Abstract
Aging is associated with appearance of white matter hyperintensities (WMH) on MRI scans. Vascular risk and inflammation, which increase with age, may contribute to white matter deterioration and proliferation of WMH. We investigated whether circulating biomarkers and genetic variants associated with elevated vascular risk and inflammation are associated with WMH volume in healthy adults (144 volunteers, 44-77 years of age). We examined association of WMH volume with age, sex, hypertension, circulating levels of total plasma homocysteine (tHcy), cholesterol (low-density lipoprotein), and C-reactive protein (CRP), and four polymorphisms related to vascular risk and inflammation: Apolipoprotein ε (ApoE ε2,3,4), Angiotensin-Converting Enzyme insertion/deletion (ACE I/D), methylenetetrahydrofolate reductase (MTHFR) C677T, C-reactive protein (CRP) -286 C>A>T, and interleukin-1β (IL-1β) C-511T. We found that larger WMH volume was associated with advanced age, hypertension, and elevated levels of homocysteine and CRP but not with low-density lipoprotein levels. Homozygotes for IL-1β -511T allele and carriers of CRP -286T allele that are associated with increased inflammatory response had larger WMH than the other allelic combinations. Carriers of the APOE ε2 allele had larger frontal WMH than ε3 homozygotes and ε4 carriers did. Thus, in healthy adults, who are free of neurological and vascular disease, genetic variants that promote inflammation and elevated levels of vascular risk biomarkers can contribute to brain abnormalities.
Keywords: brain, aging, CRP, interleukin, homocysteine, frontal lobe, ApoE, MTHFR, ACE
The aging brain undergoes multiple structural and functional changes [1-3]. One of the most prominent among them is deterioration of the cerebral white matter [4] that is evident in breakdown of myelin [5, 6] and disruption of microstructural organization [7, 8]. A common expression of white matter aging is leukoaraiosis [9], which is observed on T2-weighted MRI scans as areas of extremely high signal intensity. These bright objects (White Matter Hyperintensities, WMH) reflect multiple physiologic and pathologic changes, including ischemic lesions, loss and deformation of myelin sheath, damage to the walls of small vessels, gliosis, micro-hemorrhages, and breaches of the cerebro-spinal fluid (CSF)-brain barrier [10-12]. Since the earliest days of clinical MRI, it was clear that WMH burden is associated with advanced age [13,14] and is increased by vascular risk factors, such as hypertension [13-17]. Nonetheless, after accounting for age and manifest vascular risk, much WMH volume variance remains unexplained. A substantial share of population variability in WMH burden is heritable [18-20]. However, to date, the search for specific genes responsible for proliferation of WMH with age has born little fruit [21]. Nonetheless, because vascular risk increases WMH burden, a pool of known genetic variants that affect lipid homeostasis, blood pressure regulation, and formation of atherosclerotic pathology may yield genetic modifiers of WMH burden.
Several such genetic variants have been studied in conjunction with individual differences in WMH load. Investigations of Apolipoprotein E (APOE) gene that controls cholesterol trafficking in the blood vessels and whose alleles, ε2, ε3, and ε4, are associated with progressively higher blood levels of cholesterol [22] and increases risk for Alzheimer’s disease (AD) [23] produced mixed results [24-27]. Moreover, in some studies, ε2 allele that is associated with reduced risk for AD and lower cholesterol levels predicted increased in WMH load [28], and increase in microangiopathy-related damage [29]. Variants of genes associated with control of blood pressure, such as ACE deletion [30-31] has been linked to increased WMH burden [32-35] and lacunar strokes [36]. Studies of a variant of methylenetetrahydrofolate reductase gene (MTHFR C677T) that is associated with increased plasma levels of homocysteine, a risk factor for vascular disease and AD [37-39,42], revealed inconsistent support for its role in WMH severity [27, 40-41].
Homocysteine promotes inflammatory response [43] that may have a significant impact on brain aging [44-46] by producing neural damage and gliosis [47]. One of the most prominent proinflammatory factors associated with brain injury and elevated risk for neurodegenerative disease is interleukin-1β (IL-1β) [47-57]. The levels of IL-1β are controlled by the eponymous gene, IL-1β, a functional variant of which, IL-1β C-511T, is associated with increased proinflammatory response [55]. The TT genotype of the IL-1β has been linked to an increased risk of small vessel disease [56], seizures [57], and AD [58], which makes this variant a plausible candidate for influencing WMH proliferation. Although IL-1β C-511T polymorphisms has been linked to ventriculomegaly in schizophrenia [59] and reduction of brain volume in bipolar disorder [60], its impact on the brain structure in healthy adults is unknown. A genetic variant associated with another pro-inflammatory cytokine, IL-6, has been linked to increased WMH load in a recent study [61], but the IL-1β C-511T effect was not examined in that study.
Another inflammatory blood marker is C-reactive protein (CRP). CRP and the proinflammatory cytokines are activated in response to injury almost simultaneously [62] and act in synergy to promote atherosclerosis and plaque instability thus increasing probability of adverse cerebrovascular events [63]. Elevated plasma levels of CRP have been linked to WMH load [64, but see 65], cognitive decline, and dementia [66,67]. However, two studies of typical older adults found no associations between several CRP polymorphisms and WMH burden [61,68]. A common tri-allelic variant of the CRP gene (CRP -286 C>A>T) displays significant variations in CRP levels from the lowest in C homozygotes to the highest in persons who do not carry the C allele [69].
In this study, we examined the effect of inflammation-associated polymorphisms IL-1β C-511T and CRP -286 C>A>T on WMH volume in healthy adults of a broad age range. We compared the effect of these proinflammatory genetic variants with previously explored genetic factors linked to vascular risk: ACE insertion deletion, MTHFR C677T, and ApoE ε2, e3, and e4 variants. Furthermore, we examined the interactive effects between a known vascular risk factor, hypertension, and these genetic variants on WMH volume.
Method
Participants
The sample was part of a larger ongoing study of neural correlates of cognitive aging. Participants were residents of a major Midwestern metropolitan area in the United States and all lived independently. The exclusionary criteria were as follows: a history of cardiovascular (except treated essential hypertension), neurological, or psychiatric illness, diabetes, head trauma with a loss of consciousness for more than 5 min, thyroid problems, treatment for drug and alcohol problems, or a habit of taking three or more alcoholic drinks per day, and use of anti-seizure medication, anxiolytics, or antidepressants. The participant scored below 16 on the Center for Epidemiologic Studies Depression Scale (CES-D) [70] and above 25 on the Mini Mental State Examination (MMSE) [71]. All participants were strongly right-handed as indicated by the Edinburgh Handedness Questionnaire (75% and above) [72]; left-handers were excluded from this study. Participants were classified as hypertensive if they had a diagnosis of hypertension, were taking antihypertensive medications, or if their blood pressure averaged over three to four testing sessions exceeded 140 mm Hg (systolic) or 90 mm Hg (diastolic).
One-hundred and forty four participants (98 women and 46 men) were included in the study. Approximately 27% of participants were African-Americans. The sample included 49 persons classified as hypertensive according to the above listed criteria. Participants who had diagnosis of hypertension were taking the following medications: ACE inhibitors - 12, diuretics - 11, angiotensin II blockers -10, beta-blockers - 9, Ca channel blockers - 6, alpha-1 blockers – 1. Ten participants were taking two antihypertensive medications. Thirteen participants were taking statins for reduction of cholesterol, but there was no difference in proportion of people on statins between normotensive and hypertensive participants: 8% vs. 10%, χ2 = .13, p = .72. Eight women were on hormone-replacement therapy (HRT) and additional 24 women used HRT in the past. Among normotensive women, 35% were current or previous HRT users, whereas among hypertensive women – 43%, a nonsignificant difference: χ2= .57, p=.45.
The sample consisted of participants with a high educational attainment tha corresponded on average to almost four years of college. Men and women did not differ in age (t = −0.97, p=.33.), education (t = −0.01, p=.99.), and MMSE scores (t = 1.44, p=.15.). The demographic characteristics of the sample and descriptive statistics for blood the levels of blood markers are presented in Table 1.
Table 1.
Descriptive statistics for sample demographics, white matter hyperintensities, and levels of the blood markers.
| N | Minimum | Maximum | Mean | SD | CV | |
|---|---|---|---|---|---|---|
| Age | 144 | 44 | 77 | 58.89 | 9.09 | 0.15 |
| Education | 144 | 12 | 20 | 15.91 | 2.61 | 0.16 |
| MMSE | 144 | 26 | 30 | 28.78 | 1.09 | 0.04 |
| Systolic BP (mm Hg) | 144 | 97.67 | 156.67 | 123.85 | 12.55 | 0.10 |
| Diastolic BP (mm Hg) | 144 | 60.00 | 106.67 | 76.06 | 7.51 | 0.10 |
| Cholesterol (mg/dL) | 142 | 119.00 | 318.00 | 202.30 | 38.67 | 0.19 |
| LDL (mg/dL) | 142 | 43.00 | 214.00 | 121.64 | 33.82 | 0.28 |
| HDL (mg/dL) | 142 | 26.00 | 106.00 | 55.46 | 14.96 | 0.27 |
| Folate (ng/l) | 143 | 5.40 | 42.30 | 18.57 | 5.04 | 0.27 |
| B12 (ng/l) | 143 | 158.00 | 1,200 | 560.88 | 258.39 | 0.46 |
| Homocysteine (μmol/l) | 141 | 5.10 | 30.50 | 9.43 | 3.08 | 0.33 |
| CRP (mg/l) | 142 | 0.12 | 46.44 | 3.50 | 5.85 | 1.67 |
| Frontal WMH (mm3) | 144 | 152.00 | 27216.00 | 1667.06 | 2728.32 | 1.64 |
| Parietal WMH (mm3) | 144 | 8.00 | 5712.00 | 274.21 | 592.74 | 2.16 |
| Temporal WMH (mm3) | 144 | 54.00 | 11156.00 | 1079.61 | 1378.56 | 1.28 |
| Occipital WMH (mm3) | 144 | 376.00 | 9892.00 | 2511.69 | 1899.02 | 0.77 |
MMSE- Mini-Mental State Examination; SD-standard deviation; CV-coefficient of variation (SD/Mean); BP – blood pressure; LDL – low-density lipoprotein; HDL – high-density lipoprotein; WMH – White Matter Hyperintensities.
Procedure
MRI Acquisition
Images were acquired on a 4-Tesla MRI system (Bruker Biospin, Ettlingen, Germany) with an 8-channel RF coil. A set of 50 contiguous axial slices of fluid-attenuated inversion recovery (FLAIR) images was acquired with following parameters: TR = 8440 ms, TE =112 ms, TI=2200 ms, FA = 150°, FOV = 256×256 mm2, in plane resolution = 1 × 1 mm2, slice thickness = 2 mm, matrix size = 256 × 256. All MR scans were screened for space-occupying lesions and an experienced neuroradiologist reviewed all suspicious cases.
Quantification of white matter hyperintensities
White matter hyperintensities were identified on axial slices of the FLAIR sequence. Hyperintense regions were defined as circumscribed areas of increased signal intensity within the white matter. Because of the difficulty in differentiating WMH from emerging blood vessels and sulci in the superior convexity, the last slice on which WMH were traced was located three slices below the vertex [73]. All identifiable periventricular and deep WMH were included. WMH were measured on the frontal (FWMH), parietal (PWMH), temporal (TWMH), and occipital (OWMH) lobes. The volume of WMH for each region and each type were calculated as a sum of the areas of hyperintensities multiplied by the slice thickness. A total WMH volume was obtained by summing the volumes of hyperintensities from all of the ROIs.
Intraclass correlation (ICC) [74] was used to assess intra-rater reliability of the WMH volumes from the data collected by one rater who traced 10 randomly chosen brains on two separate occasions, seven days apart. The resulting reliability estimates for all region and all types of WMH were or exceeded 0.90. The rater, who was blind to the participants’ age, sex, and hypertension status, performed all WMH measurements. Regional demarcation was based on published rule used by Gunning-Dixon and Raz [73].
Frontal lobe
The frontal region covered the region anterior to the lateral sulcus on ventral slices. The central sulcus served as the caudal boundary on dorsal slices. The intra-rater reliability of frontal WMH was ICC = .95.
Temporal lobe
On ventral slices, the lateral fissure served as the anterior boundary of the temporal region, and the temporal–occipital incisure was the posterior boundary. The parieto–occipital sulcus appeared medially on superior slices, and a horizontal line was drawn from the parieto–occipital sulcus to the lateral surface of the cortex to separate the occipital and temporal regions. The temporal WMH was traced until the slice on which the superior temporal gyrus was no longer present (usually the slice inferior to the last slice of the corpus callosum). The intra-rater reliability of temporal WMH was ICC = .98.
Parietal lobe
The parietal region emerged on the most inferior slice where the central sulcus could first be identified. On ventral slices, the central sulcus was the anterior boundary, and the lateral fissure served as the posterior boundary separating the parietal from temporal regions. On dorsal slices (beginning with the last slice on which the corpus callosum was observed), all white matter posterior to the central sulcus was included in the parietal region. The intra-rater reliability of WMH in this region was ICC = .95.
Occipital lobe
The occipital region covered the area posterior to the temporal–occipital fissure (laterally) and the parieto–occipital sulcus (medially). The intra-rater reliability of WMH in this region was ICC = .95.
Blood Biomarker Measurements
Following an overnight fast of 12 hours, participants reported to the clinic between 9 and 10 am, and donated approximately 20 cc of whole blood for the analysis of plasma lipids, CRP, Hcy, vitamin B12, and folate. Samples for Hcy assays were placed in EDTA tubes on ice, and centrifuged to separate plasma within 30 minutes after venipuncture. The remaining samples were placed in serum separator tubes. All samples were processed in-house within four hours of venipuncture. CRP concentration was determined by immunoturbidimetry; total plasma homocysteine tHcy was assayed by fluorescence polarization immunoassay, and serum vitamin B12 and folate - by chemiluminescence. Due to laboratory errors, two lab samples were lost for tHcy and one for CRP, thus leaving ate least 142 available for blood biomarkers analyses.
Blood pressure measures
Blood pressure was measured on three or four separate days at the start of each cognitive test session by a mercury sphygmomanometer (BMS 12-S25) with a standard blood pressure cuff (Omron Professional) on the left arm with participants seated, and their forearm positioned on the table. Trained laboratory technicians conducted the measurements. The systolic and diastolic means across the measurement occasions were computed for each individual.
Genotyping
DNA was isolated from buccal samples obtained in mouthwash. Isolation was performed using a Gentra Autopure LS with the standard buccal cell protocol. For genotyping quality control, 37% direct repeats and DNA sequencing for verification were performed. Both control DNA and no-template controls were used. Success rate of genotyping at the first run was 96.25%. However, after direct repeat quality control runs with at least four successful identifications required for a call, only one sample showed an allele dropout (99.38%). After direct repeat analyses were performed seven times, the sample was genotyped with an estimated error of 0.33%. Thus, the final genotyping success rate for this study was 100%.
IL-1β C-511T (rs16944)
Polymorphism for IL-1β C-511T was interrogated with the 5′-nuclease assay using a Taqman SNP Genotyping assay (Applied Biosystems, Foster City, CA, USA). The allelic distribution of the IL-1β C-511T polymorphism fit the Hardy-Weinberg (HW) equilibrium (χ2 = 2.14, p=.14), with 38% of participants being C homozygotes (n = 55), 42% C/T heterozygotes (n = 61), whereas 28 participants had the homozygous TT genotype (19%). Variant allele frequency was 0.59. For analyses, two groups were formed: IL-1β -511T homozygotes (n = 28) and C allele carriers. The two groups did not differ in age (t=.65, p = .52), education level (t=.60, p = .54), MMSE (t=.46, p = .65).
MTHFR C677T (rs1801133)
The MTHFR C677T polymorphism was interrogated with the 5′-nuclease assay using a TaqMan SNP Genotyping Assay (Applied Biosystems, Foster City, CA, USA). The assays were run using an Applied Biosystems 7900. Genotyping revealed that 54 (37%) participants were heterozygous (CT), 11 (8%) were homozygous for the T allele (TT) and 79 (55%) were homozygous for the C allele (CC). The distribution of the alleles conformed to the HW equilibrium: χ2 = .17, p < 0.67. Variant allele frequency was .26. Because of dearth of TT homozygotes, we conducted analyses on comparison between carriers of the T allele and CC homozygotes. The carriers of the T allele differ from MTHFR C677 homozygotes in age (t=.459, p=.52), education (t=1.21, p=.23), or MMSE (t=.83, p=.41).
CRP -286C>T>A (rs3091244)
The CRP polymorphism was PCR amplified using forward 5′- AGGTGTCAGAGCTTCGGGAAGAG -3′ and reverse primers 5′-GCTCTGGGAGGAGCATGTTTGTTT-3′ in a 25 μl reaction containing 2.5 mmol/l MgCl2, 0.5 μmol/l of the primers, 1.25 U AmpliTaq Gold polymerase, and 200 μmol/l dNTPs. The mixture was denatured at 95°C for 5 minutes and amplification achieved by 15 cycles of 94°C for 30 seconds, 66°C for 30 seconds, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. The amplicons were purified using Sephadex and the purified products were analyzed on an ABI PRISM 3700 DNA Analyzer using a 50 cm capillary array. The distribution of C > A and C> T alleles met HW equilibrium requirements: χ2 =.34, p=.56; and χ2 = .002, p=.97, respectively. There was only one TT homozygote, and for the analyses, we combined all T allele carriers (N=23) and compared them to persons lacking that allele.
ACE Insertion/Deletion
Allelic discrimination of the ACE insertion/deletion was accomplished using a 5′-nuclease assay adapted from a quantitative PCR method as described by Lo et al., 2000 (Lo, YMD, Lau TK, Chan LYS, Leung TN, and Chang AMZ. Quantitative analysis of the bi-directional fetomaternal transfer of nucleated cells and plasma DNA. Clinical Chemistry 2000; 46(9): 1301-1309.). DNA was amplified using either ACE-1721F (insertion) or ACE-1428F (deletion) and ACE-1826R. Amplification was detected with the TaqMan probe 1745T. Beta-globin was utilized as a housekeeping gene for quantitation. Controls have been established by sequencing for I/I, I/D, and D/D. No template controls will also be used. The insertion/deletion heterozygotes constituted approximately half the sample (45%, 65 individuals), and 26% of the participants (N=37) were I/I homozygotes, and 29% (N=42) carried two D alleles. The frequency of the variant (D) allele was .52. The distribution of ace I/D alleles conformed to HW equilibrium: χ2 = 1.33, p = 0.25. DD homozygotes did not differ from ACE I carriers in age (t=.49, p=.63), education (t=1.31, p=.19), or MMSE (t=.06, p=.95).
ApoE ε variants
ApoE polymorphisms (rs429358 and rs7412) were preamplified with forward 5′-CAATGCTACCGAGTTTTCTTCC-3′ and reverse primers 5′-TTCAGATTCTTCACAGATGCGTA-3′ in a 25 μl reaction containing 2.5 mmol/l MgCl2, 0.5 μmol/l of the primers, 1.25 U AmpliTaq Gold polymerase, and 200 μmol/l dTTPs. The mixture was denatured at 95°C for 10 minutes and amplification achieved by 15 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. One μl of this reaction was subsequently used for rs429358 and rs7412 5′-nuclease assays under standard conditions. The primers and probes for the rs7412 assay were 5′-TCCGCGATGCCGATGAC-3′, 5′-CCCCGGCCTGGTACAC-3′, VIC-CAGGCGCTTCTGC-NFQ and FAM-CAGGCACTTCGC-NFQ. The primers and probes for the rs429358 assay were 5′-GCGGGCACGGCTGT-3′, 5′-GCTTGCGCAGGTGGGA-3′, VIC-CATGGAGGACGTGTGC-NFQ and FAM-ATGGAGGACGTGCGC-NFQ. There were 19 ε2 carriers (three homozygotes), 93 ε3 homozygotes and 32 ε4 carriers (two of them homozygotes); there were no ε2/ε4 heterozygotes. For ε4 vs. ε3, HW equilibrium was maintained: χ2=0.06, p=.81, and variant allele frequency was 0.14. However, for ε2 vs. ε3 alleles, the distribution departed from HW equilibrium: χ2=4.19, p=.04, variant allele frequency 0.10. The three allelic groupings (ε2 carriers, ε3 homozygotes, and ε4 carriers) formed a three-level ApoE variable for the analyses. There were no significant differences among the ApoE allelic groupings in age (F=1.82, p=.17), education (F=1.27, p=.29), or MMSE (F<1).
Statistical analyses
The data were analyzed within a General Linear Model (GLM) framework, with log-transformed WMH volume as the dependent variable, lobes (frontal, parietal, temporal, and occipital) as a four-level repeated measures factors, sex and hypertension diagnostic group (normotensive vs. hypertensive) as categorical predictors, and age (centered at the sample mean) as a continuous predictor. To test the effects of each of the polymorphisms, we added each as a categorical predictor into the basic model described above. Due to a small number of participants with specific combinations of alleles, we could not test for epistatic effects of the genes within the same model. All models contained first-order interactions among all predictors. We used Hyuhn-Feldt correction to adjust the probability levels of all interactions that involved repeated measures for violation of sphericity assumption.
In each analysis, a full model that included all second-order interactions was tested first. All interactions that did not reach statistical significance (p > 0.15) were removed from the model and the data were fitted to a reduced model. The GLM analyses were followed by univariate analyses of simple effects, and testing the differences among the levels of the categorical variables with Fisher’s Least Significant Difference (LSD) test, or correlation coefficients with Steiger’s Z* statistic that takes into account dependence between the compared correlations [75].
Results
The distributions of all biomarkers were skewed and application of a ln(x) transformation normalized most of them, except for folate and vitamin B12. Log-transformed values were used in all analyses.
Blood marker levels
About 50% of all participants had elevated cholesterol, but of 13 statins users, only one had cholesterol level in excess of 200 mg/dl (borderline high). Total cholesterol levels were reliably lower in persons who took statins: F(1,142)=18.44, p=.0003, after adjustment for age and sex. The difference in mean values was between 202.35 mg/dl for participants who did not take statins and 160.77 mg/dl for those who did. Most participants had normal LDL values, but 28% had borderline high (over 130 mg/dl) and 13% had elevated LDL (above 160 mg/dl).
Twenty six participants (13 women) met criteria for hyperhomocysteinemia (Hcy > 12 μmol/l), and they had lower though normal folate levels than the participants with normal Hcy levels: t(139) 4.01, p=.0001. None of the participants was deficient in folate and 45% of them exceeded the recommended range, with the sample range between 5 and 42 ng/l. The hyperhomocysteinemia group did not differ from the rest of the sample on B12 levels: t(139)=0.99, p = .32. For vitamin B12, the range was 158 - 1200 ng/l, with 38% of the participants outside of the normative 200-600 ng/l range; 37% above the normal value and one person below:158 ng/l. For 6% of the participants, CRP levels exceeded the norm (10 mg/l) and were indicative of an ongoing inflammatory process.
Effect of genetic variants on vascular risk factors
Only one of the examined polymorphisms, ACE I/D, was significantly associated with diagnosis of hypertension. Among normotensive participants, only 23% were ACE D homozygotes, whereas among their hypertensive counterparts, the ACE DD genotype was almost twice as frequent: 42%, χ2=4.88, p=.027. Notably, the distribution of persons taking any medication that directly targeted renin-angiotensin system was unrelated to the ACE genotype: χ2 = .002, p = .98. The share of homozygotes for IL-1β -511T allele was 16% among normotensive and 27% among hypertensive participants, χ2=2.38, p=.12. No difference in frequency of MTHFR 677T allele was noted: 54% vs. 57%, for normotensive and hypertensive participants, respectively, χ2=0.17, p=.69. There were 23% of ApoE ε4 carriers among normotensive vs. 30% among hypertensive participants, χ2=0.60, p=0.44. Proportions of ApoE ε2 carriers also did not differ between the groups: 11% among normotensives vs. 18% among hypertensive participants, χ2=1.74, p=0.19.
There was a significant difference in LDL levels according to ApoE ε variants: F(1,135)=4.62, p=.01. Carriers of ε4 allele had higher LDL levels than ε2 carriers did and marginally higher than those of ε3 homozygotes; there was no difference in LDL levels between the ε3 and ε4 groups. Although the trend for total cholesterol levels was in the same direction, no effect of ApoE ε variant on the level was observed: F(1,135)=2.00, p=.13.
We examined the effects of age, sex, hypertension and MTHFR C677T on the measured levels of three metabolically linked biomarkers: Hcy, folate, and vitamin B12, as repeated measures within the same model. There was a significant main effect of age: F(1,135)=9.44, p=.003. However, the effects were qualified by a significant age × sex × biomarker interaction: F(2,270)=3.55, p=.03. Decomposition of the interaction showed that among women, the levels of two biomarkers Hcy (r=.41, p =.00003) and B12 (r=.31, p=.002) correlated positively with age, with no age differences noted for the folate levels (r=.07, p=.50). For men, no significant age differences were noted in any of the biomarkers (r = .17, −.11, .16, all p > .25). Notably, no effects involving MTHFR C677T were significant (all F<1).
Carriers of T allele of the CRP -286 C>A>T polymorphism had reliably higher plasma levels of CRP: 2.76 mg/l vs. 1.47 mg/l than those who did not carry that allele; F(1,138)= 5.45, p=.02. There were neither age nor sex differences in CRP levels: F(1,138)=1.13, p=.29, and F(1,138)=1.34. p=.19, respectively. Among persons with high CRP levels indicative of inflammation, 44% were carriers of T allele, whereas among participants with normal CRP levels, only 13% carried the allele, χ2=6.15, p = .01.
Effect of age, sex, hypertension, and genetic variants on WMH volume
Age, Sex, and Hypertension
In the first model (referred hereon as the basic model), we examined age-, sex-, and hypertension-related differences in WMH volume, by lobe, without taking into account genetic variation. As none of the interactions among the between-subject factors were significant (all p>.15), they were removed from the model. The analysis of the reduced model revealed a main effect of age (F(1,140)=53.14, p <.000001), but neither sex differences (F<1), nor a main effect of hypertension (F(1,140)=1.68, p=.20, ns). However, there were three significant interactions with Lobe, indicating that each of those factors had a differential effect on WMH, depending on the location.
Lobe × Age interaction (F(3,420)=6.67, p = .0005) was due to the fact that the association with age was the weakest for occipital WMH volumes: r = .26, p=.002, followed by the parietal (r=.42, p <.00001), frontal (r=.47, p<.0001), and temporal (r=.58, p<.0001) lobes. Steiger’s test for differences in correlated correlations showed that the temporal lobe WMH had the closest association with age: Z*= 4.20, p<.0001 for temporal vs. occipital, Z*=2.08, p=.037 for temporal vs. frontal and Z*=2.80, p=.005 for temporal vs. parietal comparisons. Among all lobar WMH, the occipital showed the weakest association with age: Z*=2.28, p=.02 for comparison with the frontal, and Z*=1.89, p=.056 for comparison with the parietal. A significant Lobe × Hypertension interaction (F(3,440)= 4.48, p=.007) reflected a hypertension-related increase in WMH volume restricted to the frontal lobe: F(1,140)=4.61, p=.03, with a trend in the temporal lobe (F(1,140)=3.16, p=.077). The WMH volumes, adjusted for age and sex, were 1205.92 mm3 for hypertensive participants vs. 906.87 mm3 for their normotensive counterparts.
There were no differences in the parietal (F(1,140)=1.81, p=.18, or occipital (F(1,140)=2.08, p=.15) lobes. A significant Lobe × Sex interaction (F(3,440)=4.09, p=.01 reflected the discrepancy in the direction of sex differences trends among the lobes, with some favoring men, and other – women. However, none of the trends was significant, with the closest to the conventional 0.05 level being a trend towards a greater WMH load in the occipital lobe for men: F(1,140)=3.12, p=.08).
The next step was to examine the effects of target polymorphisms on WMH volume, while taking into account the influence of age, sex, and hypertension. The effects were tested one at time, by adding the respective allelic grouping to the model described above.
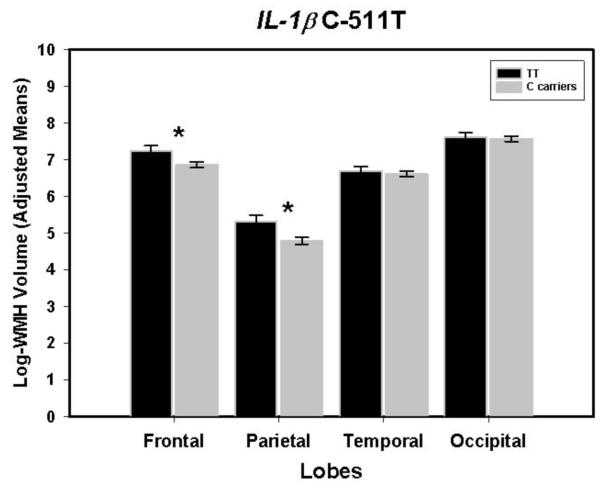
IL-1β -511T homozygotes vs. C carriers
The main effect of IL-1βT was marginally significant: F(1,139)=3.78, p=.05. It was modified by Lobe × IL-1β interaction: F(3,417)=3.69, p=.02. The effect, illustrated in Figure 1, was limited to the frontal and parietal lobes, in which T homozygotes’ WMH volumes were larger than those of the C carriers: F(1,139)=5.07 and 7.04, p = .03 and .01, respectively. After removal of one outlier (71 year old hypertensive woman; Studentized residual = 3.64), the observed effects became stronger: F(1,138)=4.56, p=.034 for the main effect, and F(3,212) = 4.08, p= .01 for IL-1B × Lobe interaction. The main effect of age and lobe × age, lobe ×hypertension, and lobe × sex interactions observed in the first model did not change with addition of the Il-1β. All the basic model effects described above (age and lobe × age, lobe ×hypertension, and lobe × sex interactions) remained significant.
Figure 1.
Volume of the white matter hyperintense regions in frontal, parietal, temporal and occipital lobes in carriers of the ancestral C allele and TT homozygotes of the single nucleotide polymorphism IL-1β C-511T. Bars are standard errors; stars indicate significant differences between the lobes.
CRP -286 C>A>T: T carriers vs. the rest
The main effect of CRP T allele was not significant F<1. However, two interactions involving the CRP factor were: CRP × Hypertension (F(1,138)=12.23, p=.004) and CRP × Hypertension × Lobe (F(3,414)=2.93, p = .04). Decomposition of the triple interaction revealed that CRP × Hypertension effect on WMH volume was significant for the frontal, temporal and parietal lobes: F(1,138)=13.56, 7.46, and 4.85, p = .0003, .01, and .03, respectively. No differences were observed in the occipital lobes. For frontal and temporal lobes, WMH volumes of persons who had hypertension and CRP T allele were significantly larger than those of participants who were normotensive, or non-carriers of T, or neither. Fisher Least-Significant Difference Test probabilities for pair-wise comparisons ranged from .0007 to .003. For parietal WMH, the differences were smaller and the Fisher’s LSD probabilities ranged from .02 to .06. The results are illustrated in Figure 2. All effects observed in the basic model remained significant.
Figure 2.
Volume of frontal, parietal, temporal and occipital white matter hyperintensities grouped according to hypertension diagnosis and presence of CRP-286T allele that is associated with highest homocysteine levels. Bars are standard errors; stars indicate significant differences between the lobes.
APOE ε variants: Comparison of ε2 carriers vs. ε3 homozygotes vs. ε4 carriers
There was no main effect of APOE ε variant: F(2,138) =1.11, p=.33. However, there was a significant Lobe × APOE interaction: F(6,414)=3.28, p=.006. Decomposition of this interaction revealed that APOE ε2 allele was associated with the largest frontal WMH, although the univariate test fell short of significance: F(2,138)=2.72, p=.07. No effect of APOE allele on WMH was observed in other lobes. In the frontal white matter, the WMH volume in ε2 carriers exceeded that of the ε3 homozygotes (Fisher’s p = .03) and ε4 carriers (Fisher’s p=.03), with the latter two groups showing no difference (Fisher’s p=.68). The main effect of age and lobe × age, lobe ×hypertension, and lobe × sex interactions observed in the first model did not change with addition of the APOE as a factor. All the basic model effects remained significant.
MTHFR 677T carriers vs. CC homozygotes and ACE Insertion/Deletion: DD homozygotes vs. I carriers
There were no significant main effect of MTHFR 677T allele or ACE DD genotype, nor were there any Lobe × polymorphism interaction: all F’s<1. All the basic model effects remained significant.
Effects of blood biomarkers on WMH volume
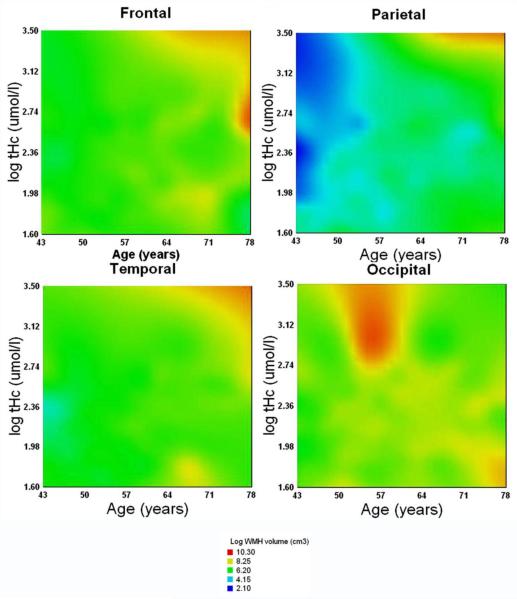
We also examined if the actual, measured levels of biomarkers – homocysteine, CRP, and LDL were associated with WMH volume. The general linear models analyses with each of the biomarkers’ levels entered (after centering at the sample mean) in separate models, revealed no effect of tHCy, LDL or CRP (all F<1). In contrast, tHcy in interaction with age was associated with WMH volume: F(1,134)=4.20, p=.04. The strength and shape of that association varied across the cerebral lobes, as indicated by Lobe × Age × Hcy interaction: F(3, 402)=6.14, p=.0001. These associations are illustrated in Figure 3 that shows on tri-variate surface plots that in frontal, parietal, and temporal lobes WMH volume was the greatest for older participants with high tHcy levels, whereas for the occipital WMH, the greatest volume was observed for middle-aged persons with elevated tHcy.
Figure 3.
Surface response plots of the tri-variate associations among age, plasma total homocysteine (log-transformed, μmol/l), and white matter hyperintensities volume (log-transformed, cm3) in four cerebral lobes (the response variable, color coded). Color temperature scale corresponds to the WMH volume: hotter colors reflect larger volumes.
Discussion
The novel finding in this study is that in healthy adults, genetic tendencies for increased inflammation are associated with increased volume of white matter hyperintensities. In the case of the CRP -286 C>A>T polymorphism, genetic and physiological risks combined to produce greater WMH burden than was associated with either of them alone. Notably, the negative impact of the risk-associated alleles was greater and more consistent in the frontal and temporal lobes, with parietal WMH showing weaker associations and occipital WMH not showing any impact at all.
In contrast, two genetic variants linked to increase in vascular risk, ACE I/D and MTHFR C677T, showed no effect on WMH burden. Among the alleles of the third vascular-risk variant, APOE ε, only ε2 was associated with increase in WMH and only in the frontal lobes. As expected from the extant literature, we observed age-related and hypertension-related increases of about 30% in WMH volume [13, 14], with both being prominent in the frontal lobe [17]. In contrast to some of the previous reports [76], we observed no clear pattern of sex differences in distribution and volume of WMH.
Differential vulnerability of the frontal lobes to aging [17, 77], and to vascular risk [17,78-80] has been well documented. Although in some studies, parietal cortices exhibit rapid age-related shrinkage as well [81], the frontal lobe differences appear with great consistency. The frontal lobes stand out as the regions, in which unlike in the other parts of the brain, almost all middle-aged and older healthy adults have at least some WMH [17,79, 82] and in which the longitudinal rate of WMH expansion is faster then in other lobes [83]. Only presence of WMH in the frontal lobe correlates with postmortem AD pathology [84], and vascular risk (hypertension) exacerbates WMH burden in the frontal lobes [17, 79-80, 82]. In acute ischemic stroke, the frontal regions withstand the worst of the damage [85]. Of course, the difference between the frontal lobe and the rest of the brain is one of a degree, not of a kind, and there are finding of differential effects of age and vascular risk on temporal and parietal WMH as well. However, the frontal lobes appear most consistently affected.
The reason for such a pattern of selective vulnerability is unclear, but it may stem from the inherent sensitivity of the regional white matter to hypoperfusion. A relatively sparse network of perforating arteries irrigates white matter and age-related changes in vascular structure, such as capillary loss, tortuosity of arterioles and accumulation of collagen in veins and venules impede cerebral blood flow (CBF) [86]. Hypertension, with its negative impact on the coronary flow reserve [87], microvasculature [87] and epithelial function [88] is accompanied by significant reduction in regional CBF [89]. Hypertension is also associated with reduced vasoreactivity, independent of WMH and stroke, the relationship is the strongest in the frontal and parietal regions and is driven mainly by elevated systolic blood pressure [90].
As WMH cluster around watershed regions of the major cerebral arteries, it is plausible that age-related decline in cerebral blood flow and chronic hypoperfusion may underpin formation and spread of the lesions [91]. Indeed, regional CBF [92, 93] is reduced within WMH borders, and persons with significant expansion of WMH evidence greater decline in CBF than those whose WMH burden evidenced lesser increases [94]. It is worth noting that in the frontal and parietal regions, anti-hypertensive therapy was the least influential in reducing age-related declines in rCBF [89]. Thus, it is plausible that the observed regional distribution of effects of age, hypertension, and polymorphisms on WMH reflects heightened sensitivity of frontal watershed areas to hypoperfusion and ischemia.
We noted an interesting pattern of relationships between an established vascular risk factor, tHcy, age and lobar WMH volumes. Jointly, older age and higher tHcy levels are associated with larger WMH volumes in frontal, parietal and temporal lobes. In the occipital lobe, middle-aged individuals with high tHcy levels have the largest WMH. Elevated tHcy is a risk factor for vascular disease [37,38], longitudinal expansion of occipital WMH may be associated with elevated vascular risk [94] and accumulation of WMH in the occipital lobe is linked to poor vascular and psychiatric outcomes [96]. Thus, it is possible that the participants who exhibited large WMH volume in the occipital lobes represent a subgroup that is at risk for cerebral and cognitive declines, a hypothesis that only a longitudinal study can test.
The effect of elevated levels CRP, especially in conjunction with hypertension may exacerbate endothelial dysfunction in poorly perfused watershed areas more than in other, better-irrigated regions. In addition, IL-1β increases blood-brain barrier permeability [52], and its elevated expression in the brain of IL-1β -511T homozygotes may promote formation of WMH in periventricular regions. CRP synthesis is influenced by multiple proinflammatory cytokines, including IL-1 [97, 98] and it would be interesting to test whether genetic predisposition to have both of them elevated is associated with incremental increase in WMH load. In this sample, there were only six participants who carried CRP T allele and were IL-1β T homozygotes. Although such a small number precludes including the CRP × IL-1β interaction in the model, comparison of the WMH volumes reveals that persons who carried this double-risk combination indeed had larger WMH volumes than the rest of the sample. Thus, a possibility of epistasis between two pro-inflammatory polymorphisms should be considered in larger samples.
The results of this study should be interpreted in the context of several limitations. First, this is a cross-sectional study, and therefore cannot elucidate age-related changes and individual differences therein. However, multiple longitudinal studies have confirm the progressive nature of age-related increase in WMH load (see [1] for a review) and it is probably safe to say that the observed age-related differences represent a snapshot of that process.
Second, the composition of the sample employed in this study imposes limits on generalization of the findings. Although the participants reported no symptoms and none of them carried a diagnosis, many of them had significant risk factors for developing vascular disease. Although our goal was to study healthy aging, and we screened the participants for history of overt disease, it would be virtually impossible to find a sample of middle-aged and older adults who would be free of all vascular risk factors, such as hypertension and hyperlipidemia.
Third, the size of the sample available for this study did not allow investigation of interactions among the genetic variants. Previous studies showed that genes that exert no significant effects on their own may affect WMH load through epistasis. For example, in one study, carriers of MTHFR 677T or ACE D alleles had increased WMH burden only when they also carried APOE ε4 or ε2 alleles [98]. In our sample, there was a possibility of epistasis between two inflammation-related SNPs, but insufficient number of participants precluded formal testing of that possibility.
Fourth, we did not assess actual blood levels of angiotensin II and interlukine-1β, and thus did not ascertain that in this sample, the genetic randomization indeed resulted in manipulation of blood biomarkers, as was the case for CRP and LDL but not for tHcy. The fact that MTHFR 677T allele was not associated with elevated homocysteine levels, may reflect uncontrolled manipulation of two significant metabolic actors in the one-carbon cycle: folate and vitamin B12 through diet. Indeed, in this sample, the levels of folate that can be reliably manipulated by nutritional means [100] were rather high. According to the Office of Dietary Supplements of the National Institutes of Health it is quite common for a breakfast cereal, a staple food for many Americans, to contain 25%-100% of daily allowance of vitamins B9 (folic acid, a synthetic form of folate), B6, B12 (http://ods.od.nih.gov/factsheets/folate/, accessed June 14, 2011). Whether such nutrition manipulation affected the possible link between WMH volume and MTHFR 677T allele remains to be investigated in studies with more precise measures of nutrition. Because we did not measure blood levels of Il-1β, we cannot confirm that the examined genetic variant indeed results in elevation of this cytokine’s levels.
Fifth, we examined only one cytokine gene polymorphism among many that can influence pro- and anti-inflammatory response. Therefore, additional candidate genes that control expression of cytokines, such as IL-6, may explain significant proportion of variance in WMH and other brain features.
In summary, genetic variants that are associated with enhanced pro-inflammatory activity increase the volume of WMH. The effects are independent of well-known risk factors for WMH, such as age and hypertension. The finding of association between one of the hallmarks of brain aging and genetic risk for enhanced inflammatory response is in accord with the theories that view aging and inflammation as inseparable aspects of the same phenomenon [48,101] dubbed “inflammaging” [102]. If this were indeed the case, in longitudinal studies one would expect to find steeper rates of neural and cognitive decline in persons carrying higher genetic load of pro-inflammatory risk. As anti-hypertensive treatment has only meager success in slowing age-related brain changes [103,104], anti-inflammatory intervention for individuals at risk may be an additional rout of intervention in attempt to stave age-related declines in brain and cognition.
Highlights.
Question: Do vascular risk and inflammation biomarkers/polymorphisms predict WMH?
WMH volume is associated with age, hypertension, and homocysteine and CRP levels.
IL-1β -511T homozygotes & CRP -286T carriers have enlarged WMH .
ApoE ε2 carriers had larger frontal WMH than ε3 homozygotes, and ε4 carriers.
Acknowledgement
This work was supported by a National Institute on Aging grant R37-AG011230 to N.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Raz N, Kennedy KM. A Systems Approach to Age-Related Change: Neuroanatomic changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. Oxford University Press; New York, NY: 2009. pp. 43–70. [Google Scholar]
- [2].Kemper TL. Neuroanatomical and neuropathological changes during aging and in dementia. In: Albert ML, Knoepfel EJE, editors. Clinical neurology of aging. Oxford University Press; New York: 1994. pp. 3–67. [Google Scholar]
- [3].Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- [4].Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psych. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- [6].Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified In vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- [8].Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hackinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- [10].Pantoni L, Garcia JH. Cognitive impairment and cellular/vascular changes in the cerebral white matter. Ann NY Acad Sci. 1997;vol. 826:92–102. doi: 10.1111/j.1749-6632.1997.tb48463.x. [DOI] [PubMed] [Google Scholar]
- [11].De Leeuw FE, De Groot JC, Achten E, Oudkerk M, Ramos LMP, Heijboer R, Hofman A, Jolles J, Van Gijn J, Breteler MMB. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosur PS. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Young VG, Halliday GM, Krill JJ. Neuropathologic correlates of white matter. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- [13].Kertesz A, Black SE, Tokar G, Benke T, Carr T, Nicholson L. Periventricular and subcortical hyperintensities on magnetic resonance imaging. ‘Rims, caps, and unidentified bright objects’. Arch Neurol. 1988;45:404–408. doi: 10.1001/archneur.1988.00520280050015. [DOI] [PubMed] [Google Scholar]
- [14].Fazekas F, Niederkorn K, Schmidt R, Offenbacher H, Horner S, Bertha G, Lechner H. White matter signal abnormalities in normal individuals: Correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- [15].Inzitari D, Diaz F, Fox A, Hachinski VC, Steingart A, Lau C, Donald A, Wade J, Mulic H, Merskey H. Vascular risk factors and leuko-araiosis. Arch Neurol. 1987;44:42–47. doi: 10.1001/archneur.1987.00520130034014. [DOI] [PubMed] [Google Scholar]
- [16].DeCarli C, Murphy, Tranh M, Grady CL, Haxby JV, Gillette JA, Salerno JA, Gonzales-Aviles A, Horwitz B, Rapoport SI, Schapiro MB. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- [17].Raz N, Rodrigue KM, Acker JD. Hypertension and the Brain: Vulnerability of the Prefrontal Regions and Executive Functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- [18].Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham study. Stroke. 2004;35:1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- [19].Kochunov P. Whole brain and regional hyperintense white matter volume and blood pressure overlap of genetic loci produced by bivariate, whole-genome linkage analyses. Stroke. 2010;41:2137–2142. doi: 10.1161/STROKEAHA.110.590943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, BL. M. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- [21].Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL, Rosamond WD, Rivadeneira F, Kelly-Hayes M, Lopez OL, Coresh J, Hofman A, DeCarli C, Heckbert SR, Koudstaal PJ, Yang Q, Smith NL, Kase CS, Rice K, Haritunians T, Roks G, de Kort PL, Taylor KD, de Lau LM, Oostra BA, Uitterlinden AG, Rotter JI, Boerwinkle E, Psaty BM, Mosley TH, van Duijn CM, Breteler MM, Longstreth WT, Jr., Wolf PA. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- [23].Roses AD. Apolipoprotein E and Alzheimer’s disease. A rapidly expanding field with medical and epidemiological consequences. Ann NY Acad Sci. 1996;802:50–57. doi: 10.1111/j.1749-6632.1996.tb32598.x. [DOI] [PubMed] [Google Scholar]
- [24].Cherbuin N, Leach LS, Christensen H, Anstey KJ. Neuroimaging and APOE genotype: A systematic qualitative review. Dement Geriatr Cogn Disord. 2007;24:348–362. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- [25].Bigler ED, Lowry CM, Kerr B, Tate DF, Hessel CD, Earl H, D, Miller MJ, Rice SA, Smith KH, Tschanz JT, Welsh-Bohmer K, Plassman B, Victoroff J. Role of white matter lesions, cerebral atrophy, and APOE on cognition in older persons with and without dementia: the Cache County, Utah, study of memory and aging. Neuropsychology. 2003;17:339–352. doi: 10.1037/0894-4105.17.3.339. [DOI] [PubMed] [Google Scholar]
- [26].He J, Iosif AM, Lee DY, Martinez O, Chu S, Carmichael O, Mortimer JA, Zhao Q, Ding D, Guo Q, Galasko D, Salmon DP, Dai Q, Wu Y, Petersen RC, Hong Z, Borenstein AR, DeCarli C. Brain structure and cerebrovascular risk in cognitively impaired patients: Shanghai Community Brain Health Initiative-pilot phase. Arch Neurol. 2010;67:1231–1237. doi: 10.1001/archneurol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paternoster L, Chen W, Sudlow CLM. Genetic determinants of white matter hyperintensities on brain scans: A systematic assessment of 19 candidate gene polymorphisms in 46 studies in 19 000 subjects. Stroke. 2009;40:2020–2026. doi: 10.1161/STROKEAHA.108.542050. [DOI] [PubMed] [Google Scholar]
- [28].Lemmens R. Association of apolipoprotein E ε2 with white matter disease but not with microbleeds. Stroke. 2007;38:1185–1188. doi: 10.1161/01.STR.0000259816.31370.44. [DOI] [PubMed] [Google Scholar]
- [29].Schmidt R, Schmidt H, Fazekas F, Schumacher M, Niederkorn K, Kapeller P, Weinrauch V, Kostner GM. Apolipoprotein E polymorphism and silent microangiopathy-related cerebral damage. Results of the Austrian Stroke Prevention Study. Stroke. 1997;28:951–956. doi: 10.1161/01.str.28.5.951. [DOI] [PubMed] [Google Scholar]
- [30].Hall J, E. The renin-angiotensin system: renal actions and blood pressure regulation. Compr Ther. 1991;17:8–17. [PubMed] [Google Scholar]
- [31].Cambien F. The angiotensin-converting enzyme (ACE) genetic polymorphism: its relationship with plasma ACE level and myocardial infarction. Clin Genet. 1994;46(1 Spec No):94–101. doi: 10.1111/j.1399-0004.1994.tb04210.x. [DOI] [PubMed] [Google Scholar]
- [32].Amar K, MacGowan S, Wilcock G, Lewis T, Scott M. Are genetic factors important in the aetiology of leukoaraiosis? Results from a memory clinic population. Int J Geriatr Psychiatry. 1998;13:585–590. doi: 10.1002/(sici)1099-1166(199809)13:9<585::aid-gps825>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [33].Sierra C, Coca A, Gómez-Angelats E, Poch E, Sobrino J, de la Sierra A. Renin-angiotensin system genetic polymorphisms and cerebral white matter lesions in essential hypertension. Hypertension. 2002;39:343–347. doi: 10.1161/hy02t2.102912. [DOI] [PubMed] [Google Scholar]
- [34].Purandare N, Voshaar R.C. Oude, Davidson Y, Gibbons L, Hardicre J, Byrne J, McCollum C, Jackson A, Burns A, Mann DM. Deletion/insertion polymorphism of the angiotensin-converting enzyme gene and white matter hyperintensities in dementia: A pilot study. J Am Geriatr Soc. 2006;54:1395–1400. doi: 10.1111/j.1532-5415.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- [35].Hou Z, Yuan Y, Zhang Z, Hou G, You J, Bai F. The D-allele of ACE insertion/deletion polymorphism is associated with regional white matter volume changes and cognitive impairment in remitted geriatric depression. Neurosci Lett. 2010;479:262–266. doi: 10.1016/j.neulet.2010.05.076. [DOI] [PubMed] [Google Scholar]
- [36].Markus HS. Genes, endothelial function and cerebral small vessel disease in man. Exp Physiol. 2008;93:121–127. doi: 10.1113/expphysiol.2007.038752. [DOI] [PubMed] [Google Scholar]
- [37].Brattström L, Israelsson B, Hultberg B. Plasma homocysteine and methionine tolerance in early-onset vascular disease. Haemostasis. 1989;19(Suppl 1):35–44. doi: 10.1159/000216094. [DOI] [PubMed] [Google Scholar]
- [38].Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Homocysteine and essential hypertension. J Clin Pharmacol. 2003;43:1299–1306. doi: 10.1177/0091270003258190. [DOI] [PubMed] [Google Scholar]
- [39].Casas JP, Hingorani AD, Bautista LE, Sharma P. Pankaj. Meta-analysis of Genetic Studies in Ischemic Stroke. Thirty-two genes involving approximately 18000 cases and 58000 controls. Arch Neurol. 2004;61:1652–1662. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- [40].de Lau LML, van Meurs JBJ, Uitterlinden AG, Smith AD, Refsum H, Johnston C, Breteler MMB. Genetic variation in homocysteine metabolism, cognition, and white matter lesions. Neurobiol Aging. 2010;31:2020–2022. doi: 10.1016/j.neurobiolaging.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [41].Watfa G, Marteau JB, Rossignol P, Kearney-Schwartz A, Fay R, Bracard S, Felblinger J, Boivin JM, Lacolley P, Visvikis-Siest S, Benetos A, Zannad F. Association study of gene polymorphisms involved in vascular alterations in elderly hypertensives with subjective memory complaints. Dement Geriatr Cogn Disord. 2010;30:440–448. doi: 10.1159/000321120. [DOI] [PubMed] [Google Scholar]
- [42].Stankovic S, Majkic-Singh N. Genetic aspects of ischemic stroke: coagulation, homocysteine, and lipoprotein metabolism as potential risk factors. Cr Rev Cl Lab Sc. 2010;47:72–123. doi: 10.3109/10408361003791520. [DOI] [PubMed] [Google Scholar]
- [43].Lazzerini P, Capecchi P, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Pasini F. Laghi. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. 2007;6:503–509. doi: 10.1016/j.autrev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [44].Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- [45].Finch C, E. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. PNAS. 2010;107:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000;49:561–570. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- [47].Schmitz T, Chew L-J. Cytokines and myelination in the Central Nervous System. Thescientificworldjo. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rothwell NJ. Cytokines - killers in the brain? J Physiol. 1999;(Pt 1):3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama K, Y Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26 doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- [50].Hayes A, Green EK, Pritchard A, Harris JM, Zhang Y, Lambert JC, Chartier-Harlin MC, Pickering-Brown SM, Lendon CL, Mann DM. A polymorphic variation in the interleukin 1A gene increases brain microglial cell activity in Alzheimer’s disease. J Neurol Neurosur PS. 2004;75:1475–1477. doi: 10.1136/jnnp.2003.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Allan SM. Cortical cell death induced by IL-1 is mediated via actions in the hypothalamus of the rat. P NATL ACAD SCI. 2000:97. doi: 10.1073/pnas.090464197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Didier N, Romero IA, Créminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;96:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- [53].Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psych Neurol. 2009 March;22:23–24. doi: 10.1177/0891988708328216. [DOI] [PubMed] [Google Scholar]
- [54].Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injection of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- [55].Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [56].Dziedzic T, Slowik A, Pera J, Szczudlik A. Interleukin 1 beta polymorphism (−511) and risk of stroke due to small vessel disease. Cerebrovasc Dis. 2005;20:299–303. doi: 10.1159/000087928. [DOI] [PubMed] [Google Scholar]
- [57].Kauffman MA, Moron DG, Consalvo D, Bello R, Kochen S. Association study between interleukin 1 beta gene and epileptic disorders: a HuGe review and meta-analysis. Genet Med. 2008;10:83–88. doi: 10.1097/GIM.0b013e318161317c. [DOI] [PubMed] [Google Scholar]
- [58].Di Bona D, Plaia A, Vasto S, Cavallone L, Lescai F, Franceschi C, Licastro F, Colonna-Romano G, Lio D, Candore G, Caruso C. Association between the interleukin-1beta polymorphisms and Alzheimer’s disease: a systematic review and meta-analysis. Brain Res Rev. 2008;59:155–163. doi: 10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [59].Papiol S, Molina V, Desco M, Rosa A, Reig S, Gispert JD, Sanz J, Palomo T, Fañanás L. Ventricular enlargement in schizophrenia is associated with a genetic polymorphism at the interleukin-1 receptor antagonist gene. NeuroImage. 2005;27:1002–1006. doi: 10.1016/j.neuroimage.2005.05.035. [DOI] [PubMed] [Google Scholar]
- [60].Papiol S, Molina V, Desco M, Rosa A, Reig S, Sanz J, Palomo T, Fañanás L. Gray matter deficits in bipolar disorder are associated with genetic variability at interleukin-1 beta gene (2q13) Genes Brain Behav. 2008;7:796–801. doi: 10.1111/j.1601-183X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- [61].Fornage M, Chiang YA, O’Meara ES, Psaty BM, Reiner AP, Siscovick DS, Tracy RP, Longstreth WTJ. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. Stroke. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon IRO, Criqui M. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice, a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- [63].Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- [64].Van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. C-reactive protein and cerebral small-vessel disease: The Rotterdam scan study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- [65].Wright CB, Moon Y, Paik MC, Brown TR, Rabbani L, Yoshita M, DeCarli C, Sacco R, Elkind MS. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke. 2009;40:3466–3471. doi: 10.1161/STROKEAHA.109.559567. Epub 2009 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. New Engl J Med. 2005;35:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- [67].Haan MN, Aiello AE, West NA, Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of Apolipoprotein APOE4 genotype. Neurobiol Aging. 2008;29:1774–1782. doi: 10.1016/j.neurobiolaging.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Reitz C, Berger K, De Maat MPM, Stoll M, Friedrichs F, Kardys I, Witteman JCM, Breteler MMB. CRP gene haplotypes, serum CRP, and cerebral small-vessel disease: The Rotterdam Scan Study and the MEMO Study. Stroke. 2007;38:2356–2359. doi: 10.1161/STROKEAHA.107.482661. [DOI] [PubMed] [Google Scholar]
- [69].Shen J, Arnett DK, Parnell LD, Peacock JM, Lai CQ, Hixson JE, Tsai MY, Province MA, Straka RJ, Ordovas JM. Association of common C-reactive protein (CRP) gene polymorphisms with baseline plasma CRP levels and fenofibrate response: the GOLDN study. Diabetes Care. 2008;31:910–5. doi: 10.2337/dc07-1687. Epub 2008 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- [71].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [72].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [73].Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- [74].Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- [75].Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- [76].Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- [77].Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in Vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- [78].Apter NS, Halstead WC, Heimburger RF. Impaired cerebral functions in essential hypertension. Am J Psychiat. 1951;107:808–813. doi: 10.1176/ajp.107.11.808. [DOI] [PubMed] [Google Scholar]
- [79].Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 2010 Dec 29; doi: 10.1016/j.neurobiolaging.2010.11.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kuller LH, Margolis KL, Gaussoin SA, Bryan NR, Kerwin D, Limacher M, Wassertheil-Smoller S, Williamson J, J.G. R, W.s.H.I.M.S.R. Group Relationship of hypertension, blood pressure, and blood pressure control with white matter abnormalities in the Women’s Health Initiative Memory Study (WHIMS)-MRI trial. J Clin Hypertens (Greenwich) 2010;12:203–212. doi: 10.1111/j.1751-7176.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- [82].Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F, L.S. Group Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- [84].Polvikoski TM, van Straaten EC, Barkhof F, Sulkava R, Aronen HJ, Niinistö L, Oinas M, Scheltens P, Erkinjuntti T, Kalaria RN. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75:2071–2078. doi: 10.1212/WNL.0b013e318200d6f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, Gonzalez RG, Lev MH. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke. 2011;42:1255–1260. doi: 10.1161/STROKEAHA.110.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropath Appl Neuro. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Levy BI, Schiffrin EL, Mourad J-J, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HAJ. Impaired Tissue Perfusion: A Pathology Common to Hypertension, Obesity, and Diabetes Mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- [88].Hummler E, Rossier BC. Physiological and pathophysiological role of the epithelial sodium channel in the control of blood pressure. Kidney Blood Press R. 1996;19:160–165. doi: 10.1159/000174065. [DOI] [PubMed] [Google Scholar]
- [89].Nobili F, Rodriguez G, Marenco S, De Carli F, Gambaro M, Castello C, Pontremoli R, Rosadini G. Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke. 1993;24:1148–1153. doi: 10.1161/01.str.24.8.1148. [DOI] [PubMed] [Google Scholar]
- [90].Hajjar I, Zhao P, Alsop D, Novak V. Hypertension and cerebral vasoreactivity: a continuous arterial spin labeling magnetic resonance imaging study. Hypertension. 2010;56:859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Enzinger C, Smith S, Fazekas F, Drevin G, Ropele S, Nichols T, Behrens T, Schmidt R, Matthews PM. Lesion probability maps of white matter hyperintensities in elderly individuals: results of the Austrian stroke prevention study. J Neurol. 2006;253:1064–1070. doi: 10.1007/s00415-006-0164-5. [DOI] [PubMed] [Google Scholar]
- [92].Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- [93].Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, Stern Y. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiat Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kraut MA, Beason-Held LL, Elkins WD, Resnick SM. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J Cereb Blood Flow Metab. 2008;28:190–197. doi: 10.1038/sj.jcbfm.9600512. [DOI] [PubMed] [Google Scholar]
- [95].Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- [96].Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry. 2004;75:1304–1308. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Katrinchak C, Fritz K. Clinical implications of C-reactive protein as a predictor of vascular risk. J Am Acad Nurse Prac. 2007;19:335–340. doi: 10.1111/j.1745-7599.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- [98].Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- [99].Szolnoki Z, Somogyvári F, Kondacs A, Szabó M, Fodor L, Bene J, Melegh B. Specific APO E genotypes in combination with the ACE D/D or MTHFR 677TT mutation yield an independent genetic risk of leukoaraiosis. Acta Neurol Scand. 2004;109:222–227. doi: 10.1046/j.1600-0404.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- [100].Brady CB, Gaziano JM, Cxypoliski RA, Guarino PD, Kaufman JS, Warren SR, Hartigan P, Goldfarb DS, Jamison RL. Homocysteine lowering and cognition in CKD: the Veterans Affairs homocysteine study. Am J Kidney Dis. 2009;54:440–449. doi: 10.1053/j.ajkd.2009.05.013. Epub 2009 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Goto M. Inflammaging (inflammtation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- [102].Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- [103].Strandgaard S, Paulson OB. Cerebral blood flow in untreated and treated hypertension. Neth J Med. 1995;47:180–184. doi: 10.1016/0300-2977(95)00065-u. [DOI] [PubMed] [Google Scholar]
- [104].Jennings JR, Mendelson DN, Muldoon M, Ryan G, P J, Raz N, Aizenstein H. the Alzheimer’s Disease Neuroimaging Initiative, Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2011 doi: 10.1038/jhh.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]