Abstract
Sensations generated by intense focused ultrasound (iFU) can occur cutaneously and/or at depth, in contrast to other forms of stimulation (heat, electricity) whose action usually occurs only at the skin surface or mechanical stimulation (von Frey hairs, calibrated forceps, tourniquets) that compress, hence stimulate all tissue. Previous work on iFU stimulation has led to the hypothesis that the tactile basis of iFU stimulation should correlate with the density of mechanoreceptors at the site of iFU stimulation. Here we tested that hypothesis, correlating a ‘two-point’ neurological exam, a standard measure of superficial mechanoreceptor density, with the intensity of superficially applied iFU necessary to generate sensations with high sensitivity and specificity. We applied iFU at 1.1 MHz for a 0.1 second to the fingertip pads of seventeen test subjects in a blinded fashion and escalating intensities until they consistently observed iFU-induced sensations. Most test subjects achieved high values of sensitivity and specificity, doing so at values of spatially and temporally averaged intensity measuring less than 100 W/cm^2. Moreover, the test subject’s sensitivity to iFU stimulation correlated with the density of mechanoreceptors as determined by a standard two-point discrimination neurological exam, consistent with earlier hypotheses.
Keywords: Intense Focused Ultrasound, Mechanoreceptors, Tactile Sensitivity, Sensation
Introduction
Intense focused ultrasound (iFU) can create a variety of sensations in human subjects – hot, cold, itching, tickling, including pain (reviewed in Gavrilov et al., 1996; Gavrilov, 2008), evoked potentials (Wright et al., 1993) as well as temporal summation (Wright et al., 2002). Recently Iwamoto and Shinoda (2006 & 2007) (reviewed in Gavrilov, 2008) have adapted ultrasound’s ability to generate sensations to the creation and design of tactile feedback displays. Ultrasound differs from other types of stimulation including heat, mechanical, or electrical stimulation (Griffin and Seah, 2008; Johansson and Vallbo, 1979; Leitgeb et al., 2007), because of its ability to induce sensations when focused upon either deep or superficial tissue, as desired (Gavrilov et al., 1996). Since both the depth as well as the focal volume of iFU can be controlled through varying its geometric focus and carrier frequency, iFU can generate its effects within small volumes of tissue. This leads to precise and focal energy deposition and therefore comparably precise and focal induction of sensations.
Several studies demonstrate that sensation induction by iFU arises through iFU’s ability to palpate tissue via the acoustic radiation force (Dalecki et al., 1995; Davies et al., 1996; Gavrilov et al., 1977a,b; Gavrilov, 1984; Gavrilov et al., 1996). The portion of these studies involving human test subjects used generally fewer than six individuals, all of whom aware of the timing of iFU delivery.
Given that the density of mechanoreceptors varies within different tissue types (skin more than muscle, fingertip pads more than the back of the calf, etc) and also varies between individuals (Martin and Jessell, 1991), Gavrilov (1984) hypothesized that the intensity of iFU necessary to generate sensations should depend upon the density of mechanoreceptors. Specifically, the lower the number of available peripheral nerve endings available to detect iFU stimulation within the focus of iFU, the greater the intensity of iFU required to generate a discernable sensation. Here we tested Gavrilov’s hypothesis by correlating the density of mechanoreceptors as measured by a standard neurological example (the ‘two-point discrimination’ test, described below) within the fingertip pads of test subjects with the intensity of iFU applied to those pads required to create consistent sensations. We worked with a relatively large number of test subjects, and applied iFU in a manner blinded to those subjects. In this fashion we tested our primary hypothesis as well as assayed the sensitivity and specificity of iFU sensation induction – in essence, the percentage of actual or sham iFU applications correctly identified, respectively.
Methods
A. Device
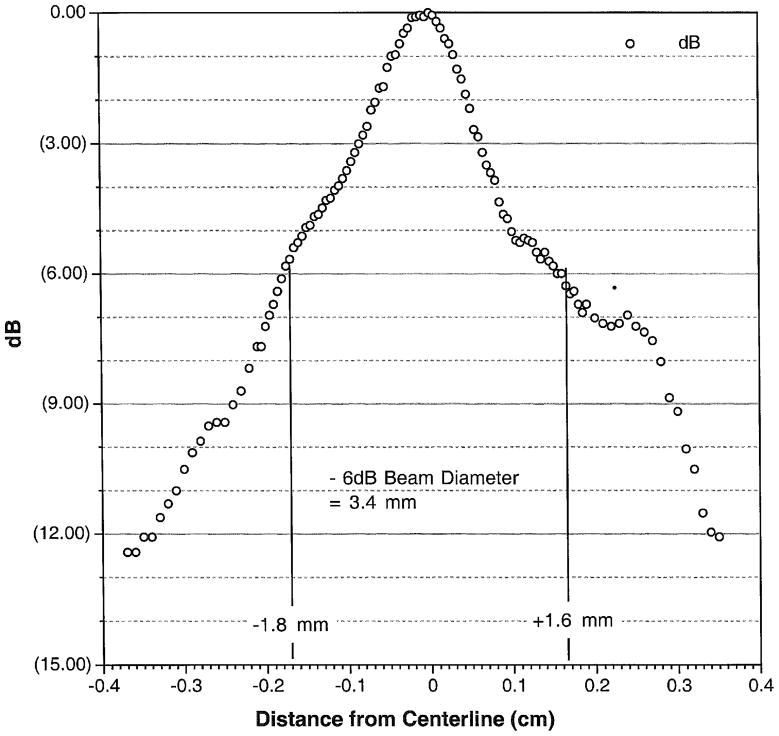
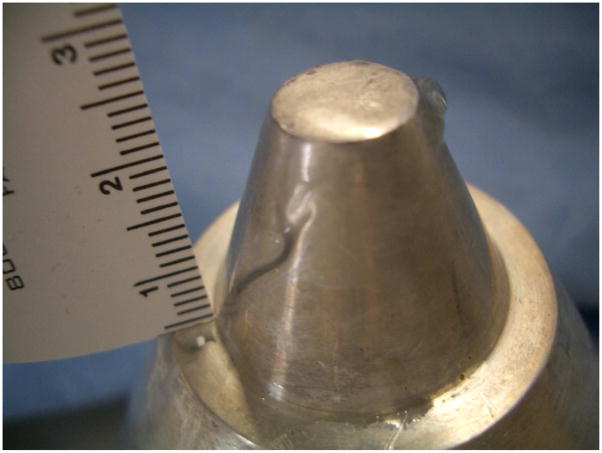
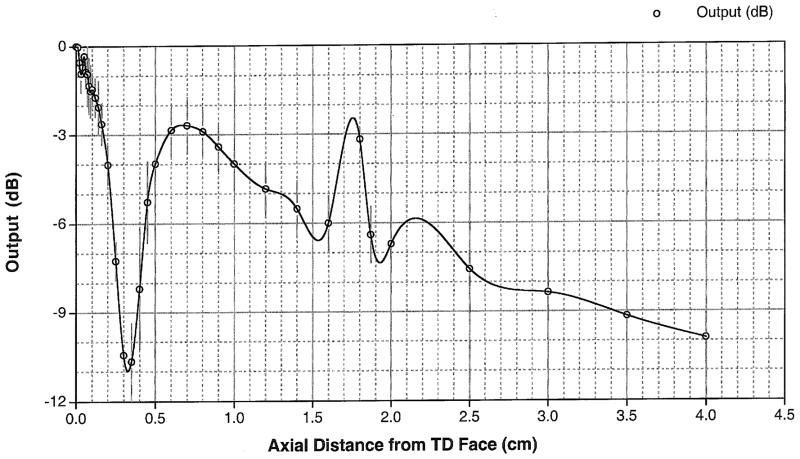
The specifics of our device are described in detail within Miao et al. (2005). Briefly, our prototype device consisted of a commercial flat, piezo-electric transducer built into a custom made, solid, cylindrical symmetric cone shaped aluminum housing (Figure 1). The focus of this ‘solid cone’ device occurred within 500 microns of its proximal surface. It has a secondary maximum of one-fourth the intensity at the surface measured 0.75 cm beyond the transducer face, assayed through use of a needle hydrophone within water (NTR, Seattle WA, with a 500 micron active measuring area). Thus the majority of the energy deposited is superficial and cutaneous. The width of the primary focus as measured at the half-pressure-maximum contour measured 1.7 mm and was also characterized via needle hydrophone (Figure 3). The solid cone device was driven by two function generators (33120A, Hewlett Packard/Agilent, Palo Alto, CA, USA) and an amplifier (A150 RF Power Amplifier, ENI, Chestnut Ridge, NY, USA). An oscilloscope (Wave Runner LT 322, LeCroy, Chestnut Ridge, NY, USA) measured the duration of the pulse, its carrier frequency and the voltage delivered to the iFU device by the amplifier during each experiment. This voltage was correlated to acoustic intensity emitted by the iFU device via a ‘force balance’ technique (Hill et al., 1994; Sutton et al., 2006). In essence, the effective weight caused by iFU beamed into an acoustic absorber placed on a scale, along with a measure of the radial extent of the iFU emitted from our device (Figure 3), translates into a temporally averaged intensity itself averaged over the area enclosed by the half-pressure-maximum contour in the focal plane: ISATA.
Figure 1.
Our ultrasound delivery device. The transducer itself is embedded within an aluminum housing.
Figure 3.
Beam plot showing the iFU intensity as a function of radial distance out from the center of the transducer face.
The fundamental frequency for our transducer was 1.117 MHz, within the range of ultrasound frequencies (0.5–3 MHz) used in other studies (Gavrilov et al., 1977a,b; Wright et al., 2002; reviewed in Gavrilov, 2008). For all experiments, we used a single pulse of ultrasound with a duration of 100 milliseconds. This choice is also consistent with the pulse length used by other researchers (typically 5–100 ms – reviewed in Gavrilov, 2008) who have successfully used iFU to produce discernable ultrasonic sensations (Dalecki et al., 1995; Gavrilov et al., 1977a,b; Wright et al., 1993, 2002).
B. Subjects
This study was approved by the University of Washington’s Human Subjects Research Board. All subjects provided written informed consent for study participation. Our study group consisted of 17 test subjects (10 male, 7 female), ages 18–56. These subjects received a nominal honorarium ($25) for their participation in each of their sessions. We conducted the iFU and the “two-point” neurological testing on two separate days, at least one week apart, as described below. All subjects were screened for any history of abnormal sensory phenomena.
We performed a two-point discrimination test, a standard neurological assay for the sensitivity skin to mechanical stimulation, here to the index finger pad of each of the subject’s hands. Specifically, we gently placed on and removed from the finger pad one or two prongs of the DISK-CRIMINATOR ™ (Dellon, Baltimore, MD, USA) in a manner blinded to the test subject. After each application we asked the test subject if they felt one or two prongs. We recorded for that test subject the set of two prongs with minimal distance between them that the test subject reliably identified as consisting of two prongs rather than a single prong. The results of the test, reported in millimeters, depends on the density of mechanoreceptors within the peripheral nerves that enervate the fingertip pads such that the greater the density, the smaller the measure (Johansson and Vallbo, 1979; Mackinnon and Dellon, 1985). The subjects were also given a questionnaire related to any recent pain in the hand or abnormal sensations that they had experienced. The questionnaire and two-point test were given both before and after iFU testing and helped to determine whether any short-term physiological or psychological perception changes were associated with the iFU stimulation tests.
After completing the questionnaire and two-point test the subject was seated and familiarized with the ultrasound device as well as the testing equipment and associated test protocol. Next, the test subjects were asked to place the pad of either the right or left index finger on the cone tip. Ultrasound gel (Aquasonic 100 Ultrasound Transmission gel, Parker Laboratories, Fairfield, NJ, USA) was used to ensure adequate acoustic transmission. The tester would alert the subjects that they were about to receive a potential iFU pulse. The participants were blinded, however, as to whether or not the iFU stimulus was actually delivered or a sham application was performed instead. The test subjects were then immediately asked if they felt a sensation. If a subject reported pain they were asked to rate it on the 11-point numeric scale (NRS 0–10). A computer program randomized each trial of 20 pulses for which finger was used (10 on left and 10 on right) and whether a sham pulse or a real pulse was given (10 real and 10 sham pulses per iFU intensity value).
We used the method of ascending limits to determine the amount of ultrasound necessary to generate a sensation with a 90% sensitivity value (Snodgrass, 1975). We call this amount the ‘90% threshold intensity of iFU’. We began testing at a sub-perceptive threshold and systematically increased the iFU intensity every trial of 20 applications until either: the subject reported 9 out of 10 true positives, the ultrasound output reached 650 W/cm2 (a value determined during separate animal studies to produce reliable and safe sensations in the paws of sensitized rats) or we ran out of time for a given subject. For graphing purposes we allocated the intensity data in bins of 30 W/cm2.
A second test session was performed a minimum of one week after the first. This second test followed the same procedures as the first. We used this second test to identify possible long-term effects on perception as well as the consistency of response by the subjects to the ultrasound stimulus.
We used the metrics of sensitivity and specificity to analyze the data (Snodgrass, 1975). To measure the sensitivity of a given test subject’s ability to discern iFU stimulation of a given intensity, we divided the number of reported iFU-stimulation experiences by the number of actual iFU applications to that finger pad plus the number of sham iFU applications incorrectly identified as actual applications. Essentially, ‘sensitivity’ refers to the number of true positives registered by the subject, reported here as a percentage of number of actual or perceived iFU applications. To measure the specificity of a given test subject’s ability to discern iFU stimulation, we divided the number of correctly identified sham applications of iFU by the number of sham iFU applications plus the number of actual iFU applications incorrectly identified as sham. Essentially, ‘specificity’ refers to the number of true negatives registered by the subject, reported here as a percentage of the number of sham or unperceived actual iFU applications.
Results
We observed no significant difference between the average two-point threshold values before and after iFU testing or between the first and second sessions. Also, we did not observe any statistically significant differences for the 50% and 90% threshold intensity value of iFU between the first and the second sessions; we therefore combined the two sessions’ worth of results for subsequent analysis. Moreover, the pre- and post-test questionnaire showed no psychological or physiological changes associated with the testing process. Out of 34 test subjects, five of them reported transient pain, generally once per testing period. The vast majority of sensations (>99%) were pain free, however, with the median pain score measuring NRS 1/10. None of the subjects complained about any longlasting effects.
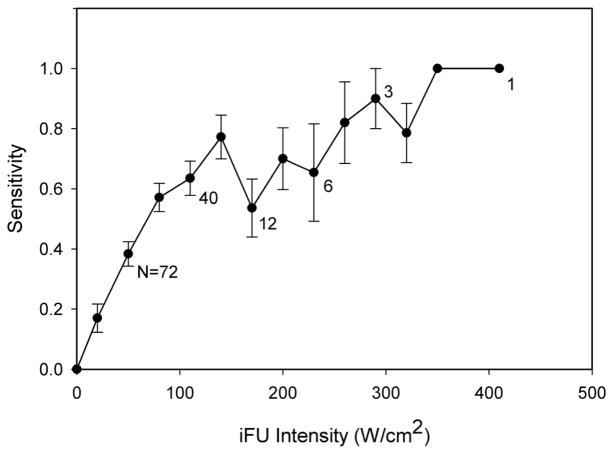
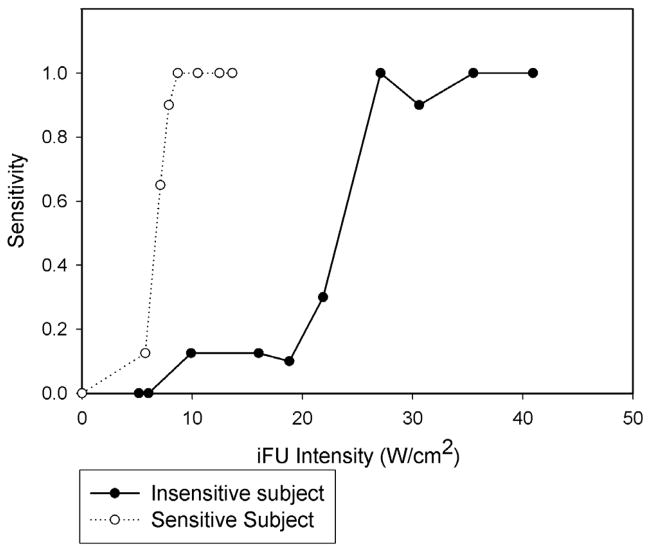
Due to time constraints, 25 of 34 tests were able to reach the 90% threshold intensity value of iFU. The average intensity for the attainment of the 90% threshold value for those members who did attain a threshold was 106.4 +/− 58.7 W/cm2. All subjects reached a sensitivity value of 50% within the time provided. The average value of iFU intensity at 50% sensitivity was 95 +/− 69.7 W/cm2. Figure 4 shows the graph of the entire population’s sensitivity data related to iFU intensity. The dip in sensitivity in the range of 170–230 W/cm2 arose due to data dropout, as we stopped collecting data from test subjects when the criteria described above were met. Therefore, successive data points on the graph contain contributions from fewer and fewer subjects, with the first data points coming from the entire test cohort and the final data point at 410 W/cm2 contributed from one test subject. Figure 5 shows an example of the sensitivity versus intensity from two separate subjects, where one test subject reached their 90% sensitivity value at a smaller value of iFU intensity than the other test subject. The specificity of subjects’ response to iFU testing remained high throughout the entire process with an average value of 94% (a 6% false positive rate).
Figure 4.
A plot of the test subjects’ sensitivity as a function of intense focused ultrasound (iFU) intensity value. The drop in sensitivity at 170 W/cm2 is due to a reduction in available data for plotting, itself caused by our cessation of data collection for many subjects due to their completion of the study. (See Figure 5 below for examples of individual contributions to this aggregate data plot.) The numbers next to data points denote the number of individual data points contributing to that average point on the graph.
Figure 5.
A plot showing the sensitivity versus the intensity values of our stimulating intense focused ultrasound (iFU), here for two of our test subjects. The first reached the 90% threshold for sensitivity at relatively low values of iFU intensity. The second reached their 90% threshold for sensitivity at a relatively large value of iFU intensity.
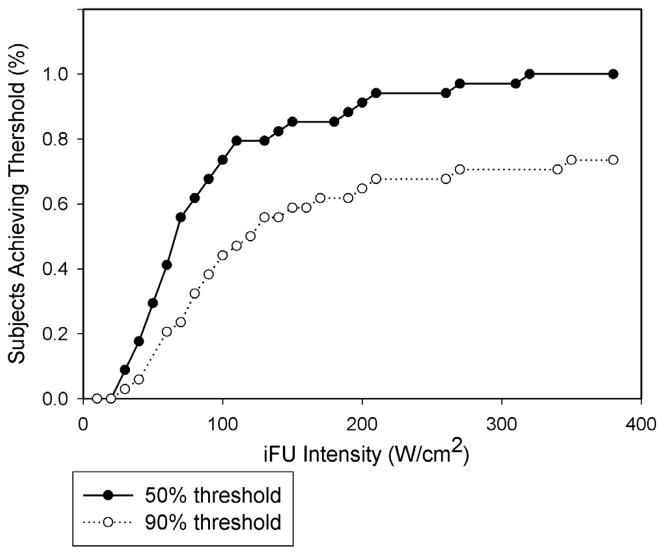
Figure 6 shows the subject population’s attainment of sensitivity threshold values versus iFU intensity, for 50% and 90% sensitivity, in the format of a Kaplan-Meyer curve (Sheean et al., 2011). In both cases, the majority of test subjects reached a given sensitivity threshold value below 100 W/cm2.
Figure 6.
A curve plotting how a given population reaches both a 90% as well as a 50% threshold as a function of intense focused ultrasound (iFU) intensity. All test subjects reached a value of 50% sensitivity while seventy percent of our test subjects reached a value of 90% sensitivity.
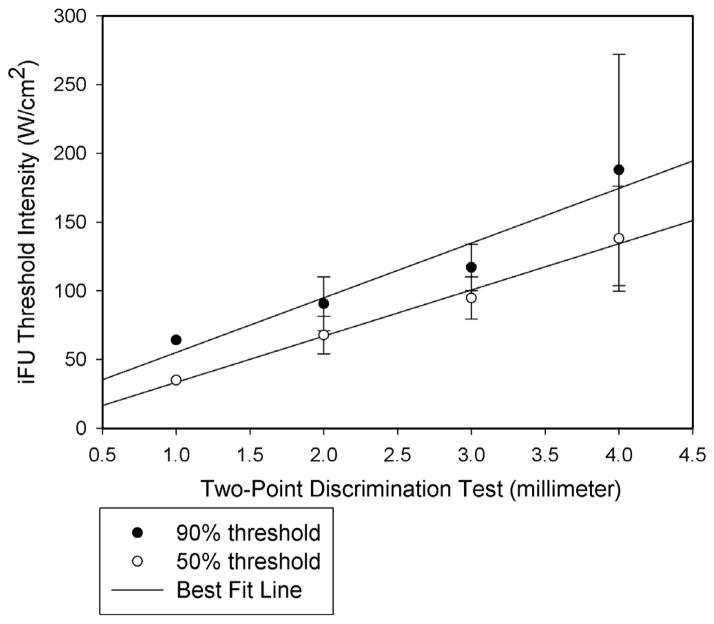
Our group of 17 subjects and 34 tests achieved an average two-point discriminant value of 2.83mm +/− 0.79, meaning that on average, the test subjects could identify a set of two prongs as actually consisting of two prongs when the average distance between the prongs was 2.83mm. Figure 7 demonstrates that there exists a statistically significant relationship between iFU threshold and twopoint discriminant value for each of 50% and 90% sensitivity threshold values. In essence, those with a low density of mechanoreceptors (therefore a relatively large distance between the prongs on the two-point DISK-CRIMINATOR™) required a relatively large amount of stimulation by iFU to reliably discern that stimulation. In contrast, those with a high density of mechanoreceptors (a relatively small distance between the prongs) required a relatively small amount of stimulation by iFU to reliably discern that stimulation.
Figure 7.
A statistically significant correlation exists between the intense focused ultrasound (iFU) intensity required for our test subjects to achieve each of a 90% and 50% sensitivity threshold value and the two-point assay of their density of peripheral nerve receptors. Here, that density is inversely proportional to the distance (reported here in millimeters) between two successfully identified stimulation points by the DISK-CRIMINATOR™.
Discussion
Discernment of iFU stimulation by our test subjects occurred in a reliable and safe manner. Also, the manner in which the test subjects became aware of the iFU stimulation as the iFU intensity increased (Figure 6) is consistent with that found for other stimuli such as mechanical vibration or thermal stimulation (Griffin and Seah, 2008; Vallbo and Johansson, 1984). In particular, the sigmoidal nature of the curve of iFU sensation threshold versus iFU intensity matches that of other tests of stimulus discernment: its initially small sensitivity to the observation of stimulation at low values of intensity; the rapid increase in sensitivity for mid-range values of stimulation; the eventual leveling out of sensitivity at large values of stimulation. The high specificity observed throughout the study suggests that iFU stimulation likely generates unique sensations not easily mistaken for standard sensations.
Our study also documents a direct relationship between iFU threshold value for reliable sensation generation and the density of peripheral mechanoreceptive terminals of our test subjects (Figure 7). These results therefore support the hypothesis of Gavrilov and colleagues that iFU’s direct interaction with mechanoreceptors contributes to ultrasound’s induction of sensation. Finally, we have demonstrated the stability of iFU finger pad responses over time and that iFU at this intensity, frequency and duration does not alter cutaneous mechanoreceptors.
Conclusion
We observed reliable discernment by our test subjects of blinded application of intense focused ultrasound in a manner consistent with observations of other stimuli. Detection of iFU stimulation varied in a way that scaled with the density of mechanoreceptors in the skin available for that detection, as was first hypothesized by Gavrilov and colleagues.
Of interest for future research may be our correlation of the density of mechanoreceptors and the intensity of ultrasound necessary to generate sensations with high sensitivity and specificity, given the variable density of mechanoreceptors throughout the human body, both cutaneous and at depth. Our study may, in particular, offer a guide to the average intensity of iFU one should consider using for future iFU studies in human subjects targeting sensation induction.
Figure 2.
A beam plot of intensity along the axis the device used in the study. The primary focus occurs near the tip of the cone, with a secondary maximum of one-fourth the intensity at the surface measured 0.75 cm below the skin.
Acknowledgments
This material is based upon work supported in part by the U.S. Department of Veterans Affairs, Office of Research and Development Rehabilitation R&D Program. We also received support from NIH (UL1 RR025014, R41 NS 049719-01), the Life Sciences Discovery Fund of the State of Washington. We thank Pavan Vaswani for his construction of the computer program that randomized the application of iFU to our test subjects. Pierre D Mourad and Michel Kliot have a significant financial interest in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dalecki D, Child SZ, Raeman CH, Cartensen EL. Tactile perception of ultrasound. J Acoust Soc Am. 1995;97(5 Pt 1):3165–3170. doi: 10.1121/1.411877. [DOI] [PubMed] [Google Scholar]
- Davies II, Gavrilov LR, Tsirulnikov EM. Application of focused ultrasound for research on pain. Pain. 1996;67:17–27. doi: 10.1016/0304-3959(96)03042-4. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinski OB, Tsirulnikov EM, Shchekanov EE. A study of reception with the use of focused ultrasound. I. Effects on the skin and deep receptor structures in man. Brain Research. 1977;135:265–277. doi: 10.1016/0006-8993(77)91030-7. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Gersuni GV, Ilyinski OB, Tsirulnikov EM, Shchekanov EE. A study of reception with the use of focused ultrasound. II. Effects on the animal receptor structures. Brain Research. 1977;135:279–285. doi: 10.1016/0006-8993(77)91031-9. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR. Use of focused ultrasound for stimulation of nerve structures. Ultrasonics. 1984;22:132–138. doi: 10.1016/0041-624x(84)90008-8. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Tsirulnikov EM, Davies II. Application of focused ultrasound for the stimulation of neural structures. Ultrasound in Med & Biol. 1996;22(2):179–192. doi: 10.1016/0301-5629(96)83782-3. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR. The possibility of generating focal regions of complex configurations in application to the problems of stimulation of human receptor structures by focused ultrasound. Acoustical Physics. 2008;54(2):269–278. [Google Scholar]
- Griffin MJ, Seah SA. Normal values for thermotactile and vibrotactile thresholds in males and females. Int Arch Occup Environ Health. 2008;81:535–543. doi: 10.1007/s00420-007-0252-6. [DOI] [PubMed] [Google Scholar]
- Hill CR, Rivens I, Vaughan MG, Ter Harr GR. Lesion development in focused ultrasound surgery: A general model. Ultrasound Med Biol. 1994;20(3):259–69. doi: 10.1016/0301-5629(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Shinoda H. Finger Ring Tactile Interface Based on Propagating Elastic Waves on Human Fingers. World Haptics; Japan: 2007. Mar, pp. 145–150. [Google Scholar]
- Iwamoto T, Shinoda H. Two Dimensional Stress Reproduction Using Ultrasound Tactile Display. Proc. SICE-ICASE International Joint Conference; 2006. pp. 4919–4921. [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanorecpetive units in glabrous skin. Journal of Physiology. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitgeb N, Schröttner J, Cech R. Perception of elf electromagnetic fields: excitation thresholds and inter-individual variability. Health Physics. 2007;92(6):591–595. doi: 10.1097/01.HP.0000243128.29337.aa. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg. 1985;10A:906–907. doi: 10.1016/s0363-5023(85)80173-8. [DOI] [PubMed] [Google Scholar]
- Martin JH, Jessell TM. In: Principles of Neuroscience. 3. Kandel ER, Schwartz JH, Jessell TM, editors. East Norwalk, CT: Appleton & Lange; 1991. pp. 340–352. [Google Scholar]
- Miao CH, Brayman AA, Loeb KR, Ye P, Zhou L, Mourad P, Crum LA. Ultrasound Enhances Gene Delivery of Human Factor IX Plasmid. Human Gene Therapy. 2005;16:893–905. doi: 10.1089/hum.2005.16.893. [DOI] [PubMed] [Google Scholar]
- Sheean PM, Bruemmer B, Gleason P, Harris J, Boushey C, Van Horn L. Publishing Nutrition Research: A Review of Multivariate Techniques-Part 1. Journal of the American Dietetic Association. 2011;111:103–110. doi: 10.1016/j.jada.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG. Psychophysics. In: Scharf B, Reynolds GS, editors. Experimental Sensory Psychology. Glenview, Ill: Scott Foresman; 1975. pp. 21–34. [Google Scholar]
- Sutton Y, McBride K, Pye S. An ultrasound mini-balance for measurement of therapy level ultrasound. Physics in medicine and biology. 2006;51(14):3397–404. doi: 10.1088/0031-9155/51/14/008. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Human Neurobiol. 1984;3:2–14. [PubMed] [Google Scholar]
- Wright A, Davies II, Riddell JG. Intra-articular ultrasonic stimulation and intracutaneous electrical stimulation: evoked potential and visual analogue scale data. Pain. 1993;52:149–55. doi: 10.1016/0304-3959(93)90126-A. [DOI] [PubMed] [Google Scholar]
- Wright A, Graven-Nielsen T, Davies II, Arendt-Nielsen L. Temporal summation of pain from skin, muscle and joint following nociceptive ultrasonic stimulation in humans. Exp Brain Res. 2002;144:475–482.2. doi: 10.1007/s00221-002-1062-4. [DOI] [PubMed] [Google Scholar]