Abstract
Aptamers can bind a wide range of biomedically relevant proteins with affinities and specificities that have therapeutic utility. Although aptamers are susceptible to nuclease-mediated degradation and cannot easily cross biological barriers, specific aptamer modification can feasibly solve these problems. To address these obstacles, Lau et al. developed a general strategy for generating natural packaging and transport vehicles for targeting agents, such as aptamers and their small-molecule ligands, by using virus-like particles (VLPs) assembled from the recombinant expression of the bacteriophage Qβ coat protein. Since RNA and DNA molecules are susceptible to nuclease-mediated degradation, it is important that Qβ VLPs protect their encapsulated aptamers from nuclease-mediated degradation and enhance their permeability. Moreover, if self-assembled using natural proteins, VLPs can guarantee the biocompatibility and biodegradability of modified aptamers in therapeutic applications. Therefore, this Perspective explores the outlook for such aptamer modification strategies for nanodrug preparation and delivery applications and the challenges that lie ahead.
Aptamers are single-stranded DNA or RNA oligonucleotides that can bind a wide range of biomedically relevant molecules, such as proteins, drugs, small molecules, and biological cells, with high affinity and specificity. Because of these properties, aptamers can serve as either biological drugs or drug carriers to treat various diseases. Although they have often been described as analogs of antibodies,1 aptamers exhibit significant advantages relative to protein therapeutics in terms of small molecular size, reproducible synthesis, and low immunity; further, they can be easily modified by chemical synthesis, making them more adaptable for different biomedical applications. Moreover, advances in chemical synthesis methods have enabled the generation of large populations of degenerate oligodeoxynucleotides, enabling the in vitro selection of aptamers using systematic evolution of ligands by exponential enrichment (SELEX), a combinatorial chemistry technique in molecular biology for producing oligonucleotides of either single-stranded DNA (ssDNA) or RNA that specifically bind to a target ligand or ligands.2–3 In view of these advantages, aptamers show considerable potential in therapeutic applications.
However, aptamers confront some application challenges. First, RNA and DNA molecules are susceptible to nuclease-mediated degradation, thus limiting their use in some therapeutic applications.4 Second, as chemicals, aptamers cannot readily cross biological barriers, such as cell membranes, to perform target-specific recognition inside cells.5 However, chemical modifications can generally be incorporated into the nucleotide sugars or internucleotide phosphodiester linkages to circumvent these problems. As shown in Table 1, aptamers can be easily assembled on the surface of carbon nanotubes, quantum dots, and metallic or silica nanoparticles by noncovalent physical adsorption or through covalent interactions.6–10 Such modifications of nucleotides can both stabilize aptamers against nuclease-mediated degradation and increase their solubility and binding affinity.11 Encapsulation-based aptamer protection and delivery using silica, polymers, or gels is another way to prevent enzymatic degradation, while being delivered across cell membranes. However, limitations, such as cell toxicity, low biocompatibility, and biophysical and chemical instability, have prevented the full realization of aptamer delivery in vivo. The ideal delivery vehicle should protect aptamers from the physiological environment and minimize metabolism and degradation during transit. It should also be biocompatible, biodegradable, stable, and able to be engineered such that aptamer molecules are concentrated at the desired site of action to increase efficacy, while, at the same time, decreasing unwanted side effects.
Table 1. Typical Aptamer-Modified Nanodrug Delivery Systems.
| Nanoparticles | Typical assembly techniques | An example application |
|---|---|---|
| Single-stranded DNA (ssDNA)-single-walled carbon nanotubes (SWNTs) | Noncovalent modification by aromatic interactions between nucleotide bases and carbon nanotube sidewalls | Protecting single-stranded DNA during cellular delivery6 |
| Aptamer-quantum dots (QDs) | Covalent conjugation between carboxyl-modified QDs and amine-labeled aptamer | A drug delivery vehicle for tumor cells7 |
| Aptamer-gold nanoparticles (GNPs) | Covalent interaction by Au-S bond | A drug carrier in tumor treatment8 |
| Aptamer-silver nanoprticles(AgNPs) | Affinity of biotin-aptamer and streptavidin stabled AgNPs | Intracellular protein imaging9 |
| Aptamer-silica nanoparticles (SiNPs) | Covalent conjugation between carboxyl-terminatal SiNPs and amine-labeled aptamer. | Targeted drug delivery10 |
Natural biomaterials, including lipids, proteins, and oligosaccharides, have been used to construct delivery platforms by encapsulating their cargos into diverse nanocontainers,12 such as cages, microspheres, nanoparticles, hydrogels, minirods, and minipellets. This type of development has especially focused on protein-based delivery platforms due to the biocompatibility, biodegradability, and low toxicity of some natural proteins. A variety of such proteins have been used and characterized, including viral capsids,13,14 ferritin/apoferritin,15,16 albumin,17 and collagen18,19 (Table 2). In the accompanying article in this issue of ACS Nano, Lau et al.20 describe a general strategy for generating natural packaging and transport vehicles for nanodrug delivery by using virus-like particles (VLPs) assembled from the recombinant expression of the bacteriophage Qβ coat protein (Figure 1).
Table 2. Comparison of Natural Biomaterials that are Used for Constructing Delivery Platforms (Updated from Lin12).
| Proteins | Delivery system | Description/characteristics |
|---|---|---|
| Collagen | Microparticles, minirods |
|
| Elastin/elastinlike polypeptide (ELP) | ELP-Dox nanoparticles, hydrogel |
|
| Ferritin/apoferritin | Protein cage |
|
| Albumin | Nanoparticles |
|
| Viral capsid | Protein cage |
|
| Whey protein | Hydrogel, whey protein beads, microspheres |
|
Figure 1.
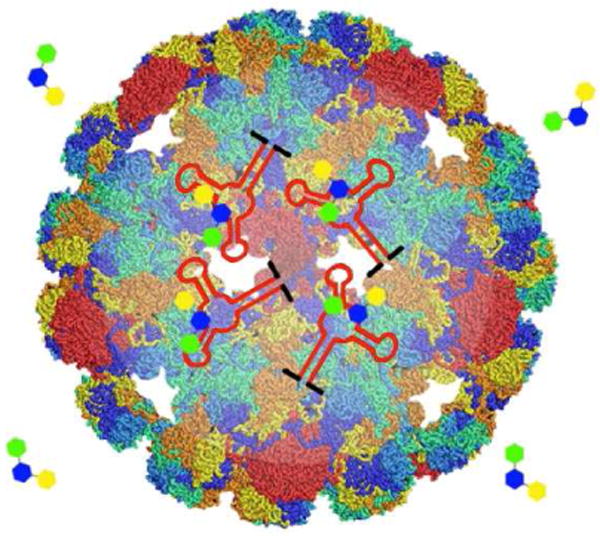
Schematic of the structure of virus-like particle encapsidating aptamers and small-molecule ligands of aptamers. The capsid shell provides channels for small-molecule ligands, as well as sufficient space for encapsulated aptamers to maintain their secondary and tertiary structures.
More specifically, the aptamer of interest is first modified with a RNA sequence from Qβ genome that can form as a hairpin structure and bind to Qβ coat protein. Next, the sequences of Qβ coat protein and modified aptamer are inserted into separate plasmids. The two plasmids are then co-transformed with E. coli to yield an elevated level of Qβ coat proteins and modified aptamers. Finally, the modified RNA hairpin sequence promotes the encapsidation of aptamers via binding to the interior surface of capsid shell in the process of Qβ coat proteins self-assembling into VLPs. Expression of Qβ coat protein and aptamer also can be performed with a single plasmid as described by Lau et al.20 Nevertheless, the authors thought that the dual-plasmid approach might have an advantage in maintaining stable copy numbers of plasmids because different plasmids contained different antibiotic resistance genes and origins of replication. While VLPs ensure the biocompatibility and biodegradability of modified aptamers by using natural proteins, the Qβ capsid shell was demonstrated to be impermeable to enzymes, thus protecting encapsulated aptamers from degradation by nucleases and enhancing their permeability for nanodrug delivery. Importantly, the capsid provides channels for small-molecule ligands while allowing sufficient space for encapsulated aptamers to maintain their secondary and tertiary structures, as revealed by the unreduced affinities of aptamers when binding with their small-molecule ligands. In addition, VLPs are stable substrates for diverse chemical and genetic modifications, enabling decoration of their exterior surfaces with targeting, immunogenic, or labeling agents. Therefore, Lau et al. have offered a convenient functional aptamer encapsulation technique that holds great potential as a promising platform for RNA aptamer delivery.
Outlook and Challenges
Lau et al.'s strategy suggests a general platform for the production of VLPs to encapsulate aptamers. Favorable biocompatibility and biodegradability, and uniform size are the advantages of VLPs for the delivery of modified aptamers to their target release sites. It has been shown that VLP encapsulation protects aptamers and their small-molecule ligands from degradation by erosion and proteolysis. Furthermore, since the flow rate of small-molecule ligands is driven by the concentration difference between the interior and exterior of the VLPs, the release rate of molecules can also be regulated by VLP channel sizes to avoid the use of high doses of drugs. Virus-like particles are a major breakthrough for aptamer delivery in protein-based templates. Specifically, VLPs have improved the pharmacokinetics and biodistribution of aptamer delivery by improving the drug release mode.
Nonetheless, some challenges still confront VLP-based aptamer delivery systems. First, the properties and applications of VLPs are still in the development stage for aptamer delivery and therapeutic purposes. Most viruses developed for bionanotechnology applications have not yet been evaluated; hence, the use of these viruses may be limited in aptamer delivery as a result of potential cytotoxicity. For example, human adenoviruses, which are typically used for gene delivery purposes, once resulted in severe hepatotoxicity in murine models.21 Such toxic effects have also been observed in non-human primates.22 Second, for clinical aptamer delivery, the biodegradability and immune response of VLPs still need to be evaluated, especially if modifications are decorated on the exterior surface of VLPs, such as folic acid and antigenic peptides. Third, fundamental questions about pharmacokinetic profiles and therapeutic efficacy of VLP-based aptamer delivery systems remain to be answered. Finally, future developments for VLP-based aptamer delivery should also focus on enhanced site-specific drug delivery by using newly developed receptor-targeting ligands, improving sustained drug release rates with enhanced permeability and retention time, minimizing damage to normal cells by directing drug molecules for site-avoidance delivery, and developing multiple drugs for sequential release.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 20975034, 21177036), the National Key Scientific Program of China (2011CB911001, 2011CB911003), and by the National Institutes of Health (GM066137, GM079359 and CA133086).
References and Notes
- 1.Keefe AD, Pai S, Ellington A. Aptamers as Therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuerk C, Gold L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 3.Ellington AD, Szostak JW. In Vitro Selection of RNA Molecules that Bind Specific Ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 4.Griffin LC, Tidmarsh GF, Bock LC, Toole JJ, Leung LL. In Vivo Anticoagulant Properties of a Novel Nucleotide-Based Thrombin Inhibitor and Demonstration of Regional Anticoagulation in Extracorporeal. Blood. 1993;81:3271–3276. [PubMed] [Google Scholar]
- 5.Ng EWM, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Pegaptanib, a Targeted Aanti-VEGF Aptamer for Ocular Vascular Disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 6.Wu YR, Phillips JA, Liu HP, Yang RH, Tan WH. Carbon Nanotubes Protect DNA Strands during Cellular Delivery. ACS Nano. 2008;2:2023–2028. doi: 10.1021/nn800325a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagalkot VS, Zhang LF, Levy-Nissenbaum E, Jon SY, Kantoff PW, Farokhzad OC. Quantum Dot-Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Jeong YY, Jon SY. A Drug-Loaded Aptamer Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano. 2010;4:3689–3696. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 9.Chen LQ, Xiao SJ, Wu T, Ling J, Li YF, Huang CZ. Aptamer-Based Silver Nanoparticles Used for Intracellular Protein Imaging and Single Nanoparticle Spectral Analysis. J Phys Chem B. 2010;114:3655–3659. doi: 10.1021/jp9104618. [DOI] [PubMed] [Google Scholar]
- 10.He XX, Hai L, Su J, Wang KM, Wu X. One-Pot Synthesis of Sustained-Released Doxorubicin Silica Nanoparticles for Aptamer Targeted Delivery to Tumor Cells. Nanoscale. 2011;3:2936–2942. doi: 10.1039/c0nr00913j. [DOI] [PubMed] [Google Scholar]
- 11.Kim YG, Cao ZH, Tan WH. Molecular Assembly for High-Performance Bivalent Nucleic Acid Inhibitor. Proc Natl Acad Sci U S A. 2008;105:5664–5669. doi: 10.1073/pnas.0711803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MaHam AH, Tang ZW, Wu H, Wang J, Lin YH. Protein-Based Nanomedicine Platforms for Drug Delivery. Small. 2009;5:1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, Stuhlmann H. Viral Nanoparticles as Tools for Intravital Vascular Imaging. Nat Med. 2006;12:354–360. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Raja KS, Janda KD, Lin T, Finn MG. Blue Fluorescent Antibodies as Reporters of Steric Accessibility in Virtus Conjugates. Bioconjug Chem. 2003;14:38–43. doi: 10.1021/bc025587g. [DOI] [PubMed] [Google Scholar]
- 15.Uchida M, Flenniken ML, Allen M, Willits DA, Crowley BE, Brumfield S, Willis AF, Jackiw L, Jutila M, Young MJ, et al. Targeting of Cancer Cells with Ferrimagnetic Ferrition Cage Nanoparticles. J Am Chem Soc. 2006;128:16626–16633. doi: 10.1021/ja0655690. [DOI] [PubMed] [Google Scholar]
- 16.Aime S, Frullano L, Geninatti Crich S. Compartmentalization of a Gadolinium Complex in The Apoferritin Cavity: A Route to Obtain High Relaxibity Contrast Agents for Magnetic Resonance Imaging. Angew Chem Int Ed. 2002;41:1017–1019. doi: 10.1002/1521-3773(20020315)41:6<1017::aid-anie1017>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Lee TK, Sokoloski TD, Royer GP. Serum Albumin Beads: An Injectable, Biodegradable System for the Sustained Release of Drugs. Science. 1981;213:233–235. doi: 10.1126/science.6787705. [DOI] [PubMed] [Google Scholar]
- 18.Kratz F. Albumin as a Drug Carrer: Design of Prodrugs, Drug Conjugates and Nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Metzmacher I, Radu F, Bause M, Knabner P, Friess W. A Model Describing the Effect of Enzymatic Degradation on Drug Release from Collagen Minirods. Eur J Pharm Biopharm. 2007;67:349–360. doi: 10.1016/j.ejpb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Lau JL, Baksh MM, Fiedler JD, Brown SD, Kussrow A, Bornhop DJ, Ordoukhanian P, Finn MG. Evolution and Protein Packaging of Small Molecule RNA Aptamers. ACS Nano. 2011 doi: 10.1021/nn2006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green NK, Herbert CW, Hale SJ, Hale AB, Mautner R, Harkins T, Ulbrich K, Fisher KD, Seymoue LW. Extended Plasma Circulation Time and Decreased Toxicity of Polymer-Coated Adenovirus. Gene Ther. 2004;11:1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 22.Schiedner G, Clemens PR, Volpers C, Kochanek S. High-Capacity “Gutless” Adenoviral Vectors: Technical Aspects and Applications. In: Curiel DT, Douglas JT, editors. Adenoviral Vectors for Gene Therapy. Academic Press; New York: 2002. pp. 429–442. [Google Scholar]


