Abstract
Scleraxis is a bHLH transcription factor that plays a central role in promoting fibroblast proliferation and matrix synthesis during the embryonic development of tendons. Mice with a targeted inactivation of scleraxis (Scx−/−) fail to properly form limb tendons, but the role that scleraxis has in regulating the growth and adaptation of tendons of adult organisms is unknown. To determine if scleraxis expression changes in response to a physiological growth stimulus to tendons, we subjected adult mice that express GFP under the control of the scleraxis promoter (ScxGFP) to a six week treadmill training program designed to induce adaptive growth in Achilles tendons. Age matched sedentary ScxGFP mice were used as controls. Scleraxis expression was sparsely observed in the epitenon region of sedentary mice, but in response to treadmill training, scleraxis was robustly expressed in fibroblasts that appeared to be emerging from the epitenon and migrating into the superficial regions of tendon fascicles. Treadmill training also led to an increase in scleraxis, tenomodulin, and type I collagen gene expression as measured by qPCR. These results suggest that in addition to regulating the embryonic formation of limb tendons, scleraxis also appears to play an important role in the adaptation of adult tendons to physiological loading.
Keywords: tendon, scleraxis, tenomodulin, collagen 1, epitenon
Introduction
Tendons are connective tissue structures responsible for the transmission of the force developed by muscles to bones, and thus enable the contraction of muscles to lead to joint movements and locomotion. Tendon tissue has a dense connective tissue matrix that is composed mainly of type I collagen, as well as type III collagen, elastin, and various proteoglycans [1]. Fibroblasts, which occupy space between collagen fibrils in tendons, are the major cellular component of tendons and are responsible for the maintenance, repair, and modification of tendon extracellular matrix (ECM) [2]. Physiological loading of tendons leads to increases in tendon mass, cross-sectional area (CSA), fibroblast density, and type I collagen content [3–5], and the failure of tendons to properly adapt to physiological loading can lead to painful tendinopathies, reduced range of motion and athletic performance, and in some cases frank tendon ruptures [6]. While fibroblasts appear to play a central role in the adaptation of tendons to mechanical loading, the molecular mechanisms that regulate fibroblast activity in adult tendons are poorly understood.
Many of the same genetic networks and signaling mechanisms that regulate musculoskeletal tissue development also direct the adaptation and regeneration of tissues in adult organisms [7]. Scleraxis (Scx) is a member of the basic helix-loop-helix (bHLH) family of transcription factors and is important for tendon formation during embryonic development [8]. Using a line of mice that express GFP under the control of 4 kb of the scleraxis promoter (ScxGFP mice), Pryce and colleagues demonstrated that scleraxis is expressed in fibroblast progenitor cells as early as E9.5, and this expression continues in tendons through the remainder of embryonic development [9]. In mice with a targeted inactivation of scleraxis (Scx−/− mice), limb tendons fail to properly form, resulting in a severe reduction in mobility after birth [8].
Injection of mesenchymal stem cells transduced with scleraxis into the injured rotator cuff muscles of rats improves healing of the bone-tendon interface [10]. Scleraxis continues to be expressed natively in tendons of adult mice [11]. Scleraxis levels increase after tendon injury in mice [12,13] and rats [14], and decrease following the complete unloading of tendons in mice [15]. Little else is known, however, about the role of scleraxis in the regulation of tendon physiology in adult organisms. Tenomodulin is another protein that plays an important role during tendon development [16] and is also expressed in adult tendon tissue [17]. Tenomodulin is a type II transmembrane protein that appears to play important roles in promoting fibroblast proliferation [16] and inhibiting angiogenesis [18]. The expression of tenomodulin is regulated, at least in part, by scleraxis [19].
To gain greater understanding of the role of scleraxis in tendon adaptation, we subjected 4 mo old male ScxGFP mice to a 6 wk progressive uphill treadmill training program. We hypothesized that, compared with sedentary control ScxGFP mice, the treadmill training protocol would lead to an increase in the mass and CSA of Achilles tendons and an increase in fibroblast density, and that fibroblast cells within the tendon matrix would demonstrate an induction of GFP expression. Additionally, we hypothesized that treadmill training would lead to an increase in the expression of scleraxis, tenomodulin, and type I collagen as measured by qPCR.
Materials and Methods
Animals
A line of transgenic mice that express the green fluorescent protein (GFP) gene under the control of 4 kb of the scleraxis promoter (ScxGFP) [9], kindly provided by Dr. Ronen Schweitzer, were used. Mice were housed under specific pathogen free conditions and provided with food and water ad libidum. All experiments were approved by the University of Michigan Committee on the Care and Use of Animals.
Treadmill Training
4 m-old male ScxGFP mice were subjected to a 5 day/wk, 6-wk-long uphill treadmill training protocol designed to provide physiological loading to the Achilles tendons (N=5). Age-matched sedentary male ScxGFP mice that were restricted to normal cage activity were used as controls (N=5). The treadmill protocol was modified from Suominen [5] and Michna [20]. Following a period of acclimatization to the treadmill environment, mice were progressed through a low intensity bout of exercise in which speeds were gradually increased from 8 to 14 m/min for a total distance of 350 to 385 m in 30 mins. Intensity was steadily increased in subsequent sessions, so that the distance eventually reached 475 to 525 m. To increase the strain on Achilles tendons through the course, treadmill elevation was gradually increased in 5o increments from 0o to 15o over the 6 wks. Each time the inclination increased, exercise intensity was decreased to initial running speeds to allow the mice to adapt, and then slowly returned back to running up to 475 to 525 m per session.
After training, mice were anesthetized by intraperitoneal injection of Avertin and prepared for tendon isolation surgery. A transcutaneous incision was made along the midline of the posterior lower hindlimb, the paratenon was reflected from the tendon, and the Achilles tendon was detached from the gastrocnemius muscle and calcaneus by transverse incisions. The tendon’s wet mass was determined, and the tendon was prepared for RNA isolation or histology.
Gene Expression
Tendons from the right hindlimbs were homogenized in QIAzol tissue lysis reagent (Qiagen). RNA was isolated using an RNeasy Mini kit (Qiagen) and treated with DNase I (Qiagen). RNA was reverse transcribed into cDNA using oligo-dT15 and random hexamer primers using Omniscript RT reagents (Qiagen), and cDNA was amplified in a CFX96 real-time thermal cycler (Bio-Rad) using a QuantiTect SYBR Green I PCR kit. Reactions were conducted in triplicate, and the methods of Livak and Schmittgen [21,22] were used to normalize target gene expression to housekeeping gene expression. The presence of single amplicons was verified by melting curve analysis. The sequences of primers used for scleraxis (Scx), tenomodulin (Tnmd), collagen type Iα2 (Col1a2) and GAPDH (GAPDH) were previously published [11].
Histology
Achilles tendons were isolated from the left hindlimbs of 2, 3, and 4 mo-old sedentary ScxGFP mice (N=5 for each age group) or from mice that underwent treadmill training or served as sedentary controls, as described above. Tendons were quickly snap frozen in Tissue Freezing Medium (Triangle Biosciences) and stored at −80°C. Tendons were sectioned at a thickness of 10μm in a cryostat and subjected to hematoxylin and eosin (H&E) staining or prepared for immunohistochemistry (IHC). For IHC, slides were briefly fixed in 4% paraformaldehyde, permeabilized in 0.2% Triton X-100, and blocked with a Mouse on Mouse blocking kit (Vector Labs). Slides were then incubated with primary mouse antibodies against β-tubulin (Developmental Studies Hybridoma Bank), secondary goat anti-mouse antibodies conjugated to AlexaFluor555 and DAPI. Prolong Gold was used to mount slides. Images were obtained using a Axioplan 2 microscope (Zeiss). Quantitative analysis of H&E stained sections to determine CSA and fibroblast density was performed using ImageJ software (NIH) [11].
Statistical Analyses
Results are presented as mean±SD. The α level was set a priori at 0.05. Prism 5.0 software (GraphPad Software) was used to conduct analyses. Differences between sedentary and trained mice were tested using t-tests, while differences between 2, 3, and 4 mo-old mice were testing using one-way ANOVA and Tukey's post hoc test.
Results
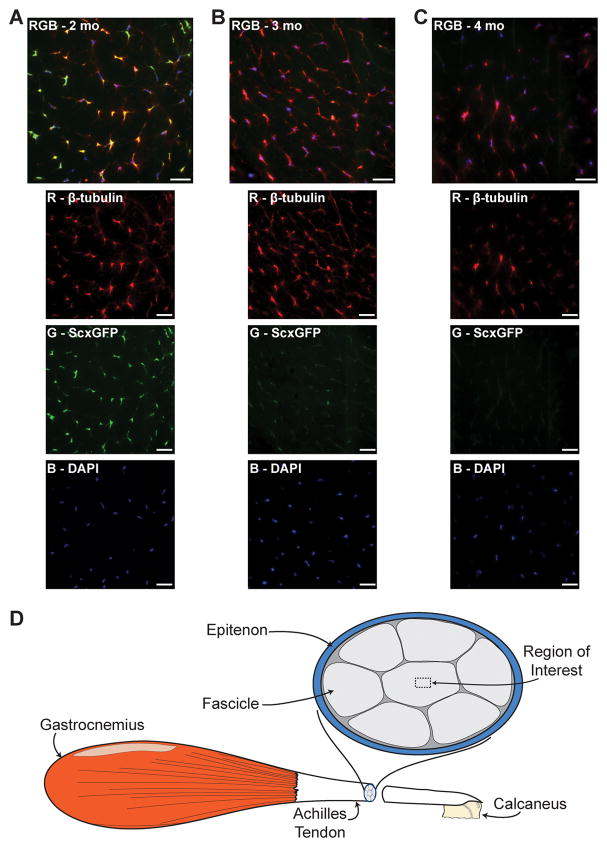
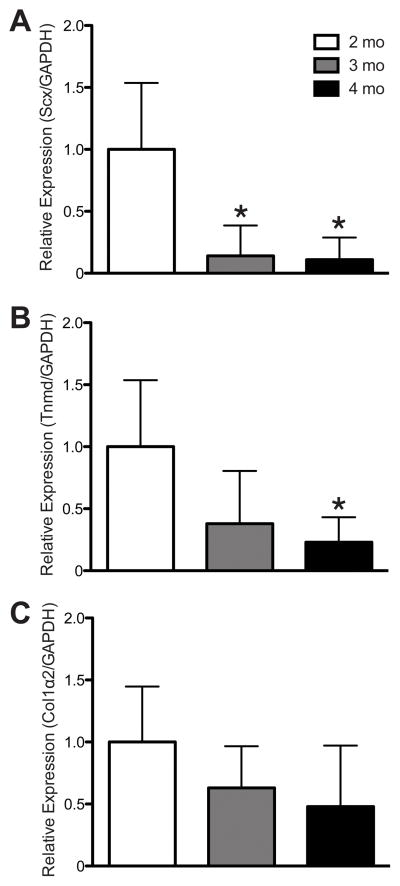
Scleraxis was strongly expressed in tendon fibroblasts from 2 mo-old mice (Fig. 1A), and this signal decreased dramatically in 3 mo-old (Fig. 1B) and 4 mo-old (Fig. 1C) mice. The change in scleraxis signal present in IHC was accompanied by quantitative changes in gene expression. Compared with 2 mo-old mice, scleraxis expression was dramatically reduced in the tendons of 3 and 4 mo-old mice (Fig. 2A). For tenomodulin, a decrease in expression occurred by 4 mos (Fig. 2B). Age-related changes in type I collagen gene expression were not observed (Fig. 2C).
Figure 1.
Scleraxis is robustly expressed in sedentary 2-mo old mice (A), and progressively decreases in 3 (B) and 4 (C) mo-old sedentary ScxGFP mice. Composite images (RGB) are in the top panel, and the individual red, green and blue channels are below. An illustration demonstrating the region of interest is shown in (D). β-tubulin, red; Scleraxis-GFP, green; nuclei, blue. Scale bars = 20μm.
Figure 2.
Compared with 2 mo-old mice, a decrease in the scleraxis (A) and tenomodulin (B) expression occurred, but no change in collagen Iα2 expression in tendons from 3 and 4 mo-old mice. Target genes were normalized to GAPDH expression, and further normalized to the 2 mo-old group using the 2−ΔΔCT method. * significantly different from 2 mo-old group
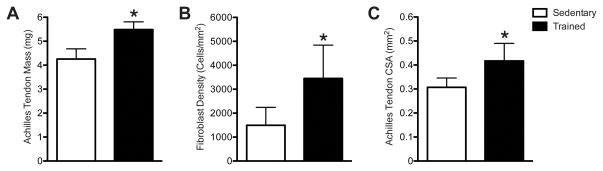
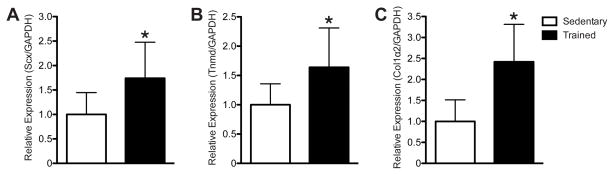
Compared with sedentary mice, mice that underwent 6 wks of treadmill training demonstrated a 29% increase in tendon mass (Fig. 3A), a 36% increase in tendon CSA (Fig. 3B), and a 131% increase in fibroblast density (Fig. 3C). To determine potential molecular mechanisms behind these changes in tendon structure, we measured the expression of scleraxis, tenomodulin, and type I collagen from the tendons. Compared with sedentary mice, treadmill training resulted in a 74% increase in scleraxis expression (Fig. 4A), a 64% increase in tenomodulin expression (Fig. 4B), and a 142% increase in type I collagen expression (Fig. 4C).
Figure 3.
Treadmill training resulted in an increase in Achilles tendon wet mass (A), CSA (B) and fibroblast density (C). * significantly different from sedentary group (P<0.05).
Figure 4.
Treadmill training resulted in an increase in scleraxis (A), tenomodulin (B), and collagen Iα2 (C) expression. Target genes were normalized to GAPDH expression, and further normalized to the sedentary group using the 2−ΔΔCT method. * significantly different from sedentary group (P<0.05).
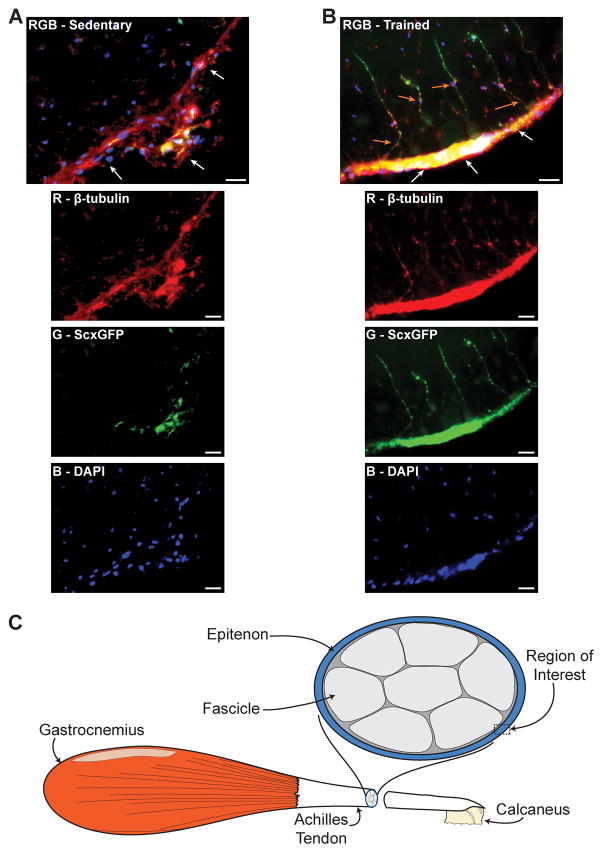
While sedentary mice displayed scleraxis expression in sparse regions throughout the epitenon and superficial layers of their tendons (Fig. 5A), mice that underwent treadmill training had dramatic increases in scleraxis expression in the epitenon regions of their tendons (Fig. 5B). Populations of scleraxis-expressing fibroblast cells that appeared to be migrating from the epitenon into tendon fascicles were also frequently noted in tendons from trained mice (Fig. 5B).
Figure 5.
Compared with sedentary mice (A), mice that underwent treadmill training (B) displayed a dramatic increase in scleraxis expression in the epitenon (white arrows) and an emergence of fibroblasts from the epitenon (orange arrows). Composite images (RGB) are in the top panel, and the individual red, green and blue channels are below. There was substantial overlap between β-tubulin and scleraxis signal in the epitenon (yellow). An illustration demonstrating the region of interest is shown in (C). β-tubulin, red; Scleraxis-GFP, green; nuclei, blue. Scale bars = 20μm.
Discussion
The significance of this study is in the new insights gained into the regional specific changes in scleraxis expression and fibroblast morphology in response to treadmill training. While we observed a substantial decrease in scleraxis expression between 2 and 4 mos of age, we demonstrated that vigorous treadmill training exercise, sufficient to increase the mass and CSA of the Achilles tendon, resulted in a dramatic increase in scleraxis expression in the tendons. Also in support of our hypothesis that scleraxis is an important regulator of tendon adaptation, the 6 wk treadmill program led to an increase in fibroblast density and an increase in scleraxis, tenomodulin, and type I collagen gene expression. Another significant finding was that the scleraxis expression was clearly enriched in the epitenon and superficial regions of fascicles in the tendons of trained mice and in fibroblasts that appeared to be emerging from the epitenon.
Scleraxis has a well described role in the embryonic development of limb tendons, and Scx−/− mice have a clearly disordered limb tendon phenotype [8]. In addition to promoting fibroblast proliferation, scleraxis binds to a tendon specific enhancer region in the type Iα1 collagen promoter to direct collagen I expression [23]. An emerging body of research suggests that scleraxis expression is regulated by members of the TGF-β superfamily of cytokines. Genetic inactivation of the TGF-β type II receptor gene in mice (Tgfbr2−/−) during development dramatically reduced scleraxis expression and disrupted the normal formation of limb tendons, resulting in a phenotype similar to that which was reported in the Scx−/− mice [24]. TGF-β directly induces the expression of scleraxis in cultured primary tendon fibroblast cells from adult mice [15]. Additional evidence for a role of TGF-β in the regulation of scleraxis was provided by the observation that treatment of mesenchymal micromass cultures with recombinant TGF-β increased scleraxis expression [25]. Moreover, myostatin (GDF-8), a member of the TGF-β superfamily, induced scleraxis expression in tendon fibroblasts [11,15], and mice that are deficient in myostatin expression (MSTN−/−) have a hypocellular tendon phenotype [11]. Scleraxis expression increased following injury to the patellar tendon [13] and FDL tendon [12], and treadmill training increases both TGF-β [26] and myostatin [27] in tendons. These previous findings along our observations are consistent with the hypothesis that scleraxis is a downstream target of TGF-β family signaling that directs the expression of genes leading to adaptive changes in tendons in response to loading.
Tenomodulin is a type II transmembrane protein that appears to play an important role in tendon development, although its precise molecular mechanisms of action are not well understood. Compared with wild type littermates, mice that are deficient in tenomodulin (Tnmd−/−) display a decrease in proliferating fibroblast cells and a reduction in fibroblast density shortly after birth [16]. Similar to scleraxis, tenomodulin expression may be regulated by members of the TGF-β superfamily, as Tgfbr2−/− mice fail to properly express tenomodulin in tendons during development [24], and treating mesenchymal micromass cultures from chick leg buds with TGF-β increased tenomodulin expression [25]. Treatment with myostatin also increased tenomodulin expression in tendon fibroblast cells [11]. However, tenomodulin expression appears to be downstream of scleraxis [19], and whether TGF-β signaling directly induces tenomodulin expression or if TGF-β indirectly induces tenomodulin expression by upregulating scleraxis expression is unclear. Tenomodulin expression levels also increased after tendon injury in mice [13] and rats [14]. The results from our study also suggest that increased expression of tenomodulin is associated with the adaptation of tendons to physiological loading.
While the fundamental cell biology of many limb tissues is well described with regards to progenitor cell localization, proliferation, and differentiation, our understanding of the biological processes that regulate tendon cell proliferation and differentiation in adult animals is less well understood. The epitenon is a region of connective tissue that contains epithelial cells and plays an important role in cell retention and the prevention of adhesion formation [28]. The epitenon has also been directly implicated as playing an important role in tendon regeneration. Using an in vitro tissue culture system, Manske [29] demonstrated that, in sections of forepaw flexor tendon explants, fibroblasts from the epitenon migrate towards the lacerated ends to form a fibrous cellular cap. Gelberman [30] and Jones [31] also demonstrated emergence of fibroblasts from the epitenon and migration to the site of injury in vivo using animal models of forepaw flexor tendon laceration and repair. Using collagenase digestion of whole tendon tissue and culture of the cells obtained from this digestion, Bi [32] identified a population of tendon stem cells that could differentiate into tenogenic, osteogenic, chondrogenic, and adipogenic lineages in vitro. A population of tendon stem cells was also identified in vitro by Zhang following collagenase digestion of whole tendon tissue [33,34]. Zhang further demonstrated that treadmill training of the animal prior to the cell isolation increased the number of tendon stem cells obtained [33], but their location within the tendon in vivo has yet to be described. The increase in cell density following treadmill training observed in our study, along with the presence of scleraxis-expressing fibroblast cells that appeared to be migrating into tendon fascicles from the epitenon suggest that a population of tendon progenitor cells exists in the epitenon and that scleraxis expression is important in the early stages of activating these cells in response to physiological loading.
While our study provided important insight into the changes in scleraxis and tenomodulin expression in response to treadmill training, there are several limitations. We only examined one time point in the treadmill training portion of the study. We did not directly identify regions of the tendon that express tenomodulin due to the lack of a commercial antibody that could recognize tenomodulin in tendon tissue. Our studies also focused on the Achilles tendon; the observed changes might not reflect other limb tendons. Finally, while we did not directly label individual fibroblast cells and track them over time, Jones and colleagues [31] used a tracking dye to demonstrate the fibroblast cells originate from the epitenon and migrate into tendon fibrils following a tenotomy. Given the close proximity of these fibroblasts to the epitenon, in many cases with portions of the cells directly in contact with the epitenon, and that their orientation was perpendicular to the direction of force transmission in tendon while most fibroblast cells are oriented in parallel with the direction of force transmission [1], these fibroblast cells are likely emerging from the epitenon and migrating into the tendon fibrils.
Injuries and diseases of tendons are a significant healthcare problem, and treatment options are limited [35]. Tendinosis is a chronic, painful overuse condition that is widely thought to occur due to a failure of the fibroblasts to regenerate damaged tendon ECM [36]. A greater understanding of the fundamental biology of tendon fibroblasts will likely improve the treatment of tendinosis, restore greater levels of function, and reduce the need for surgical intervention and the prolonged, painful and costly rehabilitation that follows surgery. As an increase in scleraxis expression is correlated with adaptive changes in tendons, a misregulation of scleraxis expression likely contributes to the development of tendinosis; this warrants further investigation. The results from our study and recent findings from Maeda et al. [15] suggest that in addition to playing an important role in the development of limb tendons, scleraxis likely plays an important role in the adaptation of tendons of adult organisms to mechanical loading.
Acknowledgments
The ScxGFP mice were a kind gift of Dr. Ronen Schweitzer. This work was supported by grants AR058920 and AR055624 from NIAMS.
References
- 1.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 2.Wang JH-C. Mechanobiology of tendon. Journal of biomechanics. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Fontana K, Almeida FM, Tomiosso TC, Pimentel ER, Höfling MAdC. Effect of high intensity aerobic exercise and mesterolone on remodeling of Achilles tendon of C57BL/6 transgenic mice. Cell Tissue Res. 2010;339:411–420. doi: 10.1007/s00441-009-0894-7. [DOI] [PubMed] [Google Scholar]
- 4.Michna H, Hartmann G. Adaptation of tendon collagen to exercise. International orthopaedics. 1989;13:161–165. doi: 10.1007/BF00268040. [DOI] [PubMed] [Google Scholar]
- 5.Suominen H, Kiiskinen A, Heikkinen E. Effects of physical training on metabolism of connective tissues in young mice. Acta Physiol Scand. 1980;108:17–22. doi: 10.1111/j.1748-1716.1980.tb06495.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. Journal of musculoskeletal & neuronal interactions. 2006;6:181–190. [PubMed] [Google Scholar]
- 7.Gayraud-Morel B, Chretien F, Tajbakhsh S. Skeletal muscle as a paradigm for regenerative biology and medicine. Regen Med. 2009;4:293–319. doi: 10.2217/17460751.4.2.293. [DOI] [PubMed] [Google Scholar]
- 8.Murchison, Price, Conner, Keene, Olson, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007 doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 9.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 10.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone Marrow-Derived Mesenchymal Stem Cells Transduced With Scleraxis Improve Rotator Cuff Healing in a Rat Model. Am J Sports Med. 2011 doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 11.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci USA. 2008;105:388–393. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loiselle AE, Bragdon GA, Jacobson JA, Hasslund S, Cortes ZE, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27:833–840. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott A, Sampaio A, Abraham T, Duronio C, Underhill TM. Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury. J Orthop Res. 2011;29:289–296. doi: 10.1002/jor.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107:399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, et al. Conversion of Mechanical Force into TGF-β-Mediated Biochemical Signals. Current biology : CB. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelinsky S, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28:289–297. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 18.Shukunami C, Oshima Y, Hiraki Y. Chondromodulin-I and tenomodulin: a new class of tissue-specific angiogenesis inhibitors found in hypovascular connective tissues. Biochem Biophys Res Commun. 2005;333:299–307. doi: 10.1016/j.bbrc.2005.05.133. [DOI] [PubMed] [Google Scholar]
- 19.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res. 1984;236:465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Léjard, Brideau, Blais, Salingcarnboriboon, Wagner, et al. Scleraxis and NFATc regulate the expression of the pro-alpha 1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007 doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 24.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, et al. Recruitment and maintenance of tendon progenitors by TGF{beta} signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem. 2009;284:29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- 27.Eliasson P, Andersson T, Kulas J, Seemann P, Aspenberg P. Myostatin in tendon maintenance and repair. Growth Factors. 2009;27:247–254. doi: 10.1080/08977190903052539. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SH, Al-Youha S, Van Agtmael T, Lu Y, Wong J, et al. Tendon Is Covered by a Basement Membrane Epithelium That Is Required for Cell Retention and the Prevention of Adhesion Formation. PLoS ONE. 2011;6:e16337. doi: 10.1371/journal.pone.0016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manske PR, Lesker PA. Histologic evidence of intrinsic flexor tendon repair in various experimental animals. An in vitro study. Clin Orthop Relat Res. 1984:297–304. [PubMed] [Google Scholar]
- 30.Gelberman RH, Steinberg D, Amiel D, Akeson W. Fibroblast chemotaxis after tendon repair. J Hand Surg Am. 1991;16:686–693. doi: 10.1016/0363-5023(91)90195-h. [DOI] [PubMed] [Google Scholar]
- 31.Jones ME, Mudera V, Brown RA, Cambrey AD, Grobbelaar AO, et al. The early surface cell response to flexor tendon injury. The Journal of hand surgery. 2003;28:221–230. doi: 10.1053/jhsu.2003.50044. [DOI] [PubMed] [Google Scholar]
- 32.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Pan T, Liu Y, Wang JH-C. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28:1178–1183. doi: 10.1002/jor.21123. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Wang JH-C. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28:198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 35.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clinics in sports medicine. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 36.Khan K, Cook J. The painful nonruptured tendon: clinical aspects. Clinics in sports medicine. 2003;22:711–725. doi: 10.1016/s0278-5919(03)00035-8. [DOI] [PubMed] [Google Scholar]