Abstract
The combination of aptamers with novel nanomaterials, including nanomaterial-based aptamer bioconjugates. has attracted considerable interest and has led to a wide variety of applications. In this review, we discuss how a variety of nanomaterials, including gold, silica and magnetic nanoparticles, as well as carbon nanotubes, hydrogels, liposomes and micelles, have been used to functionalize aptamers for a variety of applications. These aptamer functionalized materials have led to advances in amplified biosensing, cancer cell-specific recognition, high-efficiency separation, and targeted drug delivery.
Introduction
With potential applications in bioanalysis and biomedicine, nanomaterial-based technologies are making it possible to detect and diagnose cancer and infectious diseases in their early stages, and have also contributed to drug discovery, drug delivery and gene/protein delivery. The use of nanomaterials in medicine implies the development of materials and devices designed to interact with the body at sub-cellular scales with a high degree of specificity. Thus, such materials and devices may be potentially translated into targeted cellular and tissue-specific clinical applications aimed at maximal therapeutic benefit with very limited adverse effects.
The past few years have witnessed many advances in the synthesis and characterization of a variety of nanomaterials, such as metallic, silica, magnetic, hydrogel and polymeric nanoparticles and carbon nanotubes. 1,2 These nanomaterials normally have a large surface area coupled with unique size and shape, as well as composition-dependent physical and chemical properties, including surface plasmon resonance (SPR), fluorescence, magnetism, and/or loading ability. 3-5 Nanomaterial modification is the key to target specificity. By combining the inherent features of nanomaterials with the specific recognition ability of aptamers, a range of nanomaterial- aptamer conjugates have proven their utility in multiple areas. 6,7
Aptamers are single-stranded oligonucleotides, DNA or RNA, with the ability to bind to non-nucleic acid target molecules, such as peptides, proteins, drugs, organic and inorganic molecules, or even whole cells, with high affinity and specificity.8-12 They are isolated and chemically synthesized from 1012 –1015 combinatorial oligonucleotide libraries by a process known as in vitro systematic evolution of ligands by exponential enrichment (SELEX). 13,14 Over multiple rounds of selection (generally 6–18 rounds), quite large populations (>1013 different sequences) can be sieved, and the few nucleic acid species with specificity to the target can be isolated.
Aptamers can be adapted to perform a variety of functions. There are two major directions of their application: 1) Based on their specific binding pockets for the target molecule, aptamers possess high potential in biomedical applications such as drug delivery and development of new therapeutic systems. For example, Macugen, an antivascular endothelial growth factor aptamer, was approved by the U.S. FDA for the treatment of age-related macular degeneration and is now used clinically. 15,16 2) The properties of aptamers allow them to play a vital role in the development of novel biosensors. Once an aptamer sequence is identified, it can be synthesized with high purity and reproducibility at relatively low cost. Compared to antibodies, aptamers can be chemically modified with relative ease by various chemical tags, allowing them to be monitored, controlled and immobilized to various solid supports. 17
This review presents an overview of recent developments in aptamer nanomaterial hybrids, as well as their application in biosensors, specific recognition scenarios, and drug delivery, before concluding with a look to the future of nanomaterials-based aptamer bioconjugates.
Gold nanomaterials
It is well known that gold nanomaterials have unusual optical and electronic properties, high stability and biological compatibility, controllable morphology and size dispersion, and easy surface functionalization. 18-22 from the standpoints of engineering and application, aptamer-conjugated gold nanomaterials provide a powerful platform to facilitate targeted recognition, detection, and therapy, as reviewed below.
Gold nanoparticles (AuNPs) used as a colorimetric reporter rely on their unique SPR property, which causes color changes that result from both scattering and electronic dipole-dipole coupling between neighboring particles. 23, 24 Dispersed AuNPs having interparticle distances substantially greater than their average particle diameter appear red, whereas the color of the aggregates changes to purple as the interparticle distance drops below the average particle diameter. 25,26 Based on this principle, there are two general types of target-induced colorimetric assays in homogeneous solution using oligonucleotide-modified AuNPs: assembly and disassembly. 27-29
In assembly assays, the color of the AuNPs solution changes from red (dispersed particles) to purple (aggregates). A classical work from the Mirkin group employed this assembly assay to detect DNA. Two designed pieces of DNA, which were each complementary to part of the target DNA, were immobilized onto the surfaces of the AuNPs. Target DNA acted as a crosslinker, leading to aggregation of the AuNPs. 26 With the introduction of aptamers, many more kinds of analytes can be monitored using this platform, but on the basis of different mechanisms. For example, Huang et al. used aptamer-modified AuNPs for a highly specific sensing system for platelet-derived growth factors (PDGFs) and platelet-derived growth factor receptors (PDGFR) with detection limits of 3.2 nM. 30 The Apt-AuNPs solutions changed to purple in the presence of PDGFs (<400 nM), indicating that PDGF molecules acted as bridges linking Apt-AuNPs together, with the aptamer binding to PDGF in a 2:1 fashion.31
In addition to interparticle cross-linking (or bridging) mechanisms, assembly assays can take advantage of a number of other factors, such as surface charge. 32 One novel biosensing assay involving aggregation of AuNPs induced by the loss (or screening) of surface charges was reported by Zhao et al. 33 In this system, aptamer folding and unfolding on the surface of AuNPs were controlled by the addition of adenosine, K+, adenosine deaminase, and its inhibitors, in addition to changes in salt concentration. Such control made it possible to obtain distinct stages of AuNP aggregation and redispersion and to detect the substrates using specific color changes. 34
In the reverse process, disassembly assays are accompanied by color changes from purple (aggregates) to red (dispersed particles). For example, Liu et al. designed oligonucleotide-modified AuNPs that were first cross-linked by an aptamer sequence to form aggregates. Upon the addition of a target of interest, the aptamer underwent a structural switch that caused the dissociation of the AuNPs aggregates. 35
Apt-AuNPs have been incorporated into biosensors that can be used for any analyte for which an appropriate aptamer is available. Thus, in addition to the detection of ions, genes, and proteins, the surface enhancement effect of AuNPs has also been utilized for the study of cancer cells.36,37 For example, Medley et al. constructed a colorimetric assay for the direct detection of cancer cells. 38 Apt-AuNPs were targeted to assemble on the surface of a specific type of cancer cell through the recognition of the aptamer to its target on the cell membrane surface, as shown in the schematic in Fig. 1A. The assembly of the Apt-AuNPs around the cell surface causes a shift in the extinction spectra of the particles when the AuNPs are in sufficient proximity for their surface plasmon resonances to overlap (Fig. 1B). The cell-based gold structure can very efficiently interact with light and exhibits significantly increased scattering and absorption coefficients compared to individual nanoparticles, which have a weak interaction with light because of their small size. By measuring spectra of AuNPs of different sizes, the 50-nm AuNPs had the greatest measurable red shift and absorbance past 600 nm. The detection limit of the target cells was calculated to be 90 cells by spectroscopic detection and 1000 cells by the naked eye in 300 μL phosphate buffer.
Fig. 1.

(A) Schematic representation of the ACGNP-based colorimetric assay. (B) Plots depicting the absorption spectra obtained for various samples analyzed using ACGNPs. The spectra illustrate the differences in spectral characteristics observed after the ACGNPs bind to the target cells. (Adapted from Ref. 38)
Apt-AuNPs have also been adapted for heterogeneous assays. Wang et al. developed a dot-blot assay for the detection of thrombin based on Apt–AuNP conjugates. They first immobilized protein on the membrane. A color change from colorless to red was produced when the Apt-AuNPs bound to the active site of the protein. The aptasensor could be observed by the naked eye and had a detection limit of 14 pM with silver enhancement. 39 In another application, Liu et al. utilized a pair of aptamers capable of specifically binding Ramos cells for a strip-based assay. A thiolated aptamer (thiol-TD05) was immobilized on the Au-NPs, and a biotinylated aptamer (biotin-TE02) was immobilized on the strip's test zone. When a sample solution containing Ramos cells was applied to the sample pad, the solution migrated by capillary action past the conjugate pad, and then rehydrated the Apt-.AuNP conjugates. Ramos cells interacted with Apt-AuNPs and continued migrating along the strip until captured on the test zone by a second reaction between Ramos cells and the immobilized TE02 aptamers. The accumulation of Au-NPs on the test zone was visualized as a characteristic red band. Under optimal conditions and 80 μL sample loading volume, the strip was capable of detecting a minimum of 4000 Ramos cells without instrumentation (visual judgment) and 800 Ramos cells with a portable strip reader within 15 min.40
Properties and applications of colloidal AuNPs have a close relationship with their shapes. 41 For example, rod-like particles have both transverse and longitudinal extinction peaks, and anisotropy of the shape affects particle self-assembly. Recently, Lu et al. captured the signal from Apt-AuNP-based cell detection with the two-photon scattering (TPS) technique, which resulted in the highly selective and sensitive detection of breast cancer SK-BR-3 cell lines at a 100 cells/mL level using multifunctional (monoclonal anti-HER2/c-erb-2 antibody and S6 RNA aptamer) AuNP conjugates. Instead of spherical AuNPs, oval-shaped AuNPs were introduced. As the particle aspect ratio increased, TPS intensity change became higher, leading to improved sensitivity. 42
In addition to their role as a signal producer, gold nanorods (NRs) have been utilized as a platform for multiple aptamer immobilization to detect cancer cells. Through covalent linkages of fluorophore-labeled aptamers on the nanorod surface, Huang et al. 43 were able to increase the binding affinity of otherwise weak-binding aptamers by ∼26-fold compared to that of a single aptamer. It was found that ∼80 aptamer molecules were bound on each NR. Because the cell surface is much larger than the aggregate of nanoparticles and, hence, contains more binding sites, the performance of molecular recognition could be improved by synergy, in which the combined effect of two or more like-acting components exceeds the sum of the individual effects. In this case, multiple aptamers on each NR contributed to the enhanced binding affinity. In addition to targeting cells, aptamer-modified anisotropic AuNPs having different aspect ratios yield a large absorption cross section in the near-infrared (NIR) region, which has allowed the development of a novel photothermal transformer for therapy. 44 This is illustrated in another work, in which 8.5 × 10 4 W/m2 laser exposure was used to induce 93 (±11)% cell death of Apt-NRs-labeled cells. As a consequence of the selective recognition properties of aptamers, about 50 (±1) % of target (CEM) cells were damaged after laser irradiation, while 87 (±1) % of control (NB-4) cells remained intact in a suspension cell mixture.
Silica nanoparticles
In 1968, Stöber et al. reported a pioneering method for the synthesis of mono-dispersed silica nanoparticles by using the hydrolysis and condensation of silicon alkoxides in alcoholic solutions in the presence of water and using ammonia as a catalyst. 45 By simple variation in the proportions of the components, the particle diameter could be tailored from 50 nm to 2 mm. As an alternative to the Stöber method, Arriagada and Osseo-Asare developed a technique for the growth of more uniform and smaller-sized silica nanoparticles via water-in-oil reverse microemulsion (W/O) techniques. 46 Another class of silica nanomaterials, mesoporous silica nanoparticles, can be easily synthesized by using surfactant-templating methods. 47
Based on these convenient synthesis strategies and other outstanding advantages, silica nanoparticles have become an important platform in biomedical applications and bioanalysis. First, the high density of silica nanoparticles facilitates easy separation of silica nanoparticles during the synthesis, modification, and detection steps through centrifugation. 48 Second, a large number of fluorophores can be encapsulated inside single silica nanoparticles, leading to intense fluorescence when excited. Therefore, compared with single fluorophore dyes, the fluorescence signal is greatly enhanced, making it possible to push the limit of detection to lower concentrations. 49 Third, nonporous silica assures that both fluorophores and cargos are well protected from environmental oxygen when doped inside the silica network. As a result, fluorescent nanoparticles (FNPs) are highly photo-stable compared to naked dye molecules, and can be used in a wide variety of applications including photodynamic therapy and biomedical imaging. Mesoporous nanoparticles have recently been engineered into different structures, such as hollow mesoporous spheres, for the purpose of drug delivery and oxygen sensing (diagnostics). 50, 51 Fourth, the silica surface provides a universally biocompatible and versatile substrate for biomolecule immobilization, which is required for further utilization in a biosystem, e.g., for targeted recognition and delivery. Following typical DNA-silica surface conjugate chemistry,52 aptamer immobilization on silica nanoparticles has been well developed for various applications. Examples below are introduced to briefly review recent applications in protein and cell detection, as well as in drug delivery.
Wang et al. developed a sandwich assay for optical detection of thrombin and lysosome in biological media with high sensitivity and selectivity by using the signal amplification of a cationic conjugated polymer (CCP) and the separation of aptamer-functionalized silica nanoparticles (∼100 nm in diameter). 53 In the presence of the target protein, fluorescein-labeled secondary thrombin-binding aptamer is assembled on the primary aptamer-immobilized silica nanoparticles, leading to FNPs that can be further brightened upon energy transfer from a CCP to fluorescein. Under optimized conditions, the thrombin detection limit reached to 1.06 nM. In this application, aptamer-immobilized nanoparticles play two major roles. First, by providing a substrate to anchor the primary aptamer, it is easier to monitor the signal from aptamer-target binding. Second, such immobilization allows the analyte to be “extracted” from protein mixtures through centrifugation and washing.
A dye-doped silica nanoparticles-based method for sensitive and rapid detection of cancer cells has been developed, and it increases detection sensitivity in flow cytometry analyses between 10- and 100-fold compared to standard methods. 54 To fulfill specific recognition, the biotinylated aptamers are immobilized onto the surfaces of neutravidin-coated FNPs. The FNPs are then functionalized with polyethylene glycol (PEG) to prevent nonspecific interactions with neutravidin and to allow universal binding with biotinylated molecules. These aptamer-conjugated silica NPs (Apt-FNPs) have demonstrated low background signal, high signal enhancement, and efficient functionalization. As shown in Figure 2, multiplexed cancer cells can also be detected by employing the combination of fluorescence resonance energy transfer (FRET) of FNPs and the conjugation of different aptamers.55 Single-dye-, dual-dye-, and triple-dye-doped silica nanoparticles were prepared according to this method. 56 These dyes possess appropriately overlapping excitation and emission spectra, so that efficient energy transfer among them can occur. Thus, by using a single wavelength excitation, but varying the ratios of three dyes co-encapsulated into the silica nanoparticles, different emission signatures can be obtained. Zhu et al. developed an efficient cell targeting and intracellular controlled-release drug delivery system based on a conjugate consisting of mesoporous silica nanoparticles and aptamers. These mesoporous silica nanoparticles coated with polyelectrolyte multilayers served as drug containers, providing high surface area, high loading efficiency and controlled drug release. After modification by aptamers, this type of delivery vehicle could be used as a promising drug delivery system for specific intracellular delivery. 57
Fig. 2.
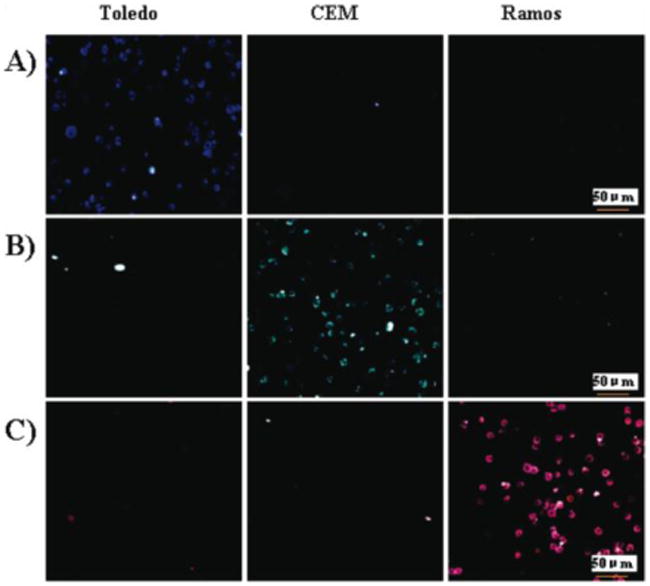
Confocal microscopy images of individual NP-aptamer conjugates with three different cells (Toledo, CEM, and Ramos): (A) NP (FAM)-T1, (B) NP (FAM-R6G)-sgc8, and (C) NP (FAM-R6G- ROX)-TDO5. (Adapted from Ref. 55)
Magnetic nanoparticles
The majority of magnetic nanoparticle (MNPs) systems utilize inorganic nanocrystals as their magnetic cores. The composition of these inorganic nanocrystals ranges from metals and alloys to metal oxides. Among the metal oxide compounds, superparamagnetic iron oxide nanoparticles, including magnetite (Fe3O4) and maghemite (g-Fe2O3), have been a major research focus during the past decade. 58 The surfaces of these particles can be modified through the creation of a few atomic layers of organic polymer or inorganic metallic (e.g., gold) or oxide surfaces (e.g., silica or alumina), suitable for further functionalization by the attachment of various bioactive molecules. 59 The biofunctional MNPs exhibit two features: specificity and magnetism. As such, they can interact with an external magnetic field and be positioned in a specific area, facilitating biomedical separation, magnetic resonance imaging (MRI), 60 drug delivery 61 and magnetic fluid hyperthermia therapy. 62
Aptamer-functionalized MNPs (Apt-MNPs) have been used for small molecule and protein detection. 63 For example, Yigit et al. combined superparamagnetic iron oxide nanoparticles and aptamers for the detection of human R-thrombin protein. The assembly of the aptamer-functionalized nanoparticles in the presence of thrombin could induce a decrease of T2 relaxation time. A detectable change in MRI signal is observed with 25 nM thrombin in human serum. Such changes were not observed with control analytes, streptavidin, or bovine serum albumin, or with inactive aptamer-functionalized nanoparticles.
Apt-MNPs have been formulated to meet the specific requirements of therapy and drug delivery, as well as magnetic imaging at the cellular level. 64 For targeting PSMA-expressing prostate cancer cells, Wang et al., assembled a bioconjugate nanoparticle with an N-terminal A10 aptamer and a 57-bp nuclease-stabilized 2′-fluoropyrimidine RNA molecule modified with C18-amine at the 3′-end,. Based on previous knowledge, 65 the GC parts in this aptamer can also act as a carrier for Doxorubicin (Dox). To fulfill the demands of this function, the design consists of MNPs coated with a carboxylic acid-PEG-derived, anti-biofouling polymer, which acts as both an MR contrast agent and as a carrier for Dox, a chemotherapeutic agent that is intercalated in the aptamer and complexed with the modified MNPs through charge interactions. A dramatic decrease in T1 and T2 (T1: 1939 ±116 to 263±23 ms; T2: 104.2±1.4 to 26.6±0.4 ms) was observed, indicating that the Apt-MNPs have the potential for highly sensitive cancer cell detection. Meanwhile, Dox decreased target cell viability after a 3-hour incubation of cells with the conjugates.
The Tan group has demonstrated early diagnosis of acute leukemia cells by using Apt-MNPs and aptamer-conjugated FNPs (Apt-FNPs). 66 An 88-base oligonucleotide sequence with specific binding properties (Kd=5 nM for CCRF-CEM acute leukemia cells) was attached to MNPs and [Ru(bipy)3]2+-doped FNPs in order to develop a specific platform for collecting and imaging intact target leukemia cells from mixed cell and whole blood samples. 67 In addition to enabling selective target extraction, Apt-MNP-based sorting eliminated the need for centrifugation of cell samples and pre-sample cleanup. As a result, the collection of unwanted aggregates of NPs and unbound materials from target cell extractions was eliminated, and a reduced background was observed. Following the magnetic separation step, Apt-FNPs were added to produce an enhanced fluorescence signal for detection. The assay based on two kinds of aptamer-conjugated particles allows selective cell collection from complex samples and blood samples within a short incubation time and with sensitive detection.
Aptamers for different cells can be combined into a two-particle assay platform for multiple cell extraction and detection. As shown in Figure 3, three sets of MNPs were respectively conjugated to aptamers for three different types of cancer cells (CCRF-CEM acute leukemia cells and cell lines for Burkitt's lymphoma (Ramos) and non-Hodgkin's B cell lymphoma (Toledo)). After cell capture from blood or FBS, FNPs conjugated to the same aptamers were added for detection and imaging. 68 Song et al. also designed a two-particle assay for rapid and ultra-sensitive detection of adenosine monophosphate (AMP). Taking advantage of separation plus signal amplification, this method allows a direct detection limit of 0.1 μM, corresponding to 3000-fold greater sensitivity compared to the colorimetric assay with AuNPs. 69 Recently, two nanoparticles were integrated into a single system (a cobalt–ferrite nanoparticle surrounded by fluorescent rhodamine within a silica shell matrix) with the AS1411 aptamer (MF-AS1411) on the surface. This system allowed multimodal targeting and imaging of nucleolin, a cellular membrane protein highly expressed in cancer. 70
Fig. 3.
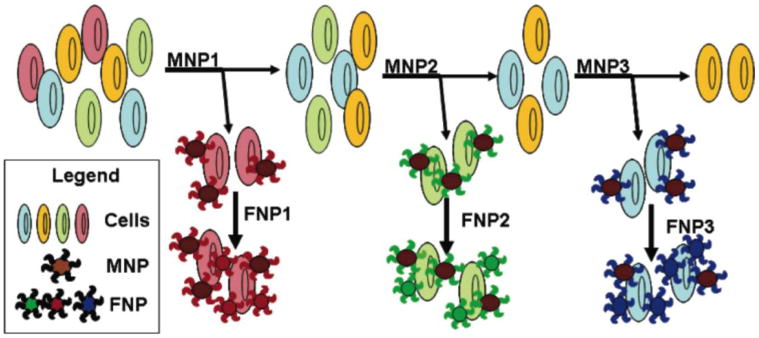
Schematic representation of the multiple extraction procedure with the MNPs being added and extracted stepwise and the corresponding FNPs being added post-magnetic extraction of cell samples. (Adapted from Ref. 66)
Single-walled carbon nanotubes
Single-walled carbon nanotubes (SWCNTs) have a high aspect ratio, high surface area, and excellent material properties, including electrical and thermal conductivity and mechanical strength.71 Such unique properties offer a broad spectrum of opportunities and application potential in biology and medicine. Here, we focus on applications using SWCNTs as field effect transistors, quenchers or reporters using aptamers as recognition elements.
SWCNTs have been extensively studied as transducer elements of biosensors because they meet the important requirements of an efficient biosensor: excellent electrical properties and a large surface-to-volume ratio. By applying a voltage to a gate electrode, the semiconducting single-walled carbon nanotubes can be switched from a conducting to an insulating state in a field-effect transistor. The first successful demonstration of a single walled carbon nanotube field effect transistor (SWCNT-FET) biosensor using aptamers as an alternative to protein-based sensing elements was described by Hye-Mi So et al. They pretreated a carbon nanotube transistor with carbodiimidazole-activated Tween 20 (CDI-Tween), and covalently immobilized thrombin aptamers onto the side wall. By measuring real-time conductance from the aptamer conjugated SWCNT-FET, the lowest detection limit achieved in this work was around 10 nM. The authors pointed out that the greatest merit of using aptamers in FET-type sensors lies in their small size. Since aptamers (1-2 nm) are much smaller than protein antibodies, it is possible that the aptamer-protein binding events can occur inside the electrical double layer in millimolar salt concentrations. 72 By comparing the detection ability of immunoglobulin E (IgE), Kenzo Maehashi further demonstrated the performance of an aptamer-conjugated SWNT-FET, which provided better results than those obtained using IgE−mAb-modified SWNT-FET under similar conditions.73 The aptamer functionalized SWNT-FET can also be used for the detection of living microorganisms in real time at ultralow concentrations. 74
Photophysical studies have found that SWCNTs can act as quenchers for fluorophores. 75 The Tan group found that a dye-labeled hairpin probe interacted with SWCNTs via non-covalent pi-stacking interactions, resulting in fluorescence quenching of the dye. 76 Upon hybridization of the probe to the target DNA, the dye was lifted from the SWCNT surface and the fluorescence signal was restored. This phenomenon has been further employed to a singlet oxygen generation (SOG) design based on a similar underlying photophysical mechanism. As shown in Figure 4, a photosensitizer, Chlorin e6 (Ce6), was covalently attached to a thrombin aptamer that wrapped onto the SWCNT surface. In the absence of a target, close proximity of the photosensitizer to the SWCNT surface caused efficient quenching of SOG. However, the conformation of the probe was altered upon target binding. Thus, in the presence of a target, the binding between the aptamer and target molecule disturbed the interaction between the aptamer and SWCNT. The DNA dissociated from the SWCNT surface, resulting in a restoration of SOG for photodynamic therapy (PDT) applications. In this general SOG regulation design, aptamers serve as a target response switch, and SWNTs can protect recognition ligands, such as ssDNA aptamers, from enzymatic digestion or degradation in the biological environment. 77
Fig. 4.

Schematic of an aptamer-photosensitizer-SWNT complex and the regulation of SOG upon target binding: (I) AP and SWNTs were mixed together to form an AP-SWNT complex. The ssDNA aptamer is wrapped on the surface of SWNTs, bringing the photosensitizer close to the SWNTs to quench SOG. (II) Target binding with aptamers disturbs the interaction between AP and SWNTs, resulting in the restoration of SOG. (Adapted from Ref. 76)
Utilizing the near-infrared absorption and photoluminescence properties of SWCNTs, another work reported a novel NIR optical protein assay based on reversible dissolution and aggregation of aptamer-wrapped SWCNTs. 78 The photoluminescence of SWNTs is observed only when they are individually dispersed. Otherwise, aggregation of individual nanotubes into bundles quenches the fluorescence through interactions with metallic tubes and substantially broadens the absorption spectra. 79 The addition of a thrombin aptamer contributed to the dissolution of SWCNTs because of the pi-stacking interactions between the aptamer bases and the SWCNT sidewalls. This step resulted in a homogeneous black solution with sharp peaks in the absorption spectrum. The subsequent introduction of thrombin released the aptamer from the SWCNTs. An observed fluorescence quenching indicated the aggregation of the SWCNTs. The fluorescence signal of the Apt–SWCNT was expected to correlate with the recognition of the target protein, thus providing a label-free and separation-free optical method for thrombin with a 0.1 nM detection limit. Since NIR photoluminescence lies within the “biological window” (700–1300 nm), where absorption and autofluorescence by tissues, blood, and water are minimized, the design is appealing for analysis of complicated sample matrices.
Hydrogels
Hydrogels are crosslinked hydrophilic polymer structures that can hold large amounts of water or biological fluids in their pore spaces. As one of the newest classes of polymer-based systems, target-responsive hydrogels have found numerous biomedical and pharmaceutical applications.80,81 Polyacrylamide/acrylate is a copolymer of acrylamide and acrylic acid (normally supplied as the sodium salt). It has been reported that acrydite-modified oligonucleotides can form into hydrogels by the introduction of proper complementary DNA as a cross-linker. 82 He et al. reported an aptamer-based reversible DNA- induced hydrogel system for molecular recognition and separation. Two kinds of acrydite-modified DNA monomers were copolymerized with acrylamide monomers to obtain the linear polyacrylamide polymers grafted with strands G1 and G2. A crosslinker DNA strand A was designed to include three functional domains: 1) segments that are complementary to G1 and G2, respectively, for hydrogel formation; 2) a toehold segment for the initiation of DNA displacement to enable a reversible hydrogel transition; and 3) an aptamer segment (e.g., adenosine aptamer). By target recognition of aptamer in strand A, the DNA hydrogel system acts as a molecular hook to fish out specific molecules in a pool of different molecules. A subsequent target separation step was realized by first washing away the nonspecific targets from the hydrogel and dissolving the hydrogel by adding a strand D, fully complementary to the crosslinker strand A, to the system.83
Besides performing recognition and separation, aptamer-conjugated hydrogels have recently been shown to have potential in developing a sustained-release system. PDGF aptamers with target protein were covalently conjugated into a polyacrylamide gel network. The results of the aptamer-conjugated hydrogels showed that the initial 24 h release was significantly decreased from 70% to 10%, when compared to the physical mixture of native hydrogel with aptamers. 84
Aptamer-incorporated hydrogels have also been considered as a novel signal transduction method, which broadens traditional fluorescence-based assays to visual detection.85 Visual detection is an increasingly attractive method for many fields requiring low cost, rapid turn-around and simplicity, because both qualitative and semi-quantitative assessment can be performed in real time without any advanced or complicated instrumentation. As the first demonstration, the sol-to-gel and gel-to-sol transitions in adenosine-responsive polyacrylamide were examined. Briefly, two acrydite-modified oligonucleotides, Strand A and Strand B, were separately copolymerized with acrylamide (4%, w/v) and incorporated into polyacrylamide chains. After mixing strand A and strand B solutions in stoichiometric concentrations, a gelatin formed by the introduction of a LinkerAdap containing three segments. The first segment could hybridize with Strand A. The second segment could hybridize with the last five nucleotides of Strand B. The third segment, which was the aptamer sequence for adenosine, could hybridize with the seven nucleotides of Strand B. Therefore, this gelatin could be dissolved and reversed to its fluid state by adding adenosine to remove the LinkerAdap. Water-soluble, citrate-modified 13-nm AuNPs were employed to prove the capability of controllable release by this approach.
In an extension to this design, a signal amplification mechanism was designed into the gel-sol transition process.86 As shown in Figure 5, a cocaine aptamer was used as a cross-linker to form the gel state. First, since amylase was added prior to the addition of the aptamer, the enzyme was trapped inside the 3-D network of the hydrogel. The substrate, amylose, was located outside of the network as the blue amylase-iodine complex. Because amylase can break down amylose into maltose, which is colorless in the presence of iodine, the physical separation of these two kinds of molecules was easily observed with the naked eye. When cocaine was added, the aptamer was removed from the network and the gel dissolved, releasing the enzyme. In the presence of amylase, the blue color disappeared. Based on this aptamer cross-linked hydrogel, 2 μM of cocaine was detected by visual observation. The incorporated amylose–I2–amylase system amplified the signal to a sufficiently distinguishable color change for visual detection.
Fig. 5.

Working principle and photograph of DNA cross-linked hydrogel for signal amplification and visual detection. In the absence of target, (A) shows enzyme (amylase) trapped inside gel on the bottom with substrate (amylose) separated on the top as a solution of the blue amylose–I2 complex. (B) binding of aptamer with target (cocaine) induces gel dissolution and enzymatic reaction making the solution colorless. (Adapted from Ref. 86)
Liposomes and Micelles
Liposomes, as the first proposed and tested drug delivery system, were explored four decades ago.87 In 1995, Gregoriadis proposed three criteria to develop an effective system based on liposomes: quantitative retention of drugs by the carrier until reaching its destination, control over the rate of clearance of vesicles from the circulatory system (or other compartments of the body when the drug is locally administered), and preferential, or at least sufficient, uptake by the target. 88 With the development of construction techniques and studies of composition, structure and function, the first two points have been widely and well addressed.89,90 Especially, long-circulating liposomes, such as polyethylene glycol (PEG)-coated liposomes, also known as STEALTHR liposomes, 91 tend to accumulate in tumors as a result of increased microvascular permeability and defective lymphatic drainage, a process also referred to as the enhanced permeability and retention (EPR) effect. 92 With respect to the third criterion, molecular recognition moieties, such as antibodies, folate, peptides and nucleic acids, for specific targets have been incorporated into liposomes. 93-95 Alberto et al. constructed both folate-targeted STEALTHR liposomal doxorubicin (FTL-Dox) and cisplatin (FTL-cisplatin), proving that folate targeting enhanced the cytotoxicity of liposomal drugs against FR-expressing tumor cells without inducing extra toxicity by folate modification. 96 To pursue more specific targeting, functionalization of liposomes with aptamers was introduced 109 In this early work, the dialkylglycerol (DAG) phosphoramidite containing a tetraethylene glycol spacer was synthesized and introduced at the 5′-end of the vascular endothelial growth factor (VEGF) aptamer to make DAG-NX213, which was further incorporated into the liposome bilayer. By using the high affinity and specificity of aptamers to guide liposomal contents to VEGF, a potent inducer of angiogenesis, this work demonstrated that: 1) the plasma residence time of the aptamer-modified liposomes is considerably longer than that of aptamer alone; and 2) this formulation observed enhanced inhibitory activity toward VEGF-induced endothelial cell proliferation in vitro, compared with the free aptamer. Recently, Cao et al. and Kang et al. have successfully extended the application of aptamer-functionalized liposomes to the cellular level by incorporation of cell-based aptamers and by simplification of the aptamer-modified liposome synthesis method. 97, 98 Each liposome had approximately 250 aptamers tethered to its surface to facilitate target binding, and approximately several thousand FITC-Dextran (FD) molecules (drug proxy) loaded inside.93 With a 30-minute incubation period, the aptamer-liposome conjugates could specifically bind with target cells and release the loaded model drug. In the two works, the targeted drug delivery was guaranteed either by sgc8 aptamer binding with its target CEM cells or AS1411 aptamer for breast cancer.
Another promising carrier system involved polymeric micelles, which were originally composed of an amphiphilic block.99,100 The self-assembly of amphiphilic block copolymers in water is based on non-polar and hydrophobic interactions between the lipophilic core-forming polymer chains. The process is concomitantly driven by a gain in entropy of the solvent molecules as the hydrophobic components withdraw from the aqueous media. 101 More recently, micelles constructed as hybrids from hydrophilic oligonucleotides and hydrophobic polymers have drawn close attention. 102,103 Theoretically, when the oligonucleotide segment is longer than the lipid core, the shape of the resulting micelles is spherical. Miyamoto et al. described a facile method for naked-eye detection of complex formation of a DNA aptamer with its target molecule as a turbidity change in the micelle dispersion. The polymeric micelle with a dehydrated poly(N-isopropylacrylamide) core surrounded by an ssDNA corona had drastically lower colloidal stability when complementary DNA was added to the dispersion. A sandwich structure was designed containing anchored DNA, its partial complementary DNA, and ATP aptamer. This fully hybrid structure induced aggregation of micelles, while the introduction of the ATP target molecule maintained the micelle dispersion by binding with the aptamer and breaking the double-stranded hybridization. 104
DNA-micelles with a modified recognition molecule comprise a novel delivery system. 105 To increase coupling efficiency between synthetic DNA and the hydrophobic moiety and to obtain uniform size distribution, Liu et al. investigated the construction of well-defined oligonucleotide–micelle structures using a DNA–diacyllipid conjugate. 106 The amphiphilic building block could be divided into three distinct segments. The first segment was a single-stranded DNA aptamer, which was highly hydrophilic. The second segment was a pyrene molecule, which acted as a fluorescence reporter. 107 The highly hydrophobic third segment was composed of two C18 hydrocarbon tails. The novelty of this design lies in the introduction of phosphoramidite chemistry which allowed synthesis of a pyrene, a diacyllipid and an aptamer phosphoramidite in a fully automated fashion, which greatly increased the reaction yield (estimated 60% lipid coupling yield). Results of fluorescence, agarose gel electrophoresis, AFM and dynamic light scattering (DLS) indicated that DNA amphiphiles could self-assemble into well-defined, homogeneous micelles with a desired size and uniform size distribution.
Efficient targeting and delivery were investigated in another recent work. The Tan group proved that aptamer-functionalized micelles (Apt-micelles) enhanced the binding ability of the aptamer moiety at physiological temperature (37 °C), even though the corresponding free aptamer had lost its binding ability under the same conditions. The merits of Apt–micelles also included greatly improved binding affinity, low koff once on the cell membrane, rapid targeting ability, high sensitivity, and effective drug delivery. These features resulted from a specific interaction-induced nonspecific insertion process which, in turn, led to the fusion between Apt-micelles and the cell membrane. Moreover, as shown in Figure 6, by flushing the Apt–micelles through a channel that included immobilized tumor cells on its surface, a system mimicking drug delivery in the blood system, the dynamic specificity of Apt-micelles in flow channel systems was demonstrated. 108
Fig. 6.

Simplified flow channel response to cell-staining assay. (A). Stepwise immobilization scheme of the flow channel. Representative images of the bright field and fluorescent images of control cells (CCRF-CEM) and target cells (Ramos) captured on the flow channel surface incubated with FITC-TDO5-micelle (B), or FITC-library-micelle (C) or free FITC-TDO5 (D) spiked in human whole blood sample under continuous flow at 300 nL/s at 37°C for 5 m. All the scale bars are 100 μm. (Adapted from Ref. 108)
Table 1 provides a summary of the aptamer-conjugated nanomaterial based biosensors described in this review.
Table 1. Aptamer-conjugated nanomaterial based biosensors described in this review.
| Aptamer-conjugated Nanomatrial | Target | Sensing system | Result | Reference |
|---|---|---|---|---|
| AuNPs | PDGFs | Colorimetric assay | 3.2 nM | 30 |
| AuNPs | Cancer cell | Colorimetric assay | 90 in 300 μL | 38 |
| AuNPs with silver enhancement | Thrombin | Dot-blot assay | 14 pM | 39 |
| AuNPs | Cancer cell | Strip-based assay | 800 in 80 μL | 40 |
| AuNPs | Cancer cell | Two-photon scattering | 100 in 1 mL | 42 |
| Silica FNPs | Thrombin | Sandwich florescence assay | 1.06 nM | 53 |
| MNPs | Thrombin | MRI | 25 nM | 63 |
| FNPs and MNPs | AMP | Florescence signal amplification | 0.1 μM | 69 |
| SWCNTs | Thrombin | FET-type conductance assay | 10 nM | 72 |
| SWCNTs | Thrombin | Florescence aasay | 0.1 nM | 79 |
| Hydrogels | Cocaine | Visual detection | 2 μM | 86 |
Conclusions
Aptamers have become increasingly important molecular tools for diagnosis and therapeutics. The studies described in this review demonstrate the broad potential of combining aptamers and nanomaterials in biosensors, bioseparation, targeted drug delivery and cancer cell treatment. There are three major advantages of these bioconjugates: 1) The specificity of aptamers guarantees a targeted detection or binding, the first and most vital step in all kinds of detection and therapy. 2) The ease of aptamer synthesis and modification can facilitate the translation of aptamer functionality into clinical practice. 3) The coupling of different nanomaterial-based amplification platforms and biocompatible loading ability dramatically enhances the intensity of the analytical signal and leads to highly efficient target cell recognition and delivery.
However, the successful realization of aptamer-conjugated nanomaterial strategies requires proper attention to nonspecific adsorption issues that commonly control not only the detectability of biosensor development, but also the toxicity to non-targeted cells in the drug delivery system. Therefore, surface physiochemical characteristics of nanomaterials must be engineered when coupling aptamers for the purpose of reducing nonspecific binding and maintaining the aptamers' active structures. Moreover, proper washing and surface blocking steps should be employed to further optimize the performance of these bioconjugates.
In the future, for the development of aptamer-conjugated nanomaterials, much more attention could be directed in both creating novel nanomaterials and strengthening the performance of the aptamers. For example, a DNA origami box is a nano-sized DNA box that can be locked or opened in response to “keys” made from short strands of DNA. By changing the nature or number of these keys, it should be possible to use these boxes as sensors and drug delivery systems. By functionalization of this DNA box with locked nucleic acid (LNA) based nuclease resistant aptamers, this new generation of powerful aptamer-conjugated nanomaterials could produce more outstanding features.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banerjee S, Wong SS. Nano Letters. 2002;2:195–200. [Google Scholar]
- 2.Lue JT. J Phys Chem Solids. 2001;62:1599–1612. [Google Scholar]
- 3.Pavlov V, Xiao Y, Shlyahovsky B, Willner I. J Am Chem Soc. 2004;126:11768–11769. doi: 10.1021/ja046970u. [DOI] [PubMed] [Google Scholar]
- 4.Slowing, Vivero-Escoto JL, Wu CW, Lin VSY. Adv Drug Deliver Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Salabas EL, Schüth F. Angew Chem Int Ed. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Yang RH, Yang L, Tan WH. ACS Nano. 2009;3:2451–2460. doi: 10.1021/nn9006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadler N, Chi C, Lelie DVD, Gang O. Nanomed. 2010;5:319–334. doi: 10.2217/nnm.10.2. [DOI] [PubMed] [Google Scholar]
- 8.Patel DJ, Suri AK, Jiang L, Fan P, Kumar RA, Nonin S. J Mol Biol. 1997;272:645–664. doi: 10.1006/jmbi.1997.1281. [DOI] [PubMed] [Google Scholar]
- 9.Mairal T, Özalp VC, Sánchez PL, Mir M, Katakis I, O'Sullivan CK. Anal Bioanal Chem. 2008;390:989–1007. doi: 10.1007/s00216-007-1346-4. [DOI] [PubMed] [Google Scholar]
- 10.Jenison RD, Gill SC, Pardi A, Polisky B. Science. 1994;263:1425–1429. doi: 10.1126/science.7510417. [DOI] [PubMed] [Google Scholar]
- 11.Bunka DHJ, Stockley PG. Nature Reviews Microbiol. 2006;4:588–596. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- 12.Sefah K, Tang ZW, Shangguan DH, Chen H, Lopez-Colon D, Li Y, Parekh P, Martin J, Meng L, Phillips JA, Kim YM, Tan WH. Leukemia. 2009;23:235–244. doi: 10.1038/leu.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson DS, Szostak JW. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- 14.Ellington D, Szostak JW. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 15.Gold L. Weinheim. Wiley-VCH; 2006. pp. 461–469. [Google Scholar]
- 16.Bouchard PR, Hutabarat RM, Thompson KM. Annu Rev Pharmacol. 2010;50:237–257. doi: 10.1146/annurev.pharmtox.010909.105547. [DOI] [PubMed] [Google Scholar]
- 17.Balamurugan S, Obubuafo A, Soper SA, Spivak DA. Anal Bioanal Chem. 2008;390:1009–1021. doi: 10.1007/s00216-007-1587-2. [DOI] [PubMed] [Google Scholar]
- 18.Daniel MC, Astruc D. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 19.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 20.Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. Chem Soc Rev. 2008;37:1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 21.Storhoff JJ, Lucas AD, Garimella V, Bao YP, Muller UR. Nat Biotechnol. 2004;22:883–887. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Chem Soc Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 23.Sandrock ML, Foss CA. J Phys Chem B. 1999;103:11398–11406. [Google Scholar]
- 24.Storhoff JJ, Lazarides AA, Mucic RC, Mirkin CA, Letsinger RL, Schatz GC. J Am Chem Soc. 2000;122:4640–4650. [Google Scholar]
- 25.Xia F, Zuo XL, Yang RQ, Xiao Y, Kang D, Vallée-Bélisle A, Gong X, Yuen JD, Hsua BBY, Heegera AJ, Plaxcob KW. Proc Natl Acad Sci USA. 2010;107:10837–10841. doi: 10.1073/pnas.1005632107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 27.Demers LM, Mirkin CA, Mucic RC, Reynolds RA, Letsinger RL, Elghanian R, Viswanadham G. Anal Chem. 2000;72:5535–5541. doi: 10.1021/ac0006627. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Lu Y. J Am Chem Soc. 2003;125:6642–6643. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Chen S, Chien Y, Chang Y, Liao H, Chang C, Jan M, Wang K, Liu C. ChemBioChem. 2005;6:1169–1173. doi: 10.1002/cbic.200500023. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Huang Y, Cao Z, Tan W, Chang H. Anal Chem. 2005;77:5735. doi: 10.1021/ac050957q. [DOI] [PubMed] [Google Scholar]
- 31.Green LS, Jellinek D, Jenison R, Ostman A, Heldin CH, Janjic N. Biochemistry-US. 1996;35:14413–14424. doi: 10.1021/bi961544+. [DOI] [PubMed] [Google Scholar]
- 32.Hunter, R. Oxford University Press, 2004, New York.
- 33.Zhao W, Chiuman W, Lam J, Brook MA, Li Y. Chem Commun. 2007;36:3729–3732. doi: 10.1039/b705335e. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Chiuman W, Lam JCF, McManus SA, Chen W, Cui Y, Pelton R, Brook MA, Li Y. J Am Chem Soc. 2008;130:3610–3618. doi: 10.1021/ja710241b. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Lu Y. Angew Chem Int Ed. 2005;118:96–100. [Google Scholar]
- 36.Lee S, Kim S, Choo J, Shin SY, Lee YH, Choi HY, Ha S, Kang K, Oh CH. Anal Chem. 2007;79:916–922. doi: 10.1021/ac061246a. [DOI] [PubMed] [Google Scholar]
- 37.Huang X, El-Sayed IH, Wei Q, El-Sayed MA. Nano Lett. 2007;7:1591–1597. doi: 10.1021/nl070472c. [DOI] [PubMed] [Google Scholar]
- 38.Medley CD, Smith JE, Tang ZW, Wu YR, Bamrungsap S, Tan WH. Anal Chem. 2008;80:1067–1072. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Li D, Ren W, Liu Z, Dong S, Wang E. Chem Commun. 2008:2520–2522. doi: 10.1039/b801055b. [DOI] [PubMed] [Google Scholar]
- 40.Liu G, Mao X, Phillips JA, Xu H, Tan W, Zeng L. Anal Chem. 2009;81:10013–10018. doi: 10.1021/ac901889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang XH, Jain PK, El-Sayed IH, El-Sayed MA. nanomedicine. 2007;2:681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 42.Lu WT, Arumugam SR, Senapati D, Singh AK, Arbneshi T, Afrin Khan S, Yu H, Ray PC. ACS nano. 2010;4:1739–1749. doi: 10.1021/nn901742q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YF, Chang HT, Tan WH. Anal Chem. 2008;80:567–572. doi: 10.1021/ac702322j. [DOI] [PubMed] [Google Scholar]
- 44.Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan WH. Langmuir. 2008;24:11860–11865. doi: 10.1021/la801969c. [DOI] [PubMed] [Google Scholar]
- 45.Stöber W, Fink A, Bohn E. J Colloid Interface Sci. 1968;26:62–66. [Google Scholar]
- 46.Osseo-Asare K, Arriagada FJ. Colloids Surf. 1990;50:321–339. [Google Scholar]
- 47.Direnzo F, Cambon H, Dutartre R. Microporous Mater. 1997;10:283–286. [Google Scholar]
- 48.Qhobosheane M, Santra S, Zhang P, Tan WH. Analyst. 2001;126:1274–1278. doi: 10.1039/b101489g. [DOI] [PubMed] [Google Scholar]
- 49.Wittenberg NJ, Haynes CL. Nanomed Nanobiotechnol. 2009;1:237–254. doi: 10.1002/wnan.19. [DOI] [PubMed] [Google Scholar]
- 50.Vallet-Regí M, Balas F, Arcos D. Angew Chem Int Ed. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 51.Cheng SH, Lee CH, Yang CS, Tseng FG, Mou CY, Lo LW. J Mater Chem. 2009;19:1252–1257. [Google Scholar]
- 52.Hilliard LR, Zhao XJ, Tan WH. Anal Chim Acta. 2002;470:51–56. [Google Scholar]
- 53.Wang YY, Liu B. Langmuir. 2009;25:12787–12793. doi: 10.1021/la901703p. [DOI] [PubMed] [Google Scholar]
- 54.Estévez MC, O'Donoghue MB, Chen XL, Tan WH. Nano Res. 2009;2:448–461. [Google Scholar]
- 55.Chen XL, Estévez MC, Zhu Z, Huang YF, Chen Y, Wang L, Tan WH. Anal Chem. 2009;81:7009–7014. doi: 10.1021/ac9011073. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Tan WH. Nano Lett. 2006;6:84–88. doi: 10.1021/nl052105b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu CL, Song XY, Zhou WH, Yang HH, Wen YH, Wang XR. J Mater Chem. 2009;19:7765–7770. [Google Scholar]
- 58.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 59.Berry CC, Curtis ASG. J Phys D: Appl Phys. 2003;36:R198–206. [Google Scholar]
- 60.Fang C, Zhang MQ. J Mater Chem. 2009;19:6258–6266. doi: 10.1039/b902182e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veiseh, Gunn JW, Zhang MQ. Adv Drug Delivery Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Müller S. Nanomed-Nanotech. 2009;5:387–393. doi: 10.1016/j.nano.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Yigit MV, Mazumdar D, Kim HK, Lee JH, Odintsov B, Lu Y. ChemBioChem. 2007;8:1675–1678. doi: 10.1002/cbic.200700323. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang LF, Shaikh M, Yuet K, Cima MJ, Langer R, Kantoff PW, Bander NH, Jon S, Farokhzad OC. ChemMedChem. 2008;3:1311–1315. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagalkot V, Farokhzad OC, Langer R, Jon S. Angew Chem Int Ed. 2006;45:8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 66.Herr JK, Smith JE, Medley CD, Shangguan DH, Tan WH. Anal Chem. 2006;78:2918–2924. doi: 10.1021/ac052015r. [DOI] [PubMed] [Google Scholar]
- 67.Zhao X, Tapec-Dytioco R, Wang K, Tan WH. Anal Chem. 2003;75:3476–3483. doi: 10.1021/ac034330o. [DOI] [PubMed] [Google Scholar]
- 68.Smith JE, Medley CD, Tang ZW, Shangguan DH, Lofton C, Tan WH. Anal Chem. 2007;79:3075–3082. doi: 10.1021/ac062151b. [DOI] [PubMed] [Google Scholar]
- 69.Song YJ, Zhao C, Ren JS, Qu XG. Chem Commun. 2009:1975–1977. doi: 10.1039/b818415a. [DOI] [PubMed] [Google Scholar]
- 70.Hwang DW, Ko HY, Lee JH, Kang H, Ryu SH, Song IC, Lee DS, Kim S. J Nucl Med. 2010;51:98–105. doi: 10.2967/jnumed.109.069880. [DOI] [PubMed] [Google Scholar]
- 71.Ajayan PM. Chem Rev. 1999;99:1787–1800. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 72.So HM, Won K, Kim YH, Kim BK, Ryu BH, Na PS, Kim H, Lee J. J Am Chem Soc. 2005;127:11906–11907. doi: 10.1021/ja053094r. [DOI] [PubMed] [Google Scholar]
- 73.Maehashi K, Katsura T, Kerman K, Takamura Y, Matsumoto K, Tamiya E. Anal Chem. 2007;79:782–787. doi: 10.1021/ac060830g. [DOI] [PubMed] [Google Scholar]
- 74.Zelada-Guillén GA, Riu J, Düzgün A, Rius F Xav. Angew Chem Int Edit. 2009;48:7334–7337. doi: 10.1002/anie.200902090. [DOI] [PubMed] [Google Scholar]
- 75.Chen RJ, Bangsaruntip S, Drouvalakis KA, Shi Kam NW, Shim M, Li MY, Kim W, Utz PJ, Dai HJ. Proc Natl Acad Sci U S A. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang RH, Jin J, Chen Y, Shao N, Kang H, Xiao Z, Tang Z, Wu Y, Zhu Z, Tan WH. J Am Chem Soc. 2008;130:8351–8358. doi: 10.1021/ja800604z. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Z, Tang ZW, Phillips JA, Yang RH, Wang H, Tan WH. J Am Chem Soc. 2008;130:10856–10857. doi: 10.1021/ja802913f. [DOI] [PubMed] [Google Scholar]
- 78.Chen H, Yu C, Jiang C, Zhang S, Liu BH, Kong JL. Chem Commun. 2009:5006–5008. doi: 10.1039/b910457g. [DOI] [PubMed] [Google Scholar]
- 79.O'Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma JP, Hauge RH, Weisman RB, Smalley RE. Science. 2002;297:593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 80.Ravi Kumar MNV, Kumar N, Kashyap N. Crit Rev Ther Drug. 2005;22:107–150. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- 81.Miyata T, Asami N, Uragami T. Nature. 1999;399:766–769. doi: 10.1038/21619. [DOI] [PubMed] [Google Scholar]
- 82.Nagahara S, Matsuda T. Polym Gels Netw. 1996;4:111–127. [Google Scholar]
- 83.He X, Wei B, Mi Y. Chem Commun. 2010;46:6308–6310. doi: 10.1039/c0cc01392g. [DOI] [PubMed] [Google Scholar]
- 84.Soontornworajit B, Zhou J, Shaw MT, Fanb TH, Wang Y. Chem Commun. 2010;46:1857–1859. doi: 10.1039/b924909e. [DOI] [PubMed] [Google Scholar]
- 85.Yang HH, Liu HP, Kang HZ, Tan WH. J Am Chem Soc. 2008;130:6320–6321. doi: 10.1021/ja801339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Z, Wu CC, Liu HP, Zou Y, Zhang XL, Kang HZ, Yang CYJ, Tan WH. Angew Chem Int Ed. 2010;49:1052–1056. doi: 10.1002/anie.200905570. [DOI] [PubMed] [Google Scholar]
- 87.Bangham D, Standish MM, Watkim JC. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 88.Gregoriadis G. Trends Biotechnol. 1995;13:527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 89.Tan ML, Choong PFM, Dass CR. Peptides. 2010;31:184–193. doi: 10.1016/j.peptides.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Rädler JO, Koltover I, Salditt T, Safinya CR. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 91.Lasic DD, Martin FJ, editors. Stealth Liposomes. CRC Press; Boca Raton: 1995. [Google Scholar]
- 92.Maeda H. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 93.Sapra P, Allen TM. Cancer Res. 2002;62:7190–7194. [PubMed] [Google Scholar]
- 94.Leamon CP, Cooper SR, Hardee GE. Bioconjugate Chem. 2003;14:738–747. doi: 10.1021/bc020089t. [DOI] [PubMed] [Google Scholar]
- 95.Jaracz S, Chen J, Kuznetsova LV, Ojima I. Bioorgan Med Chem. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 96.Gabizon, Shmeeda H, Horowitz AT, Zalipsky S. Adv Drug Deliver Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Kang HZ, O'Donoghue MB, Liu HP, Tan WH. Chem Commun. 2010;46:249–251. doi: 10.1039/b916911c. [DOI] [PubMed] [Google Scholar]
- 98.Cao ZHC, Rong T, Abhijit M, Weichen X, Wong GCL, Cheng JJ, Lu Y. Angew Chem Int Ed. 2009;48:6494–6498. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 99.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. J Control Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 100.Kakizawa Y, Kataoka K. Adv Drug Deliver Rev. 2002;54:203–222. doi: 10.1016/s0169-409x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 101.Mikhaila S, Allen C. J Control Release. 2009;138:214–223. doi: 10.1016/j.jconrel.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 102.Ding K, Alemdaroglu FE, Borsch M, Berger R, Herrmann A. Angew Chem Int Ed. 2007;46:1172–1175. doi: 10.1002/anie.200603064. [DOI] [PubMed] [Google Scholar]
- 103.Alemdaroglu FE, Alemdaroglu NC, Langguth P, Herrmann A. Macromol Rapid Comm. 2008;29:326–329. [Google Scholar]
- 104.Miyamoto D, Tang Z, Takarada T, Maeda M. Chem Commun. 2007:4743–4745. doi: 10.1039/b709775a. [DOI] [PubMed] [Google Scholar]
- 105.Alemdaroglu FE, Alemdaroglu NC, Langguth P, Herrmann A. Adv Mater. 2008;20:899–902. [Google Scholar]
- 106.Liu HP, Zhu Z, Kang HZ, Wu YR, Sefan K, Tan WH. Chem Eur J. 2010;16:3791–3797. doi: 10.1002/chem.200901546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park C, Lee IH, Song Y, Rhue M, Kim C. Proc Natl Acad Sci USA. 2006;103:1199–1203. doi: 10.1073/pnas.0505364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu YR, Sefah K, Liu HP, Wang RW, Tan WH. Proc Natl Acad Sci USA. 2010;107:5–10. doi: 10.1073/pnas.0909611107. [DOI] [PMC free article] [PubMed] [Google Scholar]


