Abstract
Needle biopsy is a routine medical procedure for examining tissue or biofluids for the presence of disease using standard methods of pathology. In this work, spray ionization directly from tissue in the biopsy needle is shown to provide highly specific molecular information through mass spectrometry analysis. The data are available within a minute after the tissue biopsy, a time scale that allows immediate medical decisions to be made. This method has been performed for tissues in a variety of organs including brain, liver, kidney, adrenal gland, stomach and spinal cord. Amino acids, hormones, fatty acids, anesthetics and phospholipids are detected from the tissues and identified using exact mass measurement and tandem mass spectrometry. Lipid profiles are rich in information and as in imaging MS methods, they have the potential to serve to distinguish diseased from healthy tissue. Needle biopsies allow a crude form of depth profiling that is demonstrated with the analysis of tissue samples taken by a needle inserted into a porcine kidney at various depths.
Needle biopsy is a diagnostic technique that is used to extract small amount of fluids, cells or tissue by inserting a hollow needle and withdrawing biofluid or tissue.1 Compared with surgical biopsy, needle biopsy reduces the risk of trauma and infection. The biopsied tissue samples are typically examined under a microscope by a pathologist after histochemical or immunohistochemical staining. An alternative to the standard pathological methods is chemical analysis by mass spectrometry and tandem mass spectrometry which can provide rich molecular information on the nature of the tissue and which through correlation with pathology, can be used to indicate the disease state of tissue.2–5 Recently, mass spectrometric analysis of tissue has been advancing rapidly and a number of ionization methods have been used to characterize tissue sections without performing chemical extraction prior to the analysis. These methods include matrix assisted laser desorption (MALDI),6, 7 desorption electrospray ionization (DESI),8, 9 secondary-ion mass spectrometry (SIMS),10 and laser ablation electrospray ionization (LAESI).11, 12 The methods have different strengths but all depend on comparing the distributions of particular compounds (e.g. phospholipids, proteins, etc.) with the known patterns characteristic of diseased vs. healthy tissue. Some other ambient ionization methods, although not applicable to tissue imaging, can also be used for direct tissue analysis without sample preparation.13 These methods include electrospray laser desorption ionization (ELDI),14 extraction electrospray ionization (EESI)15, probe electrospray ionization (PESI).16
While tissue imaging gives comprehensive information on a tissue sample, even when data is recorded at modest spatial resolution, it can take many minutes to image the surface of a small piece of tissue. The advantage is that the information obtained allows disease margins to be assessed. An alternative and more rapid procedure is to use MS imaging methodology to sample a small set of points or to perform line scans across a section, recording mass spectra continuously.17 The tissue samples taken by needle biopsy can be analyzed rapidly in a simple integrative fashion using paper spray ionization mass spectrometry.18 In this experiment, a small tissue piece is placed on paper, wetted with solvent, and an electrical potential is applied to establish a high electric field; charged droplets carrying dissolved small molecule tissue constituents are emitted. This spray ionization concept has recently been extended to allow the analytical sample itself to be used as the medium for ionization as in the leaf spray experiment in which leaves were cut to a sharp point and gave characteristic mass spectra of photochemical constituents upon application of a high voltage.19–21 In the present work, we extend this concept to animal tissue and have satisfied the need for a sharp point by examining a needle biopsy sample still within the needle. Direct needle spray ionization of tissue samples represents a simple and rapid method of chemical analysis of compounds in a particular biological sample. The procedure is highly compatible with tissue biopsy procedures which are standard in doctors’ offices and surgical rooms. Intra-surgical applications can be envisioned.
EXPERIMENTAL SECTION
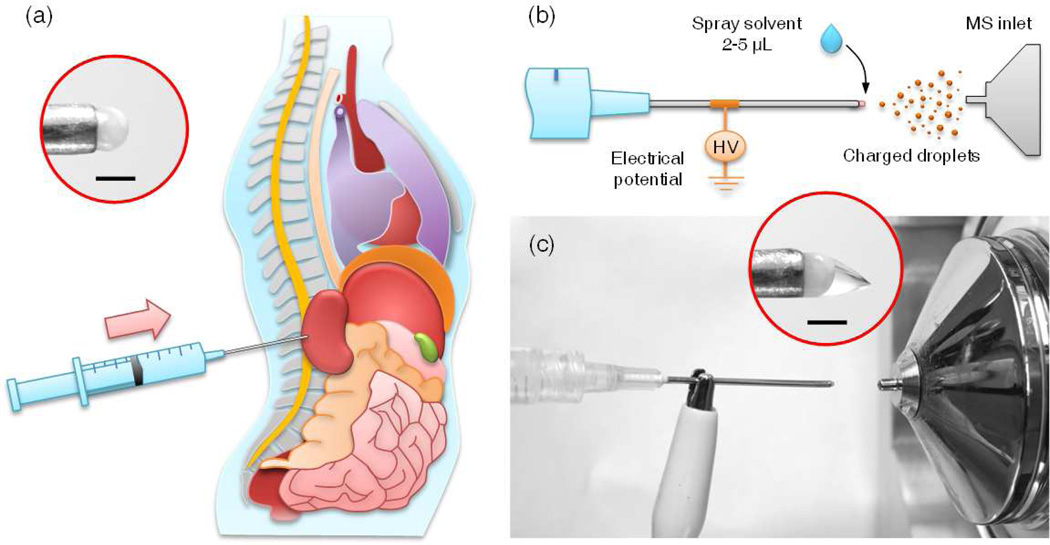
The procedure of direct tissue examination using spray ionization of needle biopsied tissues is shown schematically in Figure 1. A disposable medical syringe (3 mL, with Luer-Lok Tip) with a hypodermic needle (Precision Glide, 18G (0.838 mm I.D.) × 1.5 inch, Becton Dickinson & CO, Franklin Lakes, NJ) was used to extract the tissue. After collecting the tissue, the syringe plunger was carefully pushed forward to expose 1–3 mm of tissue at the end of the needle (Figure 1a). The syringe was then placed in front of the inlet of a mass spectrometer at a distance of 10–20 mm from the MS inlet. A high voltage (4–4.5 kV) was applied on the needle through an alligator clip. Spray solvent, methanol of 2–5 µL, was applied to the needle tip to extract the chemicals from the exposed tissue and to generate a spray and ionize endogenous compounds present in the tissue for MS analysis. Porcine adrenal gland was purchased from Pel-Freez (Rogers, AR). Porcine kidney and stomach organs were purchased from a local grocery store. Mouse brain, spinal cord and liver were obtained from School of Veterinary Medicine at Purdue University. The human normal and cancer kidney tissues were obtained from the School of Medicine at Indiana University. All human tissue samples were handled in accordance with approved institutional review board (IRB) protocols at Indiana University School of Medicine. All samples were stored at −80 °C but allowed to come to room temperature before the needle biopsy was performed. MS and MS/MS analysis were performed using Exactive Orbitrap and TSQ Quantum Access Max (Thermo Scientific Inc., San Jose, CA), respectively. The 11 peaks of the top relative intensities in m/z range 600–900 were used for PCA (principle component analysis).22
Figure 1.
Schematic showing direct spray ionization from a biopsy needle for biological tissue analysis. (a) Tissue is extracted from the organ by inserting the needle into the organ. The extracted tissue is partially released (1–3 mm) from the needle by pushing the plunger along the barrel, and it remains held by the needle tip. High voltage (4–4.5 kV) and spray solvent (2–5 µL, methanol) are applied to the needle and the exposed tissue, respectively, to generate charged droplets carrying chemicals from the tissue into the MS inlet. (b) Schematic and (c) photo of direct spray ionization with needle biopsy on tissue. Inset in (a), biological tissue held by the needle without added spray solvent. Inset in (c), Taylor cone formed at the tissue after applying high voltage and spray solvent. Scale bar is 1 mm.
RESULTS AND DISCUSSION
As shown in Figure 1c, the tissue exposed at the end of the needle serves as the sample as well as the spray tip in this ionization experiment and a Taylor cone was formed when the spray solvent and the high voltage were applied. The solvent extracts chemical and biological molecules in the tissue, carries them to the tip of Taylor cone, where charged droplets containing analyte molecules are formed. This method applies particularly well to basic and acidic compounds that can be observed readily in the positive or negative ionization modes, respectively (Figure S1a and b). A small volume of solvent is used and the spray period lasts ca. 30s for 2 µL methanol, which is sufficient time to acquire high quality mass spectral data using multiple MS and MS/MS scans. The analysis can be repeated with the same sample by adding additional solvents. The total ion intensities could vary less than 30% during each spray period of about 30 s (Figure S1c and d) while the relative intensities were found to be highly reproducible. This experiment was also repeated with samples from different extractions of the same tissue. The variation in total ion intensity was still within one order of magnitude, with similar relative intensities obtained for each analysis.
With the assistance of high resolution data acquired using the Exactive Orbitrap the presence of a wide range of fatty acids and phospholipids was revealed in the tissues examined (Figure S1a, S1b, and S2). In the positive ion mode, the dominant species are phospholipids and the main peaks are identified as protonated and potassium adducts of phosphatidylcholine (PC). In the negative ion mode, the dominant species in the lower mass range (m/z 200 – m/z 700) are identified as deprotonated fatty acids and fatty acids dimers. Identified fatty acids include palmitic acid (m/z 255.23), linoleic acid (m/z 279.23) and dimer (m/z 559.47), oleic acid (m/z 281.25) and dimer (m/z 563.50), arachidonic acid (m/z 303.23) and dimer (m/z 607.47), docosahexaenoic acid (m/z 327.23) and dimer (m/z 655.47). In the higher mass range m/z 700 – m/z 1000, the main species observed are identified tentatively based on exact mass measurements and knowledge of lipid constituents in similar tissues reported in the literature as deprotonated phosphatidylserine (PS), glycerophosphoethanolamines (PE), plasmalogens (plasm-PE) and phosphatidylinositol (PI). It is interesting to note that ketamine and xylazine used for anesthetizing the mouse were observed at much higher relative intensities in the mouse spinal cord (Figure 2a) than in other organs including the brain (Figure S1a) and liver (Figure S2a). This could be due the actual higher concentrations of these two drugs in the spinal cord or a significant matrix effect due to the difference in tissue properties. One of the main metabolites of ketamine, norketamine, was also found in these organs (Figure S3), and the relative concentration of norketamine (using ketamine as reference) was much higher in liver than that in brain and spinal cord.
Figure 2.
Spectra obtained for mouse spinal cord (a) positive and (b) negative ion modes. Mouse anesthetized with 90 mg/kg ketamine and 9 mg/kg xylazine.
Unlike the imaging mass spectrometry methods, direct tissue analysis with spray from a biopsy needle provides chemical information about a point, either on a tissue section (Figure S4) or inside a piece of tissue. However, minimal damage to the organ occurs if this method is used in vivo to analyze the samples taken from deep within the organ. As a demonstration of a crude form of depth profiling which is available using the needle biopsy spray, the biopsy needle was inserted into the porcine kidney at different depths to extract the samples of about 1 mm length from the cortex and medulla sections for direct analysis. Principal component analysis (PCA) of the spectra recorded shows a clear distinction between the samples from these two kidney sections.
As shown by the results described above, lipid profiles were ready obtained with both positive and negative ion sprays from the biopsied tissues. Lipids are important component of cell membrane and their role in cardiovascular disease is well established.23, 24 Previous studies have also shown that the lipid profile is useful in diagnosing cancer and in some cases recognizing its stage.25 Examination of the tumor and adjacent normal sections on human kidney tissue (as established by pathology)26 were carried out with the needle biopsy followed by direct spray MS analysis in negative mode (Figure 4a and b). Significantly different profiles were observed for phospholipids in the mass range m/z 700–900 for regions known independently to represent tumor and normal tissues. The relative peak intensity of plasm-PE(36:4) [M-H]− at m/z 722.51, PE(36:4) [M-H]− at m/z 738.50, PS(P-38:5) [M-H]− at m/z 792.52 and PS(38:4) [M-H]− at m/z 810.52 are much higher for the normal tissue section, while that of PE(34:1) [M-H]− at m/z 747.51 is much higher for the cancer section. These results are in good agreement with a previous analysis using DESI. The DESI imaging of the same tissue sample was performed in negative ion mode to acquire the spatial distribution of glycerophospholipids and fatty acids. The tumor section was successfully distinguished from normal tissue section using partial least square discriminate analysis and the results were verified by pathological data obtained from tissue sections stained with hematoxylin and eosin (H&E).26
Figure 4.
MS spectra of (a) normal and (b) cancer sections on a human kidney tissue, recorded using Exactive Orbitrap.
Besides the observed phospholipids and fatty acids which are structural components of cell membranes, small molecules generated from the cells and organs could also be observed with the direct spray ionization from the needle biopsied tissues. In the examination of the porcine adrenal gland, two hormones secreted from the gland, epinephrine (m/z 184.10) and norepinephrine (m/z 170.08), have been detected with needle biopsy of a porcine adrenal gland (Figure S4). The MS/MS analysis confirmed their identities (Figure S5b and c). Amino acid phenylalanine was also detected in the porcine adrenal gland (Figure S5a), which is a precursor of synthesis signaling molecules and hormones in human body.
CONCLUSIONS
Direct examination of tissues with mass spectrometry analysis can be performed with a direct spray from tissue extracted using a biopsy needle. This method can be applied for different organs and is highly compatible with in-situ needle biopsy protocol. Direct spray ionization with needle biopsy allows the identification of various endogenous chemicals of tested tissues, from small molecules to phospholipids. Amino acids, hormones, fatty acids, anesthetic and phospholipids have been observed with methanol as the spray solvent. Further optimization with other solvents will be attempted through more efficient extraction or real time derivatization to enhance the ionization efficiency of target chemicals.
Supplementary Material
Figure 3.
Depth profiling of lipids in an intact porcine kidney using needle biopsy spray. (a) PCA of the MS data acquired for needle biopsied tissues. MS spectra of tissue samples at (b) 2 mm and (c) 16 mm from the kidney surface, recorded using Exactive Orbitrap.
ACKNOWLEDGMENTS
This work was supported by NSF (Project CHE-0847205) and NIH (Project R21 RR031246). The authors thank Livia S. Eberlin, Prof. Riyi Shi and He Wang for providing tissue samples and valuable discussions.
References
- 1.Lever JV, Trott PA, Webba AJ. J. Clin. Pathol. 1985;38:1–11. doi: 10.1136/jcp.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai K, Okuyama H, Yura J, Takeyama H, Shinagawa N, Tsuruga N, Kato K, Miura K, Kawase K, Tsujimura T, et al. Carcinogenesis. 1992;13:579–584. doi: 10.1093/carcin/13.4.579. [DOI] [PubMed] [Google Scholar]
- 3.Hawkridge AM, Muddiman DC. Annu. Rev. Anal. Chem. 2009;2:265–277. doi: 10.1146/annurev.anchem.1.031207.112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer KC, Denes J, Albrecht K, Szaniszlo T, Balog J, Skoumal R, Katona M, Toth M, Balogh L, Takats Z. Angew. Chem. Int. Ed. 2009;48:8240–8242. doi: 10.1002/anie.200902546. [DOI] [PubMed] [Google Scholar]
- 5.Schafer KC, Szaniszlo T, Gunther S, Balog J, Denes J, Keseru M, Dezso B, Toth M, Spengler B, Takats Z. Anal. Chem. 2011;83:1632–1640. doi: 10.1021/ac102613m. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Nat. Med. 2001;7:493–496. doi: 10.1038/86573. [DOI] [PubMed] [Google Scholar]
- 7.Rujoi M, Estrada R, Yappert MC. Anal. Chem. 2004;76:1657–1663. doi: 10.1021/ac0349680. [DOI] [PubMed] [Google Scholar]
- 8.Eberlin LS, Ifa DR, Wu C, Cooks RG. Angew. Chem. Int. Ed. 2010;49:873–876. doi: 10.1002/anie.200906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiseman JM, Ifa DR, Song Q, Cooks RG. Angew. Chem. Int. Ed. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 10.Ostrowski SG, Bell CTV, Winograd N, Ewing AG. Science. 2004;305:71–73. doi: 10.1126/science.1099791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemes P, Woods AS, Vertes A. Anal. Chem. 2010;82:982–988. doi: 10.1021/ac902245p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha B, Nemes P, Nazarian J, Hathout Y, Hoffman EP, Vertes A. Analyst. 2010;135:751–758. doi: 10.1039/b922854c. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez FM, Harris GA, Galhena AS. Analytical Chemistry. 2011;83:4508–4538. doi: 10.1021/ac200918u. [DOI] [PubMed] [Google Scholar]
- 14.Huang MZ, Hsu HJ, Wu CI, Lin SY, Ma YL, Cheng TL, Shiea J. Rapid Commun. Mass Spectrom. 2007;21:1767–1775. doi: 10.1002/rcm.3011. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Zenobi R. Nat. Protoc. 2008;3:1467–1475. doi: 10.1038/nprot.2008.109. [DOI] [PubMed] [Google Scholar]
- 16.Chen LC, Nishidate K, Saito Y, Mori K, Asakawa D, Takeda S, Kubota T, Terada N, Hashimoto Y, Hori H, Hiraoka K. Rapid Commun. Mass Spectrom. 2008;22:2366–2374. doi: 10.1002/rcm.3626. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman JM, Puolitaival SM, Takats Z, Cooks RG, Caprioli RM. Angew. Chem. Int. Ed. 2005;44:7094–7097. doi: 10.1002/anie.200502362. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, Ouyang Z. Anal. Chem. 2011;83:1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Wang H, Cooks RG, Ouyang Z. Anal. Chem. 2011;83:7608–7613. doi: 10.1021/ac2020273. [DOI] [PubMed] [Google Scholar]
- 20.Marijinissen J, Jan vD, de Roos R. NPT Procestechnologie. 2001;8:22–24. [Google Scholar]
- 21.van der Sanden M. Delft Outlook. 1999;4:9–12. [Google Scholar]
- 22. http://faculty.fuqua.duke.edu/~kamakura/bio/WagnerKamakuraDownloads.htm.
- 23.Eberlin LS, Dill AL, Costa AB, Ifa DR, Cheng L, Masterson T, Koch M, Ratliff TL, Cooks RG. Anal. Chem. 2010;82:3430–3434. doi: 10.1021/ac9029482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G. J. Am. Med. Assoc. 1998;280:719–723. doi: 10.1001/jama.280.8.719. [DOI] [PubMed] [Google Scholar]
- 25.Eberlin LS, Dill AL, Golby AJ, Ligon KL, Wiseman JM, Cooks RG, Agar NYR. Angew. Chem. Int. Ed. 2010;49:5953–5956. doi: 10.1002/anie.201001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dill AL, Eberlin LS, Zheng C, Costa AB, Ifa DR, Cheng L, Masterson TA, Koch MO, Vitek O, Cooks RG. Anal. Bioanal. Chem. 2010;398:2969–2978. doi: 10.1007/s00216-010-4259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.