Abstract
Learning to adapt to a complex and fluctuating environment requires the ability to adjust neural representations of sensory stimuli. Through pattern completion processes, cortical networks can reconstruct familiar patterns from degraded input patterns, while pattern separation processes allow discrimination of even highly overlapping inputs. Here we show that the balance between pattern separation and completion is experience-dependent. Rats given extensive training with overlapping complex odorant mixtures show improved behavioral discrimination ability and enhanced cortical ensemble pattern separation. In contrast, behavioral training to disregard normally detectable differences between overlapping mixtures results in impaired cortical ensemble pattern separation (enhanced pattern completion) and impaired discrimination. This bidirectional effect was not found in the olfactory bulb, and may be due to plasticity within olfactory cortex itself. Thus pattern recognition, and the balance between pattern separation and completion, is highly malleable based on task demands and occurs in concert with changes in perceptual performance.
Introduction
The ability of some cortical circuits to fill in features missing from a familiar input pattern is known as pattern completion, a process essential to ensure perceptual stability in case of behaviorally irrelevant variations of stimuli. This mechanism gives way to pattern separation when the two input patterns become more distinct or as the significance of making discrimination between them increases. The CA3 region of the hippocampus1,2 and more recently the anterior piriform cortex3 have been revealed as serving critical roles in these computations. These two areas share functional and structural similarities and are both characterized as auto-associative memory networks4. The most commonly studied pattern recognition tasks in the hippocampal system involve patterns of environmental spatial cues, while the piriform cortex deals with odor-evoked olfactory bulb spatiotemporal patterns and the formation of odor objects5. In both structures, the transition between encoding patterns as similar and as different follows a nonlinear transition as stimuli are morphed from one pattern to another. The goal of the present work was to test the hypothesis derived from computational models4,6, that shifts between pattern completion and separation would reflect not only the nature of pattern overlap but also experience and ongoing task demands. That is, tasks requiring high acuity discrimination should shift cortical pattern recognition toward pattern separation, while tasks requiring stimulus grouping or reduced acuity may bias pattern recognition toward pattern completion. The olfactory cortex is an ideal model for these studies given its relatively simple architecture and proximity to the sensory epithelium, the relative ease of quantitatively manipulating sensory inputs, and the robust odor learning displayed by rodents.
Results
Previous work has demonstrated that odor learning and behavioral state can modulate odor-evoked responses as early as the olfactory bulb7–9. Here, in an effort to avoid non-specific influences such as differential behavioral state, cortical activity was recorded in urethane anesthetized animals 24 h after the final training session. Data from a total of 255 cortical neurons and 104 mitral/tufted cells are included.
Pattern completion and separation in naive rats
In odor naïve rats, single-units were isolated in the olfactory bulb (OB) mitral/tufted cell layer and anterior piriform cortex (aPCX) layer II/III and responses to a series of odorant mixtures were examined. All data presented here used the same initial mixture of ten monomolecular odorants from which components were removed (e.g., 10c-1 [10 components with 1 removed], 10c-2 [10 components with 2 removed]) or replaced (10cR1) (Fig. 1a). Typical examples of aPCX and OB single unit responding to the odorant mixtures are illustrated Supplementary Fig. 1c. These units responded to several different mixtures; however, despite the linear morphing from one mixture to the next, the response to one stimulus did not predict responsiveness to other similar, overlapping mixtures (e.g., 10c and 10c-1).
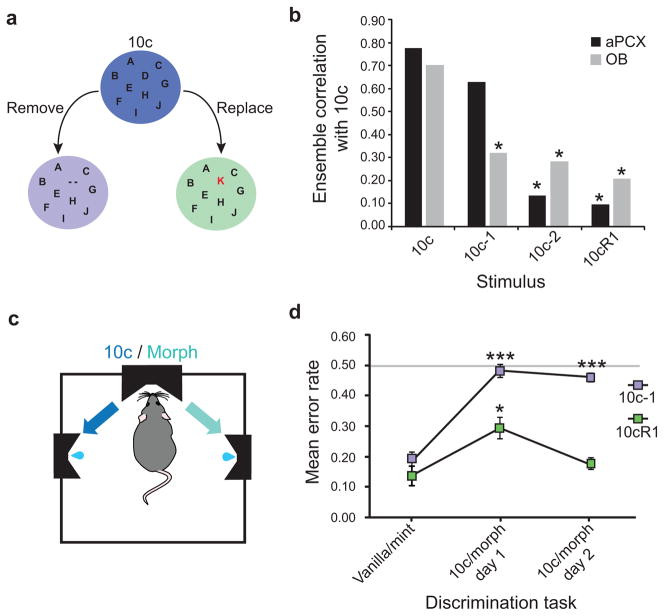
Figure 1. Sensory acuity in the naive animal.
a, Olfactory stimulus morphing: the original stimulus, a complex mixture of 10 odorant components (10c, each component symbolized by a letter), was either degraded by the progressive removal of its components (10c-1, 10c-2…) or transformed by the replacement of one component by another one (10cR1). b, Cross-correlation analyses of OB and of aPCX single-unit ensemble responses to the standard 10c mix versus its various morphs. OB mitral/tufted cell ensembles (n=28 units) showed stable de-correlation across all morphs. aPCX pyramidal cell ensembles (n=20 units) showed no de-correlation if only a single component was removed; de-correlation appeared only when more components were missing. In contrast, the addition of one single unusual component to the complex mix (10cR1) gave a clear separation of the two patterns. Asterisks indicate a significant de-correlation compared to 10c (*p<0.05 at least). c, A two alternative forced-choice task was used to evaluate the rat’s ability to discriminate the 10c full mixture from its morphed versions. d, Behavioral performances matched aPCX discrimination capacities. Rats could not detect the removal of a single component (black) while they could easily discriminate mixtures in which a novel component had been introduced (gray). For each group, asterisks indicate significant differences with the respective reference performance (vanilla/mint), *P<0.05, ***P<0.001. Error bars indicate s.e.m.
We then examined how ensembles of mitral cells or aPCX neurons3,10 differentially responded to these overlapping mixtures by studying cross-correlations between the evoked ensemble spike activity. As shown Fig. 1b, ensembles of neurons in the OB de-correlated the standard 10c mix from all of the morphed versions (pattern separation). In contrast, aPCX ensembles showed no significant decorrelation if only a single component was missing (90% overlap; difference in ensemble correlation between 10c and 10c-1, Z=0.866, P=0.1933), and responded as if the full mixture was present (pattern completion); de-correlation increased as more components were removed (80% overlap; difference in ensemble correlation between 10c and 10c-2, Z=2.617, P=0.0044) (pattern separation). Furthermore, aPCX ensembles strongly de-correlated the 10c mix from the same mixture with one component replaced (10cR1; Z=2.734, P=0.0031) (pattern separation).
Different naïve animals were trained in a two alternative forced-choice task to evaluate their ability to discriminate the 10c mixture from its morphed versions (Fig. 1c). Discrete manipulations of the standard 10c mix dramatically changed discrimination performance (Fig. 1d). After two days of training, the animals could discriminate the standard mix from the same mixture with one component replaced (10c/10cR1), while after the same period of time all animals failed to detect the removal of one component from the standard (10c/10c-1). The behavioral sensory acuity in naïve animals thus matched aPCX ensemble pattern discrimination.
Experience increases pattern separation and acuity
Animals were trained in either an “easy” 10c/10cR1 discrimination until reaching performance criterion (2 days of training; Fig. 1d) or in the more difficult 10c/10c-1 task until reaching criterion (8 days of training; Fig. 2a). Additional animals were also trained in the easy 10c/10cR1 discrimination for 8 days (Fig. 2a) to match the duration of training in the difficult task. Recordings of single unit-activity in OB and aPCX were obtained 24 h after the last training session. No change was observed in single-unit spontaneous activity after training, though there was a significant decrease in the probability of odor-evoked activity in animals trained in the difficult task (see Supplementary Results).
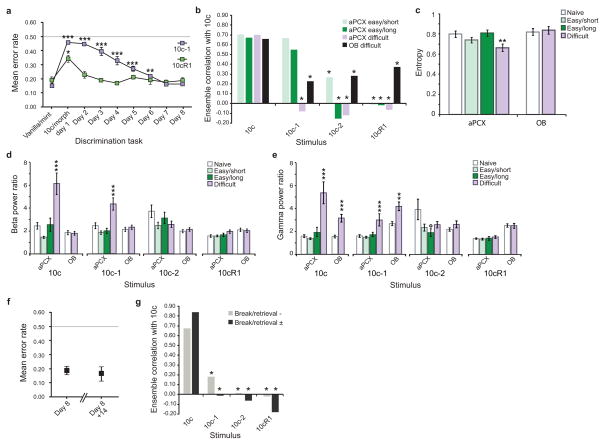
Figure 2. Learned enhancement in sensory acuity.
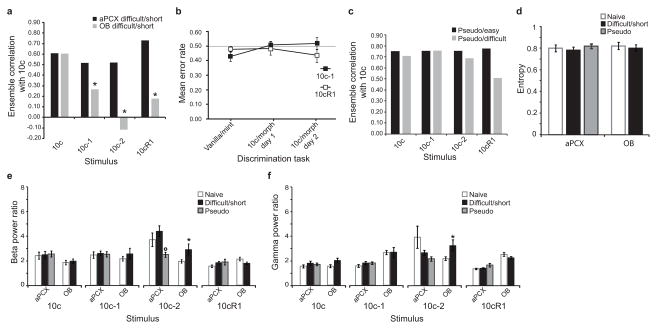
a, After extended training, rats were able to acquire the difficult (10c/10c-1) discrimination; the easy replacement detection performance was reproduced to match a similar period of training. Asterisks indicate significant differences with the reference performance (vanilla/mint), *P<0.05, **P<0.01, ***P<0.001. b–e, Electrophysiological recordings were performed after rats had reached criterion in the odor discrimination tasks: easy (replacement) for a short (2 days, see Fig. 1d) and long (8 days) training, or difficult (removal). b, Mastering a difficult but not an easy behavioral discrimination task was associated with enhanced pattern separation ability in piriform cortical ensembles, while mitral cell ensembles presented unchanged de-correlation levels (Easy/Short: n=23 aPCX units; Easy/Long: n=28 aPCX units; Difficult: n=25 units/structure). Asterisks indicate a significant de-correlation compared to 10c (*p<0.05). c, Breadth of tuning (entropy) of aPCX and OB neurons in naive and trained animals. In aPCX, the breadth of tuning in difficult but not in easy odor-experienced animals was significantly reduced (cells more selective) compared with that in naive animals (**P<0.01). The same learning did not change the selectivity of mitral cells. d,e, Power modulation of odor-evoked beta (15–35 Hz, in d) and gamma (40–80Hz, in e) oscillatory activities after discrimination learning. Mastering the difficult discrimination task was associated with enhanced gamma and beta odor-evoked activity compared to the simple tasks and naives. n=212–296 odor-evoked responses per structure and per experimental group. *Significant increase in power compared to naive animals, **P<0.01, ***P<0.001. °Significant decrease in power compared to naïve animals, P<0.05. f,g, Long-lasting memory of the difficult discrimination training. f, Maintenance of the behavioral discrimination after a two-week break in the training. g, Maintenance of aPCX neurons pattern separation ability after a two-week break in the training, either without behavioral retrieval test (in gray, n=25 cells) or with and without retrieval test (in black, n=35 cells). Data are shown as mean ±s.e.m.
Learning the difficult behavioral discrimination was associated with enhanced stimulus de-correlation (pattern separation) in aPCX ensembles (Fig. 2b). In rats trained 2 days in the easy discrimination task, aPCX cortical ensembles showed no change in mixture de-correlation compared to naïves. In contrast, in rats successfully trained to make the difficult discrimination (10c/10c-1), cortical de-correlation for this comparison was strongly significant (Z=−3.128, P=0.0009). Thus, behavioral conditioning was associated with an enhancement in pattern separation in aPCX for odors having formerly pronounced qualitative and cortical encoding similarities. Importantly, no improvement in mixture de-correlation was observed in the rats trained in the longer version of the easy training (10c still correlated to 10c-1; Z=0.603, P=0.2733. Finally, in the OB, mitral/tufted ensembles exhibited stable pattern separation after the difficult discrimination task compared to naives, suggesting a cortical origin for the observed changes in aPCX activity.
The effect of experience on breadth of tuning of single-units was also examined (Fig. 2c). Breadth of tuning was measured with entropy (Online Methods), which ranges from a value of 0.0, wherein a single-unit responds selectively to only one stimulus within a test set (here 10c, 10c-1, 10c-2, 10cR1), to 1.0, wherein a single-unit responds equally well to all stimuli in the test set. As shown Fig. 2c, in aPCX, the entropy value for units in rats trained in the easy discrimination task did not differ from those of naïve (short training: t41 = −1.384, P=0.1737; long training: t46= 0.026, P = 0.9797), whereas in rats trained in the difficult task entropy was significantly reduced (t37 = −2.714, P=0.0100). This result indicates that aPCX neurons were more narrowly tuned in animals mastering a difficult (but not an easy) olfactory discrimination. In OB, the same difficult learning did not change the selectivity of mitral cells (t42 = 0.471, P=0.6402).
In addition to single-unit activity, we also assessed whether training induced changes within OB and aPCX networks through examination of local field potentials (LFPs). In the naive anesthetized animal, mixtures evoked pronounced oscillatory activities in the beta (15–35 Hz) and gamma (40–80 Hz) frequency range in both OB and aPCX (Supplementary Fig. 1d). In animals trained in the easy discrimination task for either 2 or 8 days, no change of power in the beta or gamma bands was observed compared to naïve animals, for all odorant morphs tested (Mann-Whitney U test, P>0.05). In contrast, in animals trained in the difficult discrimination task, aPCX beta oscillations were strongly enhanced (pooled across all odorants, U548=27341, P<0.0001). More precisely, aPCX beta oscillations were selectively enhanced in response to the learned odors (10c: U137=1317, P<0.0001; 10c-1:U135=1494, P=0.0003) but not in response to the unfamiliar morphs (10c-2: U136=1999, P=0.1152; 10cR1: U134=1866, P=0.0585). No change in beta power occurred in OB (Fig. 2d, U649=48105, P=0.3229). Odor-evoked gamma band activity (Fig. 2e) showed a comparable enhancement of power both in aPCX (pooled odorants, U548=24675, P<0.0001) and OB (U649=39682, P<0.0001). For both structures, this enhancement was selective in response to the learned odors (aPCX: 10c:U137=703, P<0.0001; 10c-1: U135=1090, P<0.0001; OB: 10c: U163=1835, P<0.0001; 10c-1: U159=2325, P=0.0097).
Odor discrimination training can evoke both transient and long-term changes in olfactory cortical activity11,12. To determine if training induced a long-term change in cortical pattern separation, additional animals (n=5) were trained in the difficult 10c/10c-1 discrimination task until reaching criterion (similarly to Fig. 2a). A break of 14 days without training or odor exposure was then imposed. Long term memory was assessed afterward for some of the animals (n=2). As shown Fig. 2f, their performance after the break showed no decrement compared to the last day of the pre-break training. In parallel, the other rats (n=3) were directly recorded after the break (without new training) to avoid any reconsolidation effect that the post-break retrieval test might have induced. As shown Fig. 2g (Break/Retrieval-), the aPCX neural ensemble responses obtained for these rats verify a strong cortical de-correlation between 10c and its morphed versions, similar to what was obtained when the recordings occurred 24 h after training. The same pattern of results was obtained if the analysis pooled the cortical recordings of both behaviorally tested and non-tested rats (Break/Retrieval ±). However, although behavioral and ensemble changes were maintained for at least 2 weeks, learned changes in odor-evoked cortical oscillations were not maintained (see Supplementary Fig. 2), consistent with a more transient role for these processes during early stages of conditioning and memory13.
Experience increases pattern completion and generalization
Can animals learn to ignore differences between odors and in so doing impair olfactory cortical acuity by enhancing pattern completion? To address this question, we developed a paradigm wherein high olfactory acuity impairs performance in the behavioral task. In this modified two alternative forced-choice task, two odors A and A′ were associated to the same rewarded side in opposition to a third odor B, rewarded on the other side (Fig. 3a). Two levels of olfactory similarity between A and A′ were considered. In a first condition (“close”, Fig. 3b), the animals had to group two highly similar but nonetheless discernible mixtures (10c and 10cR1). In the second condition (“distant”, Fig. 3c), different animals had to group two very dissimilar odors (10c and limonene, a monomolecular odorant). Comparable timelines were observed for training in both tasks.
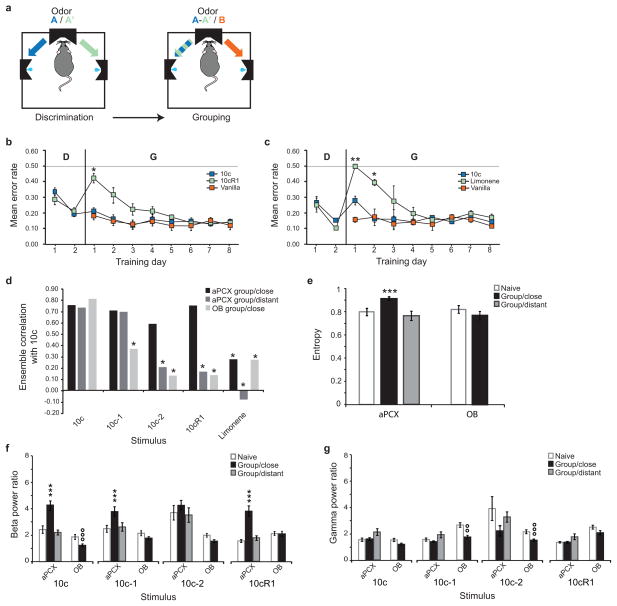
Figure 3. Experience increases cortical pattern completion.
a, Design of the grouping task. In the first phase (Discrimination), two odors A and A′ are associated with a reward in opposite ports and the rats’ ability to differentiate them is evaluated; in the second phase (Grouping), the odors A and A′ are associated to the same rewarded side and the rats’ ability to cluster them in opposition to a third odor B is evaluated. b,c, Behavioral performances detailed for each odor (same color code as in a). The animals were trained to discriminate (D) then Group (G) the original 10c stimulus either with a mixture sharing strong similarities (10cR1, in b) or with a dissimilar, single odorant (limonene, in c). Asterisks indicate a lower performance for a given odor compared to day 2 of the discrimination phase, *P<0.05, **P<0.01. d, The grouping of two close odors reversed olfactory cortical de-correlation. An enhancement of correlation between the full 10c mix and all its morphed versions was obtained in rats trained to group close stimuli (n=31 aPCX units). aPCX discrimination capacities of rats trained to group distant stimuli corresponded to those of naive (n=19 aPCX units). The grouping of close odors did not change OB pattern separation ability (n=24 mitral/tufted cells) *significant de-correlation compared to 10c, P<0.05. e, The breadth of tuning of aPCX neurons in animals trained to group close but not distant odors was significantly increased (cells less selective) compared with naive animals (***P<0.005). Mitral/tufted cells selectivity remained unchanged after the grouping task. f,g, Power modulation of odor-evoked beta (f) and gamma (g) oscillatory activities associated to grouping learning in aPCX and OB. n=156–372 odor-evoked responses per structure and per experimental groups. *significant increase in power compared to naive animals, ***P<0.001, °significant decrease in power compared to naïve animals, °°P<0.01, °°°P<0.001. Data are shown as mean ±s.e.m.
aPCX cell ensemble de-correlation was strongly affected in a task-specific manner (Fig. 3d). In the rats trained to group close mixtures, an increase in correlation between the standard and its transformed versions was observed, particularly with 10c-2 (Z=1.19, P=0.1171) and 10cR1 (Z=0.035, P=0.4859), indicating that aPCX neural ensembles were able to reduce divergence of mixture encoding (pattern completion) when high olfactory acuity would have disadvantaged behavioral performance. This suggests that aPCX ensembles merged the representation of two, formerly discriminable overlapping mixtures (10c and 10cR1) into a single representation that signaled a left reward. Note that aPCX ensembles still de-correlated 10c from a monomolecular element not present in the original mix (limonene, Z=2.703, P=0034), indicating that the observed increase in pattern completion/decrease in de-correlation was limited to similar, overlapping input patterns. Furthermore, in the rats trained to group distant odors, aPCX cortical ensemble de-correlation was unaffected compared to the naïve condition. This suggests that in this version of the task the animals solved the problem by learning that either of two distinct odors signaled a left reward, rather than merging the representations of the two. In contrast to the aPCX, OB mitral/tufted cell ensemble decorrelation was not modified for animals trained to group close odor mixtures.
The mean breadth of tuning of single-units (Fig. 3e) in animals trained to group close mixtures was significantly increased compared to the naïve condition (t45= −3.351, P=0.0016) while no change in selectivity was observed when the grouping involved distant odors (t40= −0.658, P=0.5145), showing that aPCX neurons were more broadly tuned when the animals were trained to ignore the differences between similar (but not dissimilar) olfactory stimuli. No change in the selectivity of mitral/tufted cells was observed in the close version of the grouping task (t48= −1.332, P=0.1892). Additional measures of changes in single-unit activity are presented in the supplementary information. Finally, LFP analysis revealed a significant elevation of aPCX beta power in the animals trained to group close odors (pooled odorants, U603=29102, P<0.0001), in response to the learned mixtures (10c:U154=1788, P<0.0001; 10cR1:U148=1342, P<0.0001) but also in response to some unfamiliar but overlapping versions (10c-1, U148=1913, P=0.0014) (Fig. 3f). No change in beta power was observed in aPCX in the animals trained to group distant odors (Mann-Whitney U test, P>0.05). No significant change of odour-evoked gamma power was observed after the grouping task regardless of the proximity of the odors used (Mann-Whitney U test, P>0.05) (Fig. 3g). Compared to naïve animals, a global decrease of power in response to the mixtures was observed in the OB both in the beta (all odorant considered, U626=38952, P=0.0016) and gamma frequency bands (U626=37783, P=0.0002). An important decrease was particularly observed in the beta frequency band for 10c (U156=1800, P<0.0001) and in the gamma band for 10c-1 (U156=2228, P=0.0128) and 10c-2 (U155=1788, P=0.0001).
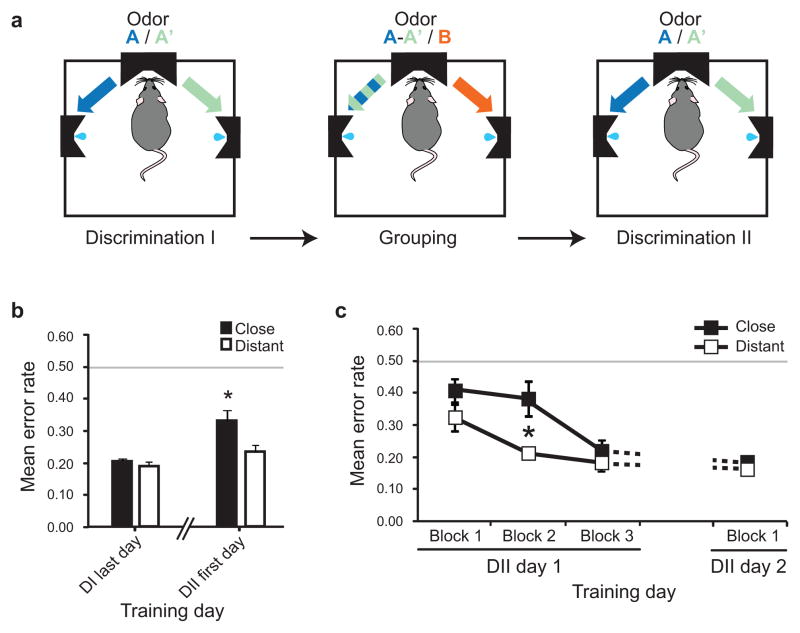
The data presented above demonstrate a shift toward enhanced cortical generalization due to the olfactory grouping learning. To identify if this change was accompanied by a behavioral impairment in discriminating the two close mixtures (10c and 10cR1), additional rats (n=4) were trained in the grouping task for 8 days as previously described (A-A′ vs. B), but were then put back in the original two alternative forced-choice task (A vs. A′) to get a new measure of their discriminative acuity (Fig. 4a). Results (Fig. 4b) indicated a significant impairment in discriminating 10c from 10cR1 after the grouping task compared to before (paired t test, t3 = −3,604, P=0.0366). In contrast, the animals that were trained in the grouping task involving distant odors (n=3 rats) presented no significant decline in discriminating 10c from limonene (t2= −1,688, P=0.2335). Repeated measures ANOVA indicated a significant effect of trial block (F1,3= 25.951, P<0.0001) but no significant effect of group (F1,5= 4.479, P=0.0879), showing that the two groups improved their performance over the course of the session (Fig. 4c). However, a significant block × group interaction (F1,3= 4.121, P=0.0256) was found: whereas the animals of the group “distant” were already at criterion by the second block of trials, the animals of the group “close” were significantly poorer for this same block (unpaired t test, t5= 2.686, P=0.0435). Together, these results suggest an impairment of olfactory acuity induced by the close version of the grouping task. After this training, and as long as it remained behaviorally relevant, the two stimuli (10c and 10cR1) were treated as the same odor object both at the cortical and the behavioral level.
Figure 4. Learned enhancement in sensory generalization.
a, Behavioral training. The rats’ discrimination capacity was evaluated again after achievement of the grouping task. A= 10c; A′= 10cR1 (group “close”) or limonene (group “distant”); B= vanilla. b, Averaged performances. The animals were poorer at discriminating close odors after training in the grouping task than they were before (* P<0.05); this impairment was not observed when the grouping task involved distant odors. c, Detailed performances over time. The error rate of the discrimination session following the grouping task is divided into three consecutive blocks of trials. Asterisks indicate a lower performance for the animals trained to group close odors compared to distant, * P <0.05. Data are shown as mean ±s.e.m.
Cortical pattern recognition during poor performance
How are OB and aPCX ensemble activity affected during periods of poor discrimination performance? Two distinct situations were considered. The first focused on the early stages of the difficult discrimination task, when the animals could not yet distinguish 10c from10c-1; OB and aPCX units were isolated from rats trained in this task when performance was still at chance levels (2 days of training; Figs. 1d and 5a). The second situation involved animals that failed to complete the simple discrimination task of the pre-training (vanilla/mint) and were thus simply exposed to the complex mixtures without behavioral consequences (pseudo-conditioning). Although it was not possible to determine the level of behavioral discrimination of these rats (Fig. 5b), they were stimulated with the same pairs of odorant mixtures (either easy-like [10c/10cR1] or difficult-like [10c/10c-1] discriminations) and during the same period of time (two days) as the conditioned animals. Importantly, we ensured that these animals were not anosmic since they were able to detect the introduction of novel odors in the cage (data not shown) and their aPCX neurons presented normal activity in response to various odorants (Fig. 5c).
Figure 5. Transient decrease in sensory acuity associated with poor behavioral performance.
a, Electrophysiological recordings were performed at the early stage of the difficult task in rats not yet able to perform the 10c/10c-1 discrimination after a short (two days) training (see Fig. 1d). Cross-correlation analysis for aPCX ensembles showed high correlations between the 10c full mixture and all its morphed versions, while OB de-correlations capacities were unaltered by the same experience (n=21 and 27 aPCX and OB units, respectively). *Significant de-correlation compared to 10c, P<0.05. b, The pseudo-conditioned animals never reached the criterion of the reference performance (error rate <0.25 for vanilla/mint) and were simply exposed, without behavioral consequence, to the same pairs of complex mixtures as the conditioned animals. c, Cross-correlation analysis for aPCX ensembles in the pseudo-conditioned animals submitted to easy-like (n=27 units) or difficult-like (n=26 units) discrimination tasks showed significant correlations between the 10c mix and all its morphed versions (*significant de-correlation, p<0.05). d, Breadth of tuning. OB and aPCX neurons recorded in rats either at the early stage of the difficult discrimination training or pseudo-conditioned showed no change of odor selectivity compared to naive conditions. e,f, Power modulation of odor-evoked beta (e) and gamma (f) oscillatory activities associated to poor behavioral performances. n=212–285 odor-evoked responses per structure and per experimental group. *significant increase in power compared to naive animals, P<0.05. °Significant decrease in power compared to naïve animals, P<0.05. Data are shown as mean ±s.e.m.
After two days of training in the difficult discrimination task, while animals performed at chance levels, aPCX ensembles showed a strong increase in pattern completion, failing to de-correlate any of the overlapping mixtures, especially 10c-2 (Z=0.395, P=0.3464) and 10cR1 (Z=−0.669, P=0.2518) (Fig. 5a), whereas for the same situation OB mitral/tufted cell ensembles de-correlated the odor mixtures similarly to naïve rats.
Analogous enhancements of cortical correlations were obtained in pseudo-conditioned animals, regardless of the pair of mixtures to which they had been exposed (easy- or difficult-like) (Fig. 5c). These results suggest a potential, experience-dependent “re-setting” of cortical acuity during failed discrimination performance, perhaps through a change in cell excitability14 or cortical synaptic inhibition15 that may be required for subsequent refinement in pattern recognition. Interestingly, this failure of ensemble de-correlation during random discrimination performance was not associated with a change in single-unit tuning selectivity (Fig. 5d). Finally, the analysis of LFPs revealed no consistent, significant change in power of the odor-evoked beta (Fig. 5e) or gamma frequency bands (Fig. 5f) for the rats presenting poor discrimination performance compared to naïve animals.
Discussion
Experience-dependent pattern recognition by networks such as the hippocampus and piriform cortex promotes both perceptual stability through pattern completion and perceptual discrimination through pattern separation. The memory of previously experienced patterns, via changes in synaptic weight within the network16,17, is a critical component of these divergent pattern recognition processes. The present results suggest that the balance between pattern separation and pattern completion is also experience-dependent. A task requiring discrimination of highly overlapping patterns induces changes in cortical activity that shift the balance toward pattern separation (enhanced decorrelation) and enhance perceptual acuity. A task requiring disregard of differences between overlapping patterns for optimal performance induces changes in cortical activity that shift the balance toward pattern completion (enhanced correlation) and impair perceptual acuity. Training in a simple task, in contrast, produces none of these changes in cortical processing or perception. Importantly, although as shown here and elsewhere18–22, odor learning induces changes in olfactory bulb odor responses, the learned changes in cortical ensembles were not associated with similar changes in the olfactory bulb ensembles, suggesting a cortical localization.
The task-dependent changes in cortical ensemble correlation and de-correlation were associated with changes in the breadth of single-unit odor receptive fields. Such cortical receptive field changes have been observed in a number of systems23,24 including the aPCX25. The breadth of tuning of neurons in many sensory systems is modulated by local inhibition. There are several classes of inhibitory interneurons in the aPCX26 mediating both feedforward and feedback inhibition27. Learning induced plasticity of these neurons15 could contribute to pyramidal cell receptive field changes. Finally, it should be noted that there are several classes of pyramidal cells in aPCX Layers II and III with different connectivity to afferent and association fiber pathways28. These cells may be differentially modified by learning.
Interestingly, during periods of impaired performance, cortical ensembles also shift toward pattern completion. Learned impairments in perception have also been described in humans29. These highly correlated ensemble responses may reflect the increased excitability observed in aPCX neurons during early stages of odor discrimination training14, though no increase in spontaneous or odor evoked activity was observed. Nonetheless, enhanced excitability may facilitate opportunities for associative synaptic plasticity which could subsequently lead to the refinement of ensemble responses and improvement in perceptual acuity in later phases of training. Top-down or modulatory inputs to aPCX providing feedback regarding response outcomes may also be necessary for induction and expression of the learned changes16,30.
In addition to changes in single-unit and ensemble plasticity, odor-evoked olfactory system LFPs were also modified by training. Although olfactory system odor-evoked activity is strongly modulated by anesthesia31 and state32, the demonstration here in anesthetized rats that olfactory system beta and gamma oscillations reflect the animal’s past olfactory experience and are selectively increased in difficult tasks, match quite well with recordings in awake animals33,34. Oscillations have been shown to support sensory perception, neural plasticity and allow transient cooperation between cerebral areas35. Gamma frequency oscillation tend to reflect local circuit activity, while beta oscillations reflect more widespread network activity36,37. In the olfactory system, oscillations have been suggested to facilitate precise temporal structure of spike trains38, which may be expected to modulate spike timing dependent plasticity in downstream targets21. Inhibitory interneurons play important roles in the temporal structure of oscillations39, and thus may be implicated in learned changes. Interestingly, while odor de-correlation by cortical ensembles was long-lasting (2 weeks), learned changes in oscillations were more transient, and were not detected after a two week delay. This suggests substantially different roles for these two measures of circuit function (oscillations and ensemble de-correlation) in storing sensory memory.
Finally, these results suggest that one consequence of learning to ignore differences between patterns is an impairment in subsequent sensory discrimination. Olfactory impairments are associated with a variety of neuropathological conditions ranging from schizophrenia40 to Alzheimer’s Disease41. Similar pathology or aging related impairments in hippocampal pattern separation have also been reported42–44. The olfactory perceptual impairments have been associated with pathology within the olfactory system45,46. However, if the pathology modifies cognitive aspects which lead to ignoring differences between stimuli, the present results suggest that an additional factor exacerbating perceptual decline could be an experience-dependent remodeling of cortical ensembles and a learned impairment in discrimination. Thus, the balance between pattern completion and separation, which can be shifted depending on task demands, could also be co-opted in disease states to impair perception and memory.
Methods
Supplementary Material
Supplementary Table 1. Cell count per experimental condition and animal
Supplementary Figure 1. Representative electrophysiological recordings
Supplementary Figure 2. Long term memory of the difficult discrimination training
Supplementary Figure 3. Cortical ensemble correlations for the different experimental conditions combined across replicates
Online methods
Acknowledgments
This work was supported by NIH grants DC003906 and DC008982 to D.A.W. and the Fyssen Foundation post-doctoral grant to J.C.
Footnotes
Contributions
D.A.W. and J.C. designed the research. J.C. performed data collection. J.C. and D.A.W. analyzed and interpreted data. J.C. and D.A.W. wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–9. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 2.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–8. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 3.Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–76. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–7. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 6.McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–65. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses. 2008;33:581–96. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher ML, Chen WR. Neural correlates of olfactory learning: Critical role of centrifugal neuromodulation. Learn Mem. 2010;17:561–570. doi: 10.1101/lm.941510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restrepo D, Doucette W, Whitesell JD, McTavish TS, Salcedo E. From the top down: flexible reading of a fragmented odor map. Trends Neurosci. 2009;32:525–31. doi: 10.1016/j.tins.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saar D, Grossman Y, Barkai E. Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. J Neurosci. 1999;19:8616–22. doi: 10.1523/JNEUROSCI.19-19-08616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–34. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Matsliah SI, Rosenblum K, Barkai E. Olfactory-learning abilities are correlated with the rate by which intrinsic neuronal excitability is modulated in the piriform cortex. Eur J Neurosci. 2009;30:1339–48. doi: 10.1111/j.1460-9568.2009.06894.x. [DOI] [PubMed] [Google Scholar]
- 15.Brosh I, Barkai E. Learning-induced enhancement of feedback inhibitory synaptic transmission. Learn Mem. 2009;16:413–6. doi: 10.1101/lm.1430809. [DOI] [PubMed] [Google Scholar]
- 16.Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. J Neurophysiol. 1992;67:1230–46. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–8. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman WJ, Schneider W. Changes in spatial patterns of rabbit olfactory EEG with conditioning to odors. Psychophysiology. 1982;19:44–56. doi: 10.1111/j.1469-8986.1982.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher ML, Wilson DA. Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. J Neurosci. 2003;23:6946–55. doi: 10.1523/JNEUROSCI.23-17-06946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol. 2008;6:e258. doi: 10.1371/journal.pbio.0060258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doucette W, et al. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69:1176–87. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SV, Choi DC, Davis M, Ressler KJ. Learning-dependent structural plasticity in the adult olfactory pathway. J Neurosci. 2008;28:13106–11. doi: 10.1523/JNEUROSCI.4465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res. 1992;577:226–35. doi: 10.1016/0006-8993(92)90278-h. [DOI] [PubMed] [Google Scholar]
- 24.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–8. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 25.Chen CF, Barnes DC, Wilson DA. Generalized Versus Stimulus-Specific Learned Fear Differentially Modifies Stimulus Encoding in Primary Sensory Cortex of Awake Rats. J Neurophysiol. 2011 doi: 10.1152/jn.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518:1670–87. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- 27.Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–65. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki N, Bekkers JM. Two layers of synaptic processing by principal neurons in piriform cortex. J Neurosci. 2011;31:2156–66. doi: 10.1523/JNEUROSCI.5430-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat Neurosci. 2011;14:791–6. doi: 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Wang LP, Shen X, Tsien JZ. Balanced dopamine is critical for pattern completion during associative memory recall. PLoS One. 2010;5:e15401. doi: 10.1371/journal.pone.0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–65. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes DC, Chapuis J, Chaudhury D, Wilson DA. Odor fear conditioning modifies piriform cortex local field potentials both during conditioning and during post-conditioning sleep. PLoS One. 2011;6:e18130. doi: 10.1371/journal.pone.0018130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27:8358–65. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 2010;104:829–39. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–18. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 36.Kay LM, et al. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009;32:207–14. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapuis J, et al. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 2009;29:10287–98. doi: 10.1523/JNEUROSCI.0505-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cenier T, et al. Respiration-gated formation of gamma and beta neural assemblies in the mammalian olfactory bulb. Eur J Neurosci. 2009;29:921–30. doi: 10.1111/j.1460-9568.2009.06651.x. [DOI] [PubMed] [Google Scholar]
- 39.Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86:2823–33. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- 40.Seckinger RA, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res. 2004;69:55–65. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 41.Murphy C. Loss of olfactory function in dementing disease. Physiol Behav. 1999;66:177–82. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- 42.Tanila H, Shapiro M, Gallagher M, Eichenbaum H. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci. 1997;17:5155–66. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanila H, Sipila P, Shapiro M, Eichenbaum H. Brain aging: impaired coding of novel environmental cues. J Neurosci. 1997;17:5167–74. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–5. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- 45.Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30:505–14. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010;133:2714–26. doi: 10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson DA. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 2010;30:1760–5. doi: 10.1523/JNEUROSCI.5636-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fletcher ML, Smith AM, Best AR, Wilson DA. High-frequency oscillations are not necessary for simple olfactory discriminations in young rats. J Neurosci. 2005;25:792–8. doi: 10.1523/JNEUROSCI.4673-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses. 1979;4:215–29. [Google Scholar]
- 50.Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002 Available from http://quantpsy.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Cell count per experimental condition and animal
Supplementary Figure 1. Representative electrophysiological recordings
Supplementary Figure 2. Long term memory of the difficult discrimination training
Supplementary Figure 3. Cortical ensemble correlations for the different experimental conditions combined across replicates
Online methods