Abstract
Bacterial conjugation is a form of type IV secretion that transports protein and DNA to recipient cells. Specific bacteriophage exploit the conjugative pili and cell envelope spanning protein machinery of these systems to invade bacterial cells. Infection by phage R17 requires F-like pili and coupling protein TraD, which gates the cytoplasmic entrance of the secretion channel. Here we investigate the role of TraD in R17 nucleoprotein uptake and find parallels to secretion mechanisms. The relaxosome of IncFII plasmid R1 is required. A ternary complex of plasmid oriT, TraD and a novel activation domain within the N-terminal 992 residues of TraI contributes a key mechanism involving relaxase-associated properties of TraI, protein interaction and the TraD ATPase. Helicase-associated activities of TraI are dispensable. These findings distinguish for the first time specific protein domains and complexes that process extracellular signals into distinct activation stages in the type IV initiation pathway. The study also provided insights into the evolutionary interplay of phage and the plasmids they exploit. Related plasmid F adapted to R17 independently of TraI. It follows that selection for phage resistance drives not only variation in TraA pilins but diversifies TraD and its binding partners in a plasmid-specific manner.
Introduction
Bacterial type IV secretion systems (T4SS) have highly versatile functions due to their ability to transmit both proteins and nucleoprotein conjugates across the cell envelope. Type IV secretion has broad clinical significance not only for delivering bacterial toxins or effector proteins directly into targeted host cells, but also for direct involvement in phenomena such as biofilm formation and the rapid horizontal spread of antibiotic resistance genes among the microbial community (de la Cruz and Davies, 2000; Ghigo, 2001; McGowan, 2001; Backert and Meyer, 2006). Conjugation systems are the largest and most widely distributed of the T4 subfamilies. These systems are responsible for plasmid conjugation in Gram-negative and Gram-positive bacteria, as well as the transfer of integrated conjugative elements, which are phage-like sequences that have been integrated into the bacterial chromosome. The extensively studied T4S system of Agrobacterium tumefaciens is related to conjugation paradigms and used by this soil borne Gram-negative bacterium to genetically transform plants. Our current understanding of the mechanistic principles of T4 secretion is due to extensive research of these DNA delivery systems (Alvarez-Martinez and Christie, 2009).
The process involves three functional substructures: cell surface pili or adhesins that mediate contact between cells, a transport channel that conducts substrates across the bacterial cell envelope, and a type IV coupling protein (T4CP) that recruits secretion substrates to the cytoplasmic entrance of the secretion channel. The general mechanism of conjugative plasmid transfer is well-characterized. Multiple proteins assemble on the plasmid origin of transfer (oriT) to form the relaxosome (de la Cruz et al., 2010). This stable complex prepares the single-strand of plasmid DNA destined for transfer (T-strand) via the nicking-closing activity of a relaxase enzyme. Initiation of transfer requires cleavage of the phosphodiester bond at a specific position, nic, within oriT. The reaction is mediated by a tyrosine residue of the relaxase, so that a covalent tyrosinyl–DNA adduct is formed. This nucleoprotein complex is specifically recognized by the plasmid-encoded T4CP and actively pumped through the transport apparatus in a reaction requiring ATP. Once in the recipient, the relaxase–ssDNA intermediate restores the original circular plasmid molecule after termination of transfer via reversion of the strand transfer reaction. Finally, stabilization of the original plasmid DNA strands by conjugative replication occurs in both donor and recipient cells.
The process has enormous importance in human health care as a major vehicle of antibiotic resistance spread among pathogens and commensal bacteria alike (Baquero, 2004; Norman et al., 2009). Accordingly research has focused on gaining detailed knowledge of the initiation stage of T4S and its control. Recent advances provide details about the recruitment and recognition process of secretion substrates by T4CPs (Nagai et al., 2005; Schulein et al., 2005; Vergunst et al., 2005; Parker and Meyer, 2007; Lang et al., 2010). T4CPs mediate multiple protein–protein interactions with cytoplasmic and inner membrane components of the secretion system (Gomis-Rüth et al., 2001; Schröder and Lanka, 2003; Cascales and Christie, 2004; Alvarez-Martinez and Christie, 2009). Experiments designed to detect the mutual modulation of protein activities, stability and localization due to these interactions will be key to defining productive docking contacts between the relaxosome and the conjugative pore, and to reconstructing the initiation pathway. Correct progression of conjugative DNA processing by the relaxosome indeed requires regulatory interactions with the T4CP (Tato et al., 2007; Mihajlovic et al., 2009; Sut et al., 2009; Wong et al., 2011). Discovering the nature of the interactions which lead to channel opening and productive entry of macromolecular secretion substrates to the transport apparatus is much more challenging, largely because macromolecules are transferred in response to cell contact and environmental cues that remain poorly defined. To move forward we sought a whole cell activity assay that involved some or all of the proteins necessary for conjugation, but what may be subject to simpler regulation. Infection by a male-specific bacteriophage (Loeb, 1960) was a promising choice for F-like paradigms because the phage life cycle depends not only on conjugative pili but also on the T4CP (Valentine et al., 1969; Schoulaker and Engelberg-Kulka, 1978).
R17 phage adhere to F-like conjugative pili via the phage attachment (A) protein (Roberts and Steitz, 1967). In a subsequent reaction known as eclipse, the viral RNA genome dissociates from its coat protein and is transiently sensitive to RNase (Paranchych, 1975). Adsorption can occur on isolated pili but eclipse requires their cellular attachment (Valentine and Strand, 1965). A processed form of protein A, covalently linked to the 3′ end of the phage RNA, pilots the nucleoprotein complex from the capsid into the cell (Krahn et al., 1972; Wong and Paranchych, 1976). Entry may occur through retraction of pili, or via the pilus lumen following pilin rearrangements at the site of attachment (Marvin and Hohn, 1969; Paranchych et al., 1971). Penetration of multiple copies of this nucleoprotein complex to the host cytosol is followed by viral replication, packaging and cell lysis.
Early work identified several plasmid proteins essential for the phage life cycle including TraA pilin, the mating pair formation (Mpf) system involved in pilus biogenesis, lytic transglycosylase P19, and importantly, the T4CP TraD (Valentine et al., 1969; Schoulaker and Engelberg-Kulka, 1978; Bayer et al., 1995). It follows that the interaction of R17 phage with a piliated host conveys exogenous signals to the cell interior that activate the T4CP for nucleoprotein trafficking. In this study, we demonstrate that T4CP-dependent uptake of R17 RNA–protein A complexes by the plasmid R1 system involves docking interactions between the T4CP and its cognate relaxosome. Phage sensitivity was then used to explore whether activation of nucleoprotein import could be uncoupled from any or all DNA processing reactions necessary for nucleoprotein export. We find that host cells are vulnerable to infecting phage only through a T4S machinery that is also competent for conjugative DNA transfer. Finally, the T4CP and a partial complex of the relaxosome was found to have a key role in transmission of exogenous signals into activation of nucleoprotein transfer.
Results
Host cell sensitivity to bacteriophage R17 requires the R1-16 relaxosome
Early research of the R17 phage life cycle used Escherichia coli hosts carrying plasmid F, and in some cases the fertility derepressed variants of R1 (i.e. R1-16 or R1-19). Plasmid-specific differences in host phage sensitivity were described in numerous studies (Willetts and Maule, 1986). We chose to develop this model rigorously for R1 proteins, and tested whether host sensitivity required the R1-16 plasmid oriT and components of the relaxosome in addition to the T4CP TraD. In F-like systems the secreted protein TraI is a bifunctional relaxase that cleaves one plasmid strand and pilots the DNA to the recipient, and a helicase that is essential for transfer (de la Cruz et al., 2010). Additional relaxosome proteins that bind oriT with sequence specificity are the E. coli IHF and plasmid proteins TraM and TraY (Mihajlovic et al., 2009). Mutant derivatives of R1-16 lacking DNA sequences essential to assembly of a functional relaxosome were generated. We deleted 34 bp of oriT spanning the site of TraI relaxase-catalysed cleavage (R1-16Δnic) (Fig. 1A). This eliminated nic, the inverted repeat (IR) and key bases for TraI recognition (Williams and Schildbach, 2006). A second construction removed 104 bp of oriT including nic and ihfA and sby binding sites for IHF and TraY (R1-16ΔoriT) (Fig. 1A). Single gene deletions in R1-16 eliminated the plasmid relaxosome protein components (ΔtraM, ΔtraY or ΔtraI). A plaque assay for host cell R17 sensitivity (R17S) confirmed the requirement for the R1-16 T4CP TraD, as expected (Table 1). Mutation of the NTP-binding Walker A box in TraDK198T blocked complementation of the R17S phenotype. The test screen of hosts carrying mutant R1-16 derivatives (Δnic, ΔoriT, ΔtraM, ΔtraY or ΔtraI) confirmed that relaxosome reconstitution is important for effective phage R17 infection (Table 1). The data argue most strongly for a role for TraI since R17S of the R1-16ΔtraI host was effectively complemented in trans. We also verified normal transfer (tra) gene expression from the R1-16Δnic and R1-16ΔoriT mutant plasmids by measuring highly efficient conjugative mobilization of a coresident oriTR1 plasmid (not shown). Complementation of the conjugative self transfer of the R1-16ΔtraM and R1-16ΔtraY with wild-type expression in trans was 10−4 to 1 transconjugant per donor cell respectively, in good agreement with prior observation (Maneewannakul et al., 1996; Pölzleitner et al., 1997). Expression in trans failed to restore efficient R17S to the host for these mutant derivatives. The transcriptionally repressed wild-type plasmid R1 normally transfers with a similar frequency of 10−3. Consistent with the mutant derivatives, plaque formation with R1-carrying hosts was below the level of detection. Results of this assay therefore cannot support or rule out a direct contribution of R1 TraM or TraY to host cell phage infection.
Fig. 1.

Schematic representation of the oriT deletion variants and the functional domains of TraI.
A. DNA sequences important to TraI binding and strand cleavage include nic (black triangle), and the inverted repeat (IR, arrows). The binding sites for the accessory proteins IHF (ihfA, ihfB), TraY (sbyA) and TraM (sbmA, sbmB, sbmC) are illustrated. To create the deletions, loxP-tetRA-loxP cassettes replaced the sequences indicated [numbering according to Graus-Goldner et al. (1990)]. Subsequent expression of Cre recombinase in trans removed the cassettes from the R1-16 deletion derivatives.
B. TraI domain N1-309 (blue) catalyses the relaxase reaction and contains the relaxase-associated ssDNA binding site. The helicase-associated ssDNA binding activity (N1-822) is independent of conserved helicase motifs (positions 990–1450). Negative cooperativity in ssDNA binding is observed between the relaxase and helicase-associated sites. Independently functional translocation signals TSA (positions 530–816) and TSB (1255–1564) mediate T4CP recognition (yellow). The C-terminal 252 residues may contain a putative interaction site for TraM (green). Positions of mutant variants used in this study are shown (*).
Table 1.
R17 phage sensitivity of E. coli[R1-16] requires a functional relaxosome and NTP binding by T4CP TraD
| Protein requirements | ||||
|---|---|---|---|---|
| Plasmid | Complementationa | Infection levelb | Plaque morphology | pfu ml−1c |
| R1-16 | − | +++ | Clear | 1.5 × 1010 |
| R1-16ΔtraD | − | − | None | n.d. |
| R1-16ΔtraD | R1 TraD | ++ | Opaque | 1.5 × 1010 |
| R1-16ΔtraD | R1 TraDK198T | − | None | n.d. |
| R1-16ΔtraM | − | − | None | n.d. |
| R1-16ΔtraM | R1 TraM | − | None | n.d. |
| R1-16 M13 | − | − | None | n.d. |
| R1-16ΔtraY | − | + | Very opaque | n.d. |
| R1-16ΔtraY | R1 TraY | + | Very opaque | n.d. |
| R1-16ΔtraI | − | − | None | n.d. |
| R1-16ΔtraI | R1 TraI | ++ | Opaque | 5.3 × 1011 |
| R1 | − | − | None | n.d. |
| pOX38 | − | +++ | Clear | 1.3 × 1014 |
| pOX38traD411 | − | − | None | n.d. |
| pOX38MK3 | − | +++ | Clear | 1.8 × 1014 |
| pOX38ΔtraI | − | ++ | Opaque | 1.2 × 1014 |
| DNA requirements | ||||
|---|---|---|---|---|
| Plasmid | Coresident plasmida | Infection levelb | Plaque morphology | pfu ml−1c |
| R1-16 | − | +++ | Clear | 1.4 × 109 |
| R1-16 | R1 oriT plasmid | + | Very opaque | n.d. |
| R1-16Δnic | − | − | None | n.d. |
| R1-16ΔoriT | − | − | None | n.d. |
Vector alone had no effect (not shown).
+++, wild-type; ++, opaque but countable; +, very opaque and not countable; −, none.
Plaque forming units per millilitre; n.d., not detected.
In comparison, phage sensitivity of cells carrying the F derivative pOX38, or mutants thereof (Table 1), confirmed a dependence on traD (Schoulaker and Engelberg-Kulka, 1978) but was independent of traI. The pOX38MK3 derivative also supported efficient plaque formation. Taken together, the sum of our initial work demonstrated that, in contrast to F-carrying cells, phage sensitivity conferred by R1-16 requires traI and activities supported by oriT, including, presumably, assembly of the relaxosome. The basis of this plasmid-specific difference may have to do with the system-specific nature of the F and R1 relaxosomes as well as marked differences in the C-terminal extensions of the two TraD proteins involved in relaxosome docking. Nonetheless, the strong phenotypes observed with the R1 model provide a means to investigate the mechanisms of R17 nucleoprotein uptake.
A TraI fragment including the relaxase domain and TSA is sufficient for cellular uptake of R17 RNA–protein A
We showed that host cell R17S required a TraD protein with an intact NTP binding site and TraI. The ability to complement the TraI function in trans enabled us to ask which domains of TraI are required for efficient nucleoprotein uptake. A very detailed functional map of F-like TraI proteins is available, as well as classes of well-defined mutations (Fig. 1B) (Haft et al., 2006; Mihajlovic et al., 2009; Sut et al., 2009; Dostal and Schildbach, 2010; Dostal et al., 2010; Lang et al., 2010; Wright et al., 2011). Cultures of E. coli host cells carrying R1-16ΔtraI and expressing wild-type or truncated alleles of traI in trans were infected with R17 phage. On the population level we visualized the progress of R17 RNA replication using agarose gel electrophoresis, and detected host cell lysis by monitoring the optical density of infected cultures (Fig. 2A and B respectively). We also used transmission electron microscopy to routinely confirm a normal progression of phage replication in single cells. Suspensions of identical hosts were exposed to phage then fixed in agar. Ultrathin sections of these blocks were prepared to reveal the cytosolic contents of individual cells from various levels. Intracellular R17 phage are readily visible as single particles that form a distinct honeycomb pattern (as illustrated in Fig. 2C and D). The cells we routinely observed were either full of hundreds of visible phage particles or lacked these altogether. We found no evidence for any mutant under any condition to indicate that the host population was uniformly infected but was delayed or dysfunctional in phage replication. We conclude therefore, that the requirement for TraD and relaxosome components observed in this study is manifest on the level of RNA entry.
Fig. 2.

TraI fragments including the relaxase domain and TSA are necessary and sufficient for nucleoprotein uptake. R17 RNA yield (A) and phage-induced cell lysis (B) are shown for plasmid R1-16 (•) or the R1-16ΔtraI (○) derivative when complemented with a wild-type R1 traI allele (▾) or fragments thereof. High RNA yields and cell lysis were observed with TraI N1-992 (relaxase + TSA) ( ). No complementation was observed with a combination of TraI fragments N1-309 (relaxase) and N310-1756 (helicase) (▵) or fragment N310-1756 alone (□). Values represent the mean of at least three experiments. Standard deviations are shown. Complementation of R1-16ΔtraI with TraI N1-992 supports normal progression of phage replication as shown by electron microscopy of the infected cells (arrows) (C). In D an enlarged region of the distinctive honeycomb pattern of R17 is highlighted.
). No complementation was observed with a combination of TraI fragments N1-309 (relaxase) and N310-1756 (helicase) (▵) or fragment N310-1756 alone (□). Values represent the mean of at least three experiments. Standard deviations are shown. Complementation of R1-16ΔtraI with TraI N1-992 supports normal progression of phage replication as shown by electron microscopy of the infected cells (arrows) (C). In D an enlarged region of the distinctive honeycomb pattern of R17 is highlighted.
Dependence of these processes on traI is shown (Fig. 2). Coexpression of a combination of TraI fragments N1-309 (relaxase) and N310-1756 (helicase), or fragment N310-1756 alone failed to complement the infection phenotype of R1-16ΔtraI (Fig. 2; open symbols), just as these fragments fail to complement R1-16ΔtraI for conjugation (Lang et al., 2010). By comparison high RNA yields (Fig. 2A) and cell lysis (Fig. 2B) were observed with the full-length TraI. Remarkably, TraI N1-992 alone was also sufficient to reconstitute phage propagation (3.6 × 1011 pfu ml−1) that was also apparent by the abundant production of R17 RNA (Fig. 2A) and the arrest of host cell culture density beginning 100 min post infection (Fig. 2B). TraI N1-992 contains the T4CP docking position TSA physically linked to the relaxase and ssDNA binding domains (Fig. 1B). This is the same fragment required – in combination with the entire helicase fragment (TraI N310-1756) – for effective conjugative gene transfer (Lang et al., 2010). Importantly, for RNA uptake, however, the helicase and C-terminal TraM interacting domains on fragment TraI N310-1756 are completely dispensable.
TraI N310-1756 carries two TS and our earlier work showed that even though this protein cannot form the TraI-T DNA adduct at nic typical for the full-length secretion substrate, it can be translocated to recipient cells (Lang et al., 2010). Thus docking of TraI N310-1756 to TraD should be normal. Nonetheless, that reaction alone is clearly not sufficient to support initiation of the phage nucleoprotein import process. Figure 2 illustrates the absence of R17 RNA synthesis in culture when R1-16ΔtraI hosts express TraI N310-1756 (Fig. 2A) and the continuous growth of cells after addition of phage similar to that of hosts carrying R1-16ΔtraI alone (Fig. 2B). In agreement with our earlier observations in conjugative transfer (Lang et al., 2010), the requirement for TraI in R17S was only met when the protein's N-terminal relaxase and ssDNA binding domains are physically linked to TSA. We propose that interactions between TraD and TraI TSA are communicated over this arm of the protein to the relaxase bound at oriT. The flow of regulatory signals appears to be crucial to early steps in both nucleoprotein uptake and secretion. We further propose that this bidirectional process alters both the conformation and the activities of the T4CP, as well as the relaxosome, in a manner that depends on oriT DNA. The observation that R1-16Δnic and R1-16ΔoriT do not support phage infection, despite the presence of all proteins, is consistent with this hypothesis.
TraI N1-992-catalysed nic-cleavage is stimulated by TraD
If the proposed model is true, it is reasonable to expect that interaction between TraD and this functional arm of TraI would affect the DNA processing reactions it catalyses. We showed previously that the purified cytosolic form of TraD enhanced TraI-catalysed nic-cleavage in vitro but, consistent with its lack of TS docking domains, the isolated relaxase TraI N1-309 was not stimulated by TraD (Mihajlovic et al., 2009). Here we purified the TraI N1-992 fragment and assayed for biochemical modulation of the nic-cleavage reaction (Fig. 3). As predicted, the activity of TraI N1-992 in this reaction was stimulated by TraD in a dose-dependent manner.
Fig. 3.
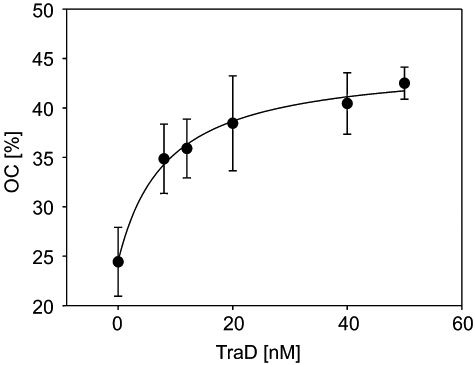
TraI N1-992-catalysed nic-cleavage is stimulated by TraD. Conversion of supercoiled oriT DNA to the open circular (OC) form by purified TraI fragment N1-992 is expressed as per cent of total DNA substrate. Nic-cleavage was measured with 100 nM TraI N1-992 alone or with increasing concentrations of TraD. Values represent the mean of at least three experiments. Standard deviations are shown.
Catalytically active relaxase and high affinity interaction with nic DNA is required for efficient nucleoprotein uptake
To help us to understand the reactions involved in nucleoprotein uptake and their control, various mutant forms of TraI N1-992 were tested. Dostal and Schildbach demonstrated that replacement of the relaxase catalytic tyrosine in F plasmid TraI eliminates nicking activity on ssDNA (Dostal et al., 2010). We replaced Y16F and Y17F on the truncated TraI N1-992. We then created a mutant allele that should alter the protein's high affinity interactions with ssDNA that are associated with the relaxase. We again drew on earlier data from the Schildbach group showing the importance of specific amino acids to this activity in the F system (Harley and Schildbach, 2003). Given that the wild-type sequences surrounding nic are identical in the R1 and F TraI binding sites, we recreated in TraIN1-992 three amino acid exchanges E153D/Q193R/R201Q previously characterized by the Schildbach group. These mutations were reported to substantially reduce the affinity of relaxase-associated interactions with ssDNA but not completely eliminate cleavage at nic. The new alleles were tested for complementation of the traI requirement in a phage infection analysis (Fig. 4). In this experiment R17 RNA yield and phage-induced cell lysis supported by TraI N1-992 and R1-16ΔtraI were in good agreement with those of plasmid R1-16. In contrast no complementation was observed with the cleavage deficient variant or the E153D/Q193R/R201Q mutant protein.
Fig. 4.

Relaxase catalysis and high affinity interaction at nic is required for efficient nucleoprotein uptake. R17 RNA yield (A) and phage-induced cell lysis (B) are shown for plasmid R1-16 (•) and R1-16ΔtraI (○) complemented with TraI N1-992 ( ). In contrast no complementation was monitored for the mutant TraI N1-992 variants, which are cleavage deficient (□) or show a reduced affinity for oriT-specific sequences (▵). Values represent the mean of at least three experiments. Standard deviations are shown.
). In contrast no complementation was monitored for the mutant TraI N1-992 variants, which are cleavage deficient (□) or show a reduced affinity for oriT-specific sequences (▵). Values represent the mean of at least three experiments. Standard deviations are shown.
A full-length TraI lacking relaxase-associated ssDNA binding activity maintains helicase activity
The amino acid exchanges introduced to TraI N1-992 were chosen because these are known to substantially reduce the affinity of the protein for sequences surrounding nic (Harley and Schildbach, 2003). Reduced affinity of the relaxase-associated binding site was confirmed for a truncated TraI N1-330 M2L/E153D/Q193R/R201Q (K. Guja and J. Schildbach, unpublished), but longer versions of the protein have not been analysed. To verify that a longer form of the mutant protein is not globally disrupted, we purified a full-length variant TraI and checked simultaneously several of its activities: DNA unwinding, T-strand cleavage and negative cooperativity between ssDNA binding sites, using an experimental system described previously (Csitkovits et al., 2004; Sut et al., 2009). The test substrates present a bubble of open duplex to support helicase loading onto ssDNA. The position of oriT DNA in single-stranded conformation centres either on the conserved IR of R1 and F, which supports high affinity ssDNA binding by the relaxase associated site of TraI (Williams and Schildbach, 2006), or a sequence outside of nic (G2028), which lacks those ssDNA recognition features (Sut et al., 2009). As expected the mutant protein exhibited robust helicase activity on both substrates (Fig. 5A and B). The activity of both proteins was identical on a substrate lacking oriT-specific sequence in single-stranded form (Fig. 5B). By contrast, wild-type TraI has lower unwinding activity on IR substrates than the mutant TraI (Fig. 5A). We believe that the sequence-specific inhibition of wild-type TraI (Sut et al., 2009) results from the negative cooperativity regulating the relaxase- and helicase-associated ssDNA binding sites (Dostal and Schildbach, 2010). The helicase activity of mutant TraI was higher than wild-type on IR DNA. This result is expected for a mutant protein deficient in ssDNA binding via the relaxase-associated site. The helicase activity of the mutant should be higher than wild-type on nic-specific DNA since the negative cooperativity that normally occurs between binding sites would be reduced in the mutant protein. The assay provides an important control confirming that TraI functions involving the central region of the mutant protein are intact. We conclude that failure of the TraI N1-992 E153D/Q193R/R201Q to support phage sensitivity is due to poor relaxase affinity for the R1 nic sequence.
Fig. 5.

Absence of relaxase-associated ssDNA binding relieves sequence-specific inhibition of oriT unwinding. Duplex unwinding catalysed by increasing concentrations of TraI (•) and TraI M2L/E153D/Q193R/R201Q (○) on heteroduplex oriT substrates that present nic and the IR for TraI binding as ssDNA (A), or constrained in dsDNA (B). The indicated per cent of substrate unwound represents the mean of three independent assays. Standard deviations are shown.
TraI TSA but no helicase-associated activities are necessary for efficient nucleoprotein uptake
We next investigated 31 residue insertion derivatives of TraI from plasmid F [TraIi (position of insertion); created by B. Traxler (Haft et al., 2006)]. These were selected because the site of insertion is known to disrupt the helicase-associated ssDNA binding site of TraI and reduced negative cooperativity between the sites [TraIi369, TraIi593 and TraIi681 (Dostal and Schildbach, 2010)]. Moreover, we compared those where the site of insertion fell within TSA – possibly compromising efficient contact with TraD (TraIi593, TraIi681) – with TraIi369 where TSA is not directly affected (Lang et al., 2010). Phage-induced cell lysis was monitored for hosts carrying R1-16ΔtraI with either the wild-type traIF allele or the insertion mutants (Fig. 6). The traIF allele complemented the lack of traIR1 efficiently, whereas both variants with disrupted TSA (TraIi593, TraIi681) did not. In contrast, effective complementation was observed with the traIF variant encoding wild-type TSA (TraIi369). Since this mutation also reduces affinity for ssDNA by the helicase-associated site and exhibits significantly reduced negative cooperativity of binding, we conclude that these activities of the wild-type protein are not important to regulation of RNA entry.
Fig. 6.

TraI TSA but not helicase-associated activities are necessary for efficient nucleoprotein uptake.
A and B. Phage-induced cell lysis was monitored for plasmid R1-16 (•) or the R1-16ΔtraI without (○) or with a wild-type traIF allele (▾) or insertion mutants (i31) thereof. traIF alleles with the insertion in TSA (TraIi593, ▵; TraIi681, □) complement the R1-16ΔtraI defect poorly (A), while traIF variant TraIi369 (♦), carrying a normal TSA, complements efficiently (B). Values represent the mean of at least three experiments. Standard deviations are shown.
In summary, TraI N1-992 is sufficient to provide a regulatory function required in host cells for efficient R17 nucleoprotein uptake. Our results pinpoint the relevant features of this functional domain to include high affinity interactions of the relaxase with ssDNA, the relaxase catalytic tyrosine, and TSA carried on the same polypeptide. Remarkably, TraI of plasmid F can provide this regulatory activity for the R1-16 machinery even though R17 uptake by the T4SS of F does not require TraI. Together, these data imply that the novel activity for TraI contributes to a larger docking and activation process involving TraD, oriT and possibly other components of the R1-16 T4SS.
Discussion
‘Male-specific’ filamentous and RNA phage exploit the presence of F-like conjugative pili and the underlying envelope spanning transport machinery to gain entry to bacterial cells. Sensitivity to distinct phage groups has been instrumental in classifying pilus types (Frost et al., 1985). Individual phage also exhibit exquisite discrimination of natural and induced variations in pilin proteins, which helped establish structure–function relationships in pilin biochemistry (Frost et al., 1985; Frost and Paranchych, 1988; Manchak et al., 2002). The route of entry taken by the R17 phage used in this study has not been determined. The tubular nature of the F-pilus offers a passive conduit for uptake, as proposed by Brinton (Brinton, 1965). Alternatively, the dynamic cycles of F pilus outgrowth and disassembly may be involved. Phage adsorption does not induce pilus retraction, but once triggered, the process draws adsorbed phage to the cell surface (Clarke et al., 2008). F-pilin subunits of retracting pili re-enter the membrane pool. The central structure of T4S components is likely to remain, and may provide the phage access to the host cell cytosol. The T4CP TraD of F-like plasmids is not involved in pilus biogenesis but is essential for host sensitivity to group I RNA phages R17, f2 and MS2, but not Qβ (Valentine et al., 1969; Schoulaker and Engelberg-Kulka, 1978). Based on what we now know about the decisive role T4CPs play in connecting the secretion channel with the cytoplasm and in recruiting and initiating (nucleo)protein secretion, further investigation of the T4CP-dependent phage infection process is warranted.
Here we develop the R17 nucleoprotein uptake model using the IncFII plasmid R1. We confirm the requirement for the T4CP and demonstrate that mutation of its NTP binding site eliminates function. We also demonstrate that the R1 relaxosome is involved in an essential manner. A novel functional domain was delineated within TraI N1-992 that is necessary and sufficient to support phage uptake by R1-16ΔtraI. The mechanism involved requires high affinity interactions of the relaxase with ssDNA, the relaxase catalytic tyrosine and the TSA region of the protein that interacts specifically with TraD. R17S was eliminated also by deletion of DNA that is important to relaxosome assembly or specific DNA recognition by the TraI relaxase. Thus a ternary complex of plasmid oriT, TraI N1-992 and TraD provides the essential activity. Normally, TraY and TraM are also bound to oriT and TraM engages in highly specific interactions with the C-terminus of TraD (Disque-Kochem and Dreiseikelmann, 1997; Beranek et al., 2004; Lu et al., 2008; Wong et al., 2011). The negative effects of traM and traY deletion on R17S could not be complemented, but we anticipate that this larger complex is involved in the nucleoprotein import mechanism. A simple binding or docking interaction between the relaxosome and the T4CP is not adequate to describe the activity, as shown by the TraI tyrosine exchange, and TraDK198T variants. What then is the subcomplex doing? T4 secretion typically depends on signals originating from contacts between cells. We showed earlier that the TraI N1-992 activating domain is sufficient to initiate plasmid DNA transfer as long as the helicase domain is provided separately (Lang et al., 2010). Taken together these data support a simple explanation where the docked complex of relaxosome and T4CP is the receptor for signals transmitted to the cell interior. Further the initial mechanisms that process the incoming signals into activation of both TraD-mediated nucleoprotein import and export processes are conserved.
In this model (Fig. 7) TraD is anchored to the base of the transfer channel while its cytosolic domain binds TraM, TraI and oriT DNA (Stage 1). The relaxosome is catalytically active at nic in the absence of TraD, but cleavage is stimulated by its presence (Mihajlovic et al., 2009). NTP hydrolysis by TraD appears to be silent. Progression from this stage requires signals communicated over the pilus from the cell exterior (Stage 2). In the case of R17 phage adsorption the productive receptor for the incoming signal is TraD docked by the R1 relaxosome (Fig. 7A). The accessory factors bound at oriT are important, but the key component is the TraI N1-992 docking and activation domain (inset). We propose that processing of the external signal through this mechanism depends on the physical link between catalytic activity at the plasmid nick site and the T4CP to modulate TraD conformation and thereby activate the essential ATPase. The activation initiates translocation of the RNA–protein A complex into the host cell (Stage 3). Activities related to TraI helicase and its C-terminal domain are dispensable. The fact that this form of translocation activation is independent of the helicase domain and that the helicase itself is not activated on the docked oriT under these conditions is logical, since phage penetration would otherwise result in plasmid DNA being extruded pointlessly into the medium. This has never been observed.
Fig. 7.

Stages of T4 nucleoprotein transfer initiation mediated by plasmid R1-16.
A and B. The T4 transfer apparatus is constitutively expressed and assembled. Stage 1: The relaxosome, containing oriT bound by TraM (green), TraY (violet), IHF (orange) and TraI (blue) is docked to the T4CP (yellow) via TraI translocation signals (TSA, TSB) and TraM binding. Stage 2: Productive pilus contacts with adherent R17 phage (A) or another cell (B) produces signals (lightning bolt) conveyed over the pilus to the T4CP-relaxosome receptor. Processing of those distinct signals into nucleoprotein translocation requires at a minimum, a conserved relaxase activation domain. TraI N1-992 comprises this domain for R1 (inset). Stage 3: During phage infection (A) downstream activation of the T4CP ATPase activity is essential for uptake of the R17 RNA–protein A complex. Alternatively, when cell contact permits plasmid self-transfer (B) initiation still requires productive interactions between the T4CP and R1 TraM bound to oriT. Signal processing is performed by the relaxase activation domain occupying the T4CP sites (inset). If TraI is bound to TraD, then the TraI N1-992 domain converts the cell contact signal into localized oriT melting (inset) and helicase activation in a separate downstream step.
In the case that the assembled pilus contacts a suitable recipient cell (Fig. 7B), the productive receptor for the incoming signal is again TraD docked by the R1 relaxosome. TraI N1-992 is fully capable of supporting initiation reactions to this point, but secretion of R1 requires a final unique step of helicase activation. Duplex unwinding can be catalysed in vitro by the truncated domain TraI N310-1756 or the full-length protein. However, the truncated helicase only supports transfer when combined with TraI N1-992, but not with the relaxase lacking TSA (TraI N1-309) (Lang et al., 2010). It follows, therefore, that the progression of activation steps when TraI N1-992 is docked to TraD also induces the localized denaturation of oriT DNA (Csitkovits et al., 2004) necessary to load and initiate helicase activity. The interaction of TraD and helicase stimulates DNA unwinding (Sut et al., 2009). In summary, we propose that the initiation cascades induced by these distinct pilus mediated stimuli progress through parallel steps mediated by identical components of the R1 machinery. The ultimate difference is that the phage mediated signals repress, or cannot induce, the final step of helicase activation. That modulation through evolution supports the survival of both the phage and the plasmid.
The results of this study raise a number of interesting points concerning the coevolution of F plasmids and the phage that target conjugative pili. Given that the phage life cycle destroys their mutual host, the evolutionary interplay can be likened to an arms race. Conjugative plasmids are subject to strong selection for mutations that confer phage resistance yet allow conjugation. Natural variation in TraA pilins alters phage sensitivity and five types are known among related F plasmids (represented by F, ColB2, R1-19, R100-1 and pED208) (Frost et al., 1985; 1994; Anthony et al., 1999; Manchak et al., 2002). Rapid selection for compensatory mutations among the phage population inevitably follows. The Paranchych laboratory noted that only 50% of a given preparation of R17 phage was active for pilus attachment, conceivably as a result of emerging mutations (Paranchych et al., 1970). In this study of R1-16, the process of R17 phage penetration was connected to assembly and function of the conjugative relaxosome in conjunction with the T4CP. It would appear that only T4SS that are maximally prepared for conjugative plasmid transfer are also vulnerable to phage uptake. This stringency benefits the conjugative plasmid. The requirement for the relaxosome in R17 exploitation of the R1 T4SS was not shared by the F derivative pOX38. The TraIF protein could provide that essential function with R1-16 even though it is not inherent to the infection mechanism via the cognate T4SS. This distinction suggests that related F plasmids may have diversified on the level of relaxosome and particularly the T4CP in response to phage-driven selection. T4CPs are generally conserved in the NTP binding domain but display variability at the N- and C-terminal domains (Alvarez-Martinez and Christie, 2009). The C-terminal extensions of TraD proteins of F-like plasmids range from 150 to 200 amino acids in length (Fig. 8). The unstructured extension is important to the specificity of substrate interaction and forms extensive contacts to TraM tetramers (Sastre et al., 1998; Lu et al., 2008; Wong et al., 2011). Perhaps, analogous to the VirB4-like protein TrwK, the C-terminal extension may also regulate ATPase activity (Pena et al., 2011). The TraD proteins of related plasmids display heterogeneity in the C-terminal extension including the length of a variable region of glutamine proline sequential repeats (QQP motif). TraDF carries just five residues (QPQQP) whereas TraDR1 contains 23. The mechanistic basis of the plasmid-specific differences revealed in this study may arise through variation in TraD. This possible explanation is under investigation. Our findings further suggest that an important factor driving diversification among the F-like T4CPs is phage resistance.
Fig. 8.

F-like T4CPs display C-terminal heterogeneity.
A. The molecular architecture of VirD4 from A. tumefaciens is compared to F-like TraD proteins according to Alvarez-Martinez and Christie (2009). The conserved NTP binding domains are indicated in grey. The TraD C-terminal extension (∼ 200 residues, violet) harbours a variable region of glutamine proline sequential repeats (QQP motif, yellow) and the TraM interaction domain (green).
B. The sequence alignment was performed by using the ClustalW2 multiple sequence alignment program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Only the last ∼ 200 residues of the aligned T4CPs are shown. T4CPs of plasmid R1 and F, investigated in this study, are highlighted in red. The TraM interaction domain according to Lu et al. (2008) is shown (green box). The T4CP designations include the protein names followed by the plasmid name, according to the GenBank database. Accession numbers for T4CPs are: YP_001096500 for TraD_NR1, NP_052980 for TraD_R100, NP_957631 for TraD_pC15-1a, YP_788090 for TraD_pO86A1, YP_538736 for TraD_pUTI89, YP_313445 for TraD_pSS_046, YP_001294757 for TraD_pSFO157, AAT85682 for TraD_R1, YP_190116 for TraD_pAPEC-02-R, YP_443957 for TraD_pAPEC_02_ColV, YP_001481211 for TraD_pAPEC-01-ColBM, ABG29548 for TraD_pMAR7, BAA97972 for TraD_F, NP_862947 for TraD_p1658/97, NP_490588 for TraD_pSLT, YP_209289 for TraD_pSCV50, NP_073256 for TraD_pKDSC50.
Experimental procedures
Strains and plasmids
All E. coli K-12 strains used in this study are described in Table S1. Plasmids are described in Table S2.
DNA preparation and PCR amplification
Plasmid DNA was purified from E. coli cells with the QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany). Restriction endonucleases and DNA modifying enzymes were obtained from Fermentas GmbH (St. Leon-Rot, Germany). DNA fragments for cloning were amplified using Phusion High-Fidelity DNA Polymerase (Finnzymes Oy, Espoo, Finland) or the Taq-Polymerase (New England Biolabs, Beverly, MA, USA). The correct DNA sequence of all amplified fragments used in cloning was verified. Enzymes were used according to manufacturers' recommendations.
Construction of traY, nic and oriT null derivatives
Primer sequences are shown in Table S3. To generate R1-16ΔtraY, R1-16Δnic and R1-16ΔoriT, the primer pairs traYko1_FW and traYko1_Rev, oriTko1_FW and oriTko1_Rev, or oriTko1_FW and oriTko2_Rev were used to amplify a loxP-TetRA-loxP cassette from CSH26Cm::LTL (Lang et al., 2010). The amplified fragments were introduced into E. coli DY330 [R1-16] and integrated via homologous recombination as previously described (Reisner et al., 2002). Introduction of the CFP B plasmid into strains carrying these null derivatives catalysed a Cre/loxP mediated recombination reaction excising the tetRA cassette.
Construction of expression plasmids
The inserts for pMSTraD_wt and pET29TraI(1–992) were amplified from R1-16 with the indicated primers (Tables S2 and S3) and ligated with pMS119EH or pET29a respectively. pET24a-TraI carries wild-type F traI while pET24a-TraIM2L/E153D/Q193R/R201Q expresses just the 36 kDa relaxase fragment of F TraI with the indicated mutations (both kindly provided by J. Schildbach, Johns Hopkins University). Reduced affinity of the mutant variant for ssDNA was confirmed by K. Guja and J. Schildbach (unpublished results). Both plasmids were cut with NdeI and StuI to introduce the mutant fragment of pET24a-TraIM2L/E153D/Q193R/R201Q into the full-length allele, resulting in pCG03. Two-step PCR was used to generate pMSTraD_A, and pRelTSAY16FY17F. In the first step primer sets 1 and 2 (Tables S2 and S3) were used to amplify two fragments from R1-16, which both carried the desired point mutations. In the second step these two fragments were annealed and amplified with primer set 3. The fragments were cut with EcoRI/HindIII or EcoRI/BamHI and religated with pMS119EH or pGZ119EH respectively. Two-step PCR was also used to generate pRelTSAFR100. The first fragment carried the mutated F traI relaxase domain from pCG03 and the second carrying bp 927–2976 from pCG02. Annealing, amplification with primer set 3 and ligation with EcoRI/BamHI cut pGZ119EH generated pRELTSAFR100 expressing the chimeric E153D/Q193R/R201Q TraI1-992.
Protein purification
TraDΔN130 was expressed and purified as described previously (Mihajlovic et al., 2009). TraI M2L/E153D/Q193R/R201Q was expressed and purified as described previously for full-length R1 TraI (Mihajlovic et al., 2009).
Escherichia coli BL21(DE3) [pet29TraI1-992] was grown in 1 l LB media supplemented with 40 µg ml−1 kanamycin to an A600 0.6. Overexpression was induced by addition of isopropyl-1-thio-α-d-galactopyranoside (IPTG) to a final concentration of 1 mM. Cells were harvested after 5 h shaking at 37°C and pellets were frozen at −80°C. Frozen cells were thawed overnight at 4°C. The pellet was resuspended in 15 ml of buffer I [50 mM sodium phosphate, 250 mM sodium chloride, 10 mM imidazole, 0.02% sodium azide (w/v), pH 8] and lysed by two passages in a French press cell. The cytoplasmic fraction was obtained by centrifugation at 21 000 g for 1 h. The supernatant was filtered (0.4 µm) and applied to a 10 ml His-Select Nickel affinity gel, equilibrated with buffer I. Adsorbed proteins were eluted with a 60 ml gradient of 0.01–0.5 M imidazole in buffer I. Fractions containing the protein were pooled and dialysed at 4°C overnight against a 100-fold volume of buffer II (50 mM sodium phosphate, 1 mM EDTA, 100 mM NaCl, pH 7.5). Soluble ammonium sulphate (AS) was added to a final concentration of 1 M to the dialysate and this fraction was loaded on two 5 ml HiTrap Phenyl HP columns, connected in tandem and equilibrated with buffer II plus 1 M AS. The column was developed with a 180 ml decreasing gradient of 1 to 0 M AS in buffer II. Peak fractions eluting between 300 and 150 mM AS were dialysed at 4°C against a 100-fold volume of buffer III [50 mM sodium phosphate, 100 mM NaCl, 0.02% sodium azide (w/v), pH 7.5], supplemented to 40% glycerol, concentrated with a Amicon filter device (Millipore) and then stored at −80°C. The apparent molecular mass of the protein of 110 kDa was confirmed by Coomassie blue staining following denaturating polyacrylamide gel electrophoresis.
R17 lysate preparation
To prepare fresh phage lysate 2 ml of an overnight culture of MS411 [R1-16] was pelleted at 4000 g for 8 min and then suspended in 1 ml 10 mM MgSO4. One hundred microlitres of cell suspension were mixed with an equal volume of R17 phage lysate (c. 109 pfu) in appropriate dilutions in ice-cold TMG-buffer [10 mM Tris-HCl, pH 7.4, 5 mM MgSO4, 0.01% (w/v) gelatine]. The phage–cell mixture was incubated for 5 min at room temperature. After incubation, 3 ml of R-top agar containing 10 mM MgSO4, 2 mM CaCl2 and 5 mM glucose were gently mixed with 200 µl of the cell–phage suspension and spread onto pre-warmed LB agar plates containing the appropriate antibiotics. Plates were incubated for at least 6 h at 37°C until plaque formation was visible. Two-millilitre ice-cold TMG-buffer was added onto plates showing confluent lysis and plates were kept at 4°C for 5 min. Top-agar and TMG-buffer was scraped off the plates, transferred to a 50 ml tube, and then centrifuged at 4000 g for 10 min. The supernatant containing the R17 phage lysate was stored at 4°C.
Infection studies with the male-specific phages
Plaque assays were performed with E. coli MS411 harbouring the desired plasmids (Table 1) under the conditions described for the phage lysate above, except that plates were incubated overnight at 37°C. Liquid infection assays were performed as described previously (Bayer et al., 1995). Briefly, 40 ml LB medium containing 2 mM CaCl2 and the appropriate antibiotics were inoculated to A600 0.05 with the desired strain. The cells were grown at 37°C to an A600 0.6 and 4 ml R17 phage lysate was added. Cultures were grown at 37°C with shaking and cell lysis was determined by measuring the A600 at the indicated time points (Figs 2, 4 and 6). Plating assays with phage Qβ, to verify these results, were abandoned, when E. coli[R1-16] supported marginal opaque plaques. Clear plaques were observed for E. coli[pOX38].
R17 RNA isolation and analysis
The increase of R17 RNA during phage maturation was measured by sampling 1.5 ml culture of cells simultaneously to measuring the optical density in the liquid infection assay described above. R17 RNA was isolated using the Fermentas GENEJet RNA purification kit according to the manufacturer's recommendations and analysed by ethidium bromide gel electrophoresis. Intensity of R17 RNA was compared to the intensity of the 23S rRNA using ImageJ (ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2011).
Electron microscopy
Plate infection assays as described above were performed and agar blocks were cut with a sterile Pasteur pipette peripherally to the plaques. Samples were fixed in 2.5% glutaraldehyde (Agar Scientific, Stansted, England) in 0.1 M cacodylate buffer, pH 7.2, for 90 min at room temperature. Samples were rinsed repeatedly in 0.1 M cacodylate buffer (pH 7.2) and post-fixed with 1% osmium tetroxide (OsO4, Gröpl, Tulln, Austria) buffered with 0.1 M cacodylate buffer, pH 7.2, for 1 h. Subsequently, the material was rinsed twice in the buffer, dehydrated in a graded series of ethanol (including en bloc staining with 1% uranyl acetate in 70% ethanol for 2 h) followed by propylene oxide and embedded in Agar 100 epoxy resin (Agar Scientific, Stansted, England). Ultrathin sections (70 nm) were cut with a Reichert Ultracut S ultramicrotome and post-stained for 5 min with lead citrate before visualization with a Philips CM10 transmission electron microscope.
Biochemical analysis
Relaxase assays on supercoiled DNA were performed as described previously (Mihajlovic et al., 2009). Heteroduplex substrates G2028 and IR were generated with primers G2028 fwd and G2028 rev or IR fwd and IR rev (Table S3) as described previously (Sut et al., 2009). T-strand cleavage and unwinding assays were performed as described (Sut et al., 2009).
Acknowledgments
This work is supported by the Austrian Science Fund (FWF) through grants P18607-B12 and W901-B05 DK: Molecular Enzymology. J.F. Schildbach, and L. Frost are gratefully acknowledged for providing plasmids and K. Guja and J. Schildbach for communicating unpublished results. We also thank S. Mihajlovic and B. Klug for their contribution to this study.
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony KG, Klimke WA, Manchak J, Frost LS. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J Bacteriol. 1999;181:5149–5159. doi: 10.1128/jb.181.17.5149-5159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol. 2006;9:207–217. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Baquero F. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat Rev Microbiol. 2004;2:510–518. doi: 10.1038/nrmicro909. [DOI] [PubMed] [Google Scholar]
- Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A, Koraimann G, Högenauer G. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek A, Zettl M, Lorenzoni K, Schauer A, Manhart M, Koraimann G. Thirty-eight C-terminal amino acids of the coupling protein TraD of the F-like conjugative resistance plasmid R1 are required and sufficient to confer binding to the substrate selector protein TraM. J Bacteriol. 2004;186:6999–7006. doi: 10.1128/JB.186.20.6999-7006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton CC., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965;27:1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science. 2004;304:1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Maddera L, Harris RL, Silverman PM. F-pili dynamics by live-cell imaging. Proc Natl Acad Sci USA. 2008;105:17978–17981. doi: 10.1073/pnas.0806786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F, Davies J. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 2000;8:128–133. doi: 10.1016/s0966-842x(00)01703-0. [DOI] [PubMed] [Google Scholar]
- de la Cruz F, Frost LS, Meyer RJ, Zechner EL. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol Rev. 2010;34:18–40. doi: 10.1111/j.1574-6976.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- Csitkovits VC, Dermic D, Zechner EL. Concomitant reconstitution of TraI-catalyzed DNA transesterase and DNA helicase activity in vitro. J Biol Chem. 2004;279:45477–45484. doi: 10.1074/jbc.M407970200. [DOI] [PubMed] [Google Scholar]
- Disque-Kochem C, Dreiseikelmann B. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J Bacteriol. 1997;179:6133–6137. doi: 10.1128/jb.179.19.6133-6137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal L, Schildbach JF. Single-stranded DNA binding by F TraI relaxase and helicase domains is coordinately regulated. J Bacteriol. 2010;192:3620–3628. doi: 10.1128/JB.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal L, Shao S, Schildbach JF. Tracking F plasmid TraI relaxase processing reactions provides insight into F plasmid transfer. Nucleic Acids Res. 2010;39:2658–2670. doi: 10.1093/nar/gkq1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Paranchych W. DNA sequence analysis of point mutations in traA, the F pilin gene, reveal two domains involved in F-specific bacteriophage attachment. Mol Gen Genet. 1988;213:134–139. doi: 10.1007/BF00333409. [DOI] [PubMed] [Google Scholar]
- Frost LS, Finlay BB, Opgenorth A, Paranchych W, Lee JS. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985;164:1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Ippen-Ihler K, Skurray RA. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo JM. Natural conjugative plasmids induce bacterial biofilm development. Nature. 2001;412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth FX, Moncalían G, Pérez-Luque R, González A, Cabezón E, de la Cruz F, Coll M. The bacterial conjugation protein TrwB resembles ring helicases and F1-ATPase. Nature. 2001;409:637–641. doi: 10.1038/35054586. [DOI] [PubMed] [Google Scholar]
- Graus-Goldner A, Graus H, Schlacher T, Hogenauer G. The sequences of genes bordering oriT in the enterotoxin plasmid P307: comparison with the sequences of plasmids F and R1. Plasmid. 1990;24:119–131. doi: 10.1016/0147-619x(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Haft RJ, Palacios G, Nguyen T, Mally M, Gachelet EG, Zechner EL, Traxler B. General mutagenesis of F plasmid TraI reveals its role in conjugative regulation. J Bacteriol. 2006;188:6346–6353. doi: 10.1128/JB.00462-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley MJ, Schildbach JF. Swapping single-stranded DNA sequence specificities of relaxases from conjugative plasmids F and R100. Proc Natl Acad Sci USA. 2003;100:11243–11248. doi: 10.1073/pnas.2035001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn PM, O'Callaghan RJ, Paranchych W. Stages in phage R17 infection. VI. Injection of A protein and RNA into the host cell. Virology. 1972;47:628–637. doi: 10.1016/0042-6822(72)90552-1. [DOI] [PubMed] [Google Scholar]
- Lang S, Gruber K, Mihajlovic S, Arnold R, Gruber CJ, Steinlechner S, et al. Molecular recognition determinants for type IV secretion of diverse families of conjugative relaxases. Mol Microbiol. 2010;78:1539–1555. doi: 10.1111/j.1365-2958.2010.07423.x. [DOI] [PubMed] [Google Scholar]
- Loeb T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960;131:932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- Lu J, Wong JJ, Edwards RA, Manchak J, Frost LS, Glover JN. Structural basis of specific TraD-TraM recognition during F plasmid-mediated bacterial conjugation. Mol Microbiol. 2008;70:89–99. doi: 10.1111/j.1365-2958.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- McGowan JE., Jr Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7:286–292. doi: 10.3201/eid0702.010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchak J, Anthony KG, Frost LS. Mutational analysis of F-pilin reveals domains for pilus assembly, phage infection and DNA transfer. Mol Microbiol. 2002;43:195–205. doi: 10.1046/j.1365-2958.2002.02731.x. [DOI] [PubMed] [Google Scholar]
- Maneewannakul K, Kathir P, Endley S, Moore D, Manchak J, Frost L, Ippen-Ihler K. Construction of derivatives of the F plasmid pOX-tra715: characterization of traY and traD mutants that can be complemented in trans. Mol Microbiol. 1996;22:197–205. doi: 10.1046/j.1365-2958.1996.00087.x. [DOI] [PubMed] [Google Scholar]
- Marvin DA, Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969;33:172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlovic S, Lang S, Sut MV, Strohmaier H, Gruber CJ, Koraimann G, et al. Plasmid R1 conjugative DNA processing is regulated at the coupling protein interface. J Bacteriol. 2009;191:6877–6887. doi: 10.1128/JB.00918-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci USA. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A, Hansen LH, Sorensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philos Trans R Soc Lond B Biol Sci. 2009;364:2275–2289. doi: 10.1098/rstb.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W. Attachment, ejection and penetration stages of the RNA phage infectious process. In: Zinder ND, editor. RNA Phages. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1975. pp. 85–111. [Google Scholar]
- Paranchych W, Krahn PM, Bradley RD. Stages in phage R17 infection. Virology. 1970;41:465–473. doi: 10.1016/0042-6822(70)90168-6. [DOI] [PubMed] [Google Scholar]
- Paranchych W, Ainsworth SK, Dick AJ, Krahn PM. Stages in phage R17 infection. V. Phage eclipse and the role of F pili. Virology. 1971;45:615–628. doi: 10.1016/0042-6822(71)90176-0. [DOI] [PubMed] [Google Scholar]
- Parker C, Meyer RJ. The R1162 relaxase/primase contains two, type IV transport signals that require the small plasmid protein MobB. Mol Microbiol. 2007;66:252–261. doi: 10.1111/j.1365-2958.2007.05925.x. [DOI] [PubMed] [Google Scholar]
- Pena A, Ripoll-Rozada J, Zunzunegui S, Cabezon E, de la Cruz F, Arechaga I. Autoinhibitory regulation of TrwK, an essential VirB4 ATPase in type IV secretion systems. J Biol Chem. 2011;286:17376–17382. doi: 10.1074/jbc.M110.208942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pölzleitner E, Zechner EL, Renner W, Fratte R, Jauk B, Högenauer G, Koraimann G. TraM of plasmid R1 controls transfer gene expression as an integrated control element in a complex regulatory network. Mol Microbiol. 1997;25:495–507. doi: 10.1046/j.1365-2958.1997.4831853.x. [DOI] [PubMed] [Google Scholar]
- Reisner A, Molin S, Zechner EL. Recombinogenic engineering of conjugative plasmids with fluorescent marker cassettes. FEMS Microbiol Ecol. 2002;42:251–259. doi: 10.1111/j.1574-6941.2002.tb01015.x. [DOI] [PubMed] [Google Scholar]
- Roberts JW, Steitz JE. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci USA. 1967;58:1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre JI, Cabezón E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol. 1998;180:6039–6042. doi: 10.1128/jb.180.22.6039-6042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoulaker R, Engelberg-Kulka H. Escherichia coli mutant temperature sensitive for group I RNA bacteriophages. J Virol. 1978;25:433–435. doi: 10.1128/jvi.25.1.433-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G, Lanka E. TraG-like proteins of type IV secretion systems: functional dissection of the multiple activities of TraG (RP4) and TrwB (R388) J Bacteriol. 2003;185:4371–4381. doi: 10.1128/JB.185.15.4371-4381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein R, Guye P, Rhomberg TA, Schmid MC, Schröder G, Vergunst AC, et al. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci USA. 2005;102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sut MV, Mihajlovic S, Lang S, Gruber CJ, Zechner EL. Protein and DNA effectors control the TraI conjugative helicase of plasmid R1. J Bacteriol. 2009;191:6888–6899. doi: 10.1128/JB.00920-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I, Matilla I, Arechaga I, Zunzunegui S, de la Cruz F, Cabezón E. The ATPase activity of the DNA transporter TrwB is modulated by protein TrwA: implications for a common assembly mechanism of DNA translocating motors. J Biol Chem. 2007;282:25569–25576. doi: 10.1074/jbc.M703464200. [DOI] [PubMed] [Google Scholar]
- Valentine RC, Strand M. Complexes of F-Pili and RNA Bacteriophage. Science. 1965;148:511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]
- Valentine RC, Silverman PM, Ippen KA, Mobach HW. The F-pilus of E. coli. Adv Microb Physiol. 1969;3:1–52. [Google Scholar]
- Vergunst AC, van Lier MC, den Dulk-Ras A, Stuve TA, Ouwehand A, Hooykaas PJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci USA. 2005;102:832–837. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N, Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986;47:1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Williams SL, Schildbach JF. Examination of an inverted repeat within the F factor origin of transfer: context dependence of F TraI relaxase DNA specificity. Nucleic Acids Res. 2006;34:426–435. doi: 10.1093/nar/gkj444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JJ, Lu J, Edwards RA, Frost LS, Glover JN. Structural basis of cooperative DNA recognition by the plasmid conjugation factor, TraM. Nucleic Acids Res. 2011;39:6775–6788. doi: 10.1093/nar/gkr296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Paranchych W. The polarity of penetration of phage R17 RNA. Virology. 1976;73:489–497. doi: 10.1016/0042-6822(76)90410-4. [DOI] [PubMed] [Google Scholar]
- Wright NT, Majumdar A, Schildbach JF. Chemical shift assignments for F-plasmid TraI (381-569) Biomol NMR Assign. 2011;5:67–70. doi: 10.1007/s12104-010-9269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


