Abstract
Physical activities undertaken in childhood, particularly activities which apply large forces quickly convey optimal benefits to bone mass, size, and structure. Evidence is accumulating that benefits persist well beyond activity cessation. This review examines the potential for early childhood activity to improve bone mineralization and structure and explores childhood activity as prevention for osteoporosis in later life.
Keywords: Osteoporosis, Bone mineral content, Bone strength, Weight-bearing physical activity, Puberty, Peak bone mass
INTRODUCTION
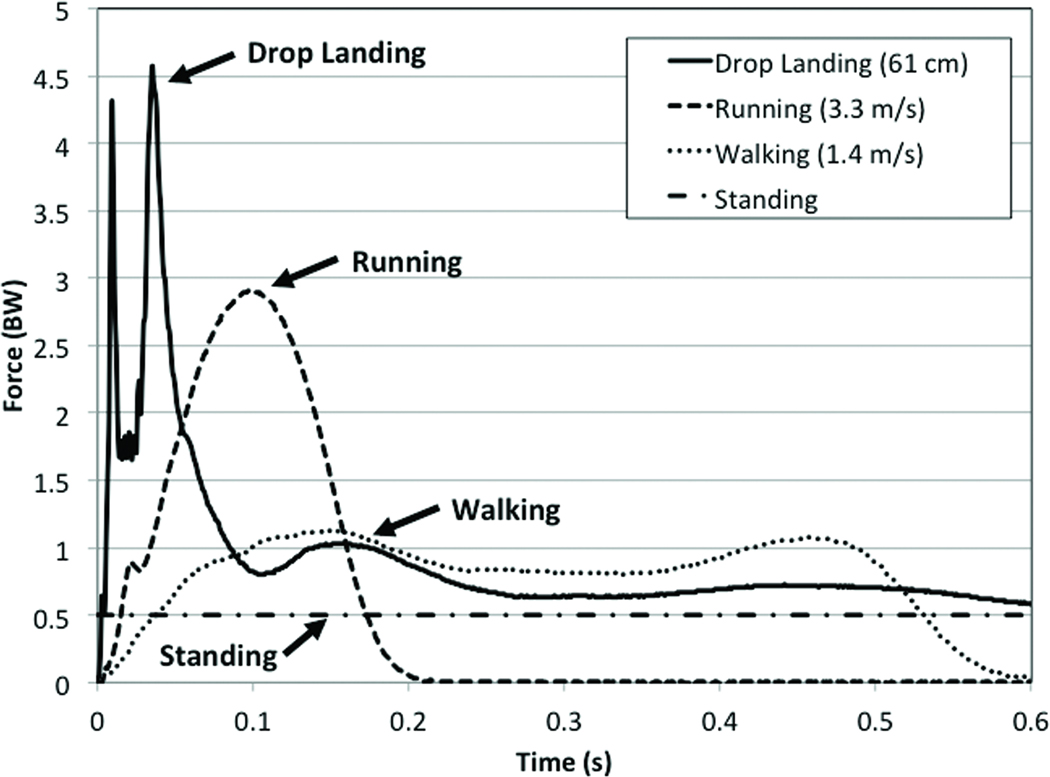
Physical inactivity is an accepted antecedent to the development of childhood obesity and is implicated in numerous chronic conditions including poor cardiovascular and metabolic health among children (30); however, childhood inactivity is rarely purported as the precursor to osteoporosis, a disease typical of old age. Recent evidence suggests that physical activity in childhood is one of the most powerful preventive strategies in the fight against osteoporosis. Based on our work (9–11, 16–18) and that of others (28, 34), we hypothesize that engaging in regular and well-designed targeted physical activity in childhood is crucial to maintaining a healthy skeleton in adulthood. In fact, considering that 60% of the risk of developing osteoporosis can be explained by the amount of bone mass accrued by early adulthood (3), physical activity undertaken during or prior to puberty may have greater positive effects on bone mass than many pharmacological interventions undertaken by adults with osteoporosis. However, as with drugs, not all physical activities have equivalent influences on skeletal development. Physical activity is a categorical term comprising everything from light leisure activities to more vigorous endeavors such as organized sport or intentional, targeted exercise. The osteogenic potential of a particular physical activity is conditional upon the magnitude of the applied load, the rate at which the load is applied, the duration of the loading bout, and the novel nature of the load (2). Physical activities shown to have the greatest osteogenic effects on the growing skeleton are those characterized by a considerable loading magnitude applied at a rapid rate. Greater forces, delivered quickly, through activities such as jumping, appear to convey the greatest benefits to bone mineralization and structure in children and adolescents. These activities are typically weight-bearing activities since body weight increases the magnitude of loading. Figure 1 depicts several weight-bearing activities (walking, running, and drop jumping) and displays the mean ground reaction forces (GRFs) in units of bodyweight (BW) as well as the time to peak force as performed by a 10-year-old girl. There is a considerable delay in the rate of force development during walking (a low-impact activity) in comparison to running, and even more so when compared to jumping (a high-impact activity). Not only does it take longer to reach peak force during walking or running, the peak force is much lower than jumping. This is important because it is the combination of force magnitude and the rate at which the force is applied that determines the impact of the activity. Based on our work (9, 10) and the available GRF data from other bone loading intervention studies (13), activities with the most osteogenic potential have GRFs greater than 3.5 times BW (per leg) with peak force occurring in less than 0.1 seconds. Data from animal studies also suggest a positive relationship between loading frequency and bone adaptation, up to a threshold (31), but there are no dose response studies that confirm this effect in humans.
Figure 1.
Ground reaction forces (measured in portions of bodyweight (BW)) and loading rates experienced by a pre-pubertal female child during quite standing, walking, running and a drop landing from a 61 cm height. All recorded values are from a single leg. Values for the drop jump were derived from a two-foot drop landing on dual force plates. Values for quiet standing reflect 0.5 BW per leg while standing on two feet; one foot on each plate.
Interventions utilizing high impact exercises (with GRFs > 3.5 times BW) show significant skeletal benefits when the exercise stimulus is delivered for a sufficient length of time (8, 9, 29). Research thus far indicates that at least 7 months of impact exercise is essential to induce a measurable change in bone mass in children (10, 13). The influence of exercise-induced loading is further dependent on maturity status with pre- and early puberty as the time when the skeleton’s response to loading is optimized. Though exposure to directed, targeted doses of high-impact activities have proven beneficial to skeletal development, data are also emerging to support the importance of lifestyle physical activity during childhood independent of exercise interventions for achieving optimal bone mass into young adulthood (4, 18). These findings are particularly relevant from a public health perspective and lend support to federal efforts directed at increasing physical activity in youth as a strategy to reduce the risk for preventable chronic conditions later in life. Our own work demonstrates that benefits to bone from early childhood activity persist into young adulthood (9, 18). If these benefits are shown to persist beyond the age of skeletal maturation as suggested by animal studies (37), we can add osteoporosis to the list of chronic conditions preventable through childhood physical activity.
The objective of this review is to examine the potential of sufficient lifestyle and targeted-impact activity during early childhood to improve bone mineralization and bone structure. We focus our review on school-based settings, emphasize protocols that have been successfully replicated (8, 10) have demonstrated long-term sustainability (23) and feature loading protocols resulting in persistent benefits beyond the growing years (9, 18, 34). Finally, we present recommendations on optimal pediatric exercise prescription based on these criteria, to reduce the risk for osteoporosis in later life. We direct readers to several reviews (13, 20, 28) for more information on the influence of physical activity on lifespan bone health.
Influence of Mechanical Loading on Skeletal Development
Impact-loading through physical activity builds bone mass and enhances bone structure to improve its overall strength. Evidence from cross-sectional, longitudinal, and randomized controlled trials support the beneficial effects of weight-bearing physical activity on bone health across the lifespan (13, 20). However, the positive effects of impact loading vary throughout life and are most evident during childhood when pre- and pubertal skeletal growth and development favors greater bone gains compared to any other time across the lifespan. The sensitivity of bone to an imposed mechanical strain created by impact loading is influenced by numerous factors including the habitual loading environment, nutritional status, and the level of circulating hormones (20, 35). Therefore, simply engaging in physical activity does not necessarily guarantee sufficient loading to influence positive skeletal changes.
During adulthood physical activity has been shown to preserve and even modestly enhance bone mass and structure (20). However, the modest gains reported in response to targeted interventions in adults require participation in relatively high-impact activities and gains are lost once the exercise stimulus is removed, confirming the old adage “use it or lose it.” Among children, this does not appear to be the case. We have found that exercise-induced bone mass gains and structural adaptations in early childhood are maintained through puberty and into adulthood (9, 10, 18); and data are emerging from others to support our findings (4, 34). This highlights the critical importance of establishing physical activity habits early in life to promote life-long skeletal health.
Effects of Childhood Physical Activity on Bone Mineral Accrual
An important marker of skeletal health is peak bone mass (PBM), which is the greatest amount of bone mass achieved at the end of the growth period. Importantly, PBM is related to adult bone strength, which is a composite construct of multiple factors including bone mass, quality and structure (20). Entering adulthood with greater bone mass (i.e., higher PBM) may reduce the proportion of fractures suffered in old age (14). Recently, Bonjour and colleagues (6) have quantified what may be gained by increasing PBM. They estimate that one standard deviation increase in population PBM would reduce fracture risk by as much as 50%. Given one third of adolescents do not participate in vigorous activity three days per week for at least 20 minutes per session, the population-attributable risk of inactivity as a factor in adult fracture risk is likely to be considerable (30).
Bone mass accrual is most often assessed via dual-energy x-ray absorptiometery (DXA) which allows for analysis of bone health at sites such as the hip, spine, radius, and whole body. Bone mineralization measured via DXA accounts for a majority of bone strength and is considered the gold standard in evaluating bone health and evaluating risk for osteoporosis (27). Research in this field may also assess surface specific bone adaptations using peripheral quantitative tomography (pQCT) or structural bone strength via magnetic resonance imaging (MRI) or hip structural analysis (HSA), the latter of which estimates bone structure by mathematically applying data derived from DXA. Structural adaptations to exercise in youth are important and should consequently be considered along with peak bone mass accrual; however published research of changes to bone geometry may be minimal for several reasons. Peripheral QCT is often a foregone technique because it does not allow for evaluation of bone health at clinically relevant skeletal sites such as the proximal femur and lumbar spine. Use of pQCT allows for analysis at bone locations, such as the distal radius and proximal tiba, which are primarily cortical in composition, where fracture is much less frequent and has fewer long term consequences in comparison to the hip and spine. In addition, use of pQCT would unnecessarily expose child research participants to at least 10 mrem of radiation for each cross-sectional analysis when < 1.0 mrem is needed for scans at several sites using the DXA. Therefore this paper will reflect the majority of the published research and present bone mass outcomes derived via DXA while acknowledging reports of bone structural adaptations as appropriate. DXA is reliably used to evaluate bone mass accrual over time with exercise interventions or regular physical activity and can therefore help us to evaluate the influence of activity on development of peak bone mass.
Evidence exists that PBM occurs by the end of the 2nd or early in the 3rd decade of life in both genders (3). That PBM is achieved so early in life and that the brief period of pubertal growth is associated with the greatest overall proportion of total bone mineral accrual indicates the importance of the circum-pubertal years in mediating osteoporosis risk in later life. For example, data from longitudinal studies of bone development indicate nearly 40% of total young adult bone mass is achieved during the two years before and the two years following accelerated linear growth or peak height velocity (PHV) (3). Examples of how early life physical activity impacts skeletal outcomes differently than physical activity undertaken later in life has been observed in cross-sectional studies of elite athletes. Kannus et al. (19) compared bone mineral content (BMC) side-to-side differences in the playing versus non-playing arms of female tennis and squash athletes and controls. Players were categorized based on the biological age when their playing careers began. Biological age was defined as the years before or after the onset of menarche. The categories included: > 5 years pre-menarche, 3–5 years pre-menarche, 0–2 years pre-menarche, 1–5 years post-menarche, and >5 years post-menarche. Adjustments were made for chronological age, anthropometric characteristics, age at menarche, training volume, daily calcium intake, and relative strength. All the athletes had greater BMC differences when compared to controls. More importantly, the athletes who began playing at-or prior to menarche had side-to-side differences nearly twice that of athletes who initiated training post-menarche. Similar outcomes were later observed by Kontulainen et al (21) who noted more favorable side-to-side differences in BMC and structure among female tennis and squash athletes initiating training prior to puberty, compared to controls and athletes initiating training at/or after puberty.
More recent findings from exercise interventions also indicate that targeted impact exercise during pre- and early-puberty confers substantial skeletal benefits; this time frame may be seen as a “window of opportunity” for bone-health programming. A systematic review of randomized and nonrandomized exercise trials in youth suggests that gains in bone mass due to physical activity range between 1–6% prior to puberty and are considerably smaller thereafter (0.3%–2%) (13). For example, our work (10) and that of Fuchs et al. (8) have shown that targeted, high-impact physical activity in early childhood conferred gains in bone mass of 3.5%–8%. These randomized, controlled studies were school-based; pre-pubertal children were randomly assigned by classroom (8) or by school (9) to a high-impact jumping intervention or a control condition. Children in the intervention groups performed drop landings from a two-foot height three times per week over 7 months. Children began by performing 40–50 jumps per session, working up to 100 jumps; GRFs were measured between 3.5 and 4.5 times BW per leg. In the initial trial the intervention took place during classroom time and was offered in addition to regular physical education (PE) (8); in the subsequent trial, jumps were incorporated into the fitness component of the regular PE curriculum (10). Participants in the control conditions were exposed to a stretching program (8) or participated in the usual PE curriculum (10). The gains in bone mass experienced by children in these interventions are among the highest reported gains observed to date over a single school year (8–10).
Others have reported moderate, but significant benefits from school-based impact programs over a single school year. In response to 10 minutes of varied jumping activities three times per week, MacKelvie and colleagues (24, 25) found early-pubertal girls gained between 1.5% and 3.1% more BMC at the femoral neck and lumbar spine, respectively, while pre-pubertal boys gained approximately 1% at the femoral neck and 1.6% at the total body (when compared to controls). Measured GRFs in this intervention ranged from 3.5 to 5 times BW. After two years of this jumping protocol, girls in the intervention group had accrued between 3.7% and 4.6% greater BMC at the lumbar spine and femoral neck region of the hip compared to controls, while boys gained 4.3% more BMC at the femoral neck compared to controls (23, 26). These findings suggest an additive effect from repeated exposure over multiple school years. In contrast, Wiebe et al. (39) exposed girls (6 to 10 years) to single-leg drop-landings (3 sessions per week; 50 landings per session) from heights of 14 cm and 28 cm and found no effect on bone mineral accrual. Peak GRFs were measured between 2.2 and 4.4 times BW per leg, similar to the GRFs reported by us (10) and Fuchs et al. (8). The discrepancy in results may be explained by differing exercise doses between Wiebe et al. (39), Fuchs et al. (8) and our study (10). In the Wiebe et al study, the number of jumps per week was fewer (50 versus 100), and the height of the drops was considerably lower (14 cm and 28 cm versus 61 cm). While the GRFs were similar between the two protocols, it is possible the muscle forces elicited by performing drops from a greater height were larger, resulting in higher strains and a better bone response. Currently, results from randomized, controlled trials of jumping interventions suggest a threshold for success that consists of GRFs that are sufficiently high (at least 3.5 times BW per leg), include a dose of 100 jumps per session requiring a 10–15-minute timeframe, and are delivered at a frequency of three times per week for 7-months or longer.
Whereas participation in activities such as jumping and competitive racquet sports appears to be effective in improving bone mass at loaded sites, the influence of other isolated activities is less well understood. Recently we examined the effects of participation in a community-based running program on bone mass in early pubertal girls (11). The intervention was brief; girls participated in the running program for the first 3 months of the 9-month study duration, spending two days per week for 75 minutes engaged in running and related activities. Bone was measured at baseline and 9-months later. The influence of physical activity outside the scope of the running program on 9-month changes in bone mass was accounted for using objective monitoring of moderate-to-vigorous physical activity (MVPA) with accelerometers; bone outcomes at follow-up were also adjusted for initial bone values, biological age, height, weight, and dietary intakes. Runners gained approximately 6% and 10% more BMC compared to controls at the femoral neck and trochanter, respectively. These preliminary results in a small sample of runners (n=31) and controls (n=11) are promising given the study intervention was a sustainable leisure activity that is likely to interest diverse participants across the life span. Future work in this area should include a randomized, controlled trial.
Although several studies, including our own, demonstrate that the skeleton of pre- and early-pubertal children responds favorably to targeted weight-bearing interventions (8, 10, 23, 26) the evidence is not as consistent for older adolescents. Several studies of late- and post-pubertal females indicate that resistance training (three times per week for 6.5 months) (5), plyometric training (three times per week for 9 months) (40), and step aerobics (two times per week for 9 months) (12) are insufficient to improve bone mass at loaded skeletal sites compared to age- and maturity-matched controls. On the other hand, there are some data to support positive effects of exercise following the pubertal growth spurt. Weeks et al. (38) employed an 8-month, randomized, controlled, school-based jumping program, three times per week in 99 males and females (13.8 ± 0.4 years). The intervention included approximately 10 minutes of varied jumping activity in place of the usual physical education class warm-up, which was performed by controls. GRFs were not reported, but participants performed ~ 300 jumps per session; average jump heights ranged from 20 cm to 40 cm. Results showed that females had greater increases in femoral neck and lumbar spine BMC compared to controls (4.9% and 1.5%, respectively) and that males gained 4.3% more total body BMC compared to controls (38). This study lends support to the notion that jumping may be a unique stimulus for healthy skeletal development throughout the growing years. However, the load must be sufficiently high and maintained for a sufficient period of time. As of this writing, among pre- and early-pubertal children, a minimum of 100 jumps per session appears to be a sufficient impact-loading dose, while older children benefit from doing more (~300/session).
Studies of young athletes and children engaged in targeted interventions are critical to understanding the potential effect of exercise on BMC accrual; however, the loads imposed during sport and targeted exercise may represent the “best case scenario” and might not generalize to the everyday physical activity choices of children. Prospective population-based observational studies provide important information on the relationship of bone accrual to the type and amount of physical activity children voluntarily choose to do. Given the timing of PHV varies considerably between individuals and systematically between boys and girls (with girls achieving PHV between 11–12 years of age and boys slightly later between 13–14 years of age), appropriate modeling of data for growth and maturation is also important in BMC accrual studies, whether they be interventions or observational. Ultimately, effective public health recommendations will require information from both study types.
Prospective observational studies examining self-selected physical activity levels and bone outcomes convincingly show that children who participate in higher levels of physical activity have greater bone mass accrual compared to less active children (1, 15). Of these studies, one of the earliest and most important is the University of Saskatchewan Paediatric Bone Mineral Accrual Study (30). This study used a mixed longitudinal design to evaluate relationships between physical activity and bone mineral accrual in a group of healthy Canadian adolescents (n ~ 200). One year after PBM, males and females who were more physically active than peers had higher total body BMC (17% and 9%, respectively) (30). Using an objective measure of physical activity (ActiGraph accelerometer) and a three-year follow-up, our work in the Iowa Bone Development Study demonstrated that the most active boys and girls had 5% and 14% more BMC at the total body and trochanter region compared to inactive peers (16). Children were 5-years old at baseline and over 470 children were followed. Similarly to the Saskatchewan study, stronger relationships were observed in boys, when compared to girls, and in both studies, boys were significantly more active than girls. The physical activity disparity between boys and girls supports increased efforts to understand why young girls are less likely to engage in high-impact-loading activity than boys and intervene accordingly. Likewise, other observational studies using objective measures of physical activity, albeit cross-sectional designs, have shown a positive association between physical activity and bone mass in children, boys more active than girls, and stronger relationships between physical activity and bone outcomes in boys when compared to girls (32, 33).
The contemporary use of objective monitors, particularly waist-worn accelerometers, is an important advancement in pediatric bone outcomes research. When an accelerometer is placed on the waist above the hip, it more directly measures the impact-loading characteristic of physical activity that influences adaptive bone modeling (when compared to other physical-activity measurement instruments). Examples of activities usually performed by children and likely to be accurately detected by waist-worn accelerometers include skipping, running, and jumping, activities with pronounced vertical movement and impact loads. Waist-worn accelerometers are also sensitive to the clinically-relevant skeletal site of the hip. In addition to demonstrating a positive association between regular physical activity and bone mass, studies using accelerometry-based measures of physical activity are helping to quantify dose-response. Our work (15, 17), and a study of similar design by Sardinha et al (32) have shown that a daily accumulation of vigorous physical activity (measured by accelerometry) of approximately 30 to 40 minutes is associated with improved femoral neck strength in children (4 to 11 years old), due to changes in bone mass and structure. Similarly, a two-year intervention by Linden and colleagues (22) showed improved bone mass and size in girls (7 yr at baseline) who participated in 40 minutes of daily physical education which did not include targeted bone loading activities. However, there is much room for further research in the area of dose-response, particularly as national priorities for increasing physical activity levels in youth are focused on combating childhood obesity. Recommended frequency, duration, and intensity for optimal metabolic health and energy balance are not necessarily optimal for skeletal health; studies are needed to identify an exercise dose that encompasses both priorities.
Influence of Childhood Physical Activity on Bone Structure and Strength
More important perhaps than the amount of bone that is accrued during childhood is the manner in which newly-acquired bone is distributed and thus influences bone structure and strength. In animal studies, rather small changes in bone quantity (<10%) resulting from impact loading are associated with rather large (>60%) changes in bone structure and subsequent strength (36). Bone adapts to the forces experienced by increasing mass and remodeling in a way to increase strength relative to the loading condition. The manner in which this occurs is two-fold, affecting either the endosteal or periosteal cortical bone surfaces, or both surfaces concurrently. The greatest immediate contribution to fracture resistance is gained when new bone is added to the periosteal surface (35). Since bone is lost from the endosteal surface during adulthood, exercise-induced increases to the periosteum are likely to help maintain the bone’s resistance to fracture with age. Periosteal apposition is the predominant effect in response to increased physical activity during growth, particularly in pre- and early puberty; results from animal studies suggest that early exercise-induced alterations to bone structure persist through senescence, significantly reducing fracture risk in older age (37).
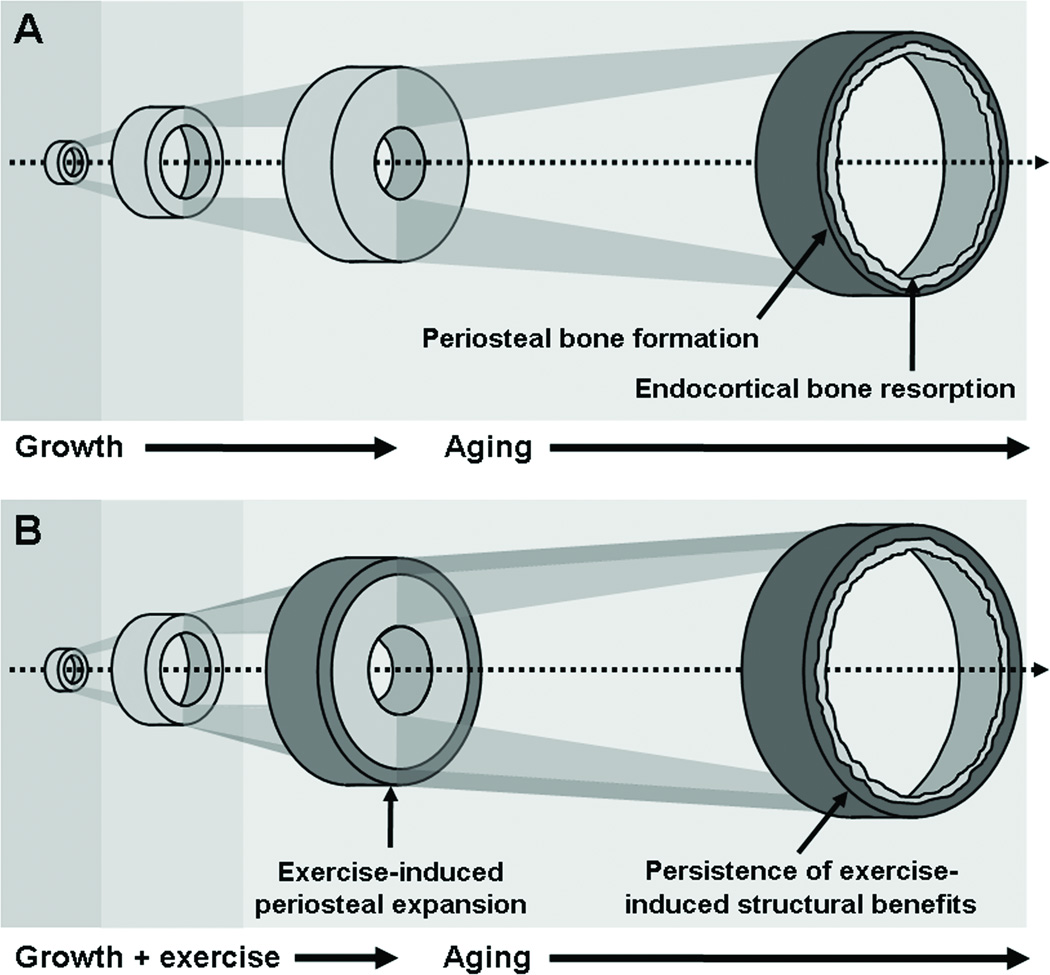
Alternatively, impact loading can result in added mass or reduced resorption on the endosteal surface (35). This is the predominant response to loading observed when exercise exposure occurs after maturity (7, 35). Further, there is a sex-specific response to loading whereby among girls, the onset of menarche and the associated increase in estrogen inhibits periosteal bone formation, limiting the diameter of the bone and simultaneously promoting bone formation on the endosteal surface (35). This too has the effect of increasing bone strength, but to a smaller degree than if bone formation occurred on the periosteal surface, further underscoring the importance of early exercise exposure to optimize bone strength, particularly among girls. Figure 2 presents a theoretical representation of the additive effect of exercise-induced periosteal expansion on growth-related changes to the skeleton and the potential protective effect this may have on risk for fracture in later life.
Figure 2.
Bone structural changes attributed to growth and aging (A) contrasted with the additive effects of exercise during growth and the subsequent benefits to lifelong skeletal health if effects persist into older adulthood (B). (Reprinted from (36). Copyright © 2009 BMJ Publishing Group Ltd.. Used with permission.
The differential effects of loading relative to biological age were recently supported in a 12-month prospective study by Ducher et al. (7) of competitive female tennis players 10 to17 years old. Players were stratified according to maturity, Pre/Peri or post-pubertal. Investigators compared the playing and non-playing humeri BMC (measured by DXA) and utilized MRI to assess total bone area (ToA), medullary area (MedA) and cortical area (CoA) and muscle cross-sectional area (MCSA). At baseline BMC, ToA, CoA and MCSA were 8–18% greater in the playing arm in all maturation groups. Among the Pre/Peri players, the relative side-to-side differences in ToA, CoA, and MCSA continued to increase over 12-months. Among the post pubertal players, only BMC and ToA increased relative to baseline. The authors reported the Pre/Peri menarcheal players exhibited 39% greater annual accrual in cortical bone area in the playing vs. non-playing arm and attributed these changes to increased periosteal apposition and not to changes in the endocortical surface, while post-pubertal girls only exhibited a smaller MedA in the playing versus non-playing arm. Further, among the Pre/Peri- pubertal girls, increases in MCSA (presumably associated with exercise training) accounted for ~32% of the variance associated with the exercise-induced benefits. The muscle-bone relationship was not observed in the older girls, suggesting that hormonal changes associated with pubertal growth may have overridden the influence of lean mass among post-pubertal girls. The study by Ducher and colleagues (7) provides some of the best evidence to date that activity-induced structural adaptations are maturity dependent.
The varying influence of exercise-induced loading according to maturity status has been explored in a handful of targeted interventions. Petit et al. (29) examined 7-month changes in bone structural properties in pre- and early pubertal girls randomized to a classroom-based jumping program (previously described) (24). Hip structural analysis (HSA) was used to assess sub-periosteal width, cross-sectional area, and cross-sectional moment of inertia of the femoral neck, intertrochanter, and femoral shaft. Section modulus, endosteal diameter, and cortical thickness were estimated from DXA data by applying mathematical assumptions associated with cross section and shape. Among the pre-pubertal girls, there were no differences for change in any bone structural outcome. Among the early pubertal girls, the observed intervention response was an increase in bone cross-sectional area and cortical thickness attributable to less endosteal expansion at the femoral neck compared to controls (29). The lack of a periosteal response is in contrast to the findings of Ducher et al, (7) who found pre/peri-pubertal racquet sport athletes had a significantly greater increase in periosteal expansion in their playing arms compared to controls. The effect of the high intensity sport training experienced by the racquet sport athletes could account for these differences or perhaps they are due to examination of very different bone sites; humerus vs proximal femur. Mackelvie et al. (26) used HSA to examine bone structural outcomes among boys, all of whom were pre-pubertal, exposed to a 2-year dose of the same exercise program employed by Petit et al (24, 29). Boys in the intervention group had close to 3% greater bone expansion on the periosteal and endosteal surfaces compared to controls. These findings are functionally important in that small gains to the periosteal surface significantly influence bone strength compared to gains on the endocortical surface and emphasize the importance of exercise prior to puberty to maximize periosteal apposition. Early interventions may be particularly crucial for girls because evidence suggests that entry into puberty introduces hormonal changes that inhibit exercise-induced changes to bone’s outermost surface (35). These data have important implications since structural changes allow for increases in bone strength without significant increases in bone mass. Given bone is predominately lost from the endosteal surface during adulthood and not from the periosteal surface, physical activity in youth may offset fracture risk through structural alterations (a benefit that would not be detected when only bone mass is measured).
Data from observational studies also show that children who spend more time in active play have improved bone structure and, therefore, bone strength compared to less active peers (17). Using accelerometers and a six-year follow-up in the Iowa Bone Development Study we measured the effect of physical activity on the proximal femur structural indices of axial and bending strength. Results indicate that physically active boys and girls have greater axial and bending strength than their less-active peers throughout childhood. On average, children ages 5 through 11 who participated in 40 minutes of at least moderate intensity physical activity per day would be expected to have 3 to 5% greater axial and bending strength than peers participating in 10 minutes per day. This finding suggests that bone geometrically adapts to increased loading during daily activity throughout childhood and that meaningful increases in bone strength are possible without extraordinary amounts of activity.
Persistent Effects of Childhood Activity on Lifelong Bone Health
The evidence is consistent as to the beneficial effects of exercise on skeletal health during the pre- and early pubertal years. Specifically, cross-sectional, observational, and intervention studies have shown that more-active children have enhanced bone mineral accrual and more optimal bone structure compared to less-active children. However, it is the potential for skeletal benefits to be sustained into older adulthood that underscores the importance of early childhood activity. Our work and that of Scerpella et al (2011) have provided intriguing data to support that even short-term bouts of skeletal loading during childhood can have a lasting effect – despite the cessation of the loading stimulus (9, 10, 34). This suggests that even if children reduce activity as they get older, skeletal changes that occurred as a result of childhood activity may persist, thereby positively influencing adult bone health.
To date, the best evidence of a long-lasting benefit from physical activity during growth comes from animal models. In one particularly well-designed study, 5-week-old growing rats were subjected to axial loading of the right forearm 3 days per week for 7 weeks, a period of time equivalent to human childhood (37). The left forearms served as internal controls and were not exercised. Bone mass and structure were assessed before and after the 7-week loading period and during 92 weeks of detraining. During detraining, DXA and PQCT scans were performed at 2-week intervals for the first 6 weeks, 4-week intervals for the next 8 weeks, and 6-week intervals thereafter. After 92 weeks of detraining (at 2 years of age), ulnas were removed and assessed for bone mineralization and strength. Following the 7-week period of axial loading, there were significant increases in bone mineralization and cortical area resulting in significant improvements in bone strength. In vivo assessments during detraining showed that loading-induced changes in bone mass diminished over time; however, the bone structural measures in exercised and non-exercised ulnas did not converge. Following detraining, in vitro measures indicated increases in bone mineralization were lost, but improvements to bone structure and strength remained. Thus, exercise during growth resulted in significant improvements in bone mineralization and structure, but only structural changes persisted into senescence.
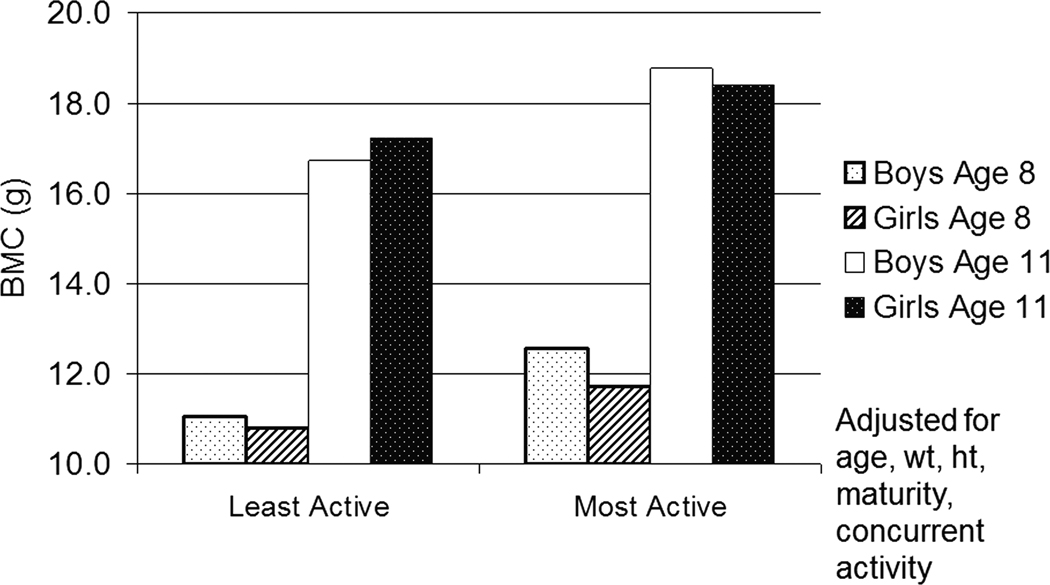
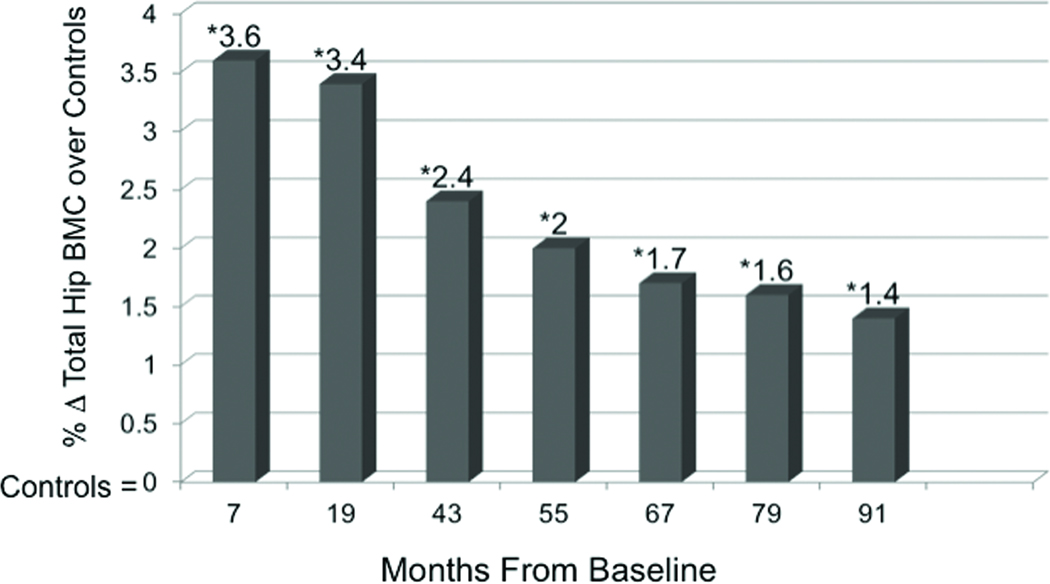
In humans we don’t yet know whether impact loading experienced while young influences bone strength in older adulthood when the likelihood of a fragility fracture is greatest. However, the sustained effect of childhood physical activity into adolescence and even young adulthood has been demonstrated in several observational studies (4, 18, 34). In the Iowa Bone Development Study, we (18) observed 333 children at ages 5, 8, and 11 years, measuring physical activity using accelerometry and bone outcomes using DXA. Children were grouped by their activity level at age 5 to investigate the effect of the age-5 physical activity on BMC at age 8 and age 11. Children in the most active quartile at age 5 had adjusted BMC values at age 8 ranging between 6% and 14% greater than those in the lowest activity quartile at age 5. By age 11, those who were most active at age 5 still had 4–7% greater BMC (adjusted for age, height, weight, and current physical activity.) While the magnitude of the benefits dampened over time, the benefits of being highly active, even at age 5, positively affected bone mass into early adolescence (Figure 3). In another prospective, longitudinal study, Baxter-Jones et al. (4) investigated whether children aged 8–15 who were most physically active at study entry, maintained higher BMC into the third decade of life compared to less active peers. One year following the attainment of peak height velocity (PHV) active males had between 7.6% and 12.5% more BMC at the lumbar spine, total body and total hip compared to inactive males, while active females had 7.8% and 14.6% more BMC at the total body and lumbar spine, respectively, compared to inactive females. In the follow-up analyses, participants were grouped according to the same physical activity levels at adolescence and BMC outcomes were evaluated adjusting for age, maturity age, height, weight, adult physical activity, calcium intake and BMC at 1 year following peak height velocity. Researchers found that males and females who were most active as children, had approximately 8%–10% more adjusted BMC at the hip as young adults. Thus, these results suggest that the effects of physical activity in youth have a greater influence than current adult physical activity. Still more evidence that impact loading during growth induces persistent changes comes from Scerpella et al. (34) who reported that ex-gymnasts exhibited enhanced BMC, areal bone mineral density, and bone size (projected area of the mid and distal radius) compared to non-gymnasts. After adjusting for age at menarche, changes in somatic growth, lean mass, and calcium intake, advantages in all measured areas among gymnasts persisted into early adulthood (4–9 years post-menarche), despite cessation of competitive gymnastics training around the time of menarche. While there were no reported differences in the mean activity levels over time between ex-gymnasts and controls, the authors did not adjust for current physical activity in the analyses which must be taken into consideration when interpreting the results. Based on evidence presented thus far, the diminishing (yet persistent) benefits associated with early exposure to physical activity may be explained by factors such as bone’s decreasing responsiveness to mechanical stimuli with age. Alternatively, the dampening influence may be a reflection of decreased physical activity with age. The latter hypothesis is supported in part by findings from two randomized, controlled trials we conducted showing that young children (7–8 years of age at baseline) who participated in high-impact jumping interventions for 7 months experienced some maintenance of increased BMC years after the cessation of training (9, 10). Building on the work of Fuchs et al. (8), we followed this cohort for another ~7 years following the cessation of the box-jumping intervention (9). Adjusting for changes in age, height, weight, and physical activity over time, we found that benefits to the hip persisted and jumpers maintained a 1.4% benefit in hip BMC compared to non-jumpers (Figure 4). The importance of physical activity throughout growth was further evidenced in this 8-year study via multi-level regression models showing that, aside from the persistent intervention effects and the influence of somatic and maturational growth, one of the most substantial contributors to the change in BMC at all measured regions of the hip over the study duration was sport participation.
Figure 3.
Mixed model least square means of Iowa Bone Development Study cohort contrasting the hip bone mineral content (BMC) (g) at ages 8 and 11 in the most active and least physically active quartiles of boys and girls at age 5 with adjustment for age, weight (wt), height (ht), maturity, and current physical activity (at age 8 and 11). N = 333 children. Findings published in (18).
Figure 4.
The effect of a jumping intervention on Δtotal hip bone mineral content (BMC) after 8 years showing percent change in total hip BMC from baseline in jumpers above that of controls. Data points are displayed after 7 mo of exercise training, 1 yr of detraining (19 mo), and 4–8 yr of detraining (43–91 mo). The intervention participants had 3.6% greater bone mass at the total hip than controls immediately after the intervention and 1.4% greater bone mass 8 yrs later. *Results are significant at each of the seven measurement intervals (p < 0.05) and are adjusted for baseline age, ΔHt, ΔWt, maturity, and sports participation. [Adapted from (9). Copyright © 2008 John Wiley and Sons. Used with permission.]
In an attempt to enhance the translation of the interventions, we replicated the effects of the box-jumping intervention with the protocol delivered by PE specialists in the schools rather than by research personnel (10). The benefits were even greater with jumpers gaining 7–8% more BMC at the hip, spine and whole body from box-jumping in comparison to controls immediately following the 7-month intervention. Three years after intervention cessation, jumpers have maintained 2–5% greater BMC than controls at the hip, spine, and whole body (after adjusting for age, maturation, and body composition) (10). What is exciting about these trials is the notion that a short, targeted dose of physical activity can have such lasting effect. This highlights the potential for all children, and not just highly-motivated athletes, to achieve optimal skeletal health through activity programs requiring minimal time and resource investments.
CONCLUSIONS and FUTURE DIRECTIONS
Several landmark longitudinal and intervention studies have provided intriguing data as to the potential for childhood physical activity to affect long-lasting skeletal health (4, 9, 10, 18), but additional follow-up beyond skeletal maturity will impart more confidence to the recommendation that appropriate physical activity during youth can permanently affect one’s skeletal future. As the evidence accumulates it is, of course, important to remember that a sedentary lifestyle does not improve skeletal health to any degree, at any age. Thus, until sufficient evidence accrues, medical and public health professionals should recommend physical activity for all children, follow the physical activity guidelines, and incorporate impact-loading activity as suggested in this review to optimize skeletal health. Specifically, all youth should participate in weight-bearing physical activity daily, accruing a minimum of 60 minutes. Our findings suggest that approximately 40 minutes of MVPA daily is necessary to convey substantial benefits to hip structure and strength (15), while results from our targeted interventions suggest that 10–15 minutes of jumping (e.g. 100 jumps from a two-foot height such that GRFs are at least 3.5 BW and higher) three times per week can substantially benefit bone mass and structure (9, 10). Furthermore, our data (10) support the importance of advocating for the promotion of sports participation across childhood and adolescence as a strategy to optimize bone mineral accrual.
In conclusion, it is essential to consider the need for population-based approaches to increase physical activity. To be effective and sustained, physical activity must be woven into the fabric of society, which requires physical and social environmental supports for increasing active lifestyles. To date, the most effective interventions to enhance skeletal development have been school-based. However, data from observational studies provide strong evidence that simply increasing moderate-to-vigorous activity may also contribute to improving bone health. Thus, the charge to researchers is to take the next difficult steps and test effective translational strategies that can be broadly disseminated in schools and communities to positively influence physical activity behaviors and subsequent life-long skeletal health.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the work of numerous scientists whose work on the influence of mechanical loading on skeletal development was not cited due to reference constraints. Dr. Gunter was supported by NIH grant RO1 AR45655-08 and the Good Samaritan Hospital Foundation. Dr. Janz was supported by NIH grants R01-DE09551, R01-DE12101, and M01-RR00059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors indicate no conflicts of interest.
References
- 1.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 2.Bauer JJ, Snow CM. What is the prescription for healthy bones? J. Musculoskelet Neuronal Interact. 2003;3:352–355. [PubMed] [Google Scholar]
- 3.Baxter-Jones AD, Faulkner RA, Forwood M, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J. Bone Miner. Res. 2011;26(8) doi: 10.1002/jbmr.412. 1729-394. [DOI] [PubMed] [Google Scholar]
- 4.Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone. 2008;43:1101–1107. doi: 10.1016/j.bone.2008.07.245. [DOI] [PubMed] [Google Scholar]
- 5.Blimkie CJ, Rice S, Webber CE, Martin J, Levy D, Gordon CL. Effects of resistance training on bone mineral content and density in adolescent females. Can. J. Physiol. Pharmacol. 1996;74:1025–1033. [PubMed] [Google Scholar]
- 6.Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud. Publica. Mex. 2009;51 Suppl 1:S5–S17. doi: 10.1590/s0036-36342009000700004. [DOI] [PubMed] [Google Scholar]
- 7.Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: A 12-month study in pre/peri- and postmenarcheal tennis players. J. Bone Miner. Res. 2011;26:1321–1329. doi: 10.1002/jbmr.323. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J. Bone Miner. Res. 2001;16:148–156. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 9.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuchs RK, Durski S, Snow C. Impact exercise increases BMC during growth: an 8-year longitudinal study. J. Bone Miner. Res. 2008;23:986–993. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuller A, Durski S, Snow C. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. 2008;42:710–718. doi: 10.1016/j.bone.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Gunter KB, Kasianchuk A. Examining the influence of participation in a community-based running program on skeletal health in growing girls. Osteoporosis Int. 2011;22 Suppl 2 S417-S39. [Google Scholar]
- 12.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos. Int. 2000;11:1010–1017. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 13.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40:14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Hui SL, Slemenda CW, Johnston CC., Jr The contribution of bone loss to postmenopausal osteoporosis. Osteoporos. Int. 1990;1:30–34. doi: 10.1007/BF01880413. [DOI] [PubMed] [Google Scholar]
- 15.Janz KF, Burns TL, Levy SM, Torner JC, Willing MC, Beck TJ, Gilmore JM, Marshall TA. Everyday activity predicts bone geometry in children: the Iowa bone development study. Med. Sci. Sports Exerc. 2004;36:1124–1131. doi: 10.1249/01.mss.0000132275.65378.9d. [DOI] [PubMed] [Google Scholar]
- 16.Janz KF, Gilmore JM, Burns TL, Levy SM, Torner JC, Willing MC, Marshall TA. Physical activity augments bone mineral accrual in young children: The Iowa Bone Development study. J Pediatr. 2006;148:793–799. doi: 10.1016/j.jpeds.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Janz KF, Gilmore JME, Levy SM, Letuchy EM, Burns TL, Beck TJ. Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone. 2007;41:216–222. doi: 10.1016/j.bone.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Torner JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med. Sci. Sports Exerc. 2010;42:1072–1078. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann. Intern. Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Khort W, Bloomsfield S, Little K, Nelson M, Yingling V. ACSM position stand: physical activity and bone health. Med. Sci. Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 21.Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J. Bone Miner. Res. 2003;18:352–359. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 22.Linden C, Ahlborg HG, Besiakov J, Gardsell P, Karlsson MK. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: Two-year data from the Pediatric Osteoporosis Prevention (POP) Study. J Bone Min Res. 2006;21:829–835. doi: 10.1359/jbmr.060304. [DOI] [PubMed] [Google Scholar]
- 23.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112(6 Pt 1):e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 24.Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J. Pediatr. 2001;139:501–508. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 25.MacKelvie KJ, McKay HA, Petit MA, Moran O, Khan KM. Bone mineral response to a 7-month randomized controlled, school-based jumping intervention in 121 prepubertal boys: associations with ethnicity and body mass index. J. Bone Miner. Res. 2002;17:834–844. doi: 10.1359/jbmr.2002.17.5.834. [DOI] [PubMed] [Google Scholar]
- 26.MacKelvie KJ, Petit MA, Khan KM, Beck TJ, McKay HA. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755–764. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 27.NIH Consensus Development Panel on Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 28.Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC. Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J. Bone Miner. Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 30.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 31.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 2002;17:1545–1554. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 32.Sardinha LB, Baptista F, Ekelund U. Objectively measured physical activity and bone strength in 9-year-old boys and girls. Pediatrics. 2008;122:e728–e736. doi: 10.1542/peds.2007-2573. [DOI] [PubMed] [Google Scholar]
- 33.Sayers A, Mattocks C, Deere K, Ness A, Riddoch C, Tobias JH. Habitual levels of vigorous, but not moderate or light, physical activity is positively related to cortical bone mass in adolescents. J. Clin. Endocrinol. Metab. 2011;96:E793–E802. doi: 10.1210/jc.2010-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos. Int. 2011;22:2205–2210. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeman E. Periosteal bone formation--a neglected determinant of bone strength. N. Engl. J. Med. 2003;349:320–323. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 36.Warden SJ, Fuchs RK. Exercise and bone health: optimising bone structure during growth is key, but all is not in vain during ageing. Br. J. Sports Med. 2009;43:885–887. doi: 10.1136/bjsm.2008.054866. [DOI] [PubMed] [Google Scholar]
- 37.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J. Bone Miner. Res. 2007;22:251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 38.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J. Bone Miner. Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 39.Wiebe PN, Blimkie CJ, Farpour-Lambert N, Briody J, Marsh D, Kemp A, Cowell C, Howman-Giles R. Effects of single-leg drop-landing exercise from different heights on skeletal adaptations in prepubertal girls: a randomized controlled study. Pediatr. Exerc. Sci. 2008;20:211–228. doi: 10.1123/pes.20.2.211. [DOI] [PubMed] [Google Scholar]
- 40.Witzke KA, Snow CM. Effects of plyometric jump training on bone mass in adolescent girls. Med. Sci. Sports Exerc. 2000;32:1051–1057. doi: 10.1097/00005768-200006000-00003. [DOI] [PubMed] [Google Scholar]