Abstract
Male germ cell maturation is governed by the expression of specific protein(s) in a precise temporal sequence during development. GRTH/Ddx25, a member of the DEAD-box protein family, is a testis-specific gonadotropin/androgen regulated RNA helicase that is present in germ cells (meiotic spermatocytes and round spermatids) and Leydig cells. GRTH is essential for completion of spermatogenesis as a posttranscriptional regulator of relevant genes during germ cell development. Male mice lacking GRTH are sterile with spermatogenic arrest due to failure of round spermatids to elongate, where striking structural changes and reduction in size of chromatoid bodies are observed. GRTH also plays a central role in preventing germ cell apoptosis. In addition to its inherent helicase unwinding/ATPase activities, GRTH binds to specific mRNAs as an integral component of RNP particles. As a shuttle protein, GRTH transports target mRNAs from nucleus to the cytoplasm for storage in chromatoid bodies of spermatids, awaiting for translation during spermatogenesis. GRTH is also associated with polyribosomes to regulate target gene translation. The finding of a missense mutation associated with male infertility where its expression associates with loss of GRTH phosphorylation supports the relevance of GRTH to human germ cell development. We conclude that the mammalian GRTH/Ddx25 is a multifunctional RNA helicase that is an essential regulator of spermatogenesis and is highly relevant for studies of male infertility and contraception.
Keywords: RNA helicase, gonadotropin, testis, spermiogenesis, messenger ribonuclear protein particle, shuttling protein, translation, phosphorylation chromatoid body, apoptosis, infertility, single nucleotide polymorphism (SNP), missense mutation
Introduction
Germ cell development in the testis is controlled by the binding of gonadotropins to specific receptors in Leydig and Sertoli cells. The receptors expressed in Leydig cells mediate the actions of Luteinizing hormone that regulate steroidogenesis predominantly through the cAMP/PKA signaling pathway (Dufau, et al., 1977, Catt, et al., 1980, Dufau, 1988). Androgens produced in the Leydig cells exert paracrine actions through androgen receptors present in Sertoli cells to regulate spermatogenesis (Chang, et al., 2004, Holdcraft and Braun, 2004, Wang, et al., 2006). Spermatogenesis is a complex process that depends on the integrated expression of an array of genes that must operate in a precise temporal sequence to produce mature spermatozoa (Steger, 2001, Eddy, 2002). It begins with mitotic proliferation of spermatogonia followed by two consecutive rounds of meiotic divisions to produce primary and 2nd spermatocytes. Haploid round spermatids emerge at the end of the 2nd meiosis, and this is followed by a series of morphological changes (spermiogenesis) to generate elongating spermatids and finally mature spermatozoa. Gene expression in haploid spermatids requires temporal uncoupling of transcription and translation in the adult mammalian testis. Post-transcriptional events including processing, export and storage of RNA have critical roles in the availability of specific transcripts for translation during the progression of spermatogenesis, where the precise regulatory mechanisms remain to be elucidated. The majority of the mRNAs of relevance in spermiogenesis are thought to be associated in messenger ribonuclear protein particles (mRNP) and stored at cytoplasmic sites of round spermatids. The Chromatoid Body (CB), a filamentous-lobular perinuclear cytoplasmic organelle present in spermatids, has long been hypothesized as the storage /processing center of mRNAs awaiting for translational activation at later stages of spermiogenesis (Parvinen and Parvinen, 1979, Hecht, 1988, Parvinen, 2005). However, to date, no definitive evidence supports the intrinsic processing mechanism of any long-lived mRNAs in this organelle.
RNA helicases members of DEAD (Glu-Asp-Ala-Glu)-box protein family are known to modulate the RNA structure that is a crucial step in many fundamental biological processes. Although these proteins participate in various aspects of RNA metabolism and translational events (de la Cruz, et al., 1999, Silverman, et al., 2003), their precise role during testicular germ cell development is poorly understood. Current knowledge of mouse PL10/Ddx3, a male germ cell-specific helicase, is limited to its functional replacement of Saccharomyces cerevisiae DED1p/mammalian translational initiation factor IF4A required for the initiation step of translation in the yeast (Chuang, et al., 1997). MVH/Ddx4, a mouse homolog of drosophila VASA that is present in the CB and cytoplasm of germ cells and is commonly used as a germ cell marker (Fujiwara, et al., 1994, Noce, et al., 2001), has been recently proposed to participate in the small RNA interfering pathway to regulate RNA processing in the CB of spermatids (Kotaja and Sassone-Corsi, 2007). However, since MVH function is confined to the pre-meiotic stage of male germ cells before the formation of CB (Tanaka, et al., 2000), it might not have a major role in the translational delay of messages during spermiogenesis.
The discovery of a hormonally regulated GRTH/Ddx25 that is a testis specific member of the DEAD-box family of RNA helicases present in Leydig cells and germ cells (Tang, et al., 1999, Dufau and Tsai-Morris, 2007), has greatly advanced understanding of the essential requirement and mechanisms underlining this RNA-helicase associated testicular functions. It has also provided insights linking the actions of gonadotropin/androgen and the post-transcriptional regulatory events during germ cell maturation. Here we summarize current status of our findings on the essential role of GRTH/Ddx25 in germ cell development with emphasis on its the multi-functional control of spermatogenesis.
GRTH structure, biochemical properties, genomic organization and tissue /cell distribution
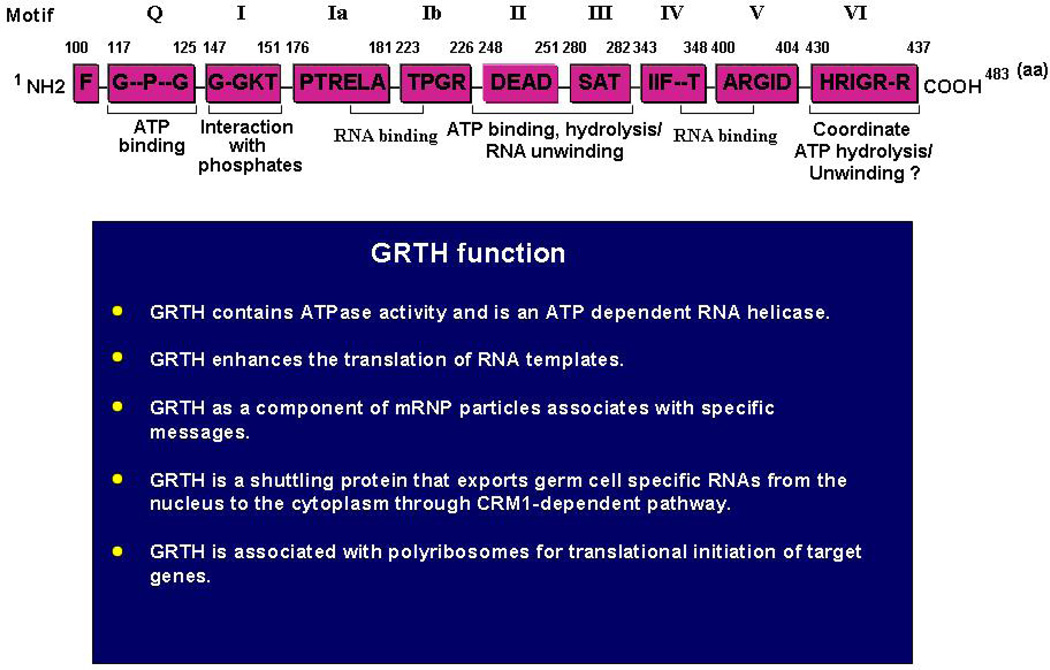
GRTH contains 483 aa and shares all conserved signature motifs of members of the DEAD-box family of RNA helicases (Tang, et al., 1999, Sheng, et al., 2003) (Fig. 1). In general, this family of proteins bind RNA through specific motifs, interact with ATP, and possess ATPase and helicase activities that unwind RNA (Cordin, et al., 2006, Linder, 2006). GRTH displays ATPase activity and is an ATP-dependent bidirectional RNA helicase. It is also a translational regulator since it enhances translation of in vitro transcribed messages in a dose-dependent manner (Tang, et al., 1999). Aside from the conserved motifs related to inherent biochemical functions, GRTH displays low amino acid sequence similarity with other members of the DEAD-box protein family. Unique 5’ and 3’ extension sequences, and the overall structure of GRTH, determine its specific physiological function in germ cells (Sheng, et al., 2003, Sheng, et al., 2006). Phylogenic comparison analysis revealed that the GRTH gene is only distantly related to other germ cells stage-specific DEAD-box RNA helicases including PL10/DDX3 (Leroy, et al., 1989) and the mouse homolog of Drosophila Vasa (MVH/DDX4) (Fujiwara, et al., 1994). GRTH is most closely related to human/mouse/yeast DBP5/DDX19, which is expressed ubiquitously and has a perinuclear localization (Gee and Conboy, 1994) that is required for its poly(A) RNA export function (Tseng, et al., 1998). The GRTH gene contains 12 coding exons and all but one of its conserved helicase motifs are contained within single exons. GRTH is a TATA-less gene and its basal promoter activity is driven by SP1/SP3 that bind GC-rich sequences at the promoter (Tsai-Morris, et al., 2004). Cell-specific transcriptional activation in expressing (pituitary α-T3 and hypothalamic GT1-7) and non-expressing cells (mouse Leydig tumor cells [MLTC]) could be accomplished by the presence or absence of as yet unidentified transcription factor(s) binding to upstream sequences.
Fig. 1. Domain structure and function of GRTH.
Top: Conserved motifs with specific intrinsic functions shared by GRTH with members of the DEAD-box family of proteins are indicated as (Q, I, Ia, Ib and II–VI). Amino acid position of each motif is indicated. Below: Summary of specific physiological function of GRTH. (Tang, et al., 1999, Sheng, et al., 2003, Sheng, et al., 2006).
The GRTH gene is transcribed as single mRNA species of 1.6 kb with almost exclusive expression in the testis. Minor transcript expression is observed in the hypothalamus, pituitary and brain of rat and in immortalized pituitary (αT3) and hypothalamic GnRH neurons (GT1-7) (Tang, et al., 1999, Tsai-Morris, et al., 2004). However, translation of this protein occurs exclusively in testicular cells. GRTH mRNA was detected in both rat and mouse Leydig and germ cells and their abundance was comparable in both cell types. However, it is not expressed in stable cultures of mouse and rat Leydig cells (MLTC and rat R2C). Testicular GRTH mRNA and protein expression is developmentally regulated. Low levels are found in immature animals of 1–2 weeks of age, and are markedly increased during early puberty (3–4 weeks) and maintained throughout adulthood (Tang, et al., 1999, Sheng, et al., 2003). Three GRTH protein species (56/61, 43/48, and minor 33 kDa) have been identified in adult testis. A significant GRTH immuno-reactive signal was present in the interstitial cells of the adult rat testis. Within the seminiferous tubules, GRTH expression is cell- and stage-specific during germ cell development. It is present in pachytene spermatocytes (SP), spermatocytes entering metaphase of meiosis and in round spermatids (RS), but not in elongated spermatids. Maximal expression in both SP and RS occurs at stage 9 of spermatogenesis. The expression of different GRTH protein species, which result from alternative utilization of ATG codons, is cell-specific and hormone-regulated. Western analysis of purified testicular cells revealed the presence of of 56/61 kDa and 33 kDa protein species in spermatocytes and round spermatids, while the 43/48 kDa species are found in Leydig cells (Sheng, et al., 2003). Three consensus Kozak ATG codons located in frame within the coding sequence of GRTH are utilized in the generation of these protein isoforms. Germ cells preferentially utilize the 1st ATG codon to produce major 56/61 kDa protein and the 3rd ATG codon for minor 33 kDa protein, while Leydig cells utilize the 2nd ATG for generation of the 43/48 kDa protein. hCG treatment of rodents caused a significant induction of the 43/48 kDa species in Leydig cells and round spermatids, but not in pachytene spermatocytes. This induction was partly prevented when animals were treated with flutamide, an androgen receptor antagonist. The androgen-induced utilization of the 2nd ATG codon in both cell types is probably regulated at the translational level through the actions of factor(s) generated by the autocrine (Leydig cells) and paracrine (Sertoli cells) effects of androgens. These induced factor(s) might promote utilization of the 2nd ATG codon through internal ribosomal entry site mechanism for the synthesis of 43/48 kDa protein in Leydig cells and round spermatids.
Regulation of GRTH gene expression
Time course studies showed up-regulation of GRTH mRNA and 43/48 kDa protein species in Leydig cells from adult rats treated with a single dose of hCG (Tang, et al., 1999, Sheng, et al., 2003). Significant increases were observed at 12 hr and reached maximal levels at 24 hrs, then returned to near basal levels at 96 hr. The increases in mRNA expression were not related to changes in message stability. Nuclear run-off studies demonstrated that in vitro newly transcribed GRTH mRNA from Leydig cell nuclear extracts was significantly increased in animals treated with hCG, indicating that the gonadotropin-induced up-regulation of GRTH gene expression occurs at the transcriptional level. The in vivo hCG effect was reproduced by in vitro exposure of Leydig cell cultures to gonadotropin (10 ng/ml) for 24 hrs, and treatment with cAMP caused comparable increases in GRTH expression. The hCG-induced increases were prevented when Leydig cells were pre-incubated with a mixture of enzyme inhibitor(s) of the steroidogenic pathway (Sheng, et al., 2003) that effectively abolished androgen production. Similar to hCG and cAMP, dihydrotestosterone but not estradiol significantly increased the expression of GRTH. This action of androgen was confirmed by in vivo studies on animals treated with the androgen receptor inhibitor flutamide, which prevented the GRTH increases induced by hCG (Sheng, et al., 2003). Taken together, these findings indicated that GRTH expression is up-regulated in Leydig cells at the transcriptional level by gonadotropin via cAMP through the actions of androgen generated by the hormonal stimulus.
GRTH targeted null mouse
Considerable information on the functional role of GRTH has been derived from studies on GRTH null mice (Tsai-Morris, et al., 2004). Adult male homozygous knockout (KO) mice are sterile but display normal sexual behavior. GRTH heterozygous male mice and null female mice and are fertile. The endocrine profiles are normal in all groups. Although Leydig cells of KO mice have reduced lipid droplets and swollen mitochondria lacking normal central cristae, normal basal circulating levels of testosterone were maintained. This excludes reduced androgen production as the cause of the spermatogenic arrrest. No morphological changes in germ cell development were observed in GRTH null mice from birth to day 21 of age. In the adult testis, round spermatids of null mice were arrested at step 8 of spermiogenesis and failed to elongate. The ultrastructure of the germ cells was generally normal up to the point of arrest. However, the CB of GRTH null mice was unusually condensed, reduced in size and lacked the typical filamentous-lobular structure during all steps of spermiogenesis. Immuno-EM gold labeling analysis showed GRTH expression in the nucleus, cytoplasm and the CB of wild type spermatids. Because the CB shares components (argonaute/RISC/dicer) of its functional somatic P-body homolog which participate in the RNA mediated gene silencing/degradation pathway (Eulalio, et al., 2007, Kotaja and Sassone-Corsi, 2007), this organelle might function an RNA storage site to control RNA processing and translation of relevant spermatogenic messages in male germ cells. The tightly compact CB of reduced size found in the GRTH KO mice with spermatogenic arrest implicates an essential requirement of GRTH to maintain the structural integrity of the CB for post-transcriptional regulation of the expression of genes that presumably control the elongating process of the germ cells.
Comparative studies in WT and KO of genes which are normally expressed early in spermatocytes, including histone H4 and high mobility group protein 2 (HMG2) showed unchanged levels of their mRNA in total cell extracts and the cytoplasmic fraction of KO mice (Sheng, et al., 2006). However, their protein expression was completely abolished, suggesting a functional role of GRTH in translational regulation. Similarly, in total extracts of round spermatocytes of KO mice no change was observed in the steady state level of transcripts of a set of genes that are normally expressed at later stages of spermatogenesis, including phosphoglycerate kinase 2 (PGK2), testicular angiotensin converting enzyme (tACE) and transition protein 2 (Tp2). In contrast, their cytosolic mRNA levels were significantly reduced and their protein expression was completely abolished. This indicated that in addition of its function as a translational regulator, GRTH is required for the nuclear export of mRNAs of specific genes during later stages of germ cell development. The lack of GRTH does not affect the expression of other proteins including acrosin, any of the cAMP response element modulator (CREM) isoforms, transcription factor SP1, steroidogenic enzymes (P450 scc and P450c17), and RNA helicases p68 and PL10 (Tsai-Morris, et al., 2004, Sheng, et al., 2006). Taken together, these data indicate that GRTH is crucial for the post-transcriptional control of the expression of subsets of stage relevant genes that might impact on histone-associated chromatin remodeling processes (H4 and Tp2) and the non-histone component of chromatin modifications (HMG2) including DNA bending and unwinding and on specific metabolic processes to generate ATP (PGK2) during spermatogenesis.
The prominent apoptosis observed in KO spermatocytes entering the metaphase of meiosis before the appearance of round spermatids indicates an additional role of GRTH in determining the survival and apoptotic fate of adult germ cells (Tsai-Morris, et al., 2004). The degree of apoptosis appears to be related to the reduction of GRTH protein expression in germ cells, since about 30% TUNEL positive cells per tubule were observed in null mice while only 8% positive cells were found in heterozygous mice and less than 2% in WT. Comparative studies on the global expression of pro- and anti-apoptotic protein profiles of individual apoptotic pathways in spermatocytes of WT and GRTH null mice indicated that GRTH acts as a negative regulator of mitochondrial, death receptor, and NF-kB pathways to prevent apoptosis in the adult testis (Gutti, et al., 2008). Lack of GRTH causes increases in the protein levels of pro-apoptotic factors and decreases of anti-apoptotic factors and reduced phospho-BAD, leading to disruption of mitochondrial integrity which promotes release of cytochrome C (Donovan and Cotter, 2004) and induction of the apoptotic cascade. This, together with the enhanced caspase 3 transcript stability, the higher level of caspase 3-binding factor, Smac, and the diminished level of binding competitor (IAP) observed in the absence of GRTH facilitates the generation of caspase 3/9 active products and PARP that induce DNA fragmentation in the apoptotic process. GRTH impacts NFκB signaling at both cytoplasmic and nuclear levels. The non-phosphorylate IκBα/β, which sequesters NF-κB dimer and prevents its translocation to the nucleus to stimulate the transcription of antiapoptotic genes, was highly elevated in the KO mice. Lack of GRTH also could alter the acetylation status of NFκB in the nucleous through the observed increases in HDAC and decreases in acetylase p300 expression in KO, which could lead to the decrease in transcription of anti-apoptotic genes and apoptosis. In regard to the TNF-R1 signaling pathway, the increase in TNFR-associated adaptor protein TRADD in GRTH KO could cause the observed activation of the downstream caspase 8 cascade that leads to apoptosis. GRTH-mediated apoptotic regulation is also indicated by its selective binding to pro-and anti-apoptotic mRNAs. Thus, as a component of mRNP particles GRTH, acts as a negative regulator of the tumor necrosis factor receptor 1 and caspase pathways, and promotes NF-κB function to control apoptosis in spermatocytes of adult mice.
GRTH function
In addition to the intrinsic biochemical function as a RNA helicase (Tang, et al., 1999), GRTH has multiple functions (Fig. 1): it 1. associates with mRNA as an integral component of messenger ribonuclear protein particles (mRNPs) (Tsai-Morris, et al., 2004); 2. is a shuttling protein that exports mRNAs from the nucleus to cytoplasmic sites (Sheng, et al., 2006); 3. is a translational regulator through its association with active elongating polyribosomes where it participates in the translation of specific RNA transcripts at certain stages in germ cell development (Sheng, et al., 2006). The GRTH protein was present in oligo-dT pull-down RNA-protein complexes from rodent testicular lysates. Also, RT-PCR analysis of RNAs extracted from immuno-precipitated testicular GRTH-mRNA complexes revealed its association with a specific set of testicular gene transcripts, including those of chromatin remodeling proteins (Tp 1 & 2 and Prm 1 & 2), cytoskeletone structural proteins (Fsc1/Odf1), and tACE. GRTH also selectively binds mRNAs of pro- and anti-apoptotic genes and mRNAs from death receptor and NF-κB pathways to mediate apoptotic regulation (Gutti, et al., 2008). Confocal studies on living cells showed that GRTH-GFP was retained in the nucleus of COS1 cells after treatment of RNA polymerase II inhibitor (DRB) that inhibits RNA synthesis, or a nuclear protein export inhibitor (LMB) that inhibits nuclear protein export through chromosome region maintenance-1 protein (CRM1)-dependent pathway. These findings indicate that GRTH functions as a transport protein to export mRNAs through the CRM1-dependent pathway, and support our studies in germ cells which demonstrated that GRTH is required for the export of long-lived transcripts (PGK2, tACE and Tp2) from the nucleus to the cytoplasm.
Individual GRTH protein species were preferentially associated with cellular compartments (Sheng, et al., 2006). The 61 kDa species is the phosphorylated form of GRTH primarily present in the cytoplasm, while the 56 kDa protein is the unphosphorylated form found predominantly in the nucleus of germ cells. GRTH protein is phosphorylated at threonine residues by cAMP-dependent protein kinase. This posttranslational species does not participate in nuclear export but is required for cytoplasmic events, including its association with polyribosomes to initiate translation of target genes. The 56 kDa protein species associates with CRM1. Nuclear export (NES) and localization signals (NLS) are present at the N-terminal of GRTH, which contains two stretches of leucine-rich sequences. The 1st leucine-rich region (aa. 61–74) functions as NES and as the binding site to CRM1, and the 2nd leucine-rich region (aa. 100–114) functions as the NLS. GRTH shuttles between cellular compartments and exports RNAs.
GRTH in the human testis
Evaluation of GRTH single nucleotide polymorphism (SNP) gene mutations in a group of infertile Japanese men with non-obstructive azoospermia (NOA) compared to fertile males revealed a missense mutation in exon 8 (R to H at aa 242) in 5.8% of infertile men and in 1 % of normal subjects. In addition, a silent mutation was identified in exon 11 of NOA patients (Tsai-Morris, et al., 2007). In COS1 cells expressing the mutated human protein only the 56 kDa non-phosphorylated species was found, whereas the 61 kDa species was absent. The R242 resides in a conserved hydrophobic pocket which that has been proposed to form an RNA binding domain and/or participate in protein-protein interaction. The reduced basicity of the mutant protein (R to H) and loss of GRTH phosphorylation could be relevant to functional aspects of the protein and cause male fertility. In another study of infertile Chinese patients with idiopathic azoospermia or oligozoospermia (Zhoucun, et al., 2006), four mutations were identified, two in introns and two others at silent mutations in exon 10 and 11. The silent mutation in exon 10 (C to T at nt 1194), within a consensus binding motif of splicing factor 2, was proposed to reduce GRTH expression and consequent impaired spermatogenesis. However, no such changes were found in the Japanese infertile group (Tsai-Morris, et al., 2008). Moreover the SNPs found in Japanese patients were not apparent in the Chinese infertile group. This suggests that the SNPs of the GRTH gene might be associated with an ethnic background of male infertility among Asian men.
Conclusion
GRTH is a testis specific-protein and is the only RNA helicase known to be hormonally regulated. Gonadotropin action via cAMP stimulates androgen and regulates GRTH in Leydig cells at the translational and transcriptional levels, and in germ cells indirectly through androgen actions on Sertoli cells. GRTH has multifunctional roles in the processing of germ-cell specific RNAs and is essential for spermatid development and completion of spermatogenesis. The phosphorylation status at designated cellular sites of GRTH determine its regulatory action. GRTH is required for the integrity of CB where it could participate in the regulation of messages awaiting for translation during germ cell development.
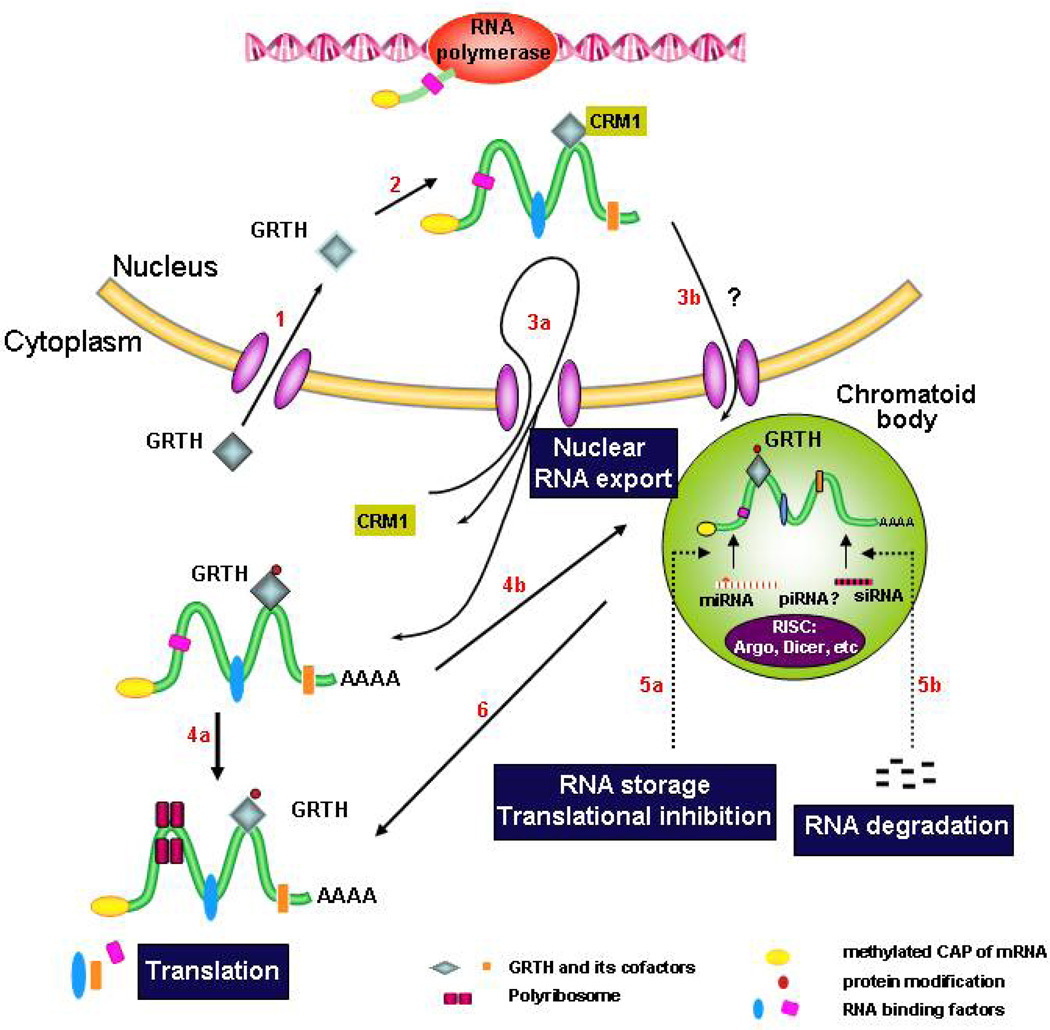
Based on current knowledge, a model of GRTH action in male germ cells during development is presented in Fig. 2. After translation in germ cells, the 56 kDa GRTH species is transported into the nucleus (route 1) where it binds selectively to subset of nuclear RNAs, and as a component of mRNP complexes associates with CRM1 (route 2) and export messages to cytoplasmic sites of germ cells through the CRM1-dependent exporting pathway via nuclear pores (route 3a). GRTH undergoes phosphorylation in the cytoplasm (61 kDa species). Phospho-GRTH associates with polyribosomes, where it regulates translation of associated mRNAs (route 4a) or transports mRNAs to the CB (route 4b) to silence/store/degrade transcripts through the small RNA silencing pathway [(RISC) and the putative si/mi/pi RNA complex] (route 5a and b). Messages are released at specific times from the CB to proceed with translation (route 6). Alternatively, GRTH exports mRNAs also through the CRM1-pathway directly to the CB via adjacent/associated nuclear pores (route 3b). GRTH is a master post-translational regulator of spermatogenesis, understanding its basic regulatory mechanisms in germ cell development provides avenues to the study of male infertility and contraception.
Fig. 2. Model of GRTH action in male germ cells during development.
Route 1–6 (in red) represent functions and transit of GRTH protein entry to the nucleous (1) where it binds messages and associates with CRM-1 (2), and as integral component of RNP exports messages through nuclear pores via CRM1-pathway to the cytoplasm (3a) and to the CB (3b & 4b) either directly (via nuclear pores adjacent or associated with the CB) (3b), or indirectly via the cytoplasmic route (4b). It is phosphorylated at cytoplasmic sites to deliver messages, associates to polyribosomes (4a) and participate in translation. In the CB, messages are potentially regulated via si/mi/pi RNA pathway (RNA storage and degradation) (5a & b). Stored messages are subsequently translated in polyribosomes (4a & 6).
Acknowledgment
This work was supported by the Intramural Research Program of the NICHD, National Instutes of Health
References
- Catt KJ, Harwood JP, Clayton RN, Davies TF, Chan V, Katikineni M, Nozu K, Dufau ML. Regulation of peptide hormone receptors and gonadal steroidogenesis. Recent Prog Horm Res. 1980;36:557–662. doi: 10.1016/b978-0-12-571136-4.50021-8. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang RY, Weaver PL, Liu Z, Chang TH. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science. 1997;275:1468–1471. doi: 10.1126/science.275.5305.1468. [DOI] [PubMed] [Google Scholar]
- Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–198. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim Biophys Acta. 2004;1644:133–147. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Dufau ML. Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol. 1988;50:483–508. doi: 10.1146/annurev.ph.50.030188.002411. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Tsai-Morris CH. Gonadotropin-regulated testicular helicase (GRTH/DDX25): an essential regulator of spermatogenesis. Trends Endocrinol Metab. 2007;18:314–320. doi: 10.1016/j.tem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Tsuruhara T, Horner KA, Podesta E, Catt KJ. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977;74:3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SL, Conboy JG. Mouse erythroid cells express multiple putative RNA helicase genes exhibiting high sequence conservation from yeast to mammals. Gene. 1994;140:171–177. doi: 10.1016/0378-1119(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Gutti RK, Tsai-Morris CH, Dufau ML. Gonadotropin-regulated testicular helicase (DDX25), an essential regulator of spermatogenesis, prevents testicular germ cell apoptosis. J Biol Chem. 2008;283:17055–17064. doi: 10.1074/jbc.M708449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht NB. Haploid gene expression and the regulation of post-meiotic structural genes. In: Parvinen M, Huhtaniemi I, Pelliniemi LJ, editors. Development and Function of the Reproductive Organs. Rome: Ares-Serono Symposia; 1988. pp. 325–334. [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Kotaja N, Sassone-Corsi P. The chromatoid body: a germ-cell-specific RNA-processing centre. Nat Rev Mol Cell Biol. 2007;8:85–90. doi: 10.1038/nrm2081. [DOI] [PubMed] [Google Scholar]
- Leroy P, Alzari P, Sassoon D, Wolgemuth D, Fellous M. The protein encoded by a murine male germ cell-specific transcript is a putative ATP-dependent RNA helicase. Cell. 1989;57:549–559. doi: 10.1016/0092-8674(89)90125-6. [DOI] [PubMed] [Google Scholar]
- Linder P. Dead-box proteins: a family affair--active and passive players in RNP-remodeling. Nucleic Acids Res. 2006;34:4168–4180. doi: 10.1093/nar/gkl468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noce T, Okamoto-Ito S, Tsunekawa N. Vasa homolog genes in mammalian germ cell development. Cell Struct Funct. 2001;26:131–136. doi: 10.1247/csf.26.131. [DOI] [PubMed] [Google Scholar]
- Parvinen M. The chromatoid body in spermatogenesis. Int J Androl. 2005;28:189–201. doi: 10.1111/j.1365-2605.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- Parvinen M, Parvinen LM. Active movements of the chromatoid body. A possible transport mechanism for haploid gene products. J Cell Biol. 1979;80:621–628. doi: 10.1083/jcb.80.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Tsai-Morris CH, Dufau ML. Cell-specific and hormone-regulated expression of gonadotropin-regulated testicular RNA helicase gene (GRTH/Ddx25) resulting from alternative utilization of translation initiation codons in the rat testis. J Biol Chem. 2003;278:27796–27803. doi: 10.1074/jbc.M302411200. Epub 22003 May 27796. [DOI] [PubMed] [Google Scholar]
- Sheng Y, Tsai-Morris CH, Gutti R, Maeda Y, Dufau ML. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J Biol Chem. 2006;281:35048–35056. doi: 10.1074/jbc.M605086200. [DOI] [PubMed] [Google Scholar]
- Silverman E, Edwalds-Gilbert G, Lin RJ. DExD/H-box proteins and their partners: helping RNA helicases unwind. Gene. 2003;312:1–16. doi: 10.1016/s0378-1119(03)00626-7. [DOI] [PubMed] [Google Scholar]
- Steger K. Haploid spermatids exhibit translationally repressed mRNAs. Anat Embryol (Berl) 2001;203:323–334. doi: 10.1007/s004290100176. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- Tang PZ, Tsai-Morris CH, Dufau ML. A novel gonadotropin-regulated testicular RNA helicase. A new member of the dead-box family. J Biol Chem. 1999;274:37932–37940. doi: 10.1074/jbc.274.53.37932. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Koh E, Dufau ML. Differences in gonadotropin-regulated testicular helicase (GRTH/DDX25) single nucleotide polymorphism between Japanese and Chinese populations. Hum Reprod. 2008;23:2611–2613. doi: 10.1093/humrep/den063. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Koh E, Sheng Y, Maeda Y, Gutti R, Namiki M, Dufau ML. Polymorphism of the GRTH/DDX25 gene in normal and infertile Japanese men: a missense mutation associated with loss of GRTH phosphorylation. Mol Hum Reprod. 2007;13:887–892. doi: 10.1093/molehr/gam065. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Lei S, Jiang Q, Sheng Y, Dufau ML. Genomic organization and transcriptional analysis of gonadotropin-regulated testicular RNA helicase--GRTH/DDX25 gene. Gene. 2004;331:83–94. doi: 10.1016/j.gene.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Sheng Y, Lee E, Lei KJ, Dufau ML. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc Natl Acad Sci U S A. 2004;101:6373–6378. doi: 10.1073/pnas.0401855101. Epub 2004 Apr 6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SS, Weaver PL, Liu Y, Hitomi M, Tartakoff AM, Chang TH. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. Embo J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Zhoucun A, Zhang S, Yang Y, Ma Y, Lin L, Zhang W. Single nucleotide polymorphisms of the gonadotrophin-regulated testicular helicase (GRTH) gene may be associated with the human spermatogenesis impairment. Hum Reprod. 2006;21:755–759. doi: 10.1093/humrep/dei388. [DOI] [PubMed] [Google Scholar]