions (%) | |
| Hypertension | 48 (100%) |
| Diabetes | 31 (65%) |
| Coronary Artery Disease | 29 (60%) |
| Obesity | 27 (56%) |
| Chronic Kidney Disease | 36 (75%) |
| Hemodynamics | |
| Blood Pressure (mm Hg) | 144±16/67±11 |
| Heart Rate (bpm) | 71±12 |
| Laboratory Results | |
| Hemoglobin (gm/dl) | 10.4±1.1 |
| B-type natriuretic peptides (pg/mL) | 397±342 |
| Creatinine (mg/dL) | 1.5±0.8 |
| Echocardiographic Values | |
| EF (%) | 57±9 |
| LV mass (grams) | 108±29 |
Values are men ± Standard deviation or number of subjects (%)
Figure 1.
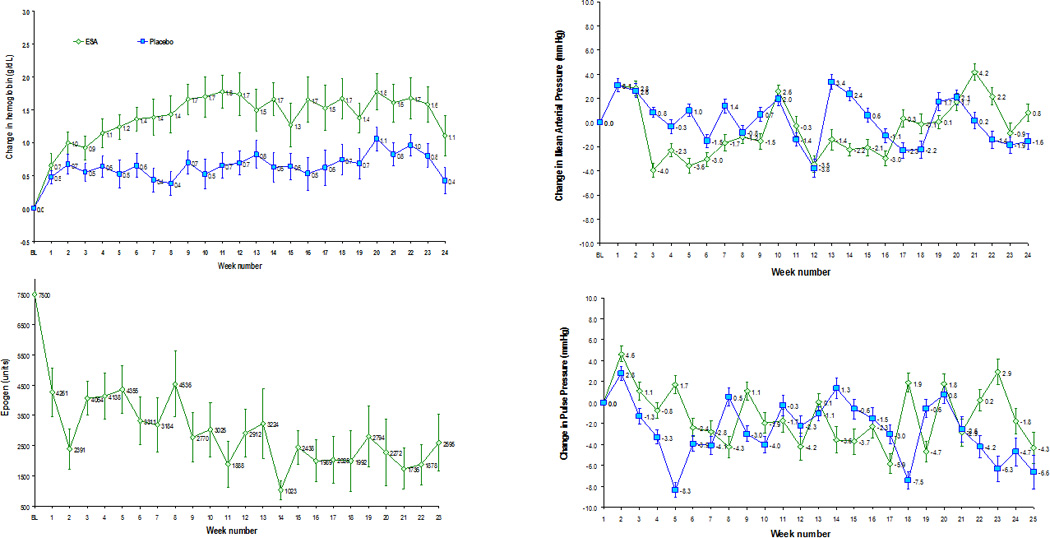
Weekly changes in hemoglobin from baseline during study period in subjects randomized to epoetin alfa or placebo (upper left panel); average weekly dose of epoetin alfa (lower left panel); changes in mean arterial blood pressure from baseline (upper right panel) and changes in pulse pressure from baseline (lower right panel) in subjects randomized to epoetin alfa or placebo. Data is mean ± standard error.
The current dosing algorithm, which mandated holding of study drug and dose reductions for too rapid a weekly rise in hemoglobin (defined as >0.4 gm/dl in any one week period [Table 1]) resulted in a holding of study drug 32% of the time. Similarly, we observed that in the placebo arm, study drug was held 24% of the time. Accordingly, we attribute these large increases in weekly hemoglobin values to changes in volume status. As a result of these large weekly fluctuations, the dose of study drug declined over the course of the trial and the final hemoglobin in the epoetin alfa cohort was ~12gm/dL, which was lower than the target of 13.0 gm/dL target of the dosing algorithm.
Determinants of Changes in Hemoglobin
Results of the repeated measures mixed models indicated that drug (p< 0.05), dose (p<0.01), change in subject’s weight (p<0.01), increase in diuretic (p<0.05) and duration of therapy (p<0.05), were all significantly associated with the absolute hemoglobin achieved and the change in hemoglobin from baseline (Table 3). The use of ESA and duration of therapy were directly associated with increases in absolute hemoglobin and changes in hemoglobin from baseline, while the dose of ESA, the change in subject’s weight and increases in diuretics were inversely associated with hemoglobin. The inverse association of ESA dose with absolute hemoglobin and change in hemoglobin are a reflection of the dosing algorithm that dictated reducing the dose as the hemoglobin increased toward targeted values in order to avoid excessive hemoglobin increases. Additionally, weekly changes in weight (which were indicative of volume overload) were the primary driver behind increases in diuretics in these patients with heart failure. A separate repeated measure mixed model using weight (kg) change as the dependent variable and time and diuretic change as the independent variables showed that, when controlling for study duration, changes in diuretic dose was significantly associated with increase in weight (β=0.5759, p<0.0001). The study duration (time) was not a significant predictor of weight change (β=−0.0051, p=0.1280). Accordingly, both an increase weight and increase diuretic dose was associated with declines in absolute and changes in hemoglobin during the duration of the trial, indicating a significant confounding effect of volume status on hemoglobin concentration. Reflective of this observation is the fact that among the subgroup of subjects who underwent evaluation of overall blood volume at baseline and after 6 months of therapy (Table 4), there was no significant difference between those assigned to ESA or placebo. However, changes in the components of blood volume differed between subjects receiving ESA as compared to placebo. Specifically, in subjects receiving ESA, increases in red cell volume and declines in plasma volume were observed which were not present in subjects assigned to placebo.
Table 3.
Determinants of Hemoglobin from Repeated Measures Mixed Models.
| Effect | Absolute Hemoglobin (gm/dl) |
Hemoglobin Change from Baseline (gm/dl) |
||
|---|---|---|---|---|
| Estimate | SE | Estimate | SE | |
| Intercept | 13.947‡ | 0.803 | 1.728‡ | 0.440 |
| Drug (ESA vs. Placebo) | 0.881† | 0.396 | 0.933‡ | 0.187 |
| Dose of ESA (per unit) | −0.106‡ | 0.009 | −0.108‡ | 0.009 |
| Time (per days) | 0.003† | 0.001 | 0.003† | 0.001 |
| Weight (per kg) | −0.039‡ | 0.010 | −0.016‡ | 0.005 |
| Increase in diuretics (Y/N) | −0.120† | 0.060 | −0.124† | 0.060 |
p < 0.05,
p < 0.01
Table 4.
Changes in Blood Volume Components from Baseline to 6 Months Stratified by Study Drug.
| Changes in Blood Volumes | ESA (n=13) |
Placebo (n=13) |
||
|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | |
| Blood Volume (mL) | 4875±255 | 4861±242 | 4662±288 | 4763±357 |
| Red Cell Volume (mL) | 1341±87 | 1509±91† | 1372±94 | 1421±108 |
| Plasma Volume (mL) | 3534±183 | 3352±176† | 3290±206 | 3342±257 |
p < 0.05,
Mean± Standard error
Effect on Blood Pressure
At baseline, systolic, diastolic, mean nor pulse blood pressure did not differ between subjects assigned to ESA versus placebo. Additionally, changes in mean arterial pressure and pulse pressure from baseline did not differ between subjects assigned to active therapy in comparison to placebo (Figure 1, right upper and lower panels). Finally, results of the repeated measures mixed model (Table 5) demonstrated that study drug and dose were not significant determinants of changes in MAP or PP even when controlling for changes in anti-hypertensive therapy. Notably, declines in pulse pressure were statistically related to duration of study and the interaction of increases in antihypertensive therapy and study duration. However, these changes were clinically minimal.
Table 5.
Determinants of Blood Pressure (Mean and Pulse Pressure) from Repeated Measures Mixed Models.
| Effect | Absolute MAP (mm Hg) |
MAP Change from Baseline (mm Hg) |
Absolute PP (mm Hg) |
PP Change from Baseline (mm Hg) |
||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Intercept | 91.953‡ | 1.862 | 1.268 | 2.227 | 72.692‡ | 3.088 | −1.489 | 3.097 |
| Drug (ESA vs. Placebo) | 1.587 | 2.637 | −1.561 | 3.182 | 7.097 | 4.409 | 1.542 | 4.422 |
| Dose of ESA (per unit) | −0.199 | 0.134 | −0.177 | 0.135 | 0.168 | 0.191 | 0.098 | 0.191 |
| Time (per week) | −0.081 | 0.045 | −0.066 | 0.045 | −0.127† | 0.064 | −0.128† | 0.064 |
| Increase in BP medications (Y/N) | 2.621 | 1.787 | 2.661 | 1.801 | −1.833 | 2.540 | −1.951 | 2.541 |
| Interaction of Time and Increase in BP medications | 0.170 | 0.136 | 0.157 | 0.1373 | 0.661‡ | 0.194 | 0.666‡ | 0.194 |
p < 0.05,
p < 0.01,
MAP = mean arterial pressure, PP = pulse pressure
Discussion
Among older adults with heart failure, a preserved ejection fraction, and concomitant anemia, the currently employed dosing algorithm which adjusts the administration of Epogen (Epoetin alpha) based on the absolute hemoglobin and weekly change in hemoglobin resulted in significant increases in hemoglobin concentration over time at relatively low weekly doses However, this algorithm did not achieve the target hemoglobin of 13 gm/dL, but rather achieved a target hemoglobin of ~12.0 gm/dl in part because of rapid weekly increases in hemoglobin resulted in mandatory holds of study drug and subsequent dose reductions. In addition to ESA dose and duration of therapy, factors indicative of volume status including weight and diuretic use were determinants of hemoglobin levels. Finally, despite the universal prevalence of hypertension in this cohort, the use of ESA and the dose of ESA were not significantly associated with changes in either steady (e.g. mean arterial pressure) or pulsatile (e.g. pulse pressure) components of blood pressure.
Dosing of ESA
The subcutaneous route of administration employed in this trial has been shown to have a lower bioavailability than intravenous ESA administration, yet paradoxically higher efficacy, hypothesized to be due to different mechanisms, including prolonged exposure of progenitor cells to the ESA(16). Accordingly, several studies have demonstrated that equivalent target hemoglobin levels can be maintained at much lower doses of epoetin alfa when administered subcutaneously than intravenously.(17, 18) Indeed some have shown that subcutaneous epoetin alfa dosing frequency can be reduced in some patients to once every 2 to 4 weeks, without compromising efficacy and potentially reducing subject burden and costs.(19, 20)
We employed weekly administration of epoetin alfa, as it has been shown to be effective in maintaining hemoglobin concentrations in the majority of stable hemodialysis patients(21), among subjects with chronic kidney disease but not on dialysis(22) and in HIV positive subjects(23). In the previous trials of heart failure patients that enrolled almost exclusively subjects with systolic heart failure, the dosing of epoetin alpha or beta range from 4000 to 10,000 Units once to three times per week(24–26) and dose adjustments in these protocols were not described. The currently employed dosing algorithm resulted in a lower weekly dose than these studies, albeit with a smaller increase in hemoglobin with active therapy. The lower doses were the result of the dosing algorithm that required, for safety reasons, holding of study drug and subsequent dose reductions in response to rapid weekly increases in hemoglobin.
Serious adverse effects (death, myocardial infarction, congestive heart failure or stroke) have been related to the dose of epoetin alfa, the rate of rise of the hemoglobin and as well as the inability to achieve a target hemoglobin(10). However, adjusted analyses suggest that patients who were able to achieve target hemoglobin had better outcomes than those who did not; and among subjects who achieved their randomized target, no increased risk associated with the higher hemoglobin goal was detected.(9, 27) Unresponsiveness to ESA has been hypothesized(28) to be a major factor associated with the excess risk in randomized clinical trials(8, 29, 30) that have demonstrated adverse outcomes because the achievement of a higher hemoglobin level itself was not associated with a higher rate of cardiovascular complications. Analyses demonstrate that the dose of ESA is independently associated with adverse outcomes(31), suggesting, but not proving, that patients with hemlogobin unresponsiveness may be particularly vulnerable to excessive ESA exposure and ESA related adverse events. Indeed, a recent analysis of a large clinical trial of ESA in patients with chronic kidney disease and diabetes demonstrated that hypo-responsiveness to ESA was associated with adverse outcomes.(32) Accordingly, the package labeling warns that “patients who have an insufficient hematologic response to ESA therapy may have an even greater risk of cardiovascular events and death than other patients.”
Notably, while epoetin alfa as administered in the current trial, resulted in an increase in hemoglobin compared to placebo, there was significant heterogeneity in the response in those randomized to active therapy (range −0.5 to 5.9 gm/dl) with 40% not achieving a >1.5 gm/dl increase. This suggests that among older adults with HFPEF and concomitant anemia, a large portion of subjects are epoetin alfa unresponsive. Accordingly, the dosing algorithm employed may have several favorable characteristics for older adults with HFPEF, a significant percentage of who could be ESA unresponsive, including achieving hemoglobin of ~12 gm/dl and not higher, limiting excessive dosing and restricting excessively rapid increases in hemoglobin concentrations.
Confounding effect of changes in Plasma volume
Treatment with epoetin alfa has been shown in healthy humans(33), in subjects with chronic renal failure(34) and subjects with severe systolic heart failure(25) to induce an elevation in hemoglobin concentration by both increasing red cell volume and by decreasing plasma volume(33), the latter of which may be mediated by a down regulation of the rennin-angiotensin-aldosterone axis(35). The observed reduction in plasma volume may be a particularly beneficial effect of ESAs, particularly for patients with volume expanded states such as heart failure. The results of our analysis of blood volume components in subjects with HFPEF suggest that while total blood volume remains unchanged during epoetin alfa therapy, there are small but significant increases in red cell volume and reduction in plasma volume that contribute to the observed changes in hemoglobin concentration with therapy.
Hemodilution appears to be the most potent factor for the development of low hemoglobin levels in patients with CHF(36) and data suggests that hemoglobin does not correlate with red cell volume in patients with anemia and HF(14). Accordingly, careful dosing of ESA in this population may be particularly important especially when the endpoint is hemoglobin. Given the confounding effects of plasma volume on hemoglobin concentration, there is the potential both for excessive dosing of ESA and an excessive rise in hemoglobin. The former can occur when ESA dose is increased in the setting when hemoglobin levels fail to rise during therapy but are actually not increasing because of a concomitant rise in plasma volume (e.g. hemodilution) and the latter can be seen during rapid reductions in plasma volume that occur during diuresis. Our analyses suggest that changes in volume status indicated by changes in diuretic dose or weekly weight changes were statistically significant and independent covariates that influenced the hemoglobin concentration, supporting the importance of accounting for these factors.
Effect of ESA on Blood Pressure
Hypertension is a known consequence of ESAs. An increase in blood pressure develops in approximately one third of patients. The mechanism of hypertension related to epoetin alfa remains uncertain. The mechanisms described have been heterogeneous including increased blood viscosity, enhanced pressor responsiveness leading to increase in total peripheral resistance(37), and declines in plasma volume.(38) Hypertension with ESAs has been demonstrated not to be related to the dose of ESAs, nor to the final hemoglobin level achieved or the rate of increase of hemoglobin.(39) One unifying factor has been a lack of a significant reduction in cardiac output in patients in whom BP increases, which may be due to abnormal cardiovascular autoregulation in these patients. The lack of effect of epoetin alfa on blood pressure in this trial is consistent with other reports(40), which found that with a dose of 4500 IU/week there was no observed pressor effect of epoetin alfa on clinic blood pressure measurements, but demonstrable differences were detected by continuous ambulatory blood pressure measurement. In this trial, we did not employ ambulatory BP measurements, and so cannot provide evidence for or against an ESA effect on continuous ambulatory blood pressure measurements.
Limitations
This study is limited by the small sample size and heterogeneous nature of the study population. However, by using a repeated measure mixed model we were able to capitalize on the strengths of our study design using each subjects as his/her own control enhancing the power of the available data. The indices related to volume status that we employed in our multivariate modeling (e.g. weight and diuretic use) are inexact and themselves could be indicative of other phenomenon that change plasma volume. Additionally, changes in dietary intake of sodium, which were not controlled for in our study and are a known contributor to expanded plasma volume in subjects with heart failure are an unmeasured confounder. However, the short term nature of the analyses we performed (e.g. weekly changes) limit the chances for confounding. Additionally, the findings were supported by the long term measures of changes in blood volume measured by a validated radionuclide technique. Finally, whether the approximately 1 gm/dl increase in hemoglobin achieved using this dosing algorithm will be associated with meaningful increases in exercise capacity or a reduction in symptoms and thus could justify the use of what is an expensive and labor intensive therapy is unknown but an important secondary endpoint of this trial.
Conclusions
The ESA dose adjustment algorithm employed in this small randomized trial resulted in increases in Hb using relatively low doses of ESA notably with increases confounded by changes in plasma volume. Accounting for the alterations in plasma volume in anemic patients with heart failure could more accurately identify subjects with a true anemia, who may be more likely to benefit from ESAs. Also, trials that employ a run-in period to evaluate for ESA responsiveness could be especially important for the population of older adults with HFPEF that were the focus of the current trial. In these subjects, ESA as employed in this trial did not significantly affect blood pressure. Whether this intervention is associated with meaningful clinical results will be determined by the outcome of this ongoing clinical trial.
Acknowledgments
The research was supported by a grant from the National Institute of Health/National Institute on Aging, RO1 AG027518-01A1
Citations
- 1.Cohen RS, Mubashir A, Wajahat R, Mani S, Hummel S, Maurer MS. The cardio-renal-anemia syndrome in elderly subjects with heart failure and a normal ejection fraction: a comparison with heart failure and low ejection fraction. Congest Heart Fail. 2006 Jul-Aug;12(4):186–191. doi: 10.1111/j.1527-5299.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- 2.He SW, Wang LX. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail. 2009 May-Jun;15(3):123–130. doi: 10.1111/j.1751-7133.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 3.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008 Sep 2;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Anand IS, Diaz R, Maggioni AP, O'Connor C, Pfeffer MA, Polu KR, Solomon SD, Sun Y, Swedberg K, Tendera M, van Veldhuisen DJ, Wasserman SM, Young JB. Design of the Reduction of Events with Darbepoetin alfa in Heart Failure (RED-HF): a Phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail. 2009 Aug;11(8):795–801. doi: 10.1093/eurjhf/hfp098. [DOI] [PubMed] [Google Scholar]
- 5.Ngo K, Kotecha D, Walters JA, Manzano L, Palazzuoli A, van Veldhuisen DJ, Flather M. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. Cochrane Database Syst Rev. 2010;(1):CD007613. doi: 10.1002/14651858.CD007613.pub2. [DOI] [PubMed] [Google Scholar]
- 6.van der Meer P, Groenveld HF, Januzzi JL, Jr, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009 Aug;95(16):1309–1314. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009 Nov 19;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 9.Szczech LA, Barnhart HX, Sapp S, Felker GM, Hernandez A, Reddan D, Califf RM, Inrig JK, Patel UD, Singh AK. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 2010 Feb;77(3):239–246. doi: 10.1038/ki.2009.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents--time for a reevaluation. N Engl J Med. 2010 Jan 21;362(3):189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 11.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992 Aug;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 12.Patwardhan VN. Nutritional anemias--WHO research program. Early developments and progress report of collaborative studies. Am J Clin Nutr. 1966 Jul;19(1):63–71. doi: 10.1093/ajcn/19.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Cohen RS, Karlin P, Yushak M, Mancini D, Maurer MS. The effect of erythropoietin on exercise capacity, left ventricular remodeling, pressure-volume relationships, and quality of life in older patients with anemia and heart failure with preserved ejection fraction. Congest Heart Fail. 2010 May;16(3):96–103. doi: 10.1111/j.1751-7133.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008 Oct 15;102(8):1069–1072. doi: 10.1016/j.amjcard.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldschuh J, Katz S. The importance of correct norms in blood volume measurement. Am J Med Sci. 2007 Jul;334(1):41–46. doi: 10.1097/MAJ.0b013e318063c707. [DOI] [PubMed] [Google Scholar]
- 16.Besarab A. Optimizing anaemia management with subcutaneous administration of epoetin. Nephrol Dial Transplant. 2005 Jun;(20) Suppl 6:vi10–vi15. doi: 10.1093/ndt/gfh1098. [DOI] [PubMed] [Google Scholar]
- 17.Locatelli F, Villa G, Messa P, Filippini A, Cannella G, De Ferrari G, Naso A, Rossi E, Formica M, Lombardi L, Rotolo U, Conte F. Efficacy and safety of once-weekly intravenous epoetin alfa in maintaining hemoglobin levels in hemodialysis patients. J Nephrol. 2008 May-Jun;21(3):412–420. [PubMed] [Google Scholar]
- 18.Pussell BA, Walker R. Dose of epoetin alfa used in haemodialysis patients when switching from subcutaneous to intravenous administration. Nephrology (Carlton) 2007 Apr;12(2):120–125. doi: 10.1111/j.1440-1797.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Pergola PE, Gartenberg G, Fu M, Sun S, Wolfson M, Bowers P. A Randomized Controlled Study Comparing Once-Weekly to Every-2-Week and Every-4-Week Dosing of Epoetin Alfa in CKD Patients with Anemia. Clin J Am Soc Nephrol. 2010 Feb 25; doi: 10.2215/CJN.06770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergola PE, Gartenberg G, Fu M, Sun S, Wolfson M, Bowers P. A randomized controlled study comparing once-weekly to every-2-week and every-4-week dosing of epoetin alfa in CKD patients with anemia. Clin J Am Soc Nephrol. 2010 Apr;5(4):598–606. doi: 10.2215/CJN.06770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barre P, Reichel H, Suranyi MG, Barth C. Efficacy of once-weekly epoetin alfa. Clin Nephrol. 2004 Dec;62(6):440–448. doi: 10.5414/cnp62440. [DOI] [PubMed] [Google Scholar]
- 22.Benz R, Schmidt R, Kelly K, Wolfson M. Epoetin alfa once every 2 weeks is effective for initiation of treatment of anemia of chronic kidney disease. Clin J Am Soc Nephrol. 2007 Mar;2(2):215–221. doi: 10.2215/CJN.02590706. [DOI] [PubMed] [Google Scholar]
- 23.Grossman HA, Goon B, Bowers P, Leitz G. Once-weekly epoetin alfa dosing is as effective as three times-weekly dosing in increasing hemoglobin levels and is associated with improved quality of life in anemic HIV-infected patients. J Acquir Immune Defic Syndr. 2003 Dec 1;34(4):368–378. doi: 10.1097/00126334-200312010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Palazzuoli A, Silverberg DS, Calabro A, Spinelli T, Quatrini I, Campagna MS, Franci B, Nuti R. Beta-erythropoietin effects on ventricular remodeling, left and right systolic function, pulmonary pressure, and hospitalizations in patients affected with heart failure and anemia. J Cardiovasc Pharmacol. 2009 Jun;53(6):462–467. doi: 10.1097/FJC.0b013e3181a6ac38. [DOI] [PubMed] [Google Scholar]
- 25.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003 Jan 21;107(2):294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 26.Palazzuoli A, Quatrini I, Calabro A, Antonelli G, Caputo M, Campagna MS, Franci B, Nuti R. Anemia correction by erythropoietin reduces BNP levels, hospitalization rate, and NYHA class in patients with cardio-renal anemia syndrome. Clin Exp Med. 2010 May 29; doi: 10.1007/s10238-010-0100-y. [DOI] [PubMed] [Google Scholar]
- 27.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008 Sep;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AK. The controversy surrounding hemoglobin and erythropoiesis-stimulating agents: what should we do now? Am J Kidney Dis. 2008 Dec;52(6 Suppl):S5–S13. doi: 10.1053/j.ajkd.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998 Aug 27;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 30.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov 16;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 31.Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006 Apr;17(4):1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 32.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010 Sep 16;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 33.Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, Calbet JA, Robach P. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol. 2007 Jan 1;578(Pt 1):309–314. doi: 10.1113/jphysiol.2006.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogh-Andersen N, Eidemak I, Lokkegaard H, Levin Nielsen S. Changes in blood and plasma volume during treatment with recombinant human erythropoietin. Scand J Clin Lab Invest Suppl. 1993;214:61–65. doi: 10.3109/00365519309090680. [DOI] [PubMed] [Google Scholar]
- 35.Olsen NV, Aachmann-Andersen NJ, Oturai P, Andersen TM, Rasmussen AB, Hulston C, Holstein-Rathlou NH, Robach P, Lundby C. Recombinant human erythropoietin in humans down-regulates proximal renal tubular reabsorption and causes a fall in glomerular filtration rate. J Physiol. 2010 Aug 19; doi: 10.1113/jphysiol.2010.194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlbrecht C, Kommata S, Hulsmann M, Szekeres T, Bieglmayer C, Strunk G, Karanikas G, Berger R, Mortl D, Kletter K, Maurer G, Lang IM, Pacher R. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J. 2008 Oct;29(19):2343–2350. doi: 10.1093/eurheartj/ehn359. [DOI] [PubMed] [Google Scholar]
- 37.Yamakado M, Umezu M, Nagano M, Tagawa H. Mechanisms of hypertension induced by erythropoietin in patients on hemodialysis. Clin Invest Med. 1991 Dec;14(6):623–629. [PubMed] [Google Scholar]
- 38.Jones MA, Kingswood JC, Dallyn PE, Andrew M, Cheetham A, Burwood R, Sharpstone P. Changes in diurnal blood pressure variation and red cell and plasma volumes in patients with renal failure who develop erythropoietin-induced hypertension. Clin Nephrol. 1995 Sep;44(3):193–200. [PubMed] [Google Scholar]
- 39.Raine AE, Roger SD. Effects of erythropoietin on blood pressure. Am J Kidney Dis. 1991 Oct;18(4) Suppl 1:76–83. [PubMed] [Google Scholar]
- 40.Imai Y, Sekino H, Fujikura Y, Munakata M, Minami N, Hashimoto J, Sakuma H, Watanabe N, Misawa S, Nishiyama A, et al. Pressor effect of recombinant human erythropoietin: results of ambulatory blood pressure monitoring and home blood pressure measurements. Clin Exp Hypertens. 1995 Apr;17(3):485–506. doi: 10.3109/10641969509037420. [DOI] [PubMed] [Google Scholar]


