Abstract
The purpose of the present study was to determine if the lacrimal gland contains 5-bromo-2’-deoxyuridine (BrdU)-label retaining cells and if they are involved in tissue repair. Animals were pulsed daily with BrdU injections for 7 consecutive days. After a chase period of 2, 4, or 12 weeks, the animals were sacrificed and the lacrimal glands were removed and processed for BrdU immunostaining. In another series of experiments, the lacrimal glands of 12-week chased animals were either left untreated or were injected with interleukin 1 (IL-1) to induce injury. Two and half day post-injection, the lacrimal glands were removed and processed for BrdU immunostaining. After 2 and 4 week of chase period, a substantial number of lacrimal gland cells were BrdU+ (11.98 ± 1.84 and 7.95 ± 1.83 BrdU+ cells/mm2, respectively). After 12 weeks of chase, there was a 97% decline in the number of BrdU+ cells (0.38 ± 0.06 BrdU+ cells/mm2), suggesting that these BrdU-label retaining cells may represent slow-cycling adult stem/progenitor cells. In support of this hypothesis, the number of BrdU labeled cells increased over 7-fold during repair of the lacrimal gland (control: 0.41 ± 0.09 BrdU+ cells/mm2, injured: 2.91 ± 0.62 BrdU+ cells/mm2). Furthermore, during repair, among BrdU+ cells 58.2 ± 3.6 % were acinar cells, 26.4 ± 4.1% were myoepithelial cells, 0.4 ± 0.4% were ductal cells, and 15.0 ± 3.0% were stromal cells. We conclude that the murine lacrimal gland contains BrdU-label retaining cells that are mobilized following injury to generate acinar, myoepithelial and ductal cells.
Keywords: Progenitor cells, BrdU-label retaining cells, Tissue repair, Lacrimal gland
Introduction
The tear film helps protect and nourish the epithelial cells of the ocular surface (Tiffany, 2008). It consists of three interacting layers: an outer lipid layer secreted by the meibomian glands, a middle aqueous layer secreted by the main lacrimal gland and an inner mucous layer secreted by the corneal and conjunctival epithelial cells (Bron, et al., 2004, Gipson and Argueso, 2003, Hodges and Dartt, 2003, Tiffany, 2008). The lacrimal gland is a tubuloacinar tissue responsible for secretion of the proteins, electrolytes and water which make up the middle aqueous layer of the tear film (Dartt, 2009, Hodges and Dartt, 2003). The lacrimal gland is composed primarily of acinar epithelial cells (>80%) but also includes ductal epithelial cells, myoepithelial cells, and plasma cells (Dartt, 2009, Hodges and Dartt, 2003).
Dry eye syndrome is the result of production of tears in inadequate quantity or of inadequate quality (Pflugfelder, 2004, Stern, et al., 1998, Zoukhri, 2006). A major subtype of dry eye syndrome is the aqueous deficient type of dry eye also called keratoconjunctivitis sicca (Schaumberg, et al., 2009, Schaumberg, et al., 2003). Chronic inflammation of the lacrimal gland can lead to insufficient tear production (Pflugfelder, 2004, Stern, et al., 1998, Zoukhri, 2006). Lacrimal gland inflammation is characterized by the presence of focal lymphocytic infiltrates, increased production of proinflammatory cytokines, and destruction of the tear-producing parenchymal cells (Pflugfelder, 2004, Stern, et al., 1998, Zoukhri, 2006). Dry eye syndrome due to lacrimal gland disease is often encountered in autoimmune diseases (such as Sjögren’s syndrome, sarcoidosis, and rheumatoid arthritis), following organ transplantation (graft-versus-host disease) or viral infections (hepatitis, HIV) (Calissendorff, et al., 1989, De Vita, et al., 2002, DeCarlo, et al., 1995, Drosos, et al., 1989, Ogawa and Kuwana, 2003, Pflugfelder, 2004, Stern, et al., 1998, Zoukhri, 2006).
Stem cells have been reported in various adult tissues including the salivary glands, the pancreas, the liver, the intestines and the mammary glands (Alison, et al., 1997, Bjerknes and Cheng, 2002, Hisatomi, et al., 2004, Okumura, et al., 2003, Zhang, et al., 2005). In the salivary glands and the pancreas, stem/progenitor cells have been identified as being active participants in tissue repair after experimentally induced injury (Hisatomi, et al., 2004, Kishi, et al., 2006, Okumura, et al., 2003, Zhang, et al., 2005). Furthermore, adult stem/progenitor cells have been demonstrated to have the capacity to differentiate into both acinar and ductal cells (Hisatomi, et al., 2004, Kishi, et al., 2006, Okumura, et al., 2003, Zhang, et al., 2005). In various tissues, stem cells have been shown to be label-retaining, slow-cycling cells. These include the pancreas, the kidney, the salivary glands, the lung, the eye, the heart, and mammary glands (Duvillie, et al., 2003, Gomperts and Strieter, 2007, Kimoto, et al., 2008, Maeshima, et al., 2003, Meinhardt, et al., 2011, Smith, 2005, Wei, et al., 1995). Stem cells are thought to both divide at a slower rate compared to transit cells and to divide asymmetrically (Kume, 2005, Poulsom, et al., 2002). The most common method for identifying slow cycling cells consists of using the 5-bromo-2’-deoxyuridine (BrdU) pulse-chase technique (Cotsarelis, et al., 1989, Cotsarelis, et al., 1990). BrdU is a thymidine analog that incorporates into the DNA of dividing cells (during the S phase of the cell cycle), rendering them detectable by immunohistochemical means. Because subsequent cell divisions in the absence of label (chase period) dilute the incorporated BrdU, only cells with the lowest replication profile are detected (Cotsarelis, et al., 1989, Cotsarelis, et al., 1990, Potten, et al., 1978).
It was recently reported that mesenchymal stem cells contribute to lacrimal gland repair following experimentally induced injury (You, et al., 2011, Zoukhri, et al., 2008). It was shown that during the repair phase, the number of stem/progenitor cells, as identified by the expression of the stem cell marker nestin, was increased (You, et al., 2011, Zoukhri, et al., 2008). In the present study, we aimed to determine the presence of BrdU-label retaining cells in the lacrimal gland. Animals were pulsed with BrdU for a period of 7 days and then chased for 2, 4 or 12 weeks, to identify the population of slow-cycling label-retaining cells. Additionally, we investigated the involvement of BrdU-label retaining cells in lacrimal gland repair following experimentally induced injury. Our results show that the lacrimal gland contains BrdU-label retaining cells that are mobilized during tissue repair to generate acinar, ductal and myoepithelial cells.
Materials and Methods
5-Bromo-2’-deoxyuridine (BrdU) solution was obtained from Invitrogen (Carlsbad, CA). All other chemicals, unless otherwise specified, were obtained from Fisher Scientific or Sigma (St. Louis, MO).
Alexa Fluor 488 and Alexa Fluor 594 conjugated secondary antibodies (1:100, Invitrogen); and FITC conjugated secondary antibodies (1:100, Jackson ImmunoResearch, Westgrove, PA) were used for detection.
In Vivo BrdU Labeling and Lacrimal Gland Injury
Female BALB/c mice (8–10 wk old) were purchased from Taconic (Germantown, NY). Animals were maintained in constant temperature rooms with fixed light/dark intervals of 12 hours’ length and were fed ad libitum. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Tufts Medical Center Animal Care and Use Committee.
BrdU (50 mg/kg body weight) was injected intraperitoneally into mice daily for 7 consecutive days. The mice were sacrificed after a 2-, 4-, or 12-week chase period, the exorbital lacrimal glands removed and processed for BrdU immunostaining. The pulse and chase time periods were chosen based on reports in the literature (Alison, et al., 1997, Bjerknes and Cheng, 2002, Chan and Gargett, 2006, Hisatomi, et al., 2004, Okumura, et al., 2003, Zhang, et al., 2005). To investigate the role of BrdU-label retaining cells in tissue repair, animals from the 12-week chase group were anesthetized and the exorbital lacrimal glands were injected, in a total volume of 2 µl, with recombinant human IL-1α (1 µg, a generous gift from the BRB Preclinical Repository of the National Cancer Institute), as previously described (Zoukhri, et al., 2008, Zoukhri, et al., 2007). Animals (from the 12-week chase group) whose lacrimal glands were left untreated were used as controls. Two and half days later (at the peak of tissue repair (Zoukhri, et al., 2008, Zoukhri, et al., 2007)), the lacrimal glands were removed and processed for histopathology (to determine tissue repair) or for BrdU immunostaining.
Histopathology and Immunostaining
Lacrimal glands were fixed, overnight at 4°C, in 4% formaldehyde made in phosphate buffered saline (PBS, containing in mM: 145 NaCl, 7.3 Na2HPO4, and 2.7 NaH2PO4 at pH 7.2). Paraffin sections of the lacrimal gland (6 µm) were deparaffinized and rehydrated using graded alcohols. For histopathology experiments, paraffin sections of the lacrimal gland were processed for hematoxylin and eosin staining. For immunostaining experiments, the slides were first subjected to microwave pretreatment (20 min) with antigen retrieval solution (citrate buffer, pH 6.0). After 3 washes in PBS, slides for single BrdU labeling were treated with proteinase K for 10 minutes while slides for double labeling experiments were treated with proteinase K for 2 minutes. After 3 washes with PBS, non-specific binding sites were blocked and tissue was permeabilized for 1 hour using 0.1% Triton-X and 10% normal donkey serum diluted in PBS. The slides were then incubated overnight at 4°C with the indicated primary antibody diluted in PBS with 0.1% normal donkey serum. After 3 washes in PBS, slides were incubated for 60 min at room temperature with the appropriate secondary antibody diluted 1:100 in 0.1% normal donkey serum. After 3 washes in PBS, coverslips were mounted with a Vectashield mounting medium containing 4',6-diamidino-2-phenylindole (DAPI to stain cell nuclei, Vector Laboratories, Burlingame, CA). Sections were viewed using a Nikon UFXII microscope equipped for epi-illumination. Omission of the primary antibody or incubation with irrelevant immunoglobulins was performed for negative control experiments.
Cell Counting
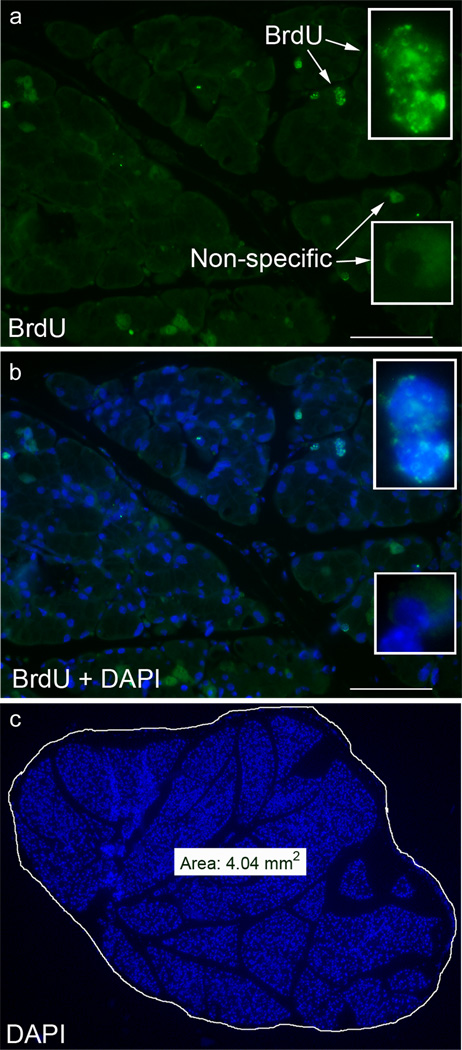
Counting of BrdU-labeled cells was conducted in a blinded manner. Multiple slides, with 4–5 sections prepared from lacrimal glands removed 2, 4, or 12 weeks post BrdU pulse were stained for BrdU immunoreactivity. All slides were processed on the same day in order to control for staining variability. Similarly, sections from control and IL-1 injected glands were processed on the same day. From each slide, one section was chosen at random and the number of BrdU labeled nuclei in the entire section counted. DAPI-stained nuclei of the entire section were then digitally captured using a Spot digital camera (Diagnostic Instruments Inc., Sterling Heights, MI) and the surface area determined using the camera’s software, as shown in Figure 1c. True positive staining was distinguished from nonspecific staining by switching between FITC (BrdU stain, green) and DAPI counterstaining (nuclei, blue) in order to ensure colocalization of both stains (Figure 1a and b). Data were then expressed as number of BrdU+ cells per mm2.
Fig. 1.
Methodology used to identify and quantify the number of BrdU+ cells. Lacrimal glands from BrdU pulsed/chased animals were fixed and stained for BrdU immunoreactivity and nuclei were counterstained with DAPI, as described in the Methods. True positive BrdU staining was defined when BrdU immunoreactivity (a, green) colocalized with DAPI (b, blue) staining. Scale bar 100 µm. The total area of the gland, visualized with DAPI (c), was determined using the SPOT camera software and then data was expressed as number of BrdU+ cells/mm2
Data Presentation and Statistical Analysis
Where appropriate, data are expressed as means ± SEM. The data were statistically analyzed using one way analysis of variance (ANOVA) followed by post-hoc t-test. Values of p < 0.05 were considered to be significant.
Results
Identification BrdU-label retaining cells in the lacrimal gland
In previous studies, it was suggested that murine lacrimal gland contains stem/progenitor cells that are mobilized during tissue repair (You, et al., 2011). These cells were identified in injured lacrimal glands by immunostaining for markers known to be expressed in stem/progenitor cells such as nestin, Sca-1 and ABCG2 (Zoukhri, et al., 2008). However, cells bearing these markers were almost undetectable in non-injured lacrimal glands and hence the presence of resident stem/progenitor cells in this tissue is still unclear (Zoukhri, et al., 2008). Therefore, we used the BrdU labeling technique in order to identify slow-cycling label-retaining cells, a unique property of stem/progenitor cells. Animals were pulsed for one week with daily injections of BrdU and after a 2-, 4-, or 12-week chasing period, the lacrimal glands were removed and the number of BrdU+ cells quantified. The pulse and chase time periods were chosen based on reports in the literature (Alison, et al., 1997, Bjerknes and Cheng, 2002, Chan and Gargett, 2006, Hisatomi, et al., 2004, Okumura, et al., 2003, Zhang, et al., 2005).
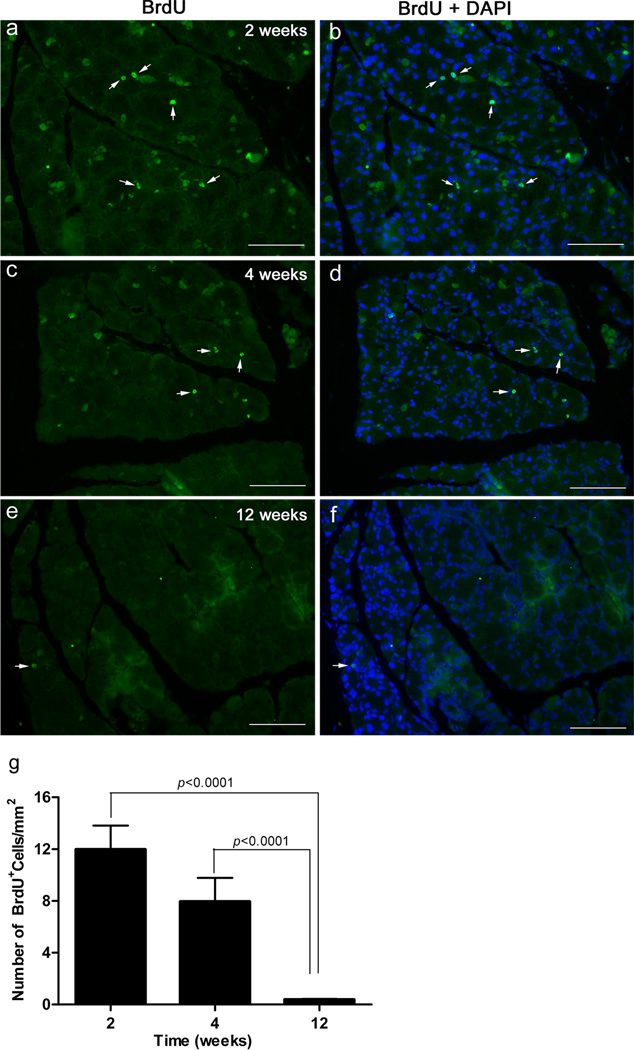
The photomicrographs depicted in Figure 2a–d show that following the 2- and 4-week chase periods, multiple BrdU+ cells could be observed in any given lacrimal gland lobule. Figure 2a–g show that the number of BrdU+ cells was high after 2 weeks of chase, decreased slightly by the 4th week and then declined dramatically at 12 weeks. After two weeks there was an average of 11.98 ± 1.84 BrdU+ cells/mm2. At 4 weeks, the average decreased by 33% to 7.95 ± 1.83 BrdU+ cells/mm2 (Fig. 2g). At 12 weeks, the average number of BrdU+ cells/mm2 declined by 97% to 0.38 ± 0.06 (Fig. 2g). Data analysis using one-way ANOVA showed a statistically significant difference between the 3 groups (p=0.0012). The post-hoc t-test found a statistically significant difference between the 2 and 12-week values and between the 4 and 12 week values (p<0.0001, Fig. 2g).
Fig. 2.
Identification of BrdU-label retaining cells in the murine lacrimal gland. Lacrimal glands were removed from 7-day BrdU pulsed animals after a chase period of 2-, 4-, or 12-weeks and processed for BrdU staining, as described in the Methods. Cell nuclei were counterstained with DAPI. a–f Representative photomicrographs of BrdU staining in glands removed from 2, 4, or 12 weeks post the 7-day BrdU pulse. Arrows point to examples of BrdU stained nuclei. Scale bar, 100 µm. g The number of BrdU+ cells was quantified as described in the Methods. Data are means ± SEM (n=3) and were statistically analyzed using one way analysis of variance (ANOVA) followed by post-hoc t-test
These results further confirm that stem/progenitor cells are present in the murine lacrimal gland. They also show that the 12 week chase period is optimal for dilution of the BrdU stain and detection of slow cycling cells in the lacrimal gland.
Involvement of BrdU-label retaining cells in lacrimal gland repair
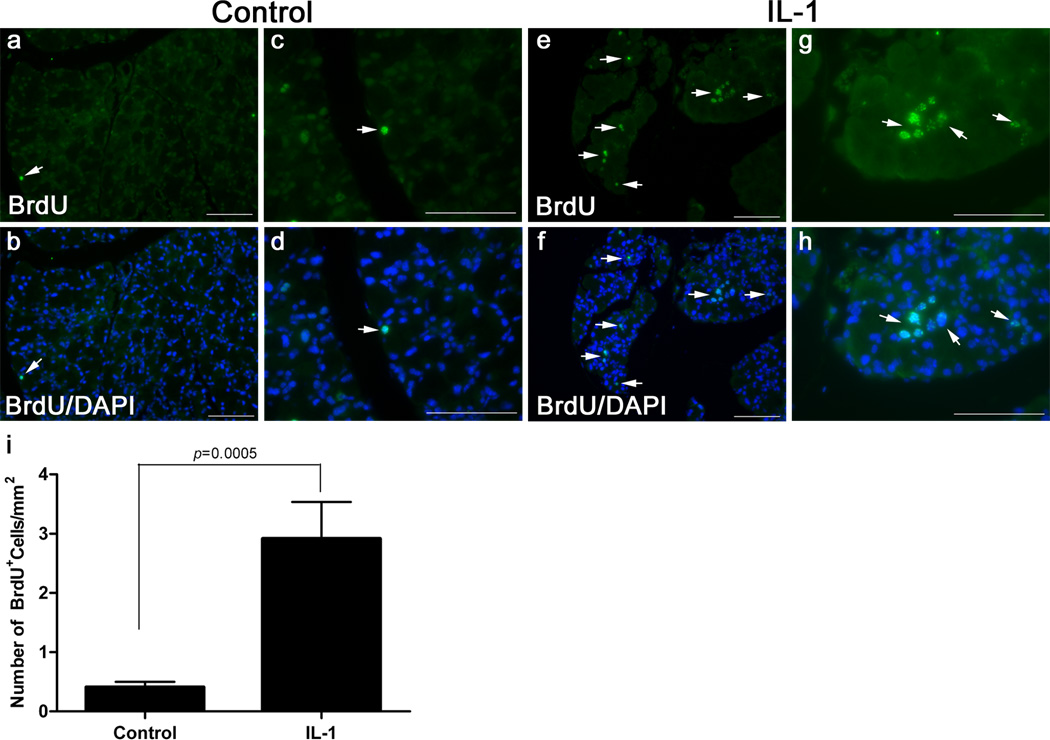
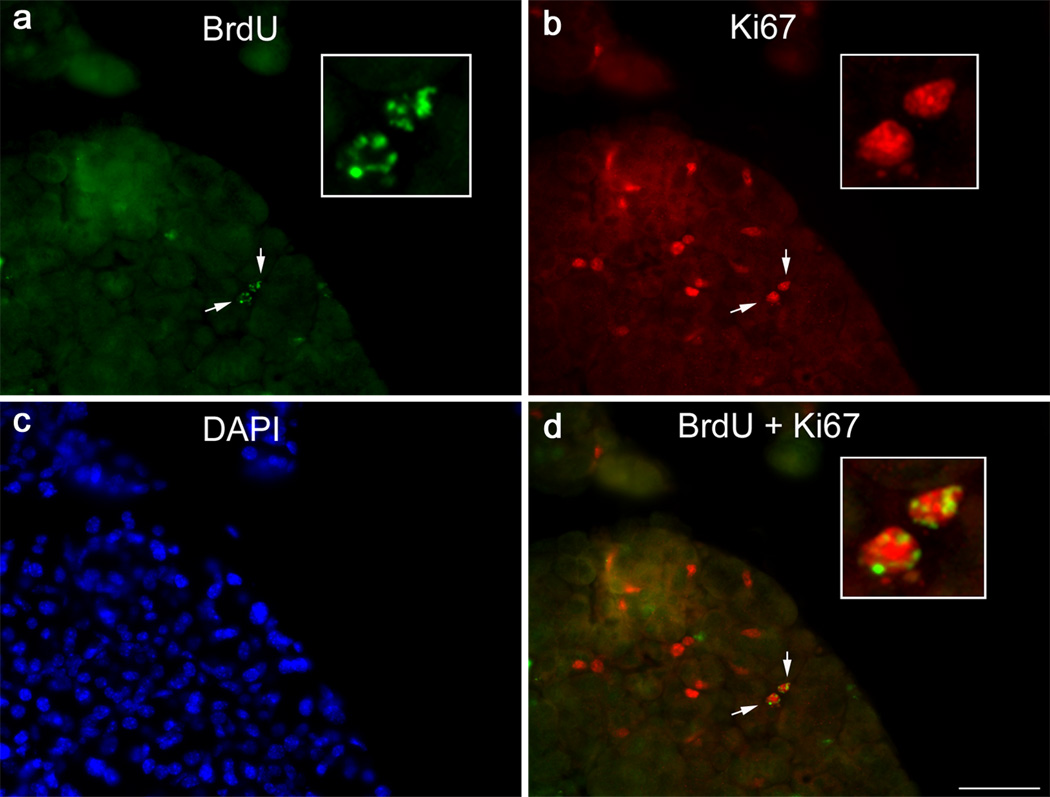
In another series of experiments we aimed to determine if the BrdU-label retaining cells were involved in lacrimal gland repair following experimentally induced injury. We previously established that a single injection of IL-1 into the lacrimal gland results in a severe inflammatory response accompanied by massive cell loss followed by mobilization of stem/progenitor cells that initiate tissue repair (You, et al., 2011, Zoukhri, et al., 2008). Lacrimal gland regeneration in female BALB/c is achieved in 6–7 days post IL-1 injection with the peak of tissue repair (i.e., mobilization of stem/progenitor cells, deposition of extracellular matrix material and induction of cell proliferation) occurring between 2 to 3 days post injury (You, et al., 2011, Zoukhri, et al., 2008). Thus we used this experimental paradigm to determine if label retaining cells were mobilized (i.e., the number of BrdU+ cells would increase) 2.5 days following injection of IL-1. We used 7-day BrdU pulsed 12-week chased animals whose lacrimal glands were either left untreated or received a single injection of IL-1. The lacrimal glands were removed 2.5 days later and processed for histopathology and immunostaining. As shown in Figure 3a–d (and in accordance with the data shown in Figure 2), in control lacrimal glands very few cells were BrdU positive (Fig. 3a–d) in contrast to glands prepared from IL-1 treated glands where the BrdU label was abundant (Fig, 3e–h). When the number of BrdU positive cells was quantified, control glands had 0.41 ± 0.09 BrdU+ cells/mm2, Fig. 3i. In injured lacrimal glands, there was a significant 7.12-fold increase in the number of BrdU labeled cells, (2.91 ± 0.62 BrdU+ cells/mm2, Fig. 3i). These results suggest that injury stimulates BrdU-label retaining cells to proliferate. To test this hypothesis, we double labeled lacrimal gland sections for BrdU and the proliferation marker, Ki67. We previously showed increased Ki67 staining during repair of the lacrimal gland (Zoukhri, et al., 2007). As shown in Figure 4, cells staining positive for both BrdU and Ki67 could be seen suggesting that BrdU positive cells are indeed capable of proliferating.
Fig. 3.
Tissue injury increases the number of BrdU+ cells. Lacrimal glands of 7-day pulsed/12 week chased animals were either left untreated (control) or were injected with IL-1 to induce tissue injury. The lacrimal glands were removed 2.5 days after injury and processed for BrdU staining, as described in the Methods. a–d Representative photomicrographs of BrdU staining in glands removed from control animals. e–h Representative photomicrographs of BrdU staining in glands removed from and IL-1 injected animals. Arrows point to examples of BrdU stained nuclei. Scale bar, 100 µm. i. The number of BrdU+ cells was quantified as described in the Methods. Data are means ± SEM (n=3) and were statistically analyzed using unpaired Student t-test
Fig. 4.
Proliferation of BrdU-label retaining cells. Lacrimal glands removed from 7-day pulsed/12-week chased animals and injected with IL-1 were processed for BrdU (a, green) and Ki67 (b, red) double-staining, as described in the Methods. Nuclei were counterstained with DAPI (c, blue). Arrows depict two BrdU and Ki67 double-labeled cells. Insets show higher magnification of the doubly-labeled cells. Scale bar, 50 µm
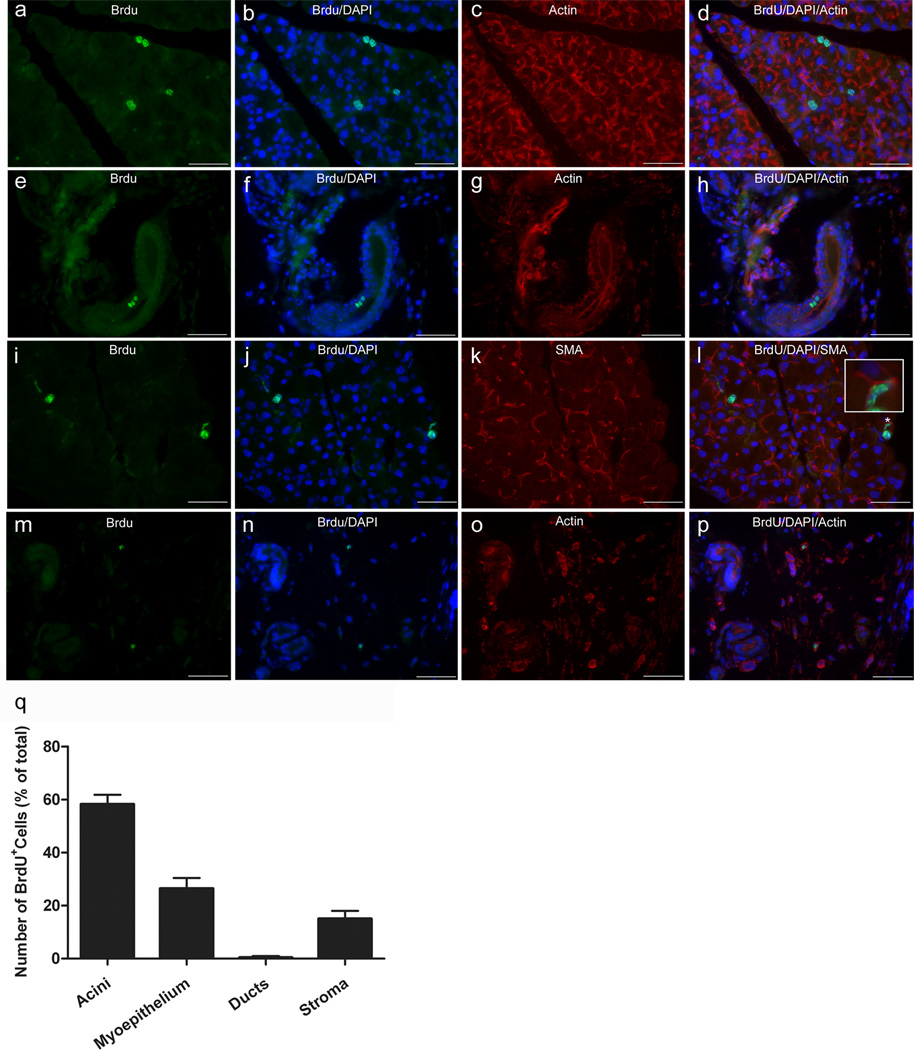
We next aimed to determine the cell type that bears the BrdU staining during tissue repair. The lacrimal gland is composed of acinar, ductal and myoepithelial cells (Hodges and Dartt, 2003). The acinar and ductal cells can be easily distinguished from each other based on their morphology but to unambiguously identify the myoepithelial cells one has to stain for α-smooth muscle actin (α-SMA) (Lemullois, et al., 1996). Lacrimal glands removed from BrdU pulsed, 12-week chased and IL-1 injected animals were processed for double immunostaining for BrdU and β-actin (acini and ducts) or BrdU and α-SMA (myoepithelial cells). Figure 5 shows that BrdU immunoreactivity can be seen associated with all three types of lacrimal gland cells: acinar (Fig. 5a–d), ductal (Fig. 5e–h) and myoepithelial cells (Fig. 5i–l). In addition, BrdU immunoreactivity was also associated with stromal cells scattered in the surrounding connective tissue (Fig. 5m–p). It should be noted that only very few ducts had BrdU+ cells within them. Indeed, when the amount of BrdU staining was quantified per cell type (acinar, myoepithelial, ductal, and stromal) only 0.4% of the BrdU stain was found to be associated with ducts (Fig. 5q). In contrast, the acinar cells accounted for most of the staining (58.2 ± 3.6 %) followed by the myoepithelial cells (26.4 ± 4.1 %) and then the stromal cells (15.0 ± 3.0 %). These findings are not surprising since acini account for >80% of the lacrimal gland parenchyma and that each acinus is surrounded by at least one myoepithelial cell (Dartt, 2009, Hodges and Dartt, 2003). Moreover, in our model of tissue injury the ductal cells are not lost (in contrast to the acinar and myoepithelial cells) following IL-1 injection and this could account for the low occurrence of BrdU staining in ductal cells observed during repair (Zoukhri, et al., 2008, Zoukhri, et al., 2007).
Fig. 5.
Distribution of BrdU label during tissue repair. Injured lacrimal glands of 7-day pulsed/12 week chased animals were removed 2.5 days after injury and processed for either BrdU/β-actin (to identify acini and ducts) or BrdU/α-SMA (to identify myoepithelial cells) double staining, as described in the Methods. a–p Representative photomicrographs of BrdU staining associated with acinar (a–d), ductal (e–h), myoepithelial (i–l) and stromal (m–p) cells. Insert in l depicts a higher magnification of the area marked by an asterisk (*) highlighting co-localization of the BrdU stain with that for α-SMA. Scale bar, 50 µm. q The number of BrdU+ cells associated with acinar, myoepithelial, ductal, and stromal cells was quantified, as described in the Methods. Data are means ± SEM (n=3)
In summary, these results suggest that in the murine lacrimal gland, the BrdU-label retaining, slow-cycling cells can differentiate, following tissue insult, into the three major cell types: acinar, myoepithelial and ductal cells.
Discussion
The BrdU pulse/chase technique has been reliably used to detect label-retaining slow-cycling cells in several adult tissues such as the pancreas, the kidney, the salivary glands, the lung, the eye, the heart, and mammary glands (Duvillie, et al., 2003, Gomperts and Strieter, 2007, Kimoto, et al., 2008, Maeshima, et al., 2003, Meinhardt, et al., 2011, Smith, 2005, Wei, et al., 1995). Some of these cells display pluripotency or a certain degree of plasticity. Indeed, several studies have reported the involvement of label-retaining cells in tissue repair (Blanpain, et al., 2007, Duvillie, et al., 2003, Forbes, et al., 2002, Gomperts and Strieter, 2007, Kimoto, et al., 2008, Poulsom, et al., 2002). In the present study, we aimed to identify BrdU-label retaining cells in the murine lacrimal gland and to investigate their role in tissue repair.
Ongoing studies in our laboratory document that the murine lacrimal gland is capable of repair following experimentally induced injury (You, et al., 2011, Zoukhri, 2010, Zoukhri, et al., 2008, Zoukhri, et al., 2007). It was reported that repair of the lacrimal gland involved the mobilization of mesenchymal stem cells (MSCs). These cells expressed the type VI intermediate filament protein nestin, which is also a marker of stem cells, whose expression was up regulated during the peak of the repair phase (2–3 days following injury) (Zoukhri, et al., 2008). More recently, it was shown that during tissue repair, lacrimal gland cells undergo epithelial-mesenchymal transition (EMT) to generate MSCs that expressed the type III intermediate filament protein vimentin along with nestin and α-SMA (You et al., In press). The results from the current studies confirm and extend those findings by showing the presence and participation of BrdU-label retaining cells in lacrimal gland repair following experimentally induced injury.
Since BrdU only labels cells which are dividing, the substantial BrdU staining after the 2 and 4 week chase period suggests that there is significant cell turnover in the murine lacrimal gland, consistent with findings in the murine submandibular gland (Kim, et al., 2008). Because subsequent cell divisions in the absence of label (chase period) dilute the incorporated BrdU, only cells with the lowest replication profile or those dividing asymmetrically are detected after a prolonged (12 week) chase period (Cotsarelis, et al., 1989, Cotsarelis, et al., 1990, Potten, et al., 1978). In other words, our data suggest the existence in the murine lacrimal gland of a population of fast-cycling transit cells that did not retain the label long term and a rare population of slow-cycling label-retaining cells that may represent adult lacrimal gland stem/progenitor cells. These results are in accordance with those reported in the murine submandibular gland where the number of BrdU+ cells after a 2 week chase amounted to 9.88 ± 2.18% and then decreased to 1.23 ± 0.09% after a seven week chase (Kim, et al., 2008).
To investigate whether the label-retaining cells had a role in tissue repair, the lacrimal glands of animals pulsed with BrdU and chased for 12-weeks were injected with IL-1 to induce injury. In mice, IL-1 injection induces a severe inflammatory response leading to destruction of acinar and myoepithelial cells followed by a repair phase which peaks between 2 and 3 days after injection (Zoukhri, et al., 2008, Zoukhri, et al., 2007). Our data show that following injection of IL-1, the number of BrdU-label retaining cells increased significantly compared to non-injured glands. This suggests that injury stimulates BrdU-label retaining cells to proliferate. Using antibodies against Ki67, a marker of proliferation, we were able to show that some of the BrdU positive cells were also positive for Ki67.
It was reported that in the submandibular gland, label-retaining cells could be observed in multiple structures including acini, ducts and myoepithelial cells (Kim, et al., 2008, Kimoto, et al., 2008). Double staining experiments for β-actin/BrdU and α-SMA/BrdU showed that label-retaining cells could be mobilized following lacrimal gland injury to differentiate into acinar, ductal and myoepithelial cells. In addition, a substantial amount of BrdU label was also seen associated with stromal cells. One limitation of the present study is that we could not determine what compartment the BrdU label was associated with since, in uninjured tissue, the number of BrdU+ cells was very low. Another limitation inherent to the BrdU pulse/technique is that one cannot rule out the contribution of circulating (i.e., non-resident lacrimal gland) BrdU+ cells in tissue repair.
In summary, our results show that the murine lacrimal gland contains BrdU-label retaining cells that are mobilized following injury to generate acinar, ductal and myoepithelial cells. Further studies are needed to determine the fate of the BrdU-label retaining cells, i.e., are these cells the same as the one identified by nestin staining and/or do they undergo EMT to generate mesenchymal stem cells during lacrimal gland repair.
Table 1.
List of primary antibodies used in these studies
| Antigen | Species | Dilution | Provider |
|---|---|---|---|
| BrdU | Sheep polyclonal | 1:200 | Novus Biologicals, Littleton, CO |
| β-actin | Rabbit Polyclonal | 1:200 | Rockland Immunochemicals, Gilbertsville, PA |
| α-SMA | Rabbit Polyclonal | 1:300 | Abcam, Cambridge, MA |
| Ki67 | Rabbit Polyclonal | 1:200 | Abcam, Cambridge, MA |
Acknowledgements
The authors gratefully acknowledge the BRB Preclinical Repository of the NCI for the generous gift of recombinant human cytokines, the assistance of Tufts Center for Neuroscience Research P30 NS047243 (Jackson), Ms. Robin Hodges and Dr. Darlene Dartt and for their critical reading of the manuscript, and Dr. Fara Sourie for her invaluable contribution to this work.
References
- Alison MR, Golding M, Sarraf CE. Liver stem cells: when the going gets tough they get going. Int J Exp Pathol. 1997;78:365–381. doi: 10.1046/j.1365-2613.1997.500375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Calissendorff B, el Azazi M, Lonnqvist B. Dry eye syndrome in long-term follow-up of bone marrow transplanted patients. Bone Marrow Transplant. 1989;4:675–678. [PubMed] [Google Scholar]
- Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita S, Damato R, De Marchi G, Sacco S, Ferraccioli G. True primary Sjögren's syndrome in a subset of patients with hepatitis C infection: a model linking chronic infection to chronic sialadenitis. Isr Med Assoc J. 2002;4:1101–1105. [PubMed] [Google Scholar]
- DeCarlo DK, Penner SL, Schamerloh RJ, Fullard RJ. Dry eye among males infected with the human immunodeficiency virus. J Am Optom Assoc. 1995;66:533–538. [PubMed] [Google Scholar]
- Drosos AA, Constantopoulos SH, Psychos D, Stefanou D, Papadimitriou CS, Moutsopoulos HM. The forgotten cause of sicca complex; sarcoidosis. J Rheumatol. 1989;16:1548–1551. [PubMed] [Google Scholar]
- Duvillie B, Attali M, Aiello V, Quemeneur E, Scharfmann R. Label-retaining cells in the rat pancreas: location and differentiation potential in vitro. Diabetes. 2003;52:2035–2042. doi: 10.2337/diabetes.52.8.2035. [DOI] [PubMed] [Google Scholar]
- Forbes SJ, Vig P, Poulsom R, Wright NA, Alison MR. Adult stem cell plasticity: new pathways of tissue regeneration become visible. Clin Sci (Lond) 2002;103:355–369. doi: 10.1042/cs1030355. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med. 2007;58:285–298. doi: 10.1146/annurev.med.58.081905.134954. [DOI] [PubMed] [Google Scholar]
- Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, Yamamoto T, Endo F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–675. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kwon HJ, Shinozaki N, Hashimoto S, Shimono M, Cho SW, Jung HS. Comparative analysis of ABCG2-expressing and label-retaining cells in mouse submandibular gland. Cell Tissue Res. 2008;334:47–53. doi: 10.1007/s00441-008-0667-8. [DOI] [PubMed] [Google Scholar]
- Kimoto M, Yura Y, Kishino M, Toyosawa S, Ogawa Y. Label-retaining cells in the rat submandibular gland. J Histochem Cytochem. 2008;56:15–24. doi: 10.1369/jhc.7A7269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Kume S. Stem-cell-based approaches for regenerative medicine. Dev Growth Differ. 2005;47:393–402. doi: 10.1111/j.1440-169X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Lemullois M, Rossignol B, Mauduit P. Immunolocalization of myoepithelial cells in isolated acini of rat exorbital lacrimal gland: cellular distribution of muscarinic receptors. Biol Cell. 1996;86:175–181. doi: 10.1016/0248-4900(96)84782-4. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Yamashita S, Nojima Y. Identification of Renal Progenitor-Like Tubular Cells that Participate in the Regeneration Processes of the Kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Spicher A, Roehrich ME, Glauche I, Vogt P, Vassalli G. Immunohistochemical and flow cytometric analysis of long-term label-retaining cells in the adult heart. Stem Cells Dev. 2011;20:211–222. doi: 10.1089/scd.2009.0203. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–S27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- Okumura K, Nakamura K, Hisatomi Y, Nagano K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto T, Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol. 2002;197:441–456. doi: 10.1002/path.1176. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Tiffany JM. The normal tear film. Dev Ophthalmol. 2008;41:1–20. doi: 10.1159/000131066. [DOI] [PubMed] [Google Scholar]
- Wei Z, Cotsarelis G, Sun T, Lavker R. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236–246. [PubMed] [Google Scholar]
- You S, Kublin CL, Avidan O, Miyasaki D, Zoukhri D. Isolation and propagation of mesenchymal stem cells from the lacrimal gland. Invest Ophthalmol Vis Sci. 2011;52:2087–2094. doi: 10.1167/iovs.10-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Avidan O, Tariq A, Ahluwalia I, Stark P, Kublin CL, Zoukhri D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland following experimentally induced injury. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.11-7893. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Kritzik M, Sarvetnick N. Identification and expansion of pancreatic stem/progenitor cells. J Cell Mol Med. 2005;9:331–344. doi: 10.1111/j.1582-4934.2005.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8:60–69. doi: 10.1016/s1542-0124(12)70070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49:4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Exp Eye Res. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]