Abstract
The National Institute on Aging Preclinical Alzheimer’s disease Workgroup (PADW) has issued a preliminary report with recommendations for classifying preclinical Alzheimer’s disease (pAD) according to 3 early disease stages. Here we examine the PADW recommendations in relation to neuropathological features in a large, consecutive series of cognitively intact elderly persons, autopsied within a year after cognitive testing (n = 126 cognitively intact patients with mean age 83.7 years at death). Subjects were grouped based on a hypothetical construct correlating pathological features with PADW stages. Many cognitively intact individuals were classifiable as pAD (53/126 or 43%), as expected based on epidemiological and biomarker studies. Of these, most (48%) were in “stage 3”, which corresponds to amyloid pathology with early neurodegeneration. As with prior studies, our data indicate that the development of neocortical neurofibrillary tangles is the key pathological event that is not observed in pAD cases: Braak stages III or IV pathology are hence not truly a substrate for “intermediate likelihood” that cognitive impairment is due to Alzheimer’s disease (AD). We also stress the importance of comorbid non-Alzheimer’s disease brain pathologies (hippocampal sclerosis, neocortical alpha-synucleinopathy, cerebrovascular disease, and brains with hippocampal neurofibrillary tangles but no cortical amyloid plaques) that can contribute to the development of cognitive impairment, or which may serve as confounds in the application of the PADW recommendations. While the final recommendations from the PADW working group have not yet been released, this preliminary analysis provides a perspective on those recommendations from a neuropathological point of view.
Keywords: Nondemented, Biomarkers, MRI, CSF, Preclinical, Neuropathology, Normal
1. Introduction
Preclinical Alzheimer’s disease (pAD) refers to individuals without antemortem cognitive changes but with some degree of confirmed Alzheimer’s disease (AD)-related pathology, amyloid plaques, and neurofibrillary tangles (NFTs) (Arriagada et al., 1992b; Bennett et al., 2006; Crystal et al., 1988; Davis et al., 1999; Haroutunian et al., 1998; Hulette et al., 1998; Katzman et al., 1988; Knopman et al., 2003; Morris and Price, 2001; Price et al., 2009; Schmitt et al., 2000; Tomlinson et al., 1968; Troncoso et al., 1996). However, precise pathological criteria for pAD have not been defined. Current neuropathological consensus guidelines for AD diagnosis refer exclusively to individuals with substantial cognitive impairment meeting criteria for dementia (1997), and so the applicability of such criteria to individuals who were cognitively intact prior to death and autopsy remains unclear. As pathological analyses provide only a cross sectional view, it is unknown whether all cognitively intact subjects with varying degrees of amyloid plaques and NFTs would eventually progress to full-blown clinical AD. If a subset of these individuals do not progress clinically, then the utility of the pAD construct may be called into question. It is also possible that early disease mechanisms may be amenable to targeting by future therapies.
Given the importance of characterizing pAD stages that may allow for the development of rational prevention strategies, the National Institutes of Health (NIH) and Alzheimer’s Association recently sponsored a panel of 14 experts, comprising the Preclinical AD Workgroup (PADW), to help define pAD (Alzheimer’s Association, 2010). These researchers and clinicians, with expertise in various areas related to pAD, provided some preliminary recommendations. A key PADW proposal was to conceive of pAD as a multistage process, with each stage being amenable to detection via particular biomarkers.
There are many neurological and systemic factors that underlie cognitive deterioration in aged individuals (Barker et al., 2002; Jellinger, 2006; Jellinger and Attems, 2010; Nelson et al., 2009b; Schneider et al., 2009a), so therapy-relevant biomarkers are surrogates for specific pathologies — not for cognitive deterioration. The search for accurate antemortem biomarkers that may prove to be surrogates for specific disease states is of prime importance and necessarily will require confirmation by autopsy. Currently, neuropathological evaluation remains the diagnostic gold standard for specific neurodegenerative disease states. Potential AD biomarkers include cerebrospinal fluid markers (amyloid, tau, phospho-tau, isoprostanes, and others) and imaging modalities such as volumetric magnetic resonance imaging (MRI), diffusion tensor imaging, functional MRI, FDG-positron emission tomography (PET), and amyloid ligand-PET; AD biomarker data have been shown to be reasonably well correlated with the neuropathologic features of AD and predictive of future cognitive decline (Beckett et al., 2010; Blennow, 2004; Braskie et al., 2010; Buerger et al., 2006; Chertkow and Black, 2007; Chong and Sahadevan, 2005; Davatzikos et al., 2010; de Leon et al., 2007; Dubois et al., 2007; Engelborghs et al., 2007; Fagan et al., 2007; Haense et al., 2009; Jack et al., 2009, 2010a; Jagust et al., 2010; Johnson et al., 1998; Kapaki et al., 2003; Li et al., 2008; Mintun et al., 2006; Misra et al., 2009; Mormino et al., 2009; Morris et al., 2009; Peskind et al., 2006; Pike et al., 2007; Risacher et al., 2009, 2010; Rowe et al., 2007; Smith et al., 2007; Stomrud et al., 2007, 2010; Walhovd et al., 2010; Weiner et al., 2010).
Prior studies using AD biomarkers appear to support a theoretical model for the progression of AD across the spectrum from normal aging to full-blown AD (Hardy and Selkoe, 2002; Jack et al., 2010b). In this model, amyloid plaques are followed successively by the development of tau pathology, neuronal dysfunction and degeneration, structural brain changes, cognitive decline and eventually the loss of function in activities of daily living that mark the transition to dementia. The recommendations of the PADW are largely based on this model of progression allowing the formation of many testable hypotheses regarding the construct of pAD and its implications for preclinical diagnosis, treatment, and prevention strategies.
Although the staging scheme for pAD as put forth by the PADW relates primarily to antemortem biomarkers, it is important to conceive of them within a framework that includes neuropathological information. The University of Kentucky Alzheimer’s Disease Center (UK-ADC) follows a large cohort of subjects who are cognitively normal on enrollment, agree to undergo extensive longitudinal annual clinical evaluations, and consent to brain autopsy at death (Schmitt et al., 2001). In the period between 1989 and 2010, 164 cognitively intact subjects have come to autopsy from this group. Detailed quantitative neuropathological and neuropsychological data proximal to death are presented in an effort to characterize the features of pAD according to the proposed PADW stages. The goal is to provide new data that may aid in consideration of pAD for clinicians, researchers, and neuropathologists.
2. Methods
2.1. Subjects
Research volunteers studied in this report were from the UK-ADC clinical cohort, representing consecutive autopsies between 1989 and April 10, 2010, in accordance with University of Kentucky Institutional Review Board (IRB) protocols (Fig. 1). Inclusion criteria are cognitive and neurological normality by enrollment examination, and willingness to undergo annual cognitive testing, physical and neurological examinations, and brain donation at death. Excluded at enrollment were individuals with a history of substance abuse, major head injury, major psychiatric illness, medical illnesses that are nonstable, impairing, or that have an effect on the central nervous system (CNS), chronic infectious diseases, stroke or transient ischemic attack (TIA), encephalitis, meningitis, or epilepsy. Annual standardized assessment includes extensive medical, cognitive, social, and functional evaluations as previously described (Schmitt et al., 2001).
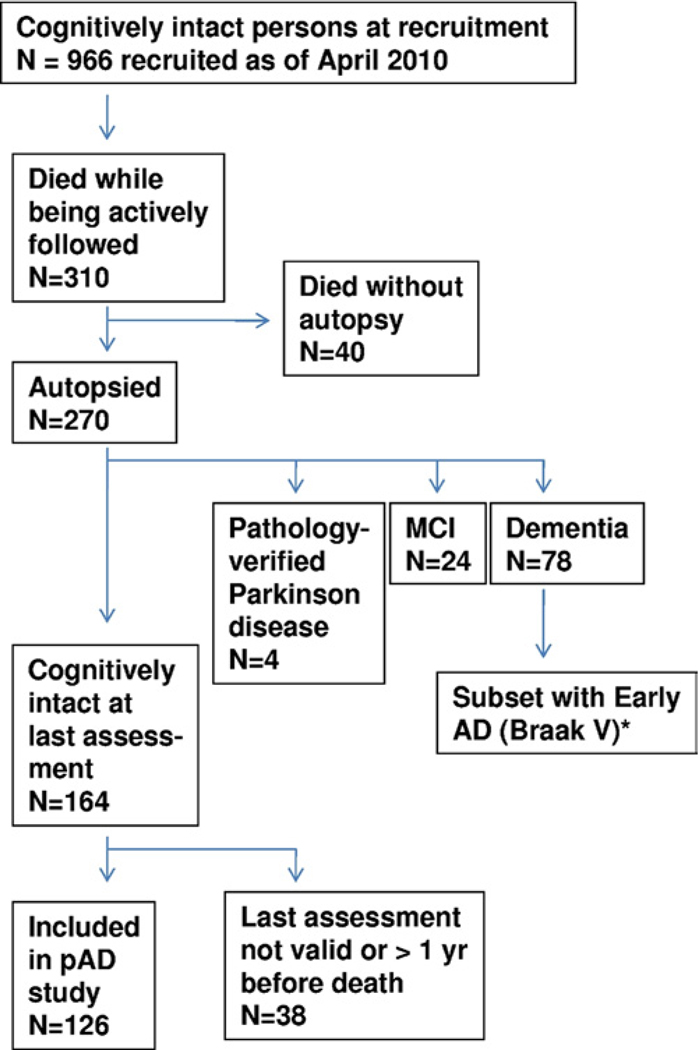
Fig. 1.
Flow chart to demonstrate the individuals included and excluded in the assessment of preclinical Alzheimer’s disease (pAD) in correlation with the pAD Workgroup recommendations (Alzheimer’s Association, 2010). Note that all individuals with eventual diagnosis of pAD or mild cognitive impairment (MCI) were recruited while initially cognitively normal. * Of the 14 individuals included as early Alzheimer’s disease (AD; Braak stage V), 12 were recruited while neurologically normal but 2 were cognitively impaired when recruited.
Overall at this research center between 1989 and April, 2010, 612 individuals came to autopsy with varying degrees of cognitive function and clinical diagnoses. Subjects who had developed dementia prior to autopsy (n = 448) were excluded from consideration of pAD. Of those followed from baseline normalcy, 270 patients were autopsied out of 310 that died while being followed (Fig. 1). Those that were still neurologically normal from this group at the time of the last evaluation (n = 164) serve as the focus of the present study. Subjects with pathologically-confirmed Parkinson’s disease (n = 4) were not included in this group. The mental status testing of UK-ADC subjects has been described previously (Schmitt et al., 2001). Since 2005, a standard test battery was required by the National Alzheimer’s Coordinating Center for all National Institute of Aging (NIA)-funded Alzheimer’s Disease Centers (Morris et al., 2006; Weintraub et al., 2009). Only scores from the last evaluation prior to autopsy were used in the present analysis. Participants included in the present study were categorized into 3 groups: “cognitively intact”, “mild cognitive impairment MCI)”, and “early AD”. “Cognitively intact” individuals had no clinical diagnosis of MCI, AD, or other dementia. Cognitively intact subjects for whom the delay between final cognitive testing and death was greater than 1 year were excluded (n = 37 individuals who were cognitively intact on last testing). One case was excluded because she was evaluated 20 days prior to death from cancer and was unable to complete the evaluation. Individuals with MCI had a clinical-pathological consensus conference diagnosis based on consensus criteria (Petersen et al., 2001; Winblad et al., 2004). For MCI, individuals who died prior to 2001 were diagnosed using retrospective chart-based evaluations as described in detail previously (Jicha et al., 2010). No person was newly diagnosed or categorized as MCI for the purposes of the present study. MCI cases were selected based solely on clinical criteria without respect to pathological features and were not subtyped. “Early AD” cases had antemortem diagnosis of probable AD, with a clinical-pathological consensus diagnosis, histopathologically confirmed AD (NIA-Reagan High-likelihood [1997], Consortium to Establish a Registry for Alzheimer’s Disease [CERAD] definite), with Braak stage V neurofibrillary pathology, and were seen within 2 years of death. All cases from our center meeting these criteria for early AD were included in this group. Using the above criteria, a total of 126 subjects displaying intact antemortem cognitive profiles were identified for inclusion in this study (Table 1). Mean days from last evaluation to autopsy was below 250 days in all cognitively intact groups. Average age at death was 83.7 years. MCI (n = 24) and early AD (n = 14) subjects, as described above, undergoing consecutive autopsy were also included for comparative purposes.
Table 1.
Application of the PADW stages to neuropathological findings at autopsy in cognitively intact subjects
| Neuropathological findings | ||||
|---|---|---|---|---|
| Stage | Khatchaturian (DPs) | CERAD (NPs) | Braak (NFTs) | Rationale and considerations |
| Stage 0 | No | Negative | 0–II | Absence of measurable pathology that might be reflected in positive antemortem biomarker results. |
| Stage 1 | Yes | Negative | 0–II | Cerebral amyloidosis may be detected using CSF amyloid measures and to a lesser degree, amyloid-PET ligand binding, although current amyloid-PET ligands may not bind DP giving false negative results in this stage. |
| Stage 2 | Yes | Possible, probable, or definite | 0–II | Cerebral amyloidosis may be detected using CSF amyloid measures and amyloid-PET ligand binding. Medial temporal lobe atrophy and positive CSF tau/p-tau provide evidence for early neuronal degeneration. |
| Stage 3 | Yes | Probable or definite | III, IV, V, or VI | NFT density is typically milder in neocortex than in clinically evident AD. Criteria for “subtle cognitive change” accounted for by modest NFT accumulation in neocortex. The authors acknowledge that MTL NFT seen in stage 2 of these operational criteria may be sufficient to produce “subtle cognitive change” in memory tests and so overlap between these stages is unavoidable. |
| Stage 0-N | No | Negative | III or IV | Although this group does not map readily on to the PADW staging, they are relevant to the discussion because they have some pathology (which may be detected using CSF biomarkers) without necessarily belonging on the AD continuum, as described previously (Nelson et al., 2009a). |
Key: AD, Alzheimer’s disease; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CSF, cerebrospinal fluid; DPs, diffuse plaques; MTL, medial temporal lobe; NPs, neuritic plaques; NFTs, neurofibrillary tangles; PADW, Preclinical Alzheimer’s Disease Workgroup; PET, positron emission tomography.
2.2. Tissue sampling and processing
Methods for neuropathological evaluations have been described in detail previously (Davis et al., 1999; Jicha et al., 2010; Nelson et al., 2007). Briefly, at least 24 different sections were sampled, and, after fixation, sectioned and stained with hematoxylin and eosin and the modified Bielschowsky method. The Gallyas stain was used for sections of the medial temporal lobe (MTL). Sections of the cortex and ventromedial temporal lobe structures were stained with 10D-5 or anti-Aβ antibody (Novacaster, Newcastle, UK).
NFTs, diffuse β-amyloid plaques (DP; plaques without surrounding dystrophic neurites), and neuritic β-amyloid plaques (NP; β-amyloid plaques surrounded or invested by argyrophilic dystrophic neurites) were counted as described previously (Nelson et al., 2007). An arithmetic mean was calculated from the count of the 5 most involved fields for DPs (number of DPs per 2.35 mm2; 100× fields), NPs (number of NPs per 2.35 mm2; 100× fields), and NFTs (number of NFTs per 0.586 mm2; 400× fields) for each region using silver-stained sections of middle frontal gyrus, middle temporal gyrus, inferior parietal lobule, and occipital lobe including primary visual area. Mean neocortical counts represent an average derived from the 4 cortical areas described above. Medial temporal lobe (MTL) plaque and tangle counts represent an average derived from the entorhinal cortex, CA1, subiculum, and amygdala. Amyloid plaque results were cross correlated with results of anti-Aβ immunostains described above. Braak staging (Braak and Braak, 1991a) and CERAD plaque scores (Gearing et al., 1995) were used to determine NIA-Reagan diagnosis of pathological AD (1997). The presence of clinical dementia required for a CERAD and subsequent NIA-Reagan diagnosis was waived in the present study, because all subjects by virtue of inclusion criteria were cognitively normal at death.
For assessment of Lewy body pathology (LBP), the alpha-synuclein mouse monoclonal antibody (Novacaster, Newcastle, UK) immunohistochemistry was used (Jicha et al., 2010; Markesbery et al., 2009). Immunohistochemistry for alpha-synuclein was performed on 10-µm sections that were pretreated with formic acid, blocked in 15% filtered horse serum in automation buffer, incubated with primary antibody for 1 hour, and developed with the avidin-biotin complex using Nova Red (Vector Laboratories, Burlingame, CA, USA) as the chromogen. The presence or absence of LBP was dichotomized for the present analysis.
Size, location, and histologic age of large and small vessel infarcts were recorded. Microinfarcts, lacunar infarcts, pale infarcts, arteriolosclerosis, cerebral amyloid angiopathy, and hemorrhagic infarcts were counted for each section. Cases in which cerebrovascular pathology was thought to be a significant contributor to antemortem clinical state were indicated by a dichotomous variable based on clinical judgment of the examining neuropathologist, as there are no current rubrics for grading the severity of cerebrovascular pathology with confident correlation to antemortem cognitive parameters.
All pathological diagnoses, semiquantitative staging, and quantitative pathologic counts were performed blinded to clinical data. Once such assessment was made, each case was brought to a neuropathology consensus conference to allow the incorporation of clinical diagnosis required for CERAD and NIA-Reagan diagnostic categorization.
2.3. Application of PADW staging criteria using extant neuropathological criteria
The PADW proposed 3 stages of pAD, hypothesized to occur in temporal sequence, that can be directly applied to autopsy specimens derived from subjects who were cognitively intact at the time of death. We applied 2 other pathological categories to define subjects lacking any appreciable AD-related pathology, and those lacking amyloid but with evidence of MTL NFTs that did not fit into the proposed PADW schema (Nelson et al., 2009a). In line with the PADW (Alzheimer’s Association, 2010), the pAD stages progress from stage 0 (insufficient number of DPs to satisfy Khatchaturian criteria for AD; Khachaturian, 1985); stage 1, asymptomatic cerebral amyloidosis; stage 2, amyloidosis plus evidence of early neurodegeneration; and stage 3, amyloidosis, evidence of neurodegeneration plus subtle cognitive change. Possibly outside of the AD spectrum were cases that we designated stage 0-N, lacking appreciable DPs (according to the Khachaturian criteria) or NPs, but Braak stage III or IV NFTs (i.e., neurofibrillary pathology predominantly confined to MTL structures). Direct application of these proposed diagnostic stages to neuropathological findings in cognitively intact subjects is operationalized in Table 1.
2.4. Cognitively impaired cases included for comparative analyses
Subjects with documented cognitive deterioration (MCI or early AD) were included if final cognitive testing occurred within 2 years of death. Average interval between final testing and autopsy for all groups was still under 10 months (Table 2). MCI cases (n = 24) for this study had a clinical-pathological consensus conference diagnosis (Petersen and Negash, 2008; Winblad et al., 2004) as described above. Subjects with early AD (n = 14) had a consensus conference AD diagnosis, met NIA-Reagan criteria for high likelihood of AD (1997), and had Braak stage V pathology (Braak and Braak, 1991b) in addition to meeting CERAD criteria for Definite AD (Gearing et al., 1995).
Table 2.
Demographic, genetic (ApoE), and neuropathological features of study groups
| Demographics and ApoE alleles |
Stage 0 (n = 59) |
Stage 0-N (n = 13) |
Stage 1 (n = 13) |
Stage 2 (n = 15) |
Stage 3 (n = 26) |
MCI (n = 24) |
Early AD (n = 14) |
|---|---|---|---|---|---|---|---|
| Age at death | 81.6 ± 8.7 | 87.6 ± 5.2 | 84.5 ± 8.5 | 83.9 ± 5.9 | 86.1 ± 6.3 | 89.1 ± 4.3 | 89.8 ± 5.9 |
| Sex (% male) | 49.2 | 61.5 | 38.5 | 40 | 50 | 40.9 | 35.7 |
| Education | 60.0 ± 2.5 | 15.8 ± 2.4 | 15.6 ± 3.3 | 17.0 ± 1.8 | 15.8 ± 2.2 | 16.3 ± 2.3 | 14.2 ± 3.0 |
| ApoE4+ (%) | 7.1 | 16.7 | 41.7 | 46.7 | 24.0 | 31.8 | 53.8 |
| MTL DPs, counted | 0.6 ± 1.8 | 1.3 ± 3.1 | 5.5 ± 4.9 | 8.0 ± 4.8 | 7.2 ± 6.3 | 6.5 ± 7.2 | 13.2 ± 5.5 |
| MTL NPs, counted | 0.04 ± 0.2 | 0.2 ± 0.4 | 1.7 ± 1.8 | 2.5 ± 2.3 | 4.0 ± 3.1 | 1.6 ± 1.9 | 3.7 ± 2.4 |
| MTL NFTs, counted | 3.1 ± 4.9 | 22.3 ± 18.6 | 5.3 ± 4.3 | 4.5 ± 4.5 | 16.4 ± 9.0 | 17.4 ± 20.3 | 42.1 ± 19.0 |
| NeoCx DPs, counted | 2.4 ± 4.6 | 2.2 ± 6.1 | 36.2 ± 10.8 | 28.7 ± 9.9 | 32.7 ± 9.5 | 21.3 ± 17.7 | 37.3 ± 8.6 |
| NeoCx NPs, counted | 0.4 ± 1.0 | 0.2 ± 0.5 | 1.6 ± 1.2 | 8.0 ± 4.7 | 12.0 ± 6.3 | 8.4 ± 5.8 | 14.8 ± 5.3 |
| NeoCx NFTs, counted | 0.1 ± 0.2 | 0.4 ± 0.5 | 0.3 ± 0.4 | 0.1 ± 0.2 | 2.1 ± 2.6 | 1.3 ± 3.1 | 6.7 ± 3.9 |
| Cortical Lewy bodies (%) | 1.7 | 0 | 15.4 | 0 | 3.9 | 12.5 | 0 |
| Hippocampal sclerosis (%) | 1.7 | 0 | 0 | 0 | 0 | 12.5 | 0 |
| Cerebrovascular pathology (%) | 1.7 | 15.4 | 30.8 | 20 | 3.9 | 29.2 | 21.4 |
Individuals tested within a year of autopsy with preclinical AD (pAD), MCI, and early AD were the basis of this study. All individuals in stages of pAD (stages 0–3) were tested within a year of death (n = 126). “NeoCx” counts refer to summed neocortical counts from inferior parietal, occipital, temporal, and frontal neocortical regions as described in 2. Methods. “MTL” counts refer to summed medial temporal lobe counts from hippocampal CA1, subiculum, entorhinal cortex, and amygdala. Note that pAD cases tend to have few, if any, neocortical NFTs. Individuals who die with clinical diagnosis of MCI tend to have exclusive or comorbid non-AD pathologies. Thus, the mean counted AD lesions are lower because some MCI cases are, for example, hippocampal sclerosis only. All cases with “early AD” are Braak stage V.
Key: AD, Alzheimer’s disease; ApoE, apolipoprotein E; DPs, diffuse amyloid plaques; MCI, mild cognitive impairment; MTL, medial temporal lobe; NFTs, neurofibrillary tangles as described in 2. Methods; NPs, neuritic amyloid plaques.
2.5. Statistical analysis
Unadjusted mean neuropsychological test scores were compared using Student or Satterthwaite t test as appropriate, and the overall alpha was preserved at 0.05 using the Bonferroni method. Adjusted means were compared using multiple linear regression. In addition, raw test scores were converted to age-corrected z scores based on the baseline performance of the entire normal cohort. Impaired performance on any test was indicated by a z score of more than 1.5 standard deviations below the mean (above for Trails A). The proportion of scores indicating impairment in each group was compared using chi-square tests. All analyses were performed using PC-SAS 9.2® or Microsoft Excel®.
Results
Persons who died without detected antemortem cognitive impairments (with final testing within a year of death) were grouped according to autopsy findings into the PADW proposed stages of pAD. Figs. 2 and 3 depict the characteristic pathological findings and distributions of PADW stages in the cohort studied. Fig. 4 shows how individuals in this research cohort fall into each of the pAD stage categories. Note that “stage 0”, without any indication of cerebral amyloidosis, is the largest case category (60/126 or 47%). There are relatively few “stage 1” (13/126, or 10%) with many DPs but few or any NPs. Among the 44 “pure” pAD cases which lack concomitant pathologies, the proportion of cases are — stage 1 (n = 8, 18%); stage 2 (n = 12, 27%); and stage 3 (n = 24, 55%).
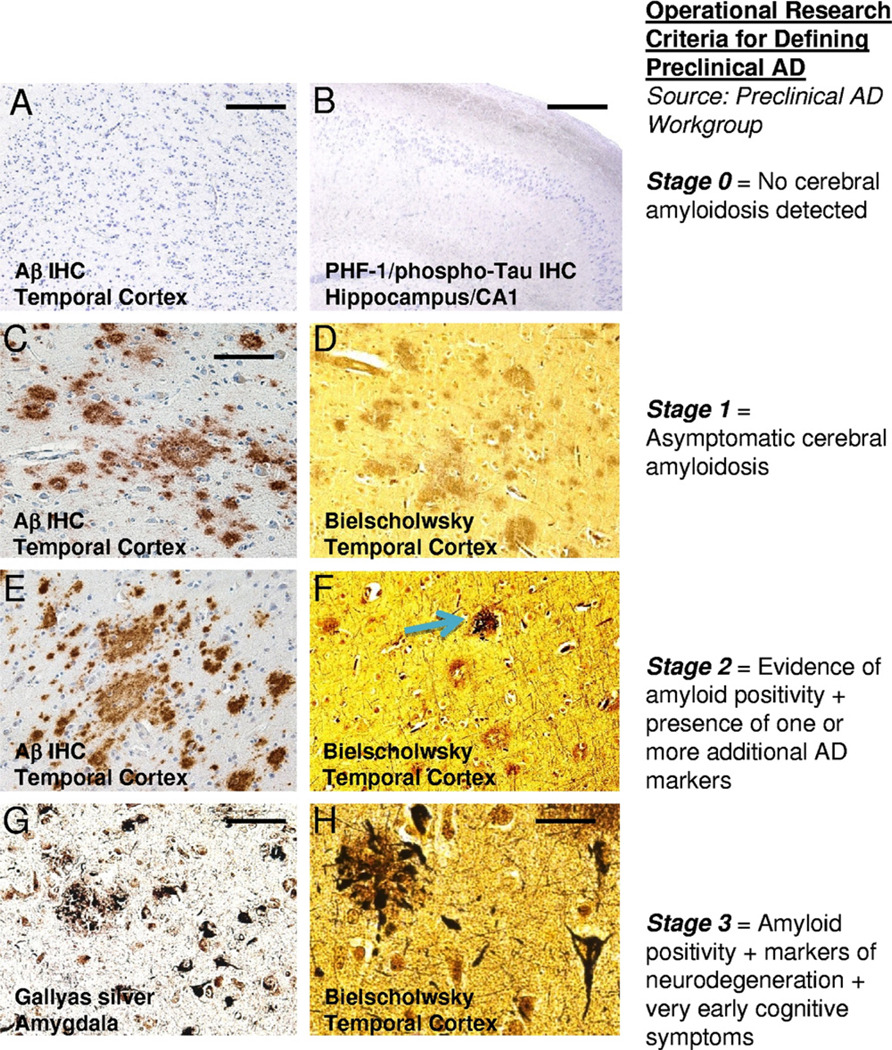
Fig. 2.
Representative histopathological features that may correspond with the evolution of preclinical Alzheimer’s disease (pAD) in correlation with the preliminary recommendations of the Preclinical Alzheimer’s Disease Workgroup (stages described at right). Stage 0 (A and B) refers to cases without cerebral amyloidosis. There are no amyloid plaques —(A) using Aβ immunohistochemistry (IHC) — or neurofibrillary changes — (B) PHF-1/phospho-tau IHC in a low-power photomicrograph of hippocampal CA1 (counterstained with hematoxylin). In stage 1, there are diffuse amyloid plaques, detectable using Aβ IHC (C), but these plaques lack neuritic component that would be detectable using a Bielschowsky silver stain (D). In stage 2, there are both diffuse amyloid plaques (E) and also some plaques with a neuritic component (blue arrow in F). By stage 3, there are typically features seen in fulminant Alzheimer’s disease (AD), but with lower densities especially in the neocortex. Medial temporal lobe regions such as the amygdala (G) may have many neuritic amyloid plaques and tangles as shown here with a sensitive Gallyas silver stain. However, one usually does not see many neuritic amyloid plaques in the neocortex (upper left of H) without also seeing some neurofibrillary tangles (lower right of H). Scale bars: (A) 500 µm; (B) 1 mm; (C–F) 100 µm; (G) 50 µm; (H) 30 µm.
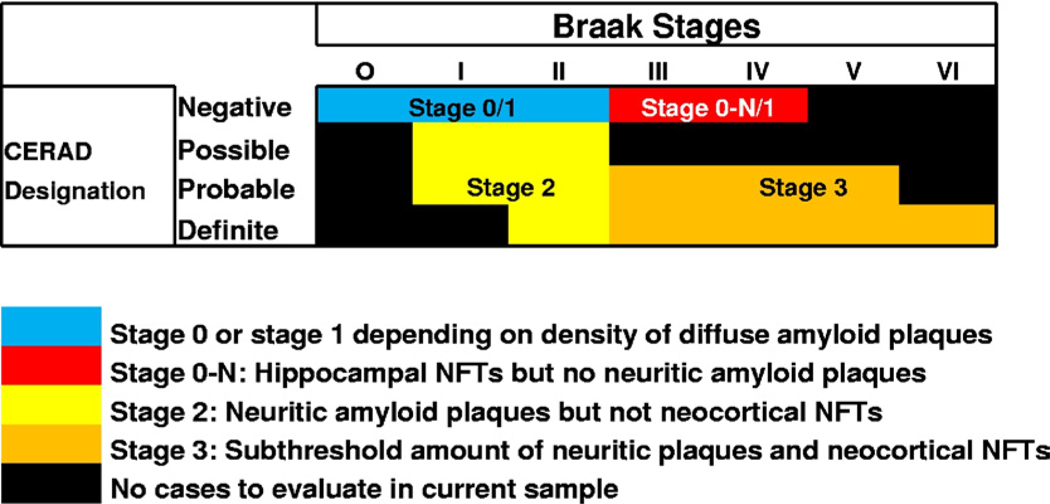
Fig. 3.
Grid with information related to how patients were classified in the current study. Only patients that died while cognitively intact with cognitive testing within a year of death were included in this study and have results that are relevant to this chart. The grid shows that individual patients can be categorized according to the extent of neurofibrillary pathology using the Braak staging. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores provide information about the density of neuritic amyloid plaques. In the earliest part of the disease, without either neurofibrillary tangles or neuritic amyloid plaques (blue portion of the chart), a case is determined to be stage 0 if there are no diffuse plaques but a case is stage 1 if the brain has enough diffuse amyloid plaques to meet the Khachaturian criteria (Khachaturian, 1985). Stage 0-N refers to cases with a modicum of neurofibrillary tangles in the hippocampus, without amyloid plaques in neocortex, as described in a prior study (Nelson et al., 2009a).
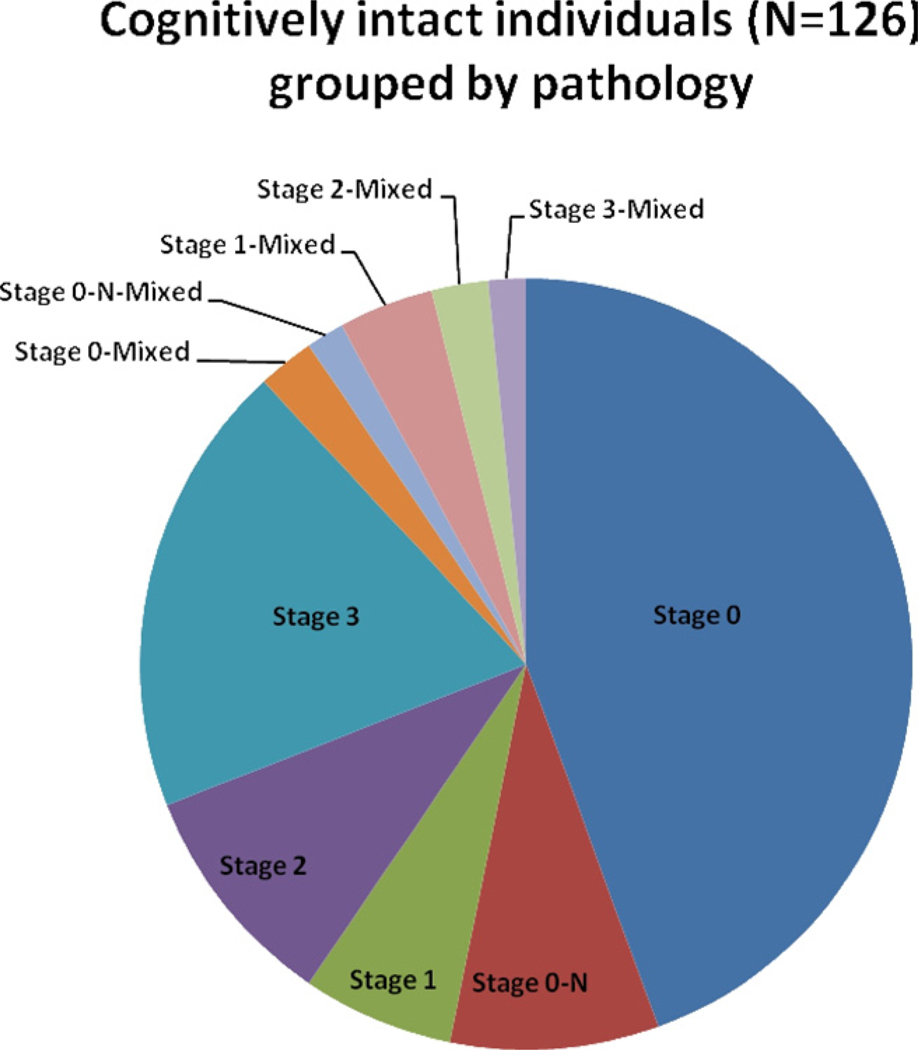
Fig. 4.
Pie chart shows relative numbers of cases that fall into each of the preclinical Alzheimer’s disease (pAD) stages (total n = 126). A relatively large proportion of are “stage 0” (59/126, or 47%) which indicates no cortical amyloidosis. Cases with diffuse plaques but lacking substantial numbers of neuritic plaques (“stage 1”) are relatively few. Cases with mixed” pathology were found in 10/54 (19%) of stage 1–3 pAD cases, indicating subclinical levels of cerebrovascular disease, cortical Lewy bodies, or hippocampal sclerosis.
Table 2 highlights demographic and neuropathological indexes relevant to each of the PADW stages cases in this series. The groups did not differ on age, education, or gender. ApoEε4 status was proportionally higher in PADW stages 1, 2, and 3 groups than in stage 0 subjects reaffirming the association of ApoEε4 status with cerebral amyloidosis in this series. Quantitative pathology (counted numbers of DPs, NPs, and NFTs in neocortical and medial temporal lobe areas as described in 2. Methods) are also presented in Table 2. Compared with early AD cases, all the pAD categories have in common a lack of appreciable neocortical NFTs. Fig. 3 and Table 3 provide information about the distribution of cases according to the CERAD and Braak staging. Note that most common Braak stage for cognitively intact patients was either Braak III or IV, whereas Braak stage 0 is relatively rarely seen in aged, cognitively intact individuals.
Table 3.
Numbers of “pure” pathology cases according to CERAD and Braak stages (n = 111)
| Braak stages | |||||||
|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | VI | |
| CERAD Designation | |||||||
| Negative | 13 | 25/1a | 18/4a | 6/2a | 5/1a | 0 | 0 |
| Possible | 0 | 1 | 3 | 0 | 0 | 0 | 0 |
| Probable | 0 | 1 | 5 | 6 | 6 | 3 | 0 |
| Definite | 0 | 0 | 2 | 1 | 4 | 3 | 1 |
The number of cases without appreciable concomitant pathologies in the present study. All cases had autopsies within a year of final clinical evaluation. Total n = 111. Note that cases with concomitant pathologies (n = 15) were not included in this table. The exceptional case with Braak stage VI and presumed preclinical AD has been described before (Nelson et al., 2009b).
Key: AD, Alzheimer’s disease; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; PADW, Preclinical Alzheimer’s disease Workgroup.
Cases without diffuse plaques/cases with diffuse plaques (i.e., PADW stage 1).
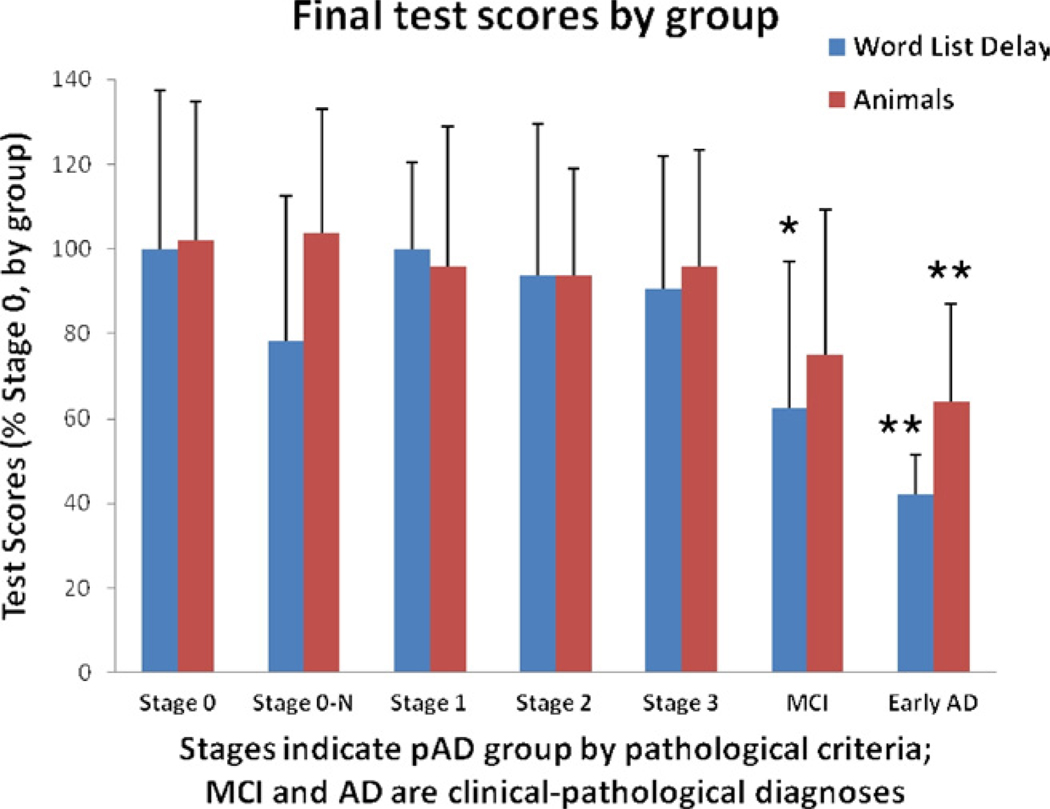
For inclusion in the study, none of the persons designated to represent pAD had antemortem diagnoses of dementia; however, we evaluated whether cognitive assessments could detect group-level differences retrospectively. Results from the last evaluation before death for all groups are presented in Table 4. Note that cognitive assessment results in pAD stages 0-N, 1, stage 2, and stage 3 are not significantly different from stage 0, for any of the tests, even prior to Bonferroni correction. Thirty-six comparisons were made in relation to stage 0 cases (6 variables and 6 groups). The alpha to preserve an overall type I error rate of 0.05 is 0.0014. The lack of significant cognitive differences between pAD groups is also seen when means are adjusted for age, education level, and apolipoprotein E (ApoE) status (data not shown) although some test scores trended down in the stage 3 pAD group (Fig. 5). These results confirm that individuals with a subthreshold burden of AD-type pathology can maintain overall cognitive capacity. However, there were some subtle changes noted on Mini Mental State Examination (MMSE) scores in stage 3 versus stage 0 cases. For subjects without concomitant pathology, the proportion of impaired MMSE scores was significantly higher in stage 3 versus stage 0 (χ2 = 4.42, p = 0.035, 1 df) as indicated by a z score of more than 1.5 standard deviations below the mean of this group. These data indicate slight differences in MMSE scores relative to the other members of the cohort but are not indicative of clinical MCI. In line with expectations, cases with the clinical diagnosis of MCI or early AD (with Braak stage V pathology) had significantly lower cognitive test scores than any of the pAD cases.
Table 4.
Cognitive test scores of study groups; cases with and without concomitant pathologies
| Stage 0 | n | Stage 0-N | n | Stage 1 | n | Stage 2 | n | Stage 3 | n | MCI | n | Early AD | n | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Including cases with concomitant pathologies | ||||||||||||||
| MMSE | 28.4 ± 1.4 | 59 | 27.7 ± 2.5 | 13 | 28.5 ± 1.5 | 13 | 28.7 ± 1.7 | 15 | 27.6 ± 2.3 | 26 | 26.0 ± 3.1**** | 23 | 20.6 ± 8.7**** | 14 |
| Animals | 16.4 ± 5.5 | 58 | 16.6 ± 4.4 | 13 | 16.2 ± 5.2 | 13 | 14.4 ± 4.4 | 15 | 15.9 ± 4.5 | 26 | 12.9 ± 5.6* | 20 | 10.7 ± 3.7*** | 11 |
| Trails A | 54.0 ± 29.2 | 51 | 55.1 ± 18.0 | 10 | 48.8 ± 14.2 | 13 | 56.9 ± 47.6 | 14 | 51.5 ± 21.1 | 22 | 66.8 ± 37.2 | 16 | 98.8 ± 34.4**** | 10 |
| Log mem immediate | 14.7 ± 4.5 | 54 | 14.4 ± 4.1 | 12 | 14.3 ± 2.9 | 13 | 14.5 ± 4.0 | 13 | 14.3 ± 3.8 | 24 | 10.7 ± 3.9*** | 19 | 6.4 ± 4.9**** | 9 |
| Praxis | 9.2 ± 1.0 | 43 | 9.2 ± 0.9 | 10 | 9.5 ± 1.1 | 13 | 9.0 ± 1.3 | 13 | 8.8 ± 0.9 | 19 | 8.6 ± 2.3 | 11 | 8.4 ± 1.3 | 5 |
| Word list delay | 6.4 ± 2.4 | 48 | 5.2 ± 2 | 11 | 6.4±1.4 | 13 | 5.6 ± 2.1 | 11 | 5.9 ± 2.0 | 21 | 4.6 ± 2.1* | 13 | 3.8 ± 1.9**** | 5 |
| Time since last eval (days) | 184.1 ± 105.6 | 59 | 213.7 ± 96.8 | 13 | 234.2 ± 101.0 | 13 | 192.6 ± 88.9 | 15 | 192.9 ± 108.4 | 26 | 318.5 ± 163.5**** | 24 | 285.0 ± 174.4 | 14 |
| Cases lacking concomitant pathologies | ||||||||||||||
| MMSE | 28.4 ± 1.3 | 56 | 27.5 ± 2.7 | 11 | 28.8 ± 1.4 | 8 | 28.8 ± 1.7 | 12 | 27.5 ± 2.3 | 24 | 26.3 ± 2.3** | 12 | 20.4 ± 8.7* | 11 |
| Animals | 16.6 ± 5.4 | 55 | 16.9 ± 4.8 | 11 | 15.6 ± 5.4 | 8 | 15.3 ± 4.1 | 12 | 15.6 ± 4.5 | 24 | 12.2 ± 5.6* | 10 | 10.4 ± 3.8*** | 9 |
| Trails A | 53.5 ± 29.8 | 48 | 54.3 ± 19.7 | 8 | 43.8 ± 21.4 | 8 | 43.8 ± 13.9 | 11 | 52.8 ± 21.6 | 20 | 70.6 ± 39.8** | 9 | 107.1 ± 33.9**** | 7 |
| Log mem immediate | 14.7 ± 4.6 | 51 | 13.6 ± 3.9 | 10 | 14.8 ± 2.7 | 8 | 14.5 ± 3.8 | 11 | 14.1 ± 3.9 | 22 | 12.2 ± 3.8* | 10 | 4.9 ± 3.9**** | 7 |
| Praxis | 9.2 ± 1.0 | 40 | 9.1 ± 1.0 | 8 | 10.0 ± 0.8* | 8 | 9.3 ± 1.1 | 10 | 8.8 ± 0.9 | 18 | 8.5 ± 2.9 | 6 | 9.0 ± 0.0 | 3 |
| Word list delay | 6.4 ± 2.4 | 45 | 5.0 ± 2.2 | 9 | 6.4 ± 1.3 | 8 | 6.0 ± 2.3 | 8 | 5.8 ± 2 | 20 | 4.0 ± 2.2* | 7 | 2.7 ± 0.6** | 3 |
| Time since last eval (days) | 181.1 ± 107.6 | 56 | 211.9 ± 98.3 | 11 | 244.5 ± 86.2 | 8 | 196.7 ± 99.2 | 12 | 191.8 ± 112.4 | 24 | 323.8 ± 168.2* | 13 | 268.1 ± 168.6 | 11 |
Cognitive test scores in all 7 groups with or without inclusion of concomitant pathologies. p values represent the results of unadjusted Student t test or Satterthwaite t test as appropriate, comparing results from that group relative to Stage 0 (minimal or no AD-type pathology) pAD group.
Key: AD, Alzheimer’s disease; eval, evaluation; Log mem, logical memory; MCI, mild cognitive impairment; MMSE, Mini Mental State Examination; pAD, preclinical Alzheimer’s disease.
p < 0.05.
p < 0.01.
p < 0.005.
p < 0.001.
Fig. 5.
Groups in the different preclinical Alzheimer’s disease (pAD) stages lack significant differences on many cognitive tests. Shown here are results for Logical Memory: Immediate Recall and Animal Fluency sections of the Universal Dataset test battery. Error bars = standard deviation. All cases with pAD were tested within a year of death. * p < 0.05 or ** p < 0.001.
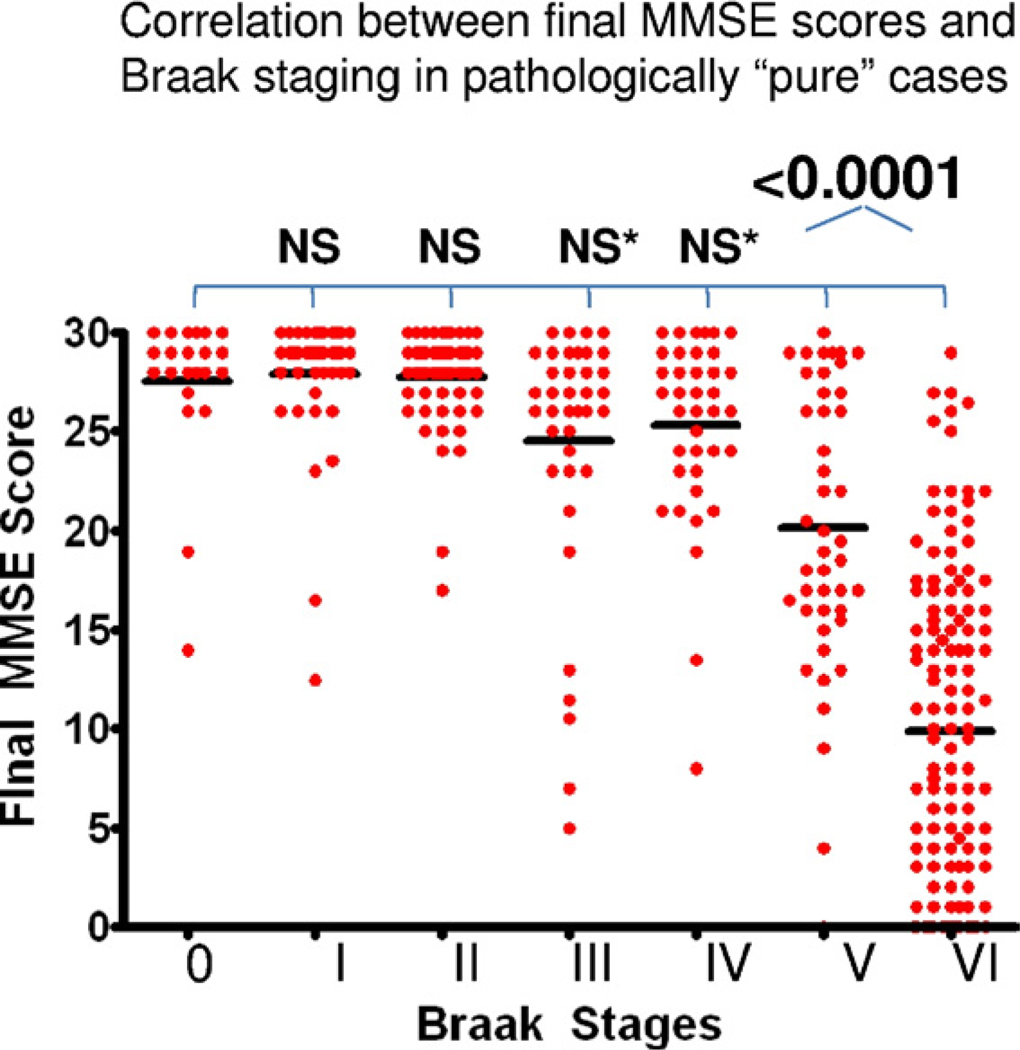
To better define clinical-pathological correlations in pAD stages, we evaluated whether some of the pathological staging criteria are applicable across the full spectrum of cognitive changes. The current criteria for neuropathological diagnosis of AD are linked primarily with neurofibrillary pathology (NPs and NFTs) (1997). We sought to test the correlation between Braak staging and global cognition in our sample that is enriched for cognitively intact individuals. A chart demonstrates the final MMSE scores of individuals in the UK-ADC cohort, stratified by their Braak stage (Fig. 6); these data incorporates also the cognitively impaired dementia. These data demonstrate that decreases in global cognition (MMSE scores) can only be seen in patients with Braak stage V or VI pathology in this sample. Braak stages I through IV do not provide a robust interval variable that can be correlated with cognitive status in pAD. This is uniform across the other cognitive tests as shown in Table 3.
Fig. 6.
Early Braak stages are not helpful interval variables in the context of preclinical or early Alzheimer’s disease (AD). Shown here is a chart of final Mini Mental State Examination (MMSE) scores plotted in correlation with Braak staging of neurofibrillary degeneration. From an initial sample of University of Kentucky Alzheimer’s Disease Center (UK-ADC) autopsies that included demented and nondemented subjects (n = 612), cases were excluded that had frontotemporal dementia pathology, alpha-synucleinopathy such as dementia with Lewy bodies, vascular dementia, and any case that lacked Braak staging or MMSE scores. Cases were not excluded from this analysis based on interval between final MMSE test and death. Although in a person with documented dementia, a Braak stage less than V may indicate some impact from the disease, this ordinal variable cannot confidently predict global cognitive status in the earliest stages of Alzheimer’s disease. This is probably because cognitive impact from AD pathology may be difficult to detect before there are abundant neocortical neurofibrillary tangles (NFTs) (Arriagada et al., 1992a). p values show result of comparing mean MMSE scores in each Braak stage versus Braak stage 0 using unpaired Student t test. NS, not significant; NS*, average final MMSE scores trended lower for Braak stage III and IV (p ~ 0.04), but this was not significant after correction for multiple comparisons.
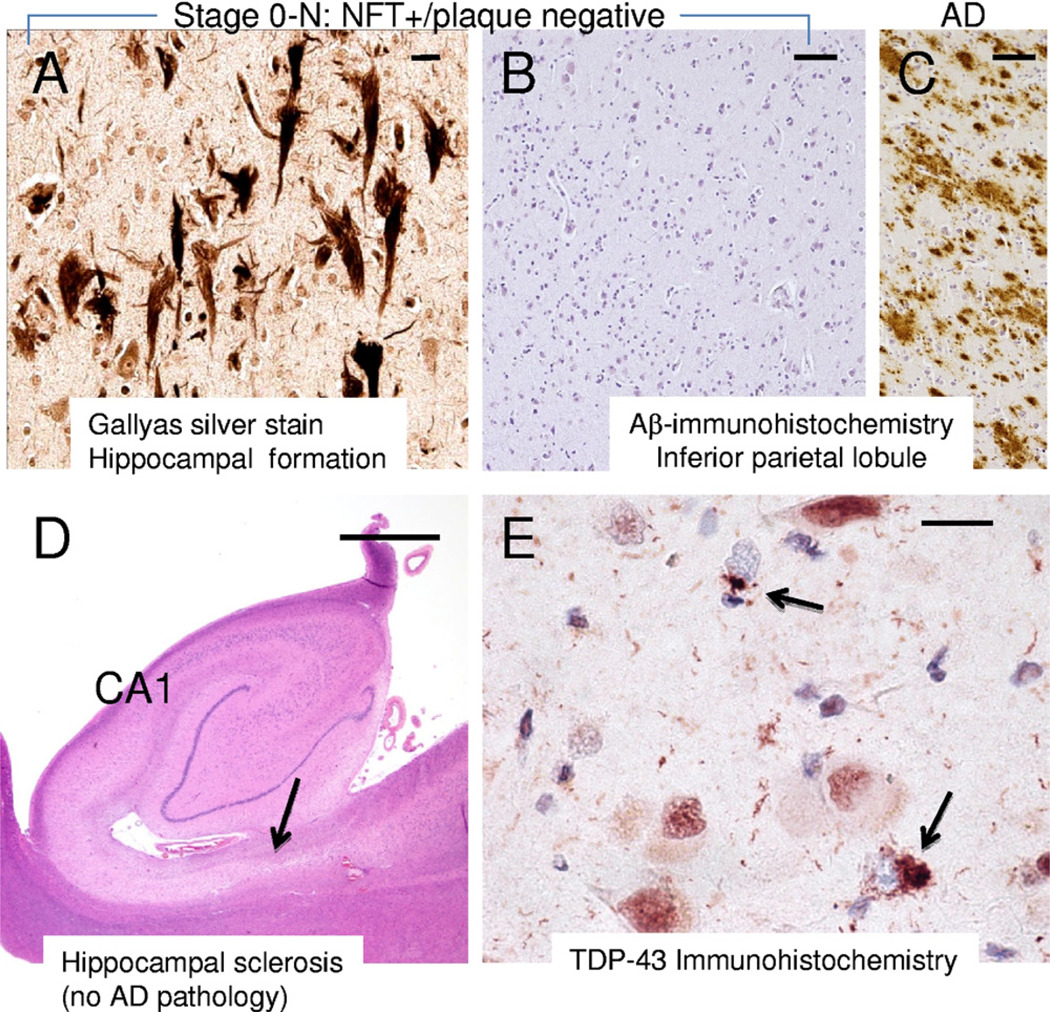
Concomitant pathologies (LBP and cerebrovascular disease) are relatively frequent in pAD (Table 2). There was a relatively high percentage of cases with incipient non-AD pathologies in pAD stages 1–3 (10/54 = 17.5% of cases). The percentage of cases with concomitant pathologies was even higher in MCI cases (11/23 = 48%) which accords with prior studies (Jicha et al., 2006; Schneider et al., 2009b). Note that more than 10% of the pAD cases had stage 0-N pathology, with MTL NFTs but lacking cortical amyloid plaques (Fig. 7). Only 1 case of hippocampal sclerosis was detected among the cognitively intact individuals.
Fig. 7.
Some prevalent brain pathologies in older individuals do not map well onto the preliminary Preclinical Alzheimer’s disease Workgroup (PADW) recommendations. Brains with medial temporal lobe neurofibrillary tangles (NFTs) but no amyloid plaques in the cortex, which we refer to as stage 0-N, comprise over 10% of preclinical Alzheimer’s disease (pAD) cases (A and B). This brain from a 78-year-old male (final Mini Mental State Examination [MMSE] score = 29) showed moderate densities of NFTs in the hippocampal formation and entorhinal cortex (A, stained with Gallyas stain) but there was no Aβ-positive plaques detected (B), in contrast to what is seen in Alzheimer’s disease (AD) brains using the same stain (C, at same magnification). Whereas stage 0-N was seen in 13/126 cognitively intact individuals, we found only a single case with hippocampal sclerosis in nondemented individuals, so this form of non-AD hippocampal atrophy appears more relevant to mild cognitive impairment and demented states. (D) An hematoxylin and eosin stained brain from an 88-year-old female with Clinical Dementia Rating (CDR) = 1 and clinical diagnosis of presumed early AD. The hippocampus appears shrunken, especially the subiculum (arrow). Immunohistochemical stain for TDP-43 (E; nuclei counterstained with hematoxylin) showed aberrant pattern of staining with cytoplasmic TDP-43 inclusions (arrows). Scale bars: (A and E) 25 µm, (B and C) 100 µm; (D) 2 mm.
4. Discussion
Here we provide a neuropathology-based construct to correlate with the preliminary PADW staging criteria, demonstrating that almost half of the cognitively intact subjects in this autopsy series met criteria for stages 1, 2, or 3 pAD. Frequency of pAD pathology progresses from stage 1 or greater (n = 53; 43%) to stage 2 or greater (n = 41; 33%), to stage 3 (n = 26; 21%). Because all the pAD groups had (by definition) intact cognition, and all lack appreciable numbers of neocortical NFTs, these data attest to the importance importance of neocortical NFTs as a key factor in AD pathobiology. However, the presence of some NFTs in hippocampal formation structures is the rule, and not the exception, in cognitively intact older individuals. ApoEε4 genotype is strongly associated with AD-type pathology in pAD, as has been shown for fulminant AD cases in many published clinical and autopsy samples, attesting again to the validity of the PADW construct of pAD stages at least when analyzed from a neuropathological perspective (Jicha et al., 2008). Comorbid pathologies represent a potential confounder to the biomarker-based clinical diagnoses.
Although many cognitively intact subjects lack substantial AD pathology, many others (43%) have at least mild pathology suggesting AD. This observation, which has been made previously (Guillozet et al., 2003; Haroutunian et al., 1999; Price et al., 2009; Schmitt et al., 2000, Thal et al., 2004, Tomlinson et al., 1968), is an important consideration for the PADW staging criteria. While AD-related pathology was present in some cognitively intact individuals, it was far milder in cognitively intact individuals than in AD, confirming that pAD is often associated with a subthreshold level of AD-type pathology. From a diagnostic perspective, it is clear that Braak neurofibrillary stages III–IV do not indicate “intermediate likelihood” of cognitive impairment related to AD pathology when clinical data are unknown. These and other data indicate that neurofibrillary pathology in the neocortex is a key event associated with cognitive decline (Arriagada et al., 1992a; Dolan et al., 2010; Nelson et al., 2009b, 2010a). The Braak staging criteria, with the first 4 stages focused on the hippocampal formation, were adapted for diagnosis of dementia subjects, but have not been validated for use in samples covering the full spectrum of disease. An increased focus on the pattern of neocortical NFT development may be merited (Markesbery et al., 2006; Nelson et al., 2009b). Correlations in our sample between Braak staging and MMSE scores more closely resemble some previously published series (Whitwell et al., 2008) than others (Jellinger and Attems, 2007b). This may reflect differences in diagnostic thresholds and also the relatively large number of clinically well-characterized, cognitively intact individuals in our sample.
The presence of DPs (stage 1) does not correlate with antemortem cognitive function in our cohort (Table 3), nor is there a substantial group of demented individuals in the UK-ADC autopsy series with DPs only (data not shown). The “Khachaturian criteria” for AD diagnosis (Khachaturian, 1985), based on DP, has been removed from AD diagnostic relevance for over a decade, and our data provide no fresh impetus to revive DP-based neuropathological diagnoses for the detection of clinically evident AD. However, for the purposes of identifying patients with pAD, at risk for eventually developing full-blown AD, and in the context of biomarker development, the presence of DPs alone (stage 1) may be directly relevant. There was subtle cognitive impairment detected in the stage 3 pAD using age-corrected z scores to study MMSE test results. These data are compatible with the PADW criteria that stage 3 cases have “subtle cognitive deterioration”. Because these cases also (by our definition) have Braak stages above II, they also satisfy the criteria of including “markers of neurodegeneration” as NFTs correlate relatively well with cell loss in AD brain (Giannakopoulos et al., 2003).
The present study also considers NFT-predominant pathology that is not well defined using the current NIA-Reagan classification system (1997, Nelson et al., 2010b). We recently described a group of autopsied patients with hippocampal NFTs but without neocortical NP, referred to as stage 0-N in the current study (Nelson et al., 2009a), which raises the question of multiple convergent pathological processes in the development of AD-type pathology. It is not known how these cases relate to the disease “tangle-predominant dementia” (Jellinger and Attems, 2007a), because these cases lack dementia and we did not find patients in our series with tangle-predominant dementia (Nelson et al., 2009a). What is clear is that NFT and amyloid plaques can develop, albeit moderately, in the absence of each other. Although many published studies suggest that NFTs are most directly associated with cell/synaptic injury in AD, NFTs are also seen in many neurodegenerative conditions, in contrast to NPs that are specific to AD (literature reviewed in Nelson et al., 2009b).
Diagnostic accuracy in pAD is critically dependent on the identification of other prevalent diseases linked to brain aging. The findings of cerebrovascular disease (CVD) and LBP in a subset of cognitively intact subjects demonstrate that, like AD, these pathologic findings exist in a preclinical state. The antemortem detection of preclinical cerebrovascular disease is possible with the use of MRI, computerized tomography (CT) scans, and vascular imaging techniques such as angiography and Doppler studies (de Leon et al., 2007; Hachinski et al., 2006; Kalaria et al., 2008; Prins et al., 2004; Yoshita et al., 2006; Zekry et al., 2002). The confident detection of preclinical dementia with Lewy bodies (DLB) is not yet achievable, but previous work demonstrated that predementia stages of dementia with Lewy bodies may be detectable through the recognition of early cognitive and importantly noncognitive symptoms (Jicha et al., 2010; Molano et al., 2010).
Hippocampal sclerosis is a prevalent neurodegenerative disease (~10% of aged brains) that causes hippocampal atrophy and cognitive impairment (Nelson et al., in press), and which raises some questions about the use of MRI-detected hippocampal atrophy as a specific biomarker for AD (Attems and Jellinger, 2006; Hua et al., 2008; Potkin et al., 2009). In fact, both hippocampal sclerosis and FTLD may show reduced MTL volumes early in the disease course (Chertkow and Black, 2007; de Leon et al., 2007). Only 1 pAD case had hippocampal sclerosis pathology, although 12.5% in the MCI subjects did, indicating that hippocampal sclerosis, when present, induces cognitive impairment as shown previously (Dickson et al., 1994; Nelson et al., 2010a).
The difficulty in detecting pAD clinically, at least cross sectionally, highlights the need for development of accurate biomarkers to detect the biological processes in AD that eventually lead to overt clinical decline and dementia (Albert et al., 2001; Chong and Sahadevan, 2005; Hulette et al., 1998; Jobst et al., 1997; Schmitt et al., 2000; Tierney et al., 1996, 2005; Watson et al., 2005). The disease process clearly begins years before the development of clinical symptoms (Arriagada et al., 1992b; Bennett et al., 2006; Braskie et al., 2010; Davis et al., 1999; Haroutunian et al., 1998; Hulette et al., 1998; Knopman et al., 2003; Morris and Price, 2001; Schmitt et al., 2000; Troncoso et al., 1996). Inevitably, a substantial proportion of “normal” control subjects have incipient AD, which is a relevant consideration for the design of research studies comparing normal and AD groups (Becker and Greig, 2008; Cummings et al., 2007; Sabbagh, 2009).
Much work remains to be done given our present level of diagnostic certainty in diverse cohorts. Cerebrospinal fluid (CSF) amyloid levels may not distinguish pAD from LBD in cognitively intact individuals given the prevalence of amyloid pathology in dementia with Lewy bodies (Aarsland et al., 2008; Clark et al., 2003; Gómez-Tortosa et al., 2003; McKeith, 2006; McKeith et al., 1998; Mollenhauer et al., 2005a, 2005b; Vanderstichele et al., 2006). On the other hand, CSF tau and phospho-tau may be reliable discriminators of these pathological disease states in “pure” disease states (Aarsland et al., 2008; Clark et al., 2003; Gómez-Tortosa et al., 2003; Mollenhauer et al., 2005a, 2005b; Vanderstichele et al., 2006) but may not enable the identification of individuals with stage 0-N pathology. Amyloid ligand-PET may fall short of identifying PADW stage 1 subjects (13/126 cases in the current series) given the low affinity of some existing amyloid ligands for DP pathology, although developments in this area appear promising as ligand binding is more fully characterized and new compounds are developed (LeVine, 2005; Lockhart et al., 2007; Thompson et al., 2009).
The good news is that the lack of AD-type pathology in many aged individuals (stage 0 and stage 0-N cases, 57% of cognitively intact persons, average age 82.6 years at death) argues that the eventual development of even mild-to-moderate AD pathology is not inevitable. There is no way to ever prove this as death in the absence of AD pathologynegates the longitudinal follow-up required to fully address this issue. Nonetheless, healthy cognitive and pathological aging appears to be an achievable goal, although the lifestyle, medical, and genetic factors allowing such a favorable pathological outcome are unknown at the present time. Identification of such factors may enable future development of effective preventive strategies for AD.
Limitations of this study include a potential inability to generalize the findings to more disparate racial and socioeconomic groups. The studied cohort, a convenience sample, is almost exclusively Caucasian and well educated (Schmitt et al., 2001). There are always biases in an autopsy sample because there never is a truly epidemiological autopsy cohort in the sense of representing without bias all members of the overall population (Zaccai et al., 2006). Because the research volunteers in the current study were motivated to be seen within a year of death, this could indicate some differences from the general population. Another issue with our dataset is the problem of missingness: not all subjects participated in every cognitive assessment because the standard battery of tests has changed over time. Further, some of the research volunteers died before or during the evolving conceptualization of clinical MCI diagnosis (Petersen et al., 2001; Winblad et al., 2004). This problem was minimized but not negated by the use of chart-based retrospective identification of some MCI patients (Jicha et al., 2010).
Another inherent shortcoming of the present study is that we did not assess all aspects of biomarkers and the pathobiology of AD in these subjects. A wealth of data supports the role of oxidative stress, inflammation, neurotransmitter alterations, synaptic loss, and other pathological neuronal changes (i.e., TDP-43, see Fig. 6) in dementia. Our focus on DPs, NPs, and NFTs in the present study in no way implies that other biochemical alterations lack importance in the development and biomarker-based detection of pAD. Further work defining the potential mechanisms in the disease process is needed.
The strengths of this study lie in the large number of clinically well-characterized, cognitively normal subjects tested cognitively near autopsy and the detailed, quantitative, neuropathologic analysis allowing the characterization of pAD staging and comparison with clinically evident AD. Assessment of non-AD pathologies, including stage 0-N cases, may have relevance to biomarker studies. The stagebased groupings that we used, in correlation to the PADW preliminary recommendations, were based on presumed correlations between biomarkers and neuropathological findings, and these will require further refinement in the future.
Supplementary Material
Acknowledgements
This study was supported by NIH/NIA R01 NS061933, P30 AG028383.
We are deeply grateful to research volunteers at the University of Kentucky Alzheimer’s Disease Center and to the late Dr William R. Markesbery for their many contributions that made the present study possible. We thank Dr Stephen Scheff for performing autopsies. We thank Paula Thomason for reviewing the manuscript, and we thank Ela Patel, Dr Huaichen Liu, and Sonya Anderson, for technical support. We also acknowledge the many researchers that have contributed to the field that could not be appropriately cited herein secondary to space limitations.
Footnotes
Disclosure statement
The authors have nothing to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2011.02.018.
References
- Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol. Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- Aarsland D, Kurz M, Beyer M, Bronnick K, Piepenstock Nore S, Ballard C. Early discriminatory diagnosis of dementia with Lewy bodies. The emerging role of CSF and imaging biomarkers. Dement. Geriatr. Cogn. Disord. 2008;25:195–205. doi: 10.1159/000113417. [DOI] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J. Int. Neuropsychol. Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. Preliminary Recommendations by Preclinical Alzheimer’s Disease Workgroup. 2010 Available at: www.alz.org/research/diagnostic_criteria/ [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992a;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992b;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;66:775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Becker RE, Greig NH. Alzheimer’s disease drug development in 2008 and beyond: problems and opportunities. Curr. Alzheimer Res. 2008;5:346–357. doi: 10.2174/156720508785132299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett LA, Harvey DJ, Gamst A, Donohue M, Kornak J, Zhang H, Kuo JH Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative: Annual change in biomarkers and clinical outcomes. Alzheimers Dement. 2010;6:257–264. doi: 10.1016/j.jalz.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991a;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991b;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM. Plaque and tangle imaging and cognition in normal aging and Alzheimer’s disease. Neurobiol. Aging. 2010;31:1669–1678. doi: 10.1016/j.neurobiolaging.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Black S. Imaging biomarkers and their role in dementia clinical trials. Can. J. Neurol. Sci. 2007;34 Suppl 1:S77–S83. doi: 10.1017/s031716710000562x. [DOI] [PubMed] [Google Scholar]
- Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, Morris JC, McKeel DW, Jr, Farlow M, Weitlauf SL, Quinn J, Kaye J, Knopman D, Arai H, Doody RS, DeCarli C, Leight S, Lee VM, Trojanowski JQ. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinicopathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J. Neuropathol. Exp. Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF studies in the preclinical diagnosis of Alzheimer’s disease. Ann. N.Y. Acad. Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133:2225–2231. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Engelborghs S, Sleegers K, Cras P, Brouwers N, Serneels S, De Leenheir E, Martin JJ, Vanmechelen E, Van Broeckhoven C, De Deyn PP. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer’s disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology. 1995;45:461–466. doi: 10.1212/wnl.45.3.461. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Gómez-Tortosa E, Gonzalo I, Fanjul S, Sainz MJ, Cantarero S, Cemillán C, Yébenes JG, del Ser T. Cerebrospinal fluid markers in dementia with lewy bodies compared with Alzheimer disease. Arch. Neurol. 2003;60:1218–1222. doi: 10.1001/archneur.60.9.1218. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch. Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2221. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Haense C, Herholz K, Jagust WJ, Heiss WD. Performance of FDG PET for detection of Alzheimer’s disease in two independent multicentre samples (NEST-DD and ADNI) Dement. Geriatr. Cogn. Disord. 2009;28:259–266. doi: 10.1159/000241879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch. Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Purohit DP, Perl DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch. Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM Alzheimer’s Disease Neuroimaging Initiative. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage. 2008;43:458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J. Neuropathol. Exp. Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, Decarli CS, Dale AM, Carmichael OW, Tosun D, Weiner MW. Update on the magnetic resonance imaging core of the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2010a;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010b;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’S Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly--an update. J. Alzheimers Dis. 2006;9(3 Suppl):61–70. doi: 10.3233/jad-2006-9s308. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007a;113:107–117. doi: 10.1007/s00401-006-0156-7. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J. Neurol. Sci. 2007b;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Cha RH, Johnson KA, Smith GE, Boeve BF, Petersen RC, Knopman DS. Age and apoE associations with complex pathologic features in Alzheimer’s disease. J. Neurol. Sci. 2008;273:34–39. doi: 10.1016/j.jns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch. Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Schmitt FA, Abner E, Nelson PT, Cooper GE, Smith CD, Markesbery WR. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol. Aging. 2010;31:1805–1813. doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst KA, Barnetson LP, Shepstone BJ. Accurate prediction of histologically confirmed Alzheimer’s disease and the differential diagnosis of dementia: the use of NINCDS-ADRDA and DSM-III-R criteria, SPECT, X-ray CT, and APO E4 medial temporal lobe dementias. The Oxford Project to Investigate Memory and Aging. Int. Psychogeriatr. 1997;9 Suppl 1:191–222. discussion 47–52. [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, Stewart R, Wimo A, Zhang ZX, Antuono P. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaki E, Paraskevas GP, Zalonis I, Zournas C. CSF tau protein and beta-amyloid (1–42) in Alzheimer’s disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. Eur. J. Neurol. 2003;10:119–128. doi: 10.1046/j.1468-1331.2003.00562.x. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch. Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J. Neuropathol. Exp. Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Multiple ligand binding sites on A beta(1–40) fibrils. Amyloid. 2005;12:5–14. doi: 10.1080/13506120500032295. [DOI] [PubMed] [Google Scholar]
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, Desanti S, Kemppainen N, Nagren K, Kim BC, Tsui W, de Leon MJ. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J. Neuropathol. Exp. Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch. Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J. Alzheimers Dis. 2006;9(3 suppl):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Ince P, Jaros EB, Fairbairn A, Ballard C, Grace J, Morris CM, Perry RH. What are the relations between Lewy body disease and AD? J. Neural Transm. Suppl. 1998;54:107–116. doi: 10.1007/978-3-7091-7508-8_10. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Misra C, Fan Y, Davatzikos C. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage. 2009;44:1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133:540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Bibl M, Trenkwalder C, Stiens G, Cepek L, Steinacker P, Ciesielczyk B, Neubert K, Wiltfang J, Kretzschmar HA, Poser S, Otto M. Follow-up investigations in cerebrospinal fluid of patients with dementia with Lewy bodies and Alzheimer’s disease. J. Neural Transm. 2005a;112:933–948. doi: 10.1007/s00702-004-0235-7. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cepek L, Bibl M, Wiltfang J, Schulz-Schaeffer WJ, Ciesielczyk B, Neumann M, Steinacker P, Kretzschmar HA, Poser S, Trenkwalder C, Otto M. Tau protein, Abeta42 and S-100B protein in cerebrospinal fluid of patients with dementia with Lewy bodies. Dement. Geriatr. Cogn. Disord. 2005b;19:164–170. doi: 10.1159/000083178. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J. Mol. Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch. Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, Smith CD, Patel E, Markesbery WR. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009a;68:774–784. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009b;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J. Neuropathol. Exp. Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J. Neuropathol. Exp. Neurol. 2010;69:449–454. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ. Hippocampal sclerosis in advanced age: clinical and pathological features in 106 cases. Brain. doi: 10.1093/brain/awr053. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Farlow MR, DeCarli C, Raskind MA, Schellenberg GD, Lee VM, Galasko DR. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch. Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, Weiner M, Macciardi F. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch. Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr. Alzheimer Res. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, Harvey DJ, Jack CR, Jr, Weiner MW, Saykin AJ. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol. Aging. 2010;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN. Drug development for Alzheimer’s disease: where are we now and where are we headed? Am. J. Geriatr. Pharmacother. 2009;7:167–185. doi: 10.1016/j.amjopharm.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. Gerontologist. 2001;41:716–722. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J. Alzheimers Dis. 2009a;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009b;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement. Geriatr. Cogn. Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Minthon L, Blennow K, Rosen I, Londos E. Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiol. Aging. 2010;31:215–223. doi: 10.1016/j.neurobiolaging.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci. Aging Knowl. Environ. 2004;23:e26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- Thompson PW, Ye L, Morgenstern JL, Sue L, Beach TG, Judd DJ, Shipley NJ, Libri V, Lockhart A. Interaction of the amyloid imaging tracer FDDNP with hallmark Alzheimer’s disease pathologies. J. Neurochem. 2009;109:623–630. doi: 10.1111/j.1471-4159.2009.05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer’s disease in memory-impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J. Neurol. Sci. 1968;7:331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol. Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Vreese K, Blennow K, Andreasen N, Sindic C, Ivanoiu A, Hampel H, Bürger K, Parnetti L, Lanari A, Padovani A, DiLuca M, Bläser M, Olsson AO, Pottel H, Hulstaert F, Vanmechelen E. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 2006;44:1472–1480. doi: 10.1515/CCLM.2006.258. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brewer J, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jr, Jennings RG, Karow D, Dale AM. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR Am. J. Neuroradiol. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA, Toga A, Green R, Walter S, Soares H, Snyder P, Siemers E, Potter W, Cole PE, Schmidt M Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211. e7. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, Vemuri P, Senjem ML, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW, Jack CR., Jr MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: Design, methods and areas of investigation--a systematic review. BMC Neurol. 2006;6:2. doi: 10.1186/1471-2377-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J. Am. Geriatr. Soc. 2002;50:1431–1438. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.