Abstract
Connexin (Cx) proteins form intercellular gap junction channels by first assembling into single membrane hemichannels that then dock to connect the cytoplasm of two adjacent cells. Gap junctions are highly specialized structures that allow the direct passage of small molecules between cells to maintain tissue homeostasis. Functional activity of nonjunctional hemichannels has now been shown in several experimental systems. Hemichannels may constitute an important diffusional exchange pathway with the extracellular space, but the extent of their normal physiological role is currently unknown. Aberrant hemichannel activity has been linked to mutations of connexin proteins involved in genetic diseases. Here, we review a proposed role for hemichannels in the pathogenesis of Keratitis-Ichthyosis-Deafness (KID) syndrome associated with connexin26 (Cx26) mutations. Continued functional evaluation of mutated hemichannels linked to human hereditary disorders may provide additional insights into the mechanisms governing their regulation in normal physiology and dysregulation in disease.
Keywords: connexin, mutation, genetic disease, channel, epidermis
INTRODUCTION
Intercellular communication is a hallmark of multicellular organisms. In chordate animals, the connexin family of structural proteins form intercellular membrane channels called gap junctions [1]. Connexin (Cx) proteins have been studied for nearly five decades in the context of these intercellular gap junction channels that facilitate electrical and biochemical coupling of adjacent vertebrate cells [2–5]. Gap junctions are well characterized with regard to their role in maintaining tissue homeostasis by enabling exchange of ions, second messengers, and metabolites [6–9]. Connexins are now known to also be capable of forming functional hemichannels in nonjunctional membranes, linking the cytoplasm of a cell with its extracellular microenvironment [10–14]. Hemichannels are thought to participate in paracrine functions and there is evidence to indicate that hemichannels mediate calcium signaling through ATP release [15, 16]. Hemichannels presently have an unclear role in normal physiology, but there is accumulating evidence showing that their activity can be altered under certain pathological conditions [17].
Mutations of connexin-encoding genes contribute to the etiology of a variety of human genetic diseases, including, but not limited to, skin disorders, congenital cataract, peripheral neuropathies, and non-syndromic sensorineural deafness [5, 18–21]. New mutations are continually discovered due to improved availability and affordability of DNA sequencing technology at academic medical centers and the high frequency of mutations in some human connexins, like connexin26 (Cx26). These findings establish a genetic basis for clinical illness, but provide little insight regarding pathophysiological mechanistic details. Connexin proteins are key players in a diverse set of fundamental cellular processes, leaving numerous possible targets for pathological interference that may include hemichannels. Filling in the gaps between connexin mutations and phenotypic consequences will help inform efforts to develop targeted therapies. Here, we focus on pathological hemichannel activation that may lead to disturbances in the normal patterns of keratinocyte proliferation and differentiation in the skin and have been implicated as a gap junction-independent mechanism of disease in the context of Keratitis-Ichthyosis-Deafness (KID) syndrome. The most frequently mutated connexin in the epidermis, Cx26, will be used as a model for discussion.
CONNEXIN HEMICHANNELS
Connexins are 4-transmembrane domain proteins that oligomerize to form hexameric structures that have been termed connexons or hemichannels [10]. Gap junctions are assembled when hemichannels in the membranes of two adjacent cells become aligned at their extracellular surfaces [2]. Hemichannels may be assembled from 6 of the same connexin to form so-called homomeric structures, or may be constituted by a combination of different connexins, producing heteromers. The connexin composition of hemichannels is dependent on cell-type and may affect channel properties, including permeability to second messengers and other solutes [20, 22].
Evidence for active hemichannels was first observed in vivo by whole-cell voltage clamp studies of solitary horizontal cells isolated from the catfish and skate retina [23, 24]. A time- and voltage-dependent outwardly rectifying membrane current was identified with behaviors consistent with half of a gap junction channel [23]. The existence of active hemichannels was initially suggested by in vitro expression of cloned connexins in single Xenopus oocytes, which resulted in increased membrane currents and permeability to fluorescent probes [25]. Single-channel conductances have now been shown by various mammalian cell-expression systems to substantiate these findings [26–28].
Hemichannels are thought to rest in a predominantly closed state in vivo, with transient openings in response to a wide range of stimuli [12]. Interestingly, hemichannel conductance is modulated by transmembrane voltage, calcium concentration, and intracellular pH as well as other variables known to regulate gap junction permeability [29–31]. Post-translational covalent modifications of connexin carboxy-terminal amino acids may also influence hemichannel open probability [32–35]. Finally, increased activity of hemichannels may also result from pathological mutations in connexin proteins. Although insights have been gained into the mechanisms of hemichannel gating, the impact of hemichannel activity on tissue homeostasis remains poorly understood at this time, making it difficult to evaluate possible physiological roles for normal hemichannel activity.
Disease-causing connexin mutations are largely single amino acid deletions or substitutions that have the potential to modify the topological and biochemical characteristics of the proteins and subsequently impact the function of the channels they form. Preliminary experimental work has suggested that mutations in connexin genes can functionally alter hemichannel properties with potentially deleterious consequences for the cell [36–40]. Constitutively active, or dysregulated ‘leaky’ hemichannels may deplete the cytoplasm of essential small molecules, depolarize the plasma membrane by permitting uncontrolled uptake of molecules, or cause lysis via osmotic pressures [40]. To date, there have been no conclusive findings showing aberrant hemichannel fluxes as causative of clinical phenotypes.
CONNEXIN26 IN EPIDERMAL PATHOLOGY
At least 9 of the known 21 human connexin isoforms are found in skin. Connexins have overlapping expression patterns in the three inner layers of the epidermis and are thought to mediate the continuous process of keratinocyte renewal [20, 41, 42]. Dye-transfer studies have confirmed the presence of gap junctional communication in human and mouse skin [43, 44]. Connexin proteins have dynamic spatial and temporal expression patterns and are most notably upregulated in states of increased keratinocyte proliferation and differentiation. For example, Cx26 is highly overexpressed in hyperproliferative psoriatic plaques [45, 46] as well as neoplastic papilloma lesions [47]. Experimentally induced wounds result in differential changes in connexin expression: upregulation in the wound proper and downregulation at the wound periphery [48, 49]. Finally, in patients with skin disorders linked to Cx26 mutations, expression of the mutant protein is greatly increased in the diseased epidermis [50]. At face value, these observations could be taken to imply an important role for Cx26 proteins in keratinocyte regulation.
Cx26 is found in keratinocytes of the stratum basale and stratum granulosum as well as other organ systems [42, 44, 48, 51]. Mutations in GJB2, the gene encoding Cx26, are linked to congenital sensorineural deafness as well as syndromic hearing loss associated with skin disorders [52]. Cx26 mutations are known to be the leading cause of autosomal recessive hearing loss, predominantly through a loss-of-function mechanism. The most common Cx26 mutation leading to non-syndromic deafness in Caucasian families is the single nucleotide deletion 35delG [53], which produces a frame shift that truncates the protein after encoding only a short segment of the amino-terminus, rendering it entirely non-functional. Similarly, the most prevalent Cx26 mutations leading to deafness in eastern Asian populations and Ashkenazi Jewish populations are 235delC and 167delT respectively, both of which also cause premature termination of the protein [54, 55]. Testing of other mutations has shown that loss of channel function ranges from partial-to-complete and may result from impaired trafficking of proteins to the plasma membrane and improper open-channel assembly. It is important to note that inherited deafness is genetically diverse and, though less common, cases are also linked to mutations in Cx26 that yield channels retaining some level of function. However, such mutations commonly produce channels with distinctly altered gating and permeability properties; it is often the case that they become impermeable to molecules regularly passed by wild-type channels [56, 57]. For example, the V84L mutation found in recessive non-syndromic deafness forms channels with similar gross unitary channel conductance to wild-type Cx26 gap junctions but with deficient permeability to inositol 1,4,5-trisphosphate [58, 59]. Thus, total or partial loss-of-function mutations are responsible for non-syndromic deafness and these patients do not suffer from defective cutaneous wound healing or skin abnormalities [60, 61], other than anecdotal reports of increased epidermal thickness [62, 63].
In contrast to the numerous Cx26 mutations causing non-syndromic deafness, those that also cause skin disease are all single amino acid changes with autosomal dominant inheritance patterns that confer either some type of pathological gain- or alteration-of-function [64–66]. These missense mutations are clustered in the amino-terminus and first extracellular loop of the protein and lead to a broad spectrum of dermatologic presentations [51]. Two main hypotheses follow: 1) Cx26 mutations that cause skin disorders do so by a novel gain- or alteration-of-function and 2) overexpression of mutated forms of Cx26 linked to KID syndrome in the epidermis in response to tissue injury [45–47] may in fact be harmful if active hemichannels are formed.
Single-cell voltage clamp experiments in Xenopus oocytes initially identified a Cx26 mutant linked to deafness and skin disease exhibiting aberrant hemichannel activity [39]. Subsequently, hemichannel activity was evaluated for additional mutations in syndromic deafness, and has currently been shown to be a common feature of the G45E, A40V, N14K, D50N, and G12R mutations causing Keratitis-Ichthyosis-Deafness (KID) syndrome (OMIM 148210) [36, 38, 39, 67–71] (Table 1). Palmoplantar Keratoderma (PPK) with Deafness (OMIM 148350) is a clinically distinct Cx26 congenital syndrome with no known role for hemichannel activity [50, 72]. The skin pathology in PPK is thought to proceed from Cx26 mutations via trans-dominant inhibition of other connexins residing in the epidermis, such as connexin43 or connexin30 [50, 73]. Notably, visualization of a three-dimensional Cx26 hemichannel [74] shows mutations in KID to be spatially oriented near pore-lining residues while those in PPK are more evenly distributed throughout the channel wall (Fig. 1), underscoring the potential of the former group to have direct implications on pore conformation and gating. There are also rare KID mutations that are not associated with hemichannel dysfunction, such as S17F [38]. In addition, several KID mutations have not yet been tested. It has been hypothesized that those lacking hemichannel activity may lead to distinct dermatologic phenotypes, but this has been difficult to conclusively establish due to the small number of cases and heterogeneous nature of the disorders [75].
Table 1.
Biophysical evidence of increased Cx26 hemichannel activity in GJB2 mutations causing KID syndrome via single-cell electrophysiology
Fig. 1.

Three-dimensional structure of a Cx26 hemichannel. Mutated residues linked to Keratitis-Ichthyosis-Deafness syndrome (yellow and orange) and Palmoplantar Keratoderma with Deafness (pink) are mapped onto three of the six subunits of the reported Cx26 crystal structure [60]. The blue protein backbone illustrates the topology of Cx26, which consists of 4 transmembrane domains, 2 extracellular loops, and 1 cytoplasmic loop. (A) View of the extracellular portion of the channel. (B) View of the cytoplasmic side of the channel, including both the amino and carboxy-termini. (C) Lateral view of the channel with 3-subunits and the protein backbone removed to simplify visualization of mutations. The yellow residues have been associated with aberrant hemichannel activity when mutated and are spatially confined to pore-lining domains.
CONNEXIN26 HEMICHANNEL PROPERTIES
The biophysical properties of hemichannels formed by two KID mutations, G45E and A40V [39, 67, 69], have been examined in the greatest detail. G45E in particular is linked to high patient mortality within the first year of life [67, 69, 76, 77]. Both show uniquely large hemichannel currents when expressed in single cells, surpassing any recorded wild-type connexin hemichannel currents that may contribute to normal homeostatic maintenance [36, 37, 39]. This finding is consistent in data derived from oocyte voltage-clamp experiments as well as patch-clamp recordings from transfected mammalian cells (Fig. 2). Furthermore, both mutations lead to rapid oocyte lysis and death in culture. Membrane depolarization and decreases in extracellular calcium have been shown to cause exaggerated activation of mutant hemichannels [36–39]. Conversely, hyperpolarization and high concentrations of divalent cations in the extracellular milieu stabilize the cell membrane and delay cell death (Fig. 3) [38, 78]. Increased extracellular calcium positively shifts the activation voltage of hemichannels, leading to tighter gating and mitigation of excessive hemichannel currents.
Fig. 2.
Membrane current recordings in Xenopus oocytes (A–C) and N2A cells expressing wild-type connexin26 (A, D) as well as Cx26-A40V (B, E) and Cx26-G45E (C, F). Oocyte expression assay is accomplished by microinjection of cloned human Cx26 mRNA. Single-cell voltage clamp (A–C) is performed with a holding potential of −40mV and voltages ranging from −30mV to +60mV are tested with 5-second pulses. Mammalian cell expression system involves transfection with plasmid vectors containing the human Cx26 coding region. Whole-cell patch-clamp data corresponds to voltages ranging from −90mV to +90mV. Cx26-G45E and Cx26-A40V show elevated hemichannel currents relative to wild-type in both Xenopus oocytes and N2A cells.
Fig. 3.
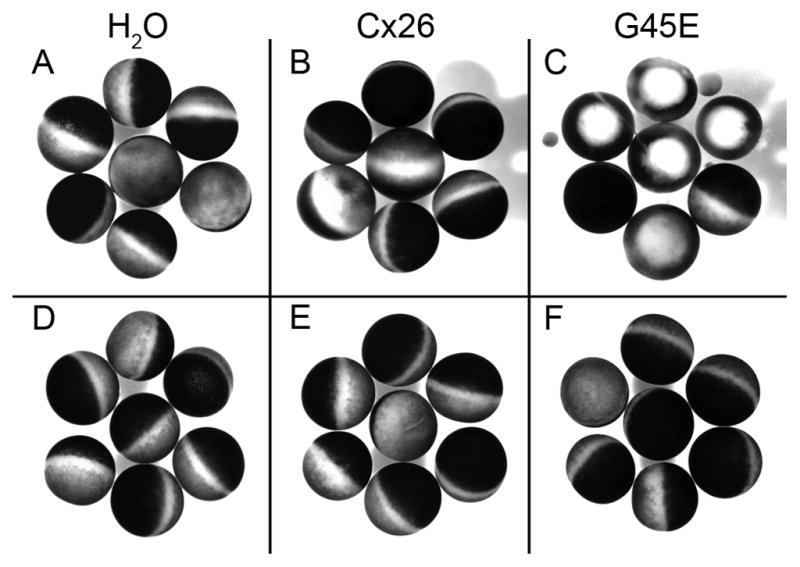
The G45E Cx26 mutant leads to accelerated cell death when expressed in Xenopus oocytes which can be rescued by culture in elevated Ca++. H2O-injected (left), wild-type Cx26-injected (middle), and G45E Cx26-injected (right) cells were incubated in 0mM (A–C) or 4mM extracellular Ca++ (D–F) for 40hrs. Cells expressing the G45E Cx26 mutant exhibit pigment disorganization, blebbing, and/or lysis in the low calcium condition but resembled the healthy negative and positive control cells in the high calcium condition.
A recent study sought to quantify hemichannel regulation by extracellular calcium for wild-type Cx26 as well as the G45E and A40V mutants [79]. Connexins were exogenously expressed in Xenopus ooctyes and macroscopic hemichannel currents were recorded by two-electrode voltage clamp during sequential perfusion with increasing concentrations of calcium. For wild-type Cx26 hemichannels held at voltages approximating normal keratinocyte membrane potentials, low extracellular calcium resulted in detectable currents that were progressively reduced as extracellular calcium was increased. The A40V mutant hemichannel showed larger currents with a shifted response curve, suggesting reduced regulation by calcium. In contrast, the G45E mutation showed increased permeability to calcium, compared to wild-type Cx26, and follow up experiments with the substituted cysteine accessibility method (SCAM) demonstrated that G45E is a pore-lining residue, implying a tentative role in channel gating and permeability [79].
Residues 40 and 45 localize to the proximal portion of the Cx26 first extracellular domain, and mutations of either result in severe forms of KID syndrome [51]. However, the development of aberrant hemichannel currents may proceed via distinct functional alterations of channel properties related to three-dimensional structure and electrochemical interactions dictated by the specific amino acid substitutions. The specificity of amino acid substitution is underscored by the apparent discrepancy in clinical phenotypes for two mutations of the same asparagine residue, N14K and N14Y. Each is associated with KID syndrome, but the skin pathologies are divergent [71, 75]. Moreover, N14K produces aberrant hemichannel currents whereas N14Y does not [80].
Whether excessive hemichannel currents are sufficient to cause reduced cell viability and epidermal pathology remains to be definitively shown. Xenopus oocyte expression systems show a consistent correlation between large single-cell transmembrane currents and accelerated cell death [37, 39]. The cellular lethality of G45E hemichannels was independently validated in transfected HEK-293 cells [36]. Recently, HeLa cell culture following transfection of the hemichannel-forming N14K Cx26 construct also showed increased cell death [75]. Mammalian cells are cultured in serum-containing media that is rich in growth factors and signaling molecules that may influence hemichannel patency and permeability. Studies suggesting that discrepancies exist in data derived from oocyte assays and data obtained from transfected mammalian cells should be cautious about drawing conclusions from subjective dye permeation assays [75], as the more quantitative biophysical methods of evaluating hemichannel activity through direct measurement of membrane current have been in good agreement (Fig. 2). Still, in vivo work with transgenic animals is needed to follow up hypotheses developed from in vitro findings to conclusively define a role for hemichannel-mediated pathology in differentiating keratinocytes.
The experimental use of pharmacological channel inhibitors may help elucidate the degree to which gained hemichannel function contributes to disease pathogenesis. The search for connexin-efficacious blockers among chemical agents known to function on other proteins involved in membrane transport has produced candidate blocker molecules such as 2-Aminoethoxydiphenyl borate (2-APB), 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), or flufenamic acid (FFA) [81–85]. As is the case with extracellular divalent cations (Ca++, Zn++, Mg++) [30], hemichannels formed from different mutant subunits may show differing sensitivities to drugs. FFA achieves rapid and reversible suppression of hemichannel currents in wild-type Cx26 as well as mutants (Fig. 4). FFA was previously shown to effectively repress the propagation of calcium waves and ATP release through connexin hemichannels in astrocytes [16]. It is important to note that the mechanisms of blockade are not well defined and may be indirect, with ancillary effects on unknown targets. Identifying or developing small molecule inhibitors of higher specificity and potency is limited by the requirement for testing of connexin mutants individually. However, this may be a worthwhile avenue to explore given the topical accessibility of connexin-expressing keratinocytes in the epidermis.
Fig. 4.

Hemichannel current suppression by flufenamic acid. A single G45E Cx26-expressing oocyte was held at −40mV and pulsed successively at 100mV for 10 minutes. Bars correspond to the average membrane current recorded during each voltage pulse. 2 minutes were allowed for membrane stabilization before perfusion with 150μM FFA for 3 minutes. Hemichannel currents shown are normalized to the initial value to monitor fractional change. > 80% hemichannel current inhibition is achieved with 150μM FFA that is partially reversed by 5 minutes of washout with the drug-free culture medium.
Further support for a generalizable role of aberrant hemichannels in epidermal pathology can be drawn from studies of mutations in GJB6, the gene that encodes Cx30. Mutations in Cx30 are linked to hidrotic ectodermal dysplasia (HED). Two mutant proteins, G11R and A88V, were reported to cause cell death when expressed in Xenopus oocytes by microinjection of the connexin mRNA [40]. Additionally, large voltage-activated currents were detected in mutant cells that were absent in wild-type controls. Transfection of HeLa cells with the Cx30 mutants resulted in increased ATP leakage into the extracellular medium [40]. Excessive release of metabolites may not only be injurious to individual cells, but could also constitute a paracrine message to propagate untoward effects throughout the tissue.
In summary, skin disease-associated mutations of connexin proteins can cause functional disturbances in hemichannel activity that may, or may not, be accompanied by changes in gap junctional intercellular conductance. It is likely that multiple disease mechanisms, including alteration of hemichannel activity, are involved across the wide spectrum of hereditary diseases involving connexin proteins. Indeed, mechanisms may even vary within individual clinical classifications, but further characterization is necessary. Experimental paradigms combining specific small molecule inhibitors with animal models will be a powerful next step toward confirming, or eliminating, the proposed role of hemichannel openings in skin disease. As an additional benefit, novel therapeutic strategies to rescue tissue integrity may emerge in the process.
HIGHLIGHTS.
Some skin disease-causing connexin mutations increase hemichannel activity.
Hemichannels may play a role in pathophysiology of skin disease.
Pharmacological blockade of hemichannels may provide novel therapeutic treatments.
Acknowledgments
Work in our laboratory is supported by NIH Grant R01 AR059505.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodenough DA. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J Cell Biol. 1974;61:557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 3.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson JD. The Occurrence of a Subunit Pattern in the Unit Membranes of Club Endings in Mauthner Cell Synapses in Goldfish Brains. J Cell Biol. 1963;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 6.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 7.Kanno Y, Loewenstein WR. Low-Resistance Coupling between Gland Cells. Some Observations on Intercellular Contact Membranes and Intercellular Space. Nature. 1964;201:194–195. doi: 10.1038/201194a0. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978;272:501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- 9.Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomembr. 1996;28:327–337. doi: 10.1007/BF02110109. [DOI] [PubMed] [Google Scholar]
- 10.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 11.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res. 316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 17.Schalper KA, Orellana JA, Berthoud VM, Saez JC. Dysfunctions of the diffusional membrane pathways mediated by hemichannels in inherited and acquired human diseases. Curr Vasc Pharmacol. 2009;7:486–505. doi: 10.2174/157016109789043937. [DOI] [PubMed] [Google Scholar]
- 18.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 19.Anand RJ, Hackam DJ. The role of gap junctions in health and disease. Crit Care Med. 2005;33:S535–538. doi: 10.1097/01.ccm.0000194035.40266.b2. [DOI] [PubMed] [Google Scholar]
- 20.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 21.Liang GS, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–754. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. American journal of physiology Cell physiology. 2005;288:C613–623. doi: 10.1152/ajpcell.00341.2004. [DOI] [PubMed] [Google Scholar]
- 23.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malchow RP, Qian H, Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res. 1993;35:237–245. doi: 10.1002/jnr.490350303. [DOI] [PubMed] [Google Scholar]
- 25.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. The Journal of cell biology. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. The Journal of general physiology. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White TW, Deans MR, O’Brien J, Al-Ubaidi MR, Goodenough DA, Ripps H, Bruzzone R. Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur J Neurosci. 1999;11:1883–1890. doi: 10.1046/j.1460-9568.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 29.DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–327. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochimica et biophysica acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke D, Koreen IV, Harris AL. Isoelectric points and post-translational modifications of connexin26 and connexin32. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:1221–1223. doi: 10.1096/fj.05-5309fje. [DOI] [PubMed] [Google Scholar]
- 33.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. The Biochemical journal. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J Biol Chem. 2001;276:8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- 36.Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–2210. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- 37.Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol. 2007;293:C337–345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 38.Lee JR, Derosa AM, White TW. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J Invest Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery JR, White TW, Martin BL, Turner ML, Holland SM. A novel connexin 26 gene mutation associated with features of the keratitis-ichthyosis-deafness syndrome and the follicular occlusion triad. J Am Acad Dermatol. 2004;51:377–382. doi: 10.1016/j.jaad.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mol Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 41.Kelsell DP, Wilgoss AL, Richard G, Stevens HP, Munro CS, Leigh IM. Connexin mutations associated with palmoplantar keratoderma and profound deafness in a single family. Eur J Hum Genet. 2000;8:469–472. doi: 10.1038/sj.ejhg.5200510. [DOI] [PubMed] [Google Scholar]
- 42.Di WL, Common JE, Kelsell DP. Connexin 26 expression and mutation analysis in epidermal disease. Cell Commun Adhes. 2001;8:415–418. doi: 10.3109/15419060109080763. [DOI] [PubMed] [Google Scholar]
- 43.Kam E, Melville L, Pitts JD. Patterns of junctional communication in skin. J Invest Dermatol. 1986;87:748–753. doi: 10.1111/1523-1747.ep12456937. [DOI] [PubMed] [Google Scholar]
- 44.Salomon D, Saurat JH, Meda P. Cell-to-cell communication within intact human skin. J Clin Invest. 1988;82:248–254. doi: 10.1172/JCI113578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivas MV, Jarvis ED, Morisaki S, Carbonaro H, Gottlieb AB, Krueger JG. Identification of aberrantly regulated genes in diseased skin using the cDNA differential display technique. J Invest Dermatol. 1997;108:188–194. doi: 10.1111/1523-1747.ep12334217. [DOI] [PubMed] [Google Scholar]
- 46.Labarthe MP, Bosco D, Saurat JH, Meda P, Salomon D. Upregulation of connexin 26 between keratinocytes of psoriatic lesions. J Invest Dermatol. 1998;111:72–76. doi: 10.1046/j.1523-1747.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- 47.Sawey MJ, Goldschmidt MH, Risek B, Gilula NB, Lo CW. Perturbation in connexin 43 and connexin 26 gap-junction expression in mouse skin hyperplasia and neoplasia. Mol Carcinog. 1996;17:49–61. doi: 10.1002/(SICI)1098-2744(199610)17:2<49::AID-MC1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Lucke T, Choudhry R, Thom R, Selmer IS, Burden AD, Hodgins MB. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J Invest Dermatol. 1999;112:354–361. doi: 10.1046/j.1523-1747.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 49.Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol Biol Cell. 1995;6:1491–1501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. Journal of cell science. 2001;114:2105–2113. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- 51.Lee JR, White TW. Connexin-26 mutations in deafness and skin disease. Expert Rev Mol Med. 2009;11:e35. doi: 10.1017/S1462399409001276. [DOI] [PubMed] [Google Scholar]
- 52.Petit C. From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med. 2006;12:57–64. doi: 10.1016/j.molmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Petit C, Levilliers J, Hardelin JP. Molecular genetics of hearing loss. Annu Rev Genet. 2001;35:589–646. doi: 10.1146/annurev.genet.35.102401.091224. [DOI] [PubMed] [Google Scholar]
- 54.Lerer I, Sagi M, Malamud E, Levi H, Raas-Rothschild A, Abeliovich D. Contribution of connexin 26 mutations to nonsyndromic deafness in Ashkenazi patients and the variable phenotypic effect of the mutation 167delT. Am J Med Genet. 2000;95:53–56. doi: 10.1002/1096-8628(20001106)95:1<53::aid-ajmg11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Martinez AD, Acuna R, Figueroa V, Maripillan J, Nicholson B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid Redox Signal. 2009;11:309–322. doi: 10.1089/ars.2008.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HL, Chang WT, Li AH, Yeh TH, Wu CY, Chen MS, Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J Neurochem. 2003;84:735–742. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- 57.Mese G, Valiunas V, Brink PR, White TW. Connexin26 deafness associated mutations show altered permeability to large cationic molecules. American journal of physiology Cell physiology. 2008;295:C966–974. doi: 10.1152/ajpcell.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci U S A. 2005;102:15201–15206. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D’Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- 61.Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209:177–186. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Adamo P, Guerci VI, Fabretto A, Faletra F, Grasso DL, Ronfani L, Montico M, Morgutti M, Guastalla P, Gasparini P. Does epidermal thickening explain GJB2 high carrier frequency and heterozygote advantage? European journal of human genetics: EJHG. 2009;17:284–286. doi: 10.1038/ejhg.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer CG, Amedofu GK, Brandner JM, Pohland D, Timmann C, Horstmann RD. Selection for deafness? Nat Med. 2002;8:1332–1333. doi: 10.1038/nm1202-1332. [DOI] [PubMed] [Google Scholar]
- 64.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Richard G. Connexin disorders of the skin. Clin Dermatol. 2005;23:23–32. doi: 10.1016/j.clindermatol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Lai-Cheong JE, Arita K, McGrath JA. Genetic diseases of junctions. J Invest Dermatol. 2007;127:2713–2725. doi: 10.1038/sj.jid.5700727. [DOI] [PubMed] [Google Scholar]
- 67.Janecke AR, Hennies HC, Gunther B, Gansl G, Smolle J, Messmer EM, Utermann G, Rittinger O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am J Med Genet A. 2005;133A:128–131. doi: 10.1002/ajmg.a.30515. [DOI] [PubMed] [Google Scholar]
- 68.Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonard L, Feldmann D, Parsy C, Freitag S, Sinico M, Koval C, Grati M, Couderc R, Denoyelle F, Bodemer C, Marlin S, Hadj-Rabia S. A familial case of Keratitis-Ichthyosis-Deafness (KID) syndrome with the GJB2 mutation G45E. Eur J Med Genet. 2008;51:35–43. doi: 10.1016/j.ejmg.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Mazereeuw-Hautier J, Bitoun E, Chevrant-Breton J, Man SY, Bodemer C, Prins C, Antille C, Saurat JH, Atherton D, Harper JI, Kelsell DP, Hovnanian A. Keratitis-ichthyosis-deafness syndrome: disease expression and spectrum of connexin 26 (GJB2) mutations in 14 patients. Br J Dermatol. 2007;156:1015–1019. doi: 10.1111/j.1365-2133.2007.07806.x. [DOI] [PubMed] [Google Scholar]
- 71.Arita K, Akiyama M, Aizawa T, Umetsu Y, Segawa I, Goto M, Sawamura D, Demura M, Kawano K, Shimizu H. A novel N14Y mutation in Connexin26 in keratitis-ichthyosis-deafness syndrome: analyses of altered gap junctional communication and molecular structure of N terminus of mutated Connexin26. Am J Pathol. 2006;169:416–423. doi: 10.2353/ajpath.2006.051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet. 1998;103:393–399. doi: 10.1007/s004390050839. [DOI] [PubMed] [Google Scholar]
- 73.Bakirtzis G, Choudhry R, Aasen T, Shore L, Brown K, Bryson S, Forrow S, Tetley L, Finbow M, Greenhalgh D, Hodgins M. Targeted epidermal expression of mutant Connexin 26(D66H) mimics true Vohwinkel syndrome and provides a model for the pathogenesis of dominant connexin disorders. Hum Mol Genet. 2003;12:1737–1744. doi: 10.1093/hmg/ddg183. [DOI] [PubMed] [Google Scholar]
- 74.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 75.de Zwart-Storm EA, Rosa RF, Martin PE, Foelster-Holst R, Frank J, Bau AE, Zen PR, Graziadio C, Paskulin GA, Kamps MA, van Geel M, van Steensel MA. Molecular analysis of connexin26 asparagine14 mutations associated with syndromic skin phenotypes. Experimental dermatology. 2011;20:408–412. doi: 10.1111/j.1600-0625.2010.01222.x. [DOI] [PubMed] [Google Scholar]
- 76.Griffith AJ, Yang Y, Pryor SP, Park HJ, Jabs EW, Nadol JB, Jr, Russell LJ, Wasserman DI, Richard G, Adams JC, Merchant SN. Cochleosaccular dysplasia associated with a connexin 26 mutation in keratitis-ichthyosis-deafness syndrome. The Laryngoscope. 2006;116:1404–1408. doi: 10.1097/01.mlg.0000224549.75161.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sbidian E, Feldmann D, Bengoa J, Fraitag S, Abadie V, de Prost Y, Bodemer C, Hadj-Rabia S. Germline mosaicism in keratitis-ichthyosis-deafness syndrome: pre-natal diagnosis in a familial lethal form. Clin Genet. 2010;77:587–592. doi: 10.1111/j.1399-0004.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 78.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol. 136:47–62. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JR, White T. American Society for Cell Biology Annual Meeting. San Diego, CA: 2009. Analysis of Mutations in Connexin26 Associated with Syndromic Deafness. [Google Scholar]
- 81.Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- 82.Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets. 2002;3:455–464. doi: 10.2174/1389450023347353. [DOI] [PubMed] [Google Scholar]
- 83.Tao L, Harris AL. 2-aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol. 2007;71:570–579. doi: 10.1124/mol.106.027508. [DOI] [PubMed] [Google Scholar]
- 84.Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) J Pharmacol Exp Ther. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- 85.Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]



