Abstract
Background
Genetic factors and early life adversity are critical in the etiology of mood disorders and substance abuse. Because of their role in the transduction of stress responses, glucocorticoid hormones and their receptors could serve as both genetic factors and mediators of environmental influences. We have shown that constitutive overexpression of the glucocorticoid receptor (GR) in forebrain results in increased emotional reactivity and “lability” in mice. Here we asked whether there was a critical period for the emergence of this phenotype.
Methods
We generated a mouse line with inducible GR overexpression specifically in forebrain (GRov). Anxiety-like behaviors and cocaine-induced sensitization were assessed in adult mice following GR overexpression during different periods in development. The molecular basis of the behavioral phenotype was examined using microarray analyses of dentate gyrus and nucleus accumbens.
Results
Transient overexpression of GR during early life led to increased anxiety and cocaine sensitization, paralleling the phenotype of lifelong GR overexpression. This increased emotional reactivity was not observed when GR overexpression was induced after weaning. GR overexpression in early life is sufficient to alter gene expression patterns for the rest of the animal’s life, with dentate gyrus being more responsive than nucleus accumbens. The altered transcripts are implicated in GR and axonal guidance signaling in dentate gyrus and dopamine receptor signaling in nucleus accumbens.
Conclusions
Transient overexpression of GR early in life is both necessary and sufficient for inducing transcriptome-wide changes in the brain and producing a lifelong increase in vulnerability to anxiety and drugs of abuse.
Keywords: Glucocorticoid receptor, early life, forebrain, anxiety, sensitization, cocaine
Introduction
Mood and addictive diseases are complex genetic disorders exacerbated by environmental stressors. Indeed, the genetic vulnerability to these illnesses may consist of enhanced reactivity to environmental adversity (1). Such a view would suggest that the best genetic candidates for vulnerability or resilience to such disorders would be those genes that modify the organism’s ability to cope with environmental demands. Here we focus on a molecule that controls reactivity to stress, the glucocorticoid receptor (GR), and ask whether there is a critical developmental period wherein GR overexpression can alter emotional responsiveness throughout life.
Stress triggers the activation of hypothalamus-pituitary-adrenal (HPA) axis, leading to the release of glucocorticoids from the adrenal cortex. Glucocorticoids exert their effects via two ligand-dependent transcription factors, the glucocorticoid receptor and the mineralocorticoid receptor (MR), which in turn regulate patterns of downstream gene expression during development and in adulthood (2–6). Extremely low or high glucocorticoid levels significantly impair brain development and function (7). Stressful events during childhood increase later risk for neuropsychiatric disorders and substance abuse (8, 9). These early adverse experiences are associated with structural and functional changes in brain regions mediating emotion and stress response, such as the hippocampus (HC), amygdala, prefrontal cortex, and nucleus accumbens (NAcc) (10–12). Both the magnitude and duration of the glucocorticoid stress response change dramatically across the lifespan (13, 14). Furthermore, changes in GR expression levels in key modulatory sites of the HPA axis contribute to the neonatal maturation of the HPA axis and other circuits involved in the regulation of emotional responses (15–17) and the development of addiction (18–20).
The role of brain GR in stress, mood disorders and drug addiction has been investigated in a number of animal models (18, 19, 21–28), though few have addressed the developmental trajectory. There are several converging lines of evidence that suggest that the degree of expression of GR in the central nervous system may be positively correlated with spontaneous anxiety behavior. Thus, GR deficient mice exhibit decreased anxiety-like behavior (23, 29, 30). Moreover, previous work in our laboratory has shown that rats that exhibit differences in anxiety and risk-taking behavior also have differences in GR expression in the hippocampus. High levels of spontaneous anxiety are associated with high levels of hippocampal GR and injection of a GR antagonist into the hippocampus caused the more anxious animals to become more exploratory (31). We have shown that constitutive glucocorticoid receptor overexpression in forebrain results in increased anxiety-like behavior and enhanced cocaine-induced sensitization in mice (19). In this study, we sought to define the critical period during development associated with increased emotional reactivity by using the CaMKIIα-tTA Tet-Off system to test the impact of the timing of the forebrain-specific induction of GR on the outcome. Our findings indicate that GR overexpression early in life is both necessary and sufficient for producing the two central features of the phenotype: high anxiety behavior and enhanced cocaine sensitization. These behavioral changes are coupled with a profound change in gene expression profiles in the dentate gyrus and nucleus accumbens.
Methods and Materials
Generation of Inducible GRov Mice
The codons encoding the influenza hemagglutinin (HA) epitope were added to 5’-end of the full length mouse GR cDNA (19). The HA-GR cDNA was cloned into the response plasmid pTRE (tetracycline-response element) of the Tet-Off inducible system. Founder TRE-HA-GR mice were crossed with CaMKIIα-tTA mice to produce bigenic mice that were treated +/− doxycycline (Dox) for various stages of lifetime. The bigenic mice express the tet-transactivator (tTA) protein only in forebrain, and tTA binds to the TRE sequence to induce transgene GR transcription in forebrain in the absence of Dox. In the presence of Dox, tTA does not bind the TRE and transgene GR mRNA is not transcribed. For detailed information, see Supplemental Methods and Materials in Supplement 1.
Behavioral Assessment
All experiments were performed with male bigenic mice as adults. The elevated plus maze, the light-dark box, and the defensive withdrawal tests were performed in the morning. For cocaine-induced behavioral sensitization, mice were first habituated to the apparatus for 15 min, then given cocaine and immediately placed back into the apparatus. After daily injections of cocaine for 5 consecutive days, mice were undisturbed for 8 days, and then given a challenge with the same dose of cocaine on Day 14. For detailed information, see Supplemental Methods and Materials in Supplement 1.
Microarray Analysis
Male adult mouse brains were collected in the morning under undisturbed condition. Laser capture microdissection (LCM) was performed and each cap contained 2 hippocampal dentate gyrus (DG) or 4 NAcc microdissected areas from serial sections for each animal. Following RNA isolation and amplification, biotinylated aRNA from each region was hybridized to Affymetrix Mouse Genome 430_2.0 GeneChips. GeneChip signal intensity data were interpreted by use of a custom chip description file (filename MM430_2_REFSEQ_v13). This file is available at http://brainarray.mbni.med.umich.edu. A detailed description is provided in Supplemental Methods and Materials in Supplement 1.
Statistical Analysis
Results are expressed as mean ± SEM. Statistical comparisons were determined by unpaired two-tailed t test and ANOVA followed by post hoc tests as necessary. Two-factor ANOVA (group × day) with repeated measures was performed on general locomotor activity in the open field. Two-factor ANOVAs (group × day) were performed on cocaine study. Ingenuity function analysis and pathway analysis were performed using Ingenuity Pathway Analysis software (IPA version 8.8).
Results
Generation of Inducible GRov Mice
To produce an animal model where we could not only target GR overexpression to the forebrain but also achieve control over the timing of the overexpression, we generated transgenic mice expressing HA-GR under the control of the TRE of the Tet-Off inducible system. In this bigenic system, Dox inhibits expression of transgene and removal of the drug allows overexpression to take place (Figure S1A in Supplement 1). To achieve forebrain-specific expression of the GR protein, the TRE-GR transgenic mouse line was bred with CaMKIIα-tTA mice in which tTA expression is targeted to neurons mainly in cortex, HC and striatum. We carried out studies with the following conditions: 1) Lifetime GRov mice: induction of GR throughout the lifetime of the animal -- bigenic mice were never treated with Dox; 2) Early Life GRov mice: induction of GR during the first 3 weeks of life -- bigenic mice were not given Dox until weaning at 3 weeks old and they were then treated with Dox for the rest of their lives; 3) Adult GRov mice: induction of GR after weaning and into adulthood -- bigenic mice were given Dox until weaning, including the gestation period. Dox treatment was then omitted for the rest of their lives; 4) No-GRov mice: no induction of GR at any time -- bigenic mice were treated with Dox throughout their lives, including the gestation period. Using No-GRov mice as control proved to be important as the CaMKIIα-tTA mice in this study showed decreased locomotor activity (32).
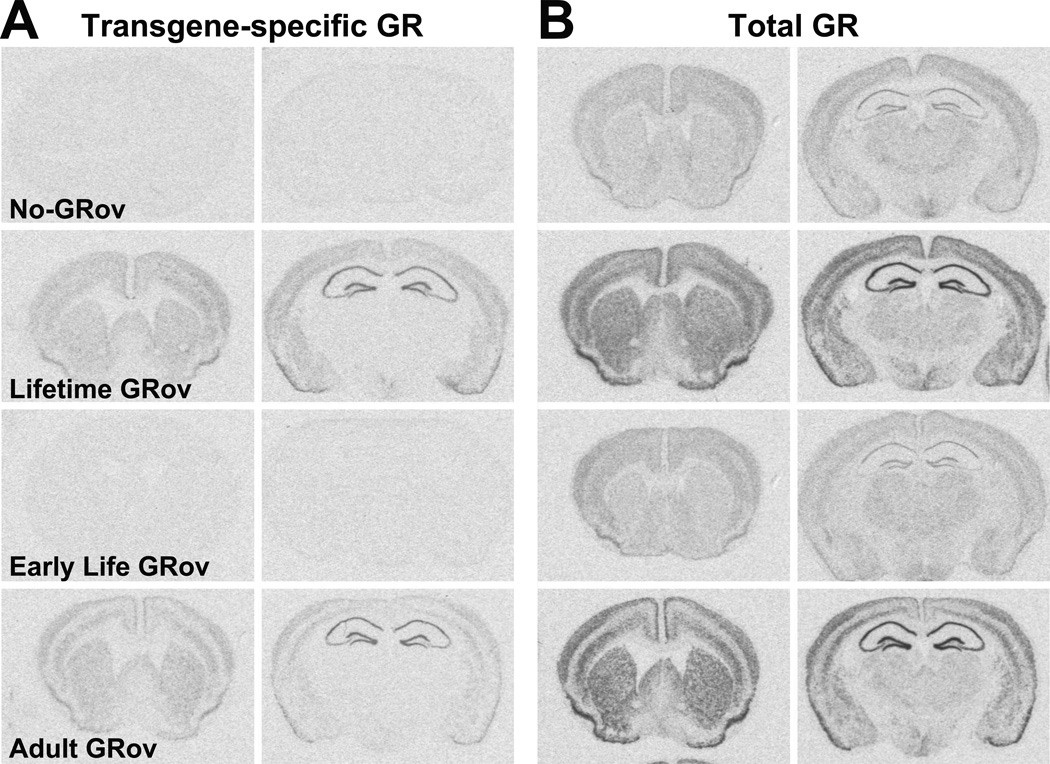
In situ hybridization (ISH) experiments using a probe specific to the transgene showed that the distribution of transgene-specific GR mRNA in adult bigenic mice was primarily forebrain-specific (Figure 1A, second row). Transgene-specific GR was expressed in cerebral cortex, olfactory nuclei, caudate-putamen, nucleus accumbens, septum, HC (CA1, CA2, CA3, and DG), some of the nuclei in the amygdala complex, as well as some nuclei in the hypothalamus. The most intense hybridization signals for the transgene-specific GR mRNA were observed in HC. Transgene-specific GR overexpression in forebrain could be reliably induced in the absence of Dox (Figure 1A, second and fourth rows) or turned off in the presence of Dox (Figure 1A, first and third rows). Furthermore, transgene-specific HA-GR (Figure S1B in Supplement 1, left) and overexpression of GR (Figure S1B in Supplement 1, right) proteins in HC were confirmed by Western blot.
Figure 1.
Generation of inducible glucocorticoid receptor overexpression in forebrain (GRov) mice. Representative sections showing transgene-specific glucocorticoid receptor (GR) mRNA expression (A) and overexpression of total GR mRNA (B) from bigenic mice under different induction conditions when examined as adults (first row: No-GRov; second row: Lifetime GRov; third row: Early Life GRov; fourth row: Adult GRov). Representative images are shown at Bregma 1.18 mm (left) and −1.94 mm (right). n = 5–7 mice per group.
As can be seen in Figure 1A (third row), there was no detectable transgene-specific GR mRNA in Early Life GRov mice when examined as adults. However, Early Life GRov mice exhibited comparable distribution patterns of the transgene-specific GR mRNA at postnatal day 21 (weaning) compared to the Lifetime GRov mice (Figure S2A in Supplement 1). By contrast, there was no detected transgene-specific GR mRNA at postnatal day 21 in bigenic mice that were treated with Dox before weaning (Figure S2B in Supplement 1, top row).
ISH analysis using a probe for GR mRNA showed that Lifetime GRov mice exhibited significantly higher levels of total GR mRNA in forebrain compared to No-GRov mice (Figure 1B) (Table S1 in Supplement 1). GR mRNA levels across forebrain regions were comparable between Lifetime and Adult GRov mice, except for regions of the laminar layers of the cortex and the amygdala (Figure 1B) (Table S1 in Supplement 1). By contrast, the distribution pattern and levels of GR mRNA were comparable between Early Life GRov mice tested as adults and No-GRov mice (Figure 1B) (Table S1 in Supplement 1), indicating no profound changes in GR mRNA expression in Early Life GRov mice when they reach adulthood, even in the face of overexpression of the transgene-specific GR early in life.
Normal Basal HPA Axis Activity
There were no significant differences in basal circulating adrenocorticotropic hormone (ACTH) and corticosterone (CORT) levels among No-GRov, Lifetime GRov, Early Life GRov, and Adult GRov mice in the morning (Figure S3 in Supplement 1). ISH experiments revealed no group differences in basal mRNA expression of MR in HC (Table S2 in Supplement 1). These results suggest that overexpression of GR in the forebrain early in life, post-weaning, or throughout the animal’s lifetime does not substantially alter basal HPA axis activity.
Increased Anxiety-Like Behavior in Early Life GRov Mice
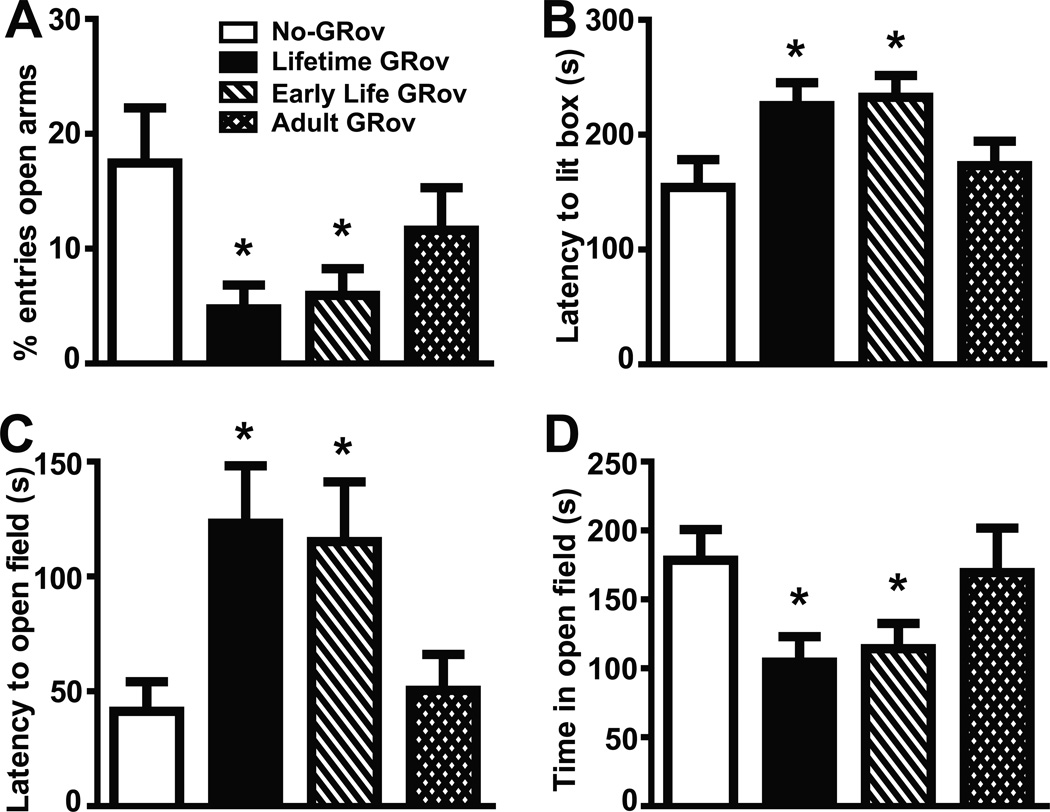
General locomotor activity was measured in adult mice for 30 min in the open field with repeated measures for 3 consecutive days. The results did not reveal any group differences in locomotor activity (Figure S4 in Supplement 1), indicating there is no significant effect of genotype or Dox treatment on this behavior. To determine the behavioral consequences of forebrain-specific GR overexpression during different phases of the life on reactivity in an anxiogenic environment, adult mice were examined by the elevated plus maze, the light-dark box, and the defensive withdrawal tests. Lifetime GRov mice exhibited significantly increased anxiety responses in these tests (p < .05 for each test) (Figure 2) compared to No-GRov mice, replicating our finding of increased anxiety-like behavior in constitutive forebrain-specific GRov mice (19). Interestingly, Early Life GRov mice, with GR overexpression induced only during the first 3 weeks of life, demonstrated the same pattern of increased anxiety-like behavior (p < .05 for each test) (Figure 2) compared to No-GRov controls. By contrast, Adult GRov mice whose GR overexpression was induced after weaning and remained induced at the time of behavioral evaluation, showed no evidence of increased anxiety behavior (Figure 2).
Figure 2.
Increased anxiety-like behavior in Early Life and Lifetime GRov mice. (A) Both Early Life and Lifetime GRov mice entered the open arms significantly fewer times than No-GRov mice in the elevated plus maze. (B) Both Early Life and Lifetime GRov mice had a significantly longer latency to enter the lit side of the light-dark box. (C and D) Both Early Life and Lifetime GRov mice had a significantly longer latency to exit from the dark chamber into the open field (C) and spent significantly less time in the open field (D) than No-GRov mice in the defensive withdrawal test. GRov, glucocorticoid receptor overexpression in forebrain. *p < .05 versus No-GRov group. Elevated plus maze: n = 18–20 mice per group. Light-dark box: n = 26–30 mice per group. Defensive withdrawal: n = 7–10 mice per group.
These results demonstrate that overexpression of GR in forebrain during early life is necessary and sufficient for triggering increased anxiety-like behavior in mice. By contrast, overexpression of GR in forebrain after weaning is neither necessary nor sufficient to produce the anxiety phenotype. These findings suggest that early developmental programs surrounding stress responsiveness are set in place early during development and have lifelong consequences for anxiety behavior.
Enhancement of Cocaine-Induced Sensitization in Early Life GRov Mice
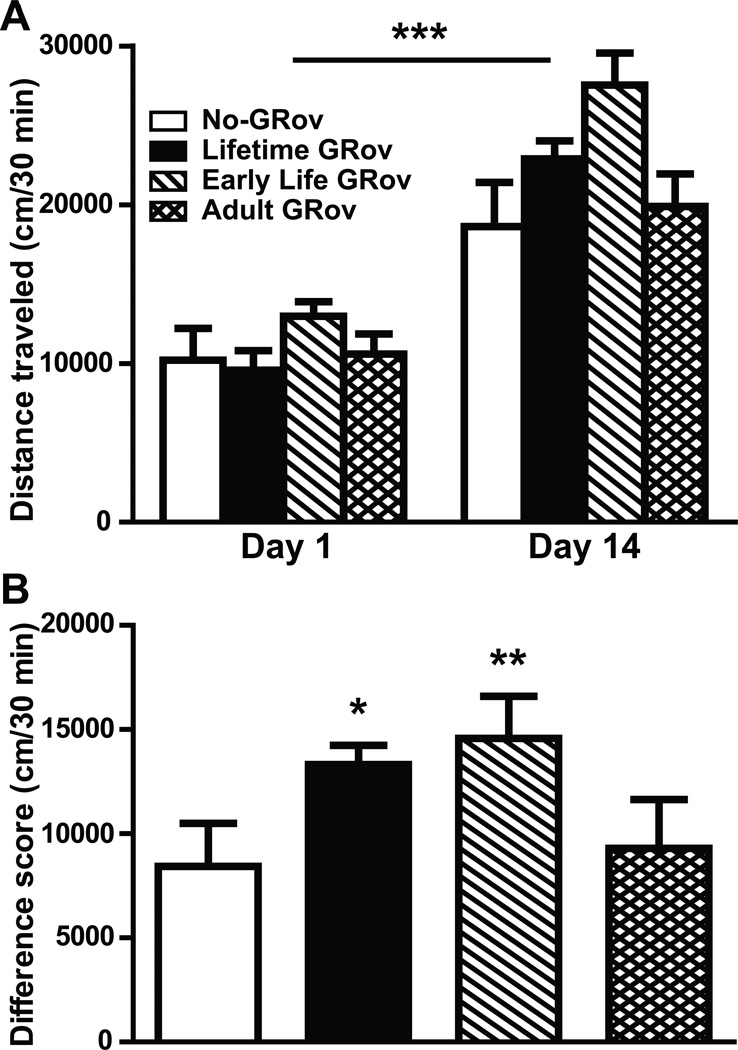
To evaluate the role of forebrain-specific GR overexpression on the locomotor response produced by cocaine, we administered i.p. cocaine to all four groups of bigenic mice and measured locomotion in the open field. Locomotor activity in response to the cocaine challenge on Day 14 was significantly enhanced relative to Day 1 in all groups of mice (p < .001) (Figure 3A) (Figure S5 in Supplement 1), indicating the development of behavioral sensitization. ANOVA revealed a significant group × day interaction among No-, Lifetime, and Early Life GRov mice (p < .05). To further assess the “degree” of sensitization, we calculated the difference in distance traveled between Day 14 and Day 1 in these bigenic mice (Figure 3B). Both Lifetime and Early Life GRov mice showed greater sensitization than No-GRov mice (p < .05 and p < .01, respectively). Once again, Adult GRov mice did not differ significantly from No-GRov controls. These results demonstrate that overexpression of GR in forebrain during early life also plays a role in causing enhanced cocaine-induced sensitization in mice, indicating that early developmental events represent a critical antecedent for the vulnerability for drug abuse.
Figure 3.
Enhancement of cocaine-induced sensitization in Early Life and Lifetime GRov mice. (A) Distance traveled in response to the cocaine challenge was significantly increased at Day 14 relative to Day 1 in all four groups of bigenic mice. ANOVA revealed a significant group × day interaction among No-, Lifetime, and Early Life GRov mice (p < .05). ***p < .001 versus respective group on Day 1. (B) The difference in distance traveled between Day 14 and Day 1. Both Lifetime and Early Life GRov mice showed a greater degree of sensitization using this measure compared to No-GRov mice. GRov, glucocorticoid receptor overexpression in forebrain. *p < .05 and **p < .01 versus No-GRov group. n = 9–16 mice per group.
Altered Gene Expression Profiling in DG of HC and NAcc
Given the above behavioral results, we undertook an investigation of the molecular mechanisms that may lead to the increased emotional reactivity and drug vulnerability seen with GR overexpression. We focused on the two groups that produced a similar behavioral phenotype -- Lifetime and Early Life GRov and asked what they have in common in terms of gene expression changes relative to No-GRov controls. We carried out gene expression profiling studies using laser capture microdissection combined with microarray to evaluate global change in basal gene expression in DG and in NAcc. These two regions were selected for several reasons: a) the hippocampus in general and the DG in particular were sites of normal high expression of GR and of overexpression in GRov; b) our previous work on constitutive GRov had shown significant changes in hippocampal gene expression (26); c) there is increasing recognition of the role of the DG in anxiety-like behavior (33, 34); d) the changes in cocaine sensitization suggested possible alterations in the reward pathway, particularly in the nucleus accumbens. These selections do not imply that these were the only regions where alterations in gene expression might be taking place, but rather that they represent key components of the neuronal circuitry of relevance to the behaviors under study.
Dentate Gyrus
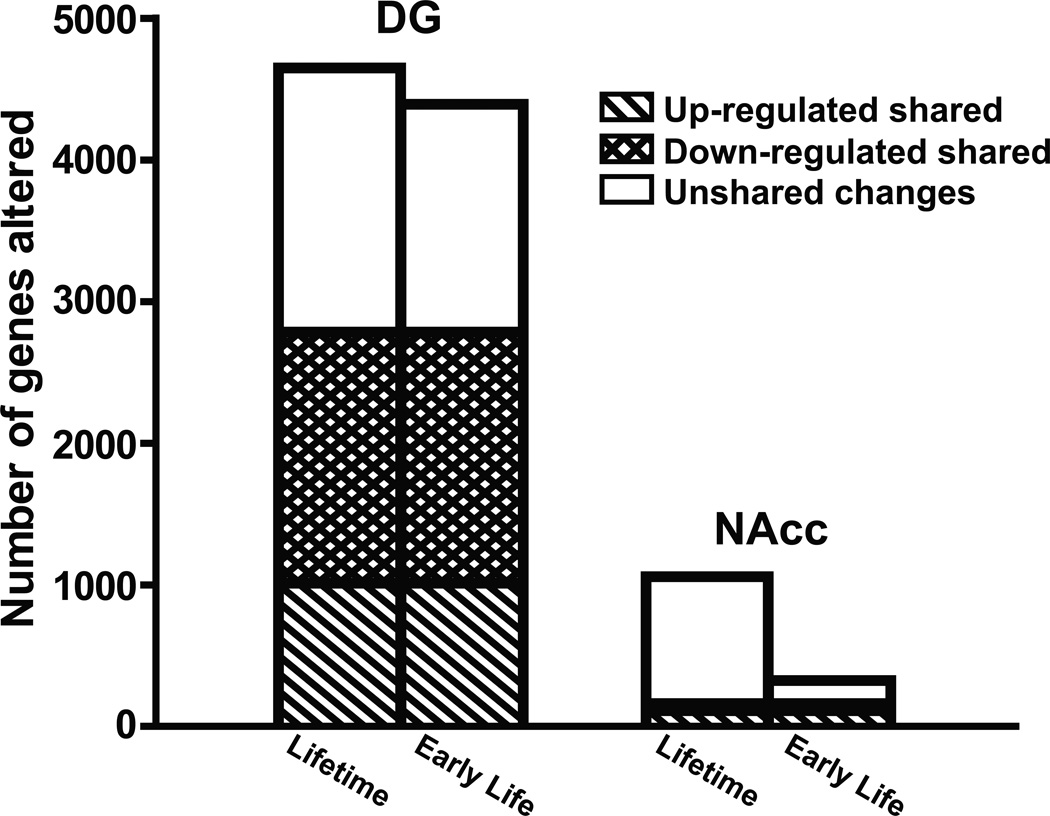
As indicated in Figure 4, there were ~4500 genes significantly altered in expression in DG in Early Life or Lifetime GRov when compared to No-GRov mice (4393 and 4648 genes, respectively). Interestingly, 2785 transcripts, or 62% of the observed changes, were shared between Early Life and Lifetime GRov mice (1774 genes down-regulated and 1011 genes up-regulated). Since the behavioral phenotypes of the two transgenic animals were similar, a working hypothesis is that these shared transcripts were altered early in life, remained altered throughout the life of the animal and contributed to the observed behavioral phenotype.
Figure 4.
Altered gene expression profiling in DG and NAcc. The number of genes changed in Lifetime or Early Life GRov mice when compared to No-GRov mice was divided by three categories: 1) transcripts that were up-regulated and shared by Early Life and Lifetime GRov mice; 2) transcripts that were down-regulated and shared by Early Life and Lifetime GRov mice; 3) transcripts that were regulated but not shared by Early Life and Lifetime GRov mice. GRov, glucocorticoid receptor overexpression in forebrain; DG, dentate gyrus; NAcc, nucleus accumbens. DG: n = 6 mice per group. NAcc: n = 6–7 mice per group.
The majority of the shared altered transcripts are implicated in axonal guidance, growth, synaptogenesis, neurotransmitter signaling, developmental process, and learning and memory. Ingenuity function analysis revealed that overexpression of GR early in life or throughout life clearly affected the expression of genes involved in transcription (528 and 582 genes, respectively) (p = 1.4E-9 and 1.8E-12, respectively) and transactivation (200 genes and 209 genes, respectively) (p = 3.4E-5 and 1.4E-4, respectively). Ingenuity pathway analysis demonstrated that the GR signaling canonical pathway was altered in DG of the Early Life GRov mice (p < .002). In these mice, 75 genes involved in GR signaling pathway were substantially changed in DG (Figure S6 in Supplement 1). Similarly in Lifetime GRov mice, 69 genes involved in GR signaling were significantly altered in DG (Figure S6 in Supplement 1). Of these two pools, 43 transcripts implicated in GR signaling were shared between Early Life and Lifetime GRov mice (Table 1), likely contributing to the common behavioral phenotype. There were 32 genes uniquely altered in Early Life GRov compared to No-GRov mice (Figure S6 in Supplement 1). On the other hand, in the Lifetime GRov group, 26 genes involved in GR signaling were uniquely changed (Figure S6 in Supplement 1). It can be argued that the transcripts altered in only one of the groups and not in the other might not play a direct role in the common behavioral phenotype. Alternatively, it could be argued that the two observed patterns of GR signaling might represent two different albeit overlapping types of alteration of the GR pathway, either of which is sufficient to produce the behavior phenotype. Using qRT-PCR, we confirmed 14 out of 22 genes altered and shared by Early Life and Lifetime GRov groups (Table S3 in Supplement 1). Moreover, Ingenuity pathway analysis revealed that the axonal guidance signaling canonical pathway was altered in DG in both Early Life and Lifetime GRov mice (96 and 122 genes, respectively) (p < .005 and p = 3.6E-6, respectively). 67 genes implicated in the axonal guidance signaling were shared between Early Life and Lifetime GRov mice (Table S4 in Supplement 1). These results indicate that transient early exposure to GR overexpression set in motion changes in gene expression patterns in DG, comparable in magnitude to lifelong overexpression, with a highly significant overlap in the profile under the two conditions.
Table 1.
Alterations in GR Signaling Pathway in DG
| Early Life vs No-GRov | Lifetime vs No-GRov | ||||
|---|---|---|---|---|---|
| Gene | RefSeq ID | FC | p | FC | p |
| CCL2 | NM_011331 | −1.19 | .018 | −1.19 | .011 |
| CCL11 | NM_011330 | −1.26 | .001 | −1.36 | .001 |
| CD3E | NM_007648 | −1.19 | .003 | −1.18 | .001 |
| CSN2 | NM_009972 | −1.19 | .001 | −1.17 | .007 |
| GTF2A2 | NM_001039519 | 1.19 | .031 | 1.17 | .046 |
| GTF2H1* | NM_008186 | −1.24 | .029 | −1.25 | .055 |
| GTF2H5 | NM_181392 | 1.16 | .049 | 1.19 | .016 |
| HSP90AA1* | NM_010480 | 1.40 | .003 | 1.27 | .022 |
| HSP90AB1* | NM_008302 | 1.84 | .002 | 1.60 | .046 |
| HSPA2* | NM_001002012 | 1.67 | .006 | 1.77 | .007 |
| IL2 | NM_008366 | −1.17 | .007 | −1.11 | .011 |
| IL1RN | NM_001039701 | −1.10 | .023 | −1.10 | .015 |
| JAK1* | NM_146145 | 1.16 | .124 | 1.20 | .004 |
| JAK3 | NM_010589 | −1.19 | .004 | −1.21 | .003 |
| KRT35 | NM_016880 | −1.12 | .044 | −1.15 | .011 |
| JUN* | NM_010591 | 1.32 | .110 | 1.72 | .001 |
| MAP2K1* | NM_008927 | 1.25 | .082 | 1.30 | .017 |
| MAP3K7IP1* | NM_025609 | −1.14 | .014 | −1.11 | .057 |
| MAPK1* | NM_001038663 | 1.31 | .096 | 1.22 | .178 |
| MAPK11 | NM_011161 | −1.24 | .001 | −1.20 | .006 |
| MAPK13 | NM_011950 | −1.23 | .028 | −1.23 | .029 |
| MED1 | NM_134027 | 1.27 | .004 | 1.25 | .002 |
| NCOR1 | NM_011308 | 1.38 | .001 | 1.30 | .001 |
| NFKBIA | NM_010907 | 1.24 | .033 | 1.28 | .045 |
| NRAS | NM_010937 | 1.11 | .045 | 1.11 | .040 |
| NRIP1 | NM_173440 | 1.26 | .044 | 1.28 | .012 |
| PCK2 | NM_028994 | −1.27 | .018 | −1.28 | .005 |
| PGR | NM_008829 | −1.30 | .013 | −1.31 | .020 |
| PIK3C2G | NM_011084 | −1.14 | .002 | −1.12 | .004 |
| PIK3R4 | NM_001081309 | 1.38 | .002 | 1.32 | .001 |
| POLR2A* | NM_009089 | −1.35 | .007 | −1.52 | .001 |
| POLR2H | NM_145632 | 1.20 | .047 | 1.33 | .002 |
| POU2F2 | NM_001163554 | −1.40 | .001 | −1.31 | .001 |
| RRAS* | NM_009101 | −1.14 | .043 | −1.18 | .005 |
| SLPI | NM_011414 | −1.17 | .042 | −1.22 | .020 |
| SMARCA2 | NM_011416 | 1.59 | .001 | 1.53 | .004 |
| SRA1 | NM_001164406 | −1.29 | .003 | −1.16 | .042 |
| STAT5A | NM_001164062 | −1.13 | .006 | −1.14 | .015 |
| TAF10* | NM_020024 | −1.44 | .006 | −1.33 | .015 |
| TAF11 | NM_026836 | −1.18 | .035 | −1.14 | .050 |
| TAF7L | NM_001161855 | −1.12 | .033 | −1.13 | .012 |
| TGFB1* | NM_011577 | −1.26 | .012 | −1.20 | .086 |
| TRAF2* | NM_009422 | −1.19 | .048 | −1.22 | .017 |
This list includes genes that are altered in DG and shared by Lifetime and Early Life GRov mice when compared to No-GRov mice. Fold changes (FC) of gene expression represent either Lifetime or Early Life GRov mice compared with No-GRov animals. GR, glucocorticoid receptor; GRov, glucocorticoid receptor overexpression in forebrain; DG, dentate gyrus. Genes with asterisk mark have been confirmed by qRT-PCR. n = 6 mice per group.
Nucleus Accumbens
There were 562 and 1058 genes altered in expression in NAcc in Early Life and Lifetime GRov mice, respectively, when compared to No-GRov controls (Figure 4). Only 162 transcripts (51 genes down-regulated and 111 genes up-regulated) showed shared changes in expression between Early Life and Lifetime GRov mice. Thus, the majority of the altered transcripts in NAcc were uniquely changed based on the duration of GR induction -- 71% of Early Life GRov changes and 85% of Lifetime GRov changes were distinct. This indicates that lifelong exposure to GR overexpression in NAcc induces a more dynamic process than in DG.
The majority of the genes that were significantly altered in NAcc in both Early Life and Lifetime GRov are implicated in axonal guidance and neurotransmitter signaling (e.g. β-adrenergic and dopaminergic). Ingenuity function analysis revealed that GR overexpression early in life or throughout life affected the expression of genes involved in transcription (80 and 165 genes, respectively) (p < .05 and p = 8.9E-6, respectively). Ingenuity pathway analysis identified that the GR signaling pathway was altered in NAcc when comparing Lifetime and No-GRov mice (p < .05) (Table S5 in Supplement 1), but not profoundly changed between Early Life and No-GRov mice. It therefore appears that constant overexpression of GR is needed for the alteration of GR signaling pathway in NAcc. While 69 GR signaling pathway-related genes were changed in DG, only 21 genes involved in GR signaling pathway were altered in NAcc between Lifetime and No-GRov mice. Ingenuity pathway analysis revealed the dopamine receptor signaling canonical pathway was altered in NAcc in both Early Life and Lifetime GRov mice (p < .01 and p < .05, respectively) (Table 2). Surprisingly, altered dopamine receptor signaling was differentially regulated between Early Life and Lifetime GRov in comparison with No-GRov. A number of transcripts have been validated by qRT-PCR (Table S6 in Supplement 1). Furthermore, Ingenuity pathway analysis revealed that the axonal guidance signaling canonical pathway was altered in NAcc in both Early Life and Lifetime GRov mice (19 and 30 genes changed, respectively) (p < .05, respectively) (Table S7 in Supplement 1). However, only 6 transcripts implicated in the axonal guidance signaling were shared in NAcc between groups.
Table 2.
Altered Dopamine Receptor Signaling in NAcc
| Early Life vs No-GRov | Lifetime vs No-GRov | ||||
|---|---|---|---|---|---|
| Gene | RefSeq ID | FC | p | FC | p |
| ADCY1 | NM_009622 | 1.21 | .067 | −1.15 | .039 |
| ADCY7 | NM_001037724 | 1.14 | .031 | NC | |
| MAOA | NM_173740 | 1.14 | .029 | NC | |
| PPP1CA* | NM_031868 | −1.53 | .003 | NC | |
| PPP1R1B | NM_144828 | −1.37 | .001 | −1.21 | .012 |
| PPP2R5C* | NM_001081458 | −1.45 | .119 | NC | |
| PPP2R5D | NM_009358 | −1.17 | .024 | NC | |
| PRKAG1* | NM_016781 | −1.22 | .024 | NC | |
| AADC* | NM_016672 | NC | 1.75 | .029 | |
| IL4I1 | NM_010215 | NC | −1.17 | .012 | |
| PPP1CB* | NM_172707 | NC | 1.12 | .122 | |
| PPP1R12A* | NM_027892 | NC | 1.23 | .002 | |
| PPP1R14C* | NM_133485 | NC | 1.21 | .022 | |
| PPP1R3C* | NM_016854 | NC | 1.11 | .010 | |
| PPP2R5A | NM_144880 | NC | −1.10 | .017 | |
| SMOX | NM_001177836 | NC | 1.10 | .019 | |
Fold changes (FC) of gene expression represent either Lifetime or Early Life GRov mice compared with No-GRov controls. GRov, glucocorticoid receptor overexpression in forebrain; NAcc, nucleus accumbens; NC, no change. Genes with asterisk mark have been confirmed by qRT-PCR. n = 6–7 mice per group.
Taken together, the expression results demonstrate that short term overexpression of GR during early life is sufficient to change the gene expression profile in at least two brain regions for the rest of the animal’s life. Moreover, the dentate gyrus is more vulnerable to enhanced GR activation relative to the nucleus accumbens.
Discussion
In this study, transient early-life GR overexpression in forebrain causes lifelong alterations in reactivity to the environment as indexed by increased anxiety behavior and enhanced sensitization to cocaine. Enhanced GR expression prior to weaning sets in motion a cascade of molecular and neural events that cause profound changes in the transcriptome, in a region specific manner. The existence of a critical time window is consistent with the notion that GR activity is involved in a highly orchestrated biological program of spatial and temporal gene expression that controls the birth, trajectory, final location, and connectivity of neurons and brain circuits (10, 12, 35–37) -- a structural elaboration that determines all aspects of brain function. Given that the glucocorticoid receptor is a ligand-activated transcription factor, it has the potential to modify this unfolding developmental program either because of its endogenous levels of activity or as a result of environmental stress. Indeed, an optimal level of corticosteroids is critical for normal brain maturation (7). Thus, GR and its ligands are powerful modulators of neural development while being mediators of environmental impact. It is therefore not surprising that early traumatic experiences are associated with long-term alterations in neuroendocrine responsiveness, emotional regulation, and expression levels of genes that have been related to anxiety and mood disorders, and substance abuse (10–12, 35, 37).
The hippocampus is critical for both the evaluation and the termination of the stress response (3, 38–42). Furthermore, the hippocampus, and the DG in particular, show a remarkable degree of structural and functional plasticity (43). The HC also plays a pivotal role in emotion regulation with lesion and neuropharmacological studies demonstrating a direct and important role for the hippocampus in the expression of anxiety (33, 34, 44, 45). We focused on the DG because it is the subregion of the hippocampus that undergoes the greatest degree of development postnatally, showing maximal plasticity during early life. Moreover, manipulations of glucocorticoid levels have profound effects on the development of that region (46). It therefore represented the best candidate area for studying the lifelong impact of inducing glucocorticoid receptors early in life. Indeed, gene expression profiling revealed that transient overexpression of GR early in life results in ~4400 transcripts being altered in DG. Remarkably, there is a very high degree of overlap in gene expression changes in DG between Early Life and Lifetime GRov mice that share a common behavioral phenotype. This underscores the idea that most of the critical changes took place during early development and remained constant into adulthood. Although the impact of these changes in gene expression on functional circuitry and signaling of DG remains to be elucidated, the findings demonstrate a profound level of cellular reorganization induced by increasing the expression of the glucocorticoid receptor in early life, and suggest that the lasting alterations of behavior may derive in large part from the reprogramming of this region of the hippocampus.
Stress and glucocorticoids have also been implicated in the determination of the propensity to develop substance abuse, and our own findings support the strong interplay between the stress system and reactivity to psychostimulants. We used cocaine sensitization as an index of vulnerability to addictive behavior, as sensitization has been suggested to indicate a progressive increase in the incentive salience of psychoactive drugs, and is associated with the development of addiction (47). Stress is known to influence the dopaminergic system in the forebrain and modulates responsiveness to drugs of abuse (48). Selective GR ablation in mouse dopaminoceptive neurons expressing dopamine receptor 1a decreases the motivation of mice to self-administer cocaine (20), suggesting that GR increases the propensity to self-administer cocaine by acting on the postsynaptic neurons of the dopaminergic system. While the dopamine receptor signaling pathway was altered in NAcc in both Early Life and Lifetime GRov, different sets of dopamine receptor signaling-related genes are involved in the dysregulation. Thus, it appears that the early changes induced by GR in NAcc are further modified by the ongoing induction of GR. This makes it difficult to pinpoint a shared set of molecular changes in NAcc that account for the increased cocaine sensitization in the two groups. It is conceivable that the enhancement of cocaine-induced sensitization in Early Life and Lifetime GRov is mediated by different molecular mechanisms within the NAcc and these could include differences in dopamine receptor signaling. In the present study, we only conducted microarray analyses in the ventral striatum. However, it is quite likely that gene expression differences in the dorsal striatum also contribute to the behavioral phenotype -- especially given GR mRNA differences in the dorsal striatum and the known relationship between glucocorticoid activity and dopamine signaling (20, 48). It is also possible that changes elsewhere in the forebrain, for example in the hippocampus or prefrontal cortex, are responsible for the shared phenotype.
In summary, this mouse model involved a genetic manipulation that may mirror naturally occurring conditions that lead to individual differences in emotionality: increased GR levels resulting from genetic predisposition, or increased GR activity that is induced by severe environmental stressors. Both of these conditions, or their interactions, can trigger a cascade of molecular events that has a lifelong impact on brain structure and on behavior. Thus, this inducible mouse model can serve as a valuable tool for investigating the interplay of genes and environment during development, and for defining the molecular and epigenetic mechanisms that underlie individual differences in emotional reactivity and vulnerability to substance abuse.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Conte Center Grant L99MH60398, National Institute on Drug Abuse Program Project Grant 5P01DA021633-02 to HA and SJW, Office of Naval Research Grant N00014-09-1-0598, and the Nancy Pritzker Network for Research on Depression.
We thank Dr. Shelly Flagel for helpful discussion of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information: supplement 1, including the supplemental Methods and Materials and the supplemental Results (6 Figures S1–S6, 7 Tables S1–S7)
Financial Disclosure
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession # GSE 30187).
References
- 1.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 2.Akil H, Morano M. Stress. In: Bloom F, Kupfer D, editors. Psychopharmacology: the fourth generation of progress. New York: Raven; 1995. pp. 773–785. [Google Scholar]
- 3.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 4.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 5.Akil H. Stressed and depressed. Nat Med. 2005;11:116–118. doi: 10.1038/nm0205-116. [DOI] [PubMed] [Google Scholar]
- 6.De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 7.Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 8.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 9.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 11.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oitzl MS, Champagne DL, van der Veen R, De Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM, Krey LC, McEwen BS. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol. 1983;18:55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 15.Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. I. Ontogeny and autoregulation. Brain Res. 1985;350:159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- 16.Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. II. An autoradiographic study. Brain Res. 1985;350:165–168. doi: 10.1016/0165-3806(85)90260-3. [DOI] [PubMed] [Google Scholar]
- 17.Romeo RD, Tang AC, Sullivan RM. Early life experiences: enduring behavioral, neurological and endocrinological consequences. In: Pfaff DW, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. New York: Elsevier; 2009. pp. 1975–2004. [Google Scholar]
- 18.Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA. 2004;101:11851–11856. doi: 10.1073/pnas.0402208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, et al. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- 21.Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 23.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 24.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Q, Hebda-Bauer EK, Pletsch A, Luo J, Hoversten MT, Osetek AJ, et al. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt MV, Sterlemann V, Wagner K, Niederleitner B, Ganea K, Liebl C, et al. Postnatal glucocorticoid excess due to pituitary glucocorticoid receptor deficiency: differential short- and long-term consequences. Endocrinology. 2009;150:2709–2716. doi: 10.1210/en.2008-1211. [DOI] [PubMed] [Google Scholar]
- 29.Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, et al. Long-term antidepressant treatment reduces behavioural deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol. 1995;7:841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 30.Rochford J, Beaulieu S, Rousse I, Glowa JR, Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharmacology. 1997;132:145–152. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- 31.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinney BC, Schneider JS, Schafer GL, Lowing JL, Mohan S, Zhao MX, et al. Decreased locomotor activity in mice expressing tTA under control of the CaMKII alpha promoter. Genes Brain Behav. 2008;7:203–213. doi: 10.1111/j.1601-183X.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 33.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 34.Eren-Koçak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry. 2011;69:534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 36.Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 37.Champagne DL, de Kloet ER, Joëls M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin Fetal Neonatal Med. 2009;14:136–142. doi: 10.1016/j.siny.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 40.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 42.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 44.Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116:494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- 45.Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- 46.Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TE, Berridge KC. Addition. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 48.Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.