Abstract
Retinoic acid receptors (RARs) α, β and γ are key regulators of embryonic development. Hematopoietic differentiation is regulated by RARα, and several types of leukemia show aberrant RARα activity. Through microarray expression analysis, we identified transcripts differentially expressed between F9 wild-type (Wt) and RARα knockout cells cultured in the absence or presence of the RAR-specific ligand all trans retinoic acid (RA). We validated the decreased Mest, Tex13, Gab1, Bcl11a, Tcfap2a and HMGcs1 transcript levels, and increased Slc38a4, Stmn2, RpL39l, Ref2L, Mobp and Rlf1 transcript levels in the RARa knockout cells. The decreased Mest and Tex13 transcript levels were associated with increased promoter CpG-island methylation and increased repressive histone modifications (H3K9me3) in RARα knockout cells. Increased Slc38a4 and Stmn2 transcript levels were associated with decreased promoter CpG-island methylation and increased permissive histone modifications (H3K9/K14ac, H3K4me3) in RARα knockout cells. We demonstrated specific association of RARα and RXRα with the Mest promoter. Importantly, stable expression of a dominant negative, oncogenic PML–RARα fusion protein in F9 Wt cells recapitulated the decreased Mest transcript levels observed in RARα knockout cells. We propose that RARα plays an important role in cellular memory and imprinting by regulating the CpG methylation status of specific promoter regions.
INTRODUCTION
Retinoic acid (RA) receptors α, β and γ are nuclear receptors that function as ligand-activated regulators of embryonic development. Retinoic acid receptor α (RARα) is the major RA receptor involved in hematopoietic differentiation (1). In vitro, RARα was shown to regulate the transcriptional activities of RA target genes by dimerizing with retinoid X receptors (RXRs) and binding to retinoic acid response elements (RAREs) (2,3), but little is known about transcriptional regulation by endogenous RARα.
Translocation events targeting the RARα gene is a recurring theme in acute promyelocytic leukemia (APL) (4–6), thus pointing to oncogenic functions of the resulting RARα fusion proteins. The most common translocation generates a PML–RARα fusion protein which is sufficient to induce APL in mouse models (7). The expression of leukemic RARα fusion proteins attenuates the transcriptional induction by RA (2,4) and induces DNA hypermethylation of specific genes (8). Importantly, aberrant promoter methylation is a key feature in APL patients (9,10). The deregulated promoter methylation in cells from APL patients and the binding of RARα to genomic regions devoid of RA inducible genes (11,12) strongly argue for a role of RARα in regulating cellular memory and imprinting through CpG methylation of promoter regions.
The nuclear receptor co-regulatory protein TIF1α (Trim24) modulates the function of RARα in a ligand-dependent manner, and loss of TIF1α increases the incidence of hepatocellular carcinomas in animal models (13), indicating that unmodulated RARα activity in certain contexts can drive tumorigenesis. Studies from MMTV-wnt1 animal models demonstrated that agonist activated RARα inhibited mammary tumor formation and growth, but that the loss of RARα delayed mammary tumorigenesis (14), thus supporting roles of RARα as both a tumor suppressor and protooncogene. Current models of nuclear receptor action suggest that the different functions of RARα are dictated by cofactor recruitment, resulting in epigenetic changes, e.g. histone modifications (15).
Histone modifications are important hallmarks of epigenetic activity. Acetylation of histone 3 lysines 9 and 14 (H3K9/K14ac) and trimethylation of histone 3 lysine 4 (H3K4me3) are frequently increased in the promoter proximal regions of actively transcribed genes relative to silenced genes (16,17). In contrast, trimethylation of histone 3 lysine 9 (H3K9me3) is associated with imprinting and transcriptional repression (16,18). H3K9me3 also marks genes differentially expressed in human cancers (19). The inverse relationship between H3K4me3 and H3K9me3 is emphasized by the observation that HP1, a key factor in the formation of heterochromatin, binds with high affinity to histone H3 methylated at lysine 9 but not at lysine 4 (18). Consequently, the transcriptional status is reflected by various epigenetic marks.
The heritable silencing and imprinted regulation of mammalian transcription relies in part on the symmetric methylation of CpG dinucleotides (20). In humans, imprinting defects have been associated with Beckwith-Wiedemann, Prader-Willi and Angelman syndromes (21,22). The processes of hypermethylation and de novo DNA methylation have been extensively studied in development as well as in cancer, but less is known about the role of DNA demethylation (23). This may be because enzymes catalyzing the active removal of cytosine methylation have not been identified, and because appropriate model systems have not been established (24). As a consequence, it is not clear how specific promoters are targeted for methylation or demethylation.
The F9 embryonal carcinoma stem cell system is a well-established model for RA signaling (25). Importantly, F9 cells are genomically stable and closely resemble embryonic stem cells in morphology, growth behavior and marker expression (25,26). We show here that in F9 cells the knockout of RARα is associated with reduced Mest transcript levels and gene-specific epigenetic changes in the Mest promoter region, an effect that is partially rescued by restoring RARα2 expression. Furthermore, a similar decrease in Mest transcript level can be seen by overexpression of the dominant negative PML–RARα oncoprotein. Our findings demonstrate that the loss of a single transcription factor can induce extensive, gene-specific changes in DNA methylation and thus alter the epigenetic signature of the cell. Our findings yield new insights into the mechanisms of APL and hereditary disorders resulting from defective genetic imprinting. We conclude that in F9 stem cells RARα sustains the transcription of Mest and Tex13 and prevents the transcription of Slc38a4 and Stmn2 by maintaining promoter specific epigenetic signatures independent of the RA ligand.
MATERIALS AND METHODS
Cell culture and RA treatment of F9 teratocarcinoma cells
F9 Wt and RARα−/− cells were propagated as described (27,28). A batch of F9 RARα−/− cells with low passage number was revived from liquid nitrogen cryo-storage and the genotype of the RARα−/− cell line was confirmed by western blot (Figure 1F). For microarray analyses 2.0 × 106 cells were plated 16 h prior to drug treatment. All-trans RA (Cat. #R2625, Sigma, MD, USA) and cycloheximide (chx) (Cat. #C7698, Sigma, MD, USA) were dissolved in 100% ethanol (EtOH). The cells were pretreated with 1 µg/ml chx for 30 min before 7.5 h treatment with RA (1 µM) or vehicle (EtOH, 0.1%). For gene expression analyses F9 cells were cultured in RA (1 µM) or vehicle (0.1%, EtOH) 24 or 8 h prior to RNA harvest.
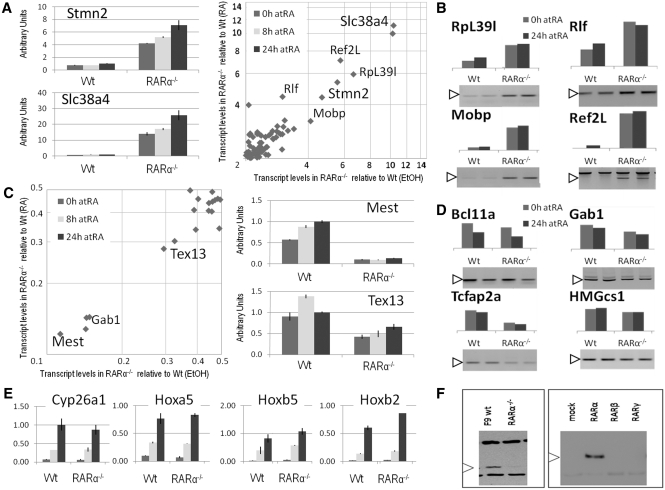
Figure 1.
Gene expression analyses of wild-type and RARα knockout cells. Relative transcript levels were identified by microarray analysis and the genes differentially expressed (2-fold or more difference in transcript levels between wild-type and RARα knockout cells) were plotted as fold difference in presence of RA against the fold difference in vehicle-treated cells (A right panel, transcript levels in RARα−/− cells >2-fold transcript levels in Wt cells; C left panel, transcript levels in RARα−/− cells <0.5-fold transcript levels in Wt cells). Selected genes with increased transcript levels in RARα−/− cells (Slc38a4 and Stmn2) and with decreased transcript levels in RARα−/− cells (Mest and Tex13) were validated by real-time PCR (A left panel and C right panel, respectively) to be differentially expressed between Wt and RARα−/− cells in a ligand-independent manner (measured in arbitrary units correlated with 36B4 expression, note the logarithmic scale). The duration of the RA treatment is indicated by the bar color (0 h; gray, 8 h; light gray, and 24 h; dark gray). Additional genes were validated by semi-quantitative PCR to be differentially expressed between Wt and RARα−/− cells in a ligand-independent manner. Genes with increased transcript levels (B) and genes decreased transcript levels (D). Different time points of RA treatment are indicated by the bar color (0 h; gray, and 24 h; dark gray). The specific bands (white arrowheads) and the relative transcript levels (bars above gels) are indicated. Assessment of household gene (36B4 and HPRT1) transcript levels confirmed similar amounts of cDNA in all samples (data not shown). (E) Transcript levels of RA inducible genes (Cyp26a1, Hoxa5, Hoxb5 and Hoxb2) assessed by real-time PCR at 0, 8 and 24 h of RA treatment of Wt and RARα−/− cells (correlated with 36B4 transcript levels). Each graph is a compilation of three independent biological replicates. (F) Western blot validation of the RARα knockout cell line. A band of the expected size is detected by an RARα specific antibody in Wt, but not in the RARα−/− cell line (left). In lysates from transfected COS cells RARα, but not RARβ or RARγ, is detected by the Ab (Santa Cruz, sc-551). The data represent three independent assays (microarray and real-time PCR), harvesting new RNA for each experiment, or a representative assay out of at least three independent assays (semi-quantitative PCR and western blot).
Purification of RNA, microarray analysis and statistical analysis
Total RNA was extracted and on-column DNase treatment was carried out using a RNAeasy mini kit (Cat. #74104, Qiagen, MD, USA) according to the manufacturers’ specifications. Preparation of cRNA, gel electrophoresis quality control, chip hybridization and scanning were carried out by the Microarray Core Facility at Weill Cornell Medical College (WCMC). The microarray analyses were performed following the Affymetrix Genechip expression analysis technical manual. The fragmented cRNA was hybridized to the microarray chips (MG-430.2, Cat. #900496, Affymetrix, CA, USA), which include over 45 000 transcripts representing 34 000 substantiated mouse genes. The hybridization assays were performed using biological triplicates (e.g. independently propagated cells). The data were analyzed using Genespring v7.0 software (Agilent Technologies, CA, USA). Briefly, the data sets were summarized using the GC-RMA algorithm, followed by a three-step normalization process consisting of: (i) data transformation, converting values <0.01–0.01, (ii) chip normalization to the 50th percentile of total intensity and (iii) per gene normalization to median intensity. Genes were filtered by expression level to exclude genes with a raw signal below 100. In order to identify RA-induced genes that were differentially expressed between Wt and RARα−/− cell line, treatment (RA+chx) versus control (EtOH+chx) comparisons were performed for each cell line. To determine which genes were differentially expressed at statistically significant levels between EtOH- and RA-treated cells, a one-way ANOVA (P < 0.05 cutoff) was performed on each of the two groups (Wt and RARα−/− cells). To identify genes which were differentially expressed between Wt and RARα−/− cell lines independent of RA treatment, Wt versus RARα−/− cell line comparisons were performed for each of the two conditions (RA+chx and vehicle+chx), and a one-way ANOVA (P < 0.05 cutoff) was performed on each of the two groups (EtOH and RA-treated cells). The data have been deposited in the GEO database (accession #GSE31280). For reverse transcriptase (RT) reactions total RNA was extracted using Trizol reagent (Cat. #15596, Invitrogen, CA, USA). The RNA was quantitated by optical density at 260 nm.
Generation of cDNA, semi-quantitative and real-time PCR reaction
Total RNA (1.5 µg) isolated from F9 cells was reverse transcribed (Cat. #95048, Quanta Biosciences, MD, USA), then diluted 1:10 with H2O. Subsequent PCR reactions were set up using 3–5 µl as template. Semi-quantitative PCRs were performed with Taq polymerase (0.5 U, Cat. #CB4050, Denville Scientific, NJ, USA) in a BioRad iCycler: (95°C, 120 s)×1, (94°C, 15 s; 55–65°C, 30 s; 72°C, 30 s)×28–35, (72°C, 4 min)×1. Annealing temperature and number of repeats were determined empirically for each primer pair such that the PCR was in the linear range. Amplification in the linear range was demonstrated by including a 3-fold serial dilution of cDNA from RA-treated Wt cells in each reaction (1:1, 1:3, 1:9). An RT reaction without RT enzyme served as negative controls for gDNA template. The PCR products were subjected to electrophoresis on TAE gels with 1.5% (w/v) agarose and 0.3 mg/ml ethidium bromide. The bands were visualized and quantified using a FluorChem 8800 system (Alpha Innotech, CA). Real-time PCR was performed using SYBR Green Supermix (Cat. #84091, Quanta Biosciences, MD, USA) in a 15 µl reaction containing reaction-mix (×1), 0.25 μM of each primer and 3 µl of cDNA template. The reactions were run on a Bio-Rad MyiQTM Single Color Real-time PCR Detection System (BioRad, CA, USA). Amplification in the linear range was demonstrated by a serial dilution of cDNA from RA-treated Wt cells included in each reaction (1:1, 1:5, 1:10, 1:50, 1:100, 1:500). Reactions with H2O and template without RT enzyme, respectively, served as negative controls for primer–dimer and for amplification of residual genomic DNA (gDNA). Primer sequences are listed in the Supplementary Appendix S1A. Each expression analysis was performed using biological triplicates (e.g. independently propagated cells, repeated three times).
Purification of gDNA and Bisulfite methylation assay
gDNA was isolated from untreated F9 Wt and RARα−/− cells and purified (29,30). Promoter-specific methylation was assessed by bisulfite sequencing (31). The regions assayed were Mest: −337; +116, Slc38a4: −123; +374, Stmn2: −103; +224, Tex13: −248; +29 and Cyp26a1: −67; +152 relative to the transcriptional start site of each gene, see Supplementary Appendix S1A for details (Supplementary Data). In brief, cytosine to uracil conversion was performed on 1.0 µg of gDNA according to the manufacturer (Cat. #D5001, Zymo Research, CA, USA). PCR reactions were performed on the converted DNA with promoter-specific primers (Supplementary Data) designed using the online Methprimer software (32). PCR reactions were set up using 3–5 µl cytosine to uracil converted gDNA as template with Taq polymerase (Cat. #10342, Invitrogen, CA, USA) in 15 µl reactions containing PCR buffer [final concentration of 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2], 0.2 mM concentration of each deoxynucleoside triphosphate, 0.2 μM concentration of each primer, and 3–5 µl of cDNA template. Sequential PCR reactions were performed using converted gDNA (3–5 µl), and then 1 µl of the initial PCR, as templates in a BioRad iCycler using following protocol: (95°C, 120 s)×1, (94°C, 15 s; 55°C, 30 s; 72°C, 30 s)×35–40, (72°C, 4 min)×1. In the second PCR reaction, semi-nested primer pairs were utilized for increased fidelity in the amplification of Mest and Stmn2 promoter region. The PCR products were purified using PCR purification kit (Cat. # 28106, Qiagen, MD, USA), and the eluted DNA was recovered by ligation into the pGEM-T easy vector (Cat. #A1360, Promega, WI, USA). The ligated products were transformed into competent DH5α bacteria, and plated on LB agar plates containing ampicillin for overnight incubation at 37°C. DNA was isolated from resistant colonies and confirmed by NotI restriction digest (Cat. #R0189, New England Biolabs, MA, USA) to contain inserts of the expected sizes. From each F9 cell line (Wt and RARα−/−) a total of 10n independent clones containing each of the desired PCR products were sequenced using T7(+) and SP6(+) primers (Supplementary Data).
Western blots
The SDS–PAGE and western blot analysis were performed as described (30,33) using RARα primary Ab (Cat. #sc-551, Santa Cruz, CA, USA) in a 1:1000 dilution and HPR-conjugated anti-rabbit secondary Ab (Cat. #sc-2030, Santa Cruz, CA, USA) in a 1:10 000 dilution. Each Ab was diluted in PBS with 5% Blotto (Biorad, CA, USA) and 0.1% Tween-20. The membranes were developed with Supersignal Substrate (Cat. #34080, Pierce, IL, USA) for 5 min and exposed to Biomax film (Eastman Kodak, NY, USA).
Chromatin immunoprecipitation assays
A one-step ChIP protocol which utilizes formaldehyde cross-linking was employed for histone ChIP assays. For transcription factor ChIP assays (RARα and RXRα), we used a two-step ChIP protocol. IPs of sonicated chromatin from 5.0× 106 F9 cells were performed with 2 µg of Ab per IP (30,31). The regions amplified were Mest: −337; −197, Slc38a4: −123; +171, Stmn2: −103; +22, Tex13: −132; +32 and Cyp26a1: −97; −10 relative to the transcriptional start site of each gene (Supplementary Data). Each ChIP assay was performed using biological triplicates (e.g. independently propagated cells). Abs: H3K9me3 (ab8898, Abcam, MA, USA), H3K4me3 (07-473, Upstate, MA, USA), H3K9/K14ac (06-599, Upstate, MA, USA), RARα (sc-551, Santa Cruz, CA, USA), RXRα (sc-553, Santa Cruz, CA, USA), Rabbit-IgG (sc-2027, Santa Cruz, CA, USA).
Generation of stable clones
The pSG5 mRARα2 and pSG5 mPML–RARα expression vectors were stably introduced into F9 Wt and RARα−/− cells, respectively (Supplementary Data). In brief, the expression vector and a selection vector, pPGK Hygromycin (34), were co-transfected into F9 cells using lipofectamine2000 (Cat. #11668, Invitrogen, CA, USA) as described (35). Colonies were picked and screened (28). PCR screening was performed using the mRARαE6(+)/mRARαE7(−) primer pair. Successful gDNA purification was evident by a 414-bp PCR product, whereas integration of the transgene was evident by an additional PCR product of 239 bp. Transgene expression in positive clones was verified by the presence of a 162-bp PCR product using the rβ-globin5′C/rβ-globin3′B primer pair, which spans the β-globin intron of the pSG5 vector (primer sequences (Supplementary Data)).
Accession of data sets
Gene expression profiles were deposited at GEO with the accession code GSE31280 (http://www.ncbi.nlm.nih.gov/geo/querry/acc.cgi?acc=GSE31280).
Statistical analysis
Biological triplicates were analyzed using one-way ANOVA in the expression and ChIP analyses. The data was analyzed by a two step approach; the first step assessed the effect of RA (vehicle versus RA-treated cells). The second step assessed the effect of RARα (Wt versus RARα−/− cells). If the difference between vehicle and RA-treated cells (first step) was not significant the data was collectively assessed when assessing the effect of the genotype (second step). The standard deviation was determined for each of the data sets (plotted as error bars in the graphs), and ANOVA values of P < 0.05 between compared samples were assigned statistical significance.
RESULTS
Transcriptome profiling of F9 Wt and RARα knockout cells reveals a ligand-independent role of RARα
We aimed to identify novel genes whose expression depends on RARα. Therefore, we performed a comparative microarray analysis of F9 Wt and F9 RARα knockout (RARα−/−) cells cultured in the presence or absence of RA. Through pair-wise comparison between the Wt and RARα−/− cell lines in either the presence or absence of RA (see supplementary material for details (Supplementary Data)), we identified several genes that were differentially expressed (Figure 1A and C display genes increased and decreased, respectively, in RARα−/− relative to Wt cells). Excluding multiple hits of the same genes, we found 63 transcripts with elevated levels in RARα−/− cells and 14 transcripts with reduced levels in RARα−/− cells relative to levels in Wt cells.
We reviewed the functions of the differentially expressed genes to elucidate the pathways in which RARα may be implicated. Slc38a4 (solute carrier family 38, member 4; Ata3) is a paternally expressed gene regulated by imprinting (36). Slc38a4 encodes an arginine transporter found in placental tissue and in adult liver and muscle (37). Stmn2 (Statmin 2; Scg10) is differentially expressed during neoplastic conversion of human prostate epithelial cells and may be an early marker of cancer initiation (38). Ref2L (similar to RNA and export factor-binding protein 1–II) may, by similarity to Thoc4, function as a co-factor for PML (39). RpL39l (ribosomal protein L39-like) is involved in spermatogenesis and the human homolog is overexpressed in various types of cancer, including ovarian cancer (40). Mobp (myelin-associated oligodendrocytic basic protein) stabilizes the myelin sheath (41). Rlf (rearranged l-myc fusion sequence) is the intrachromosomal recipient of the l-myc rearrangement. Expression of the resulting fusion protein inhibits embryonic stem cell differentiation and embryoid body formation (42).
Mest (mesoderm specific transcript; Peg1) is a paternally expressed gene regulated by imprinting; the Mest promoter is unmethylated in sperm, but highly methylated in oocytes (43,44). Decreased Mest expression has been associated with glioblastoma (45), whereas invasive breast and lung cancer show increased Mest expression (46). Tex13 (testis expressed gene 13B) is located on the X chromosome and may play a role in spermatogenesis (47). Gab1 (growth factor receptor-bound protein 2 associated protein 1) is a key factor in endothelial development (48). Tcfap2a (transcription factor AP-2, alpha) is induced during RA-mediated differentiation of primary astrocytes (49). Abnormal expression of the human homolog was reported in breast cancer, acute lymphoblastic leukemia, and head and neck squamous cell carcinomas (50–52). HMGcs1 (3-hydroxy-3-methylglutaryl-CoA synthase 1) is involved in cholesterol biosynthesis (53).
The genes that are differentially expressed between Wt and RARα−/− cells are predominantly expressed in the placenta, in the brain and in the testis (Supplementary Data). These are tissues where imprinting plays a major role in transcriptional regulation (54,55) thus suggesting that RARα could be involved in regulating genetic imprinting.
We verified transcript levels of a subset of the differentially expressed genes identified in our microarray analysis. The Slc38a4 and Stmn2 transcript levels were assessed by real-time PCR upon 0, 8 and 24 h of RA treatment (Figure 1A, left panel). Relative to F9 Wt cells the RARα−/− cells exhibited increased levels of Slc38a4 and Stmn2 at all three time points. The observed increases in transcript levels of Ref2L, Rlf, RpL39l and Mobp in RARα−/− cells relative to Wt cells were validated by semi-quantitative PCR (Figure 1B). The decreased Mest and Tex13 transcript levels in RARα−/− cells relative to F9 Wt cells were validated by real-time PCR after 0, 8 and 24 h of RA treatment (Figure 1C). The observed decreases in Bcl11a, Gab1, HMGcs1 and Tcfap2a transcript levels in RARα−/− cells relative to Wt cells were validated by semi-quantitative PCR (Figure 1B). Consistent with the microarray data, the transcript levels were affected by the knockout of RARα, but were relatively insensitive to RA.
The transcript levels of 11 out of 12 differentially expressed genes were not changed by addition of RA. This was somewhat unexpected as RARα is an RA activated nuclear receptor. In contrast, the microarray analyses identified several known RA-inducible target genes (Cyp26a1, Hoxa5, Cdx1, Tmtc1, Csn3, Aurkc, MP11, Pdgfrβ, RARβ2, Hoxb5 and Hoxb2 (Supplementary Data)) as showing >3-fold increases in transcript levels upon addition of RA to F9 Wt cells for 8 h.
We validated four of the RA responsive genes (Cyp26a1, Hoxa5, Hoxb5 and Hoxb2) by real-time PCR, and demonstrated a similar induction of these genes in F9 Wt and RARα−/− cells upon addition of RA to F9 Wt cells for 8 h and for 24 h (Figure 1E). This demonstrates a functional transcriptional response to the RA treatment, possibly mediated by RARβ and/or RARγ. Thus, in the F9 RARα−/− cells the addition of RA induces transcription of several known RA responsive genes. Specifically, the transcript levels of Cyp26a1, Hoxa5, Hoxb5 and Hoxb2 were increased by 10-fold or more while transcript levels of Mest, Tex13, Slc38a4 and Stmn2 were increased by <2-fold upon 24 h of RA treatment of F9 Wt and RARα−/− cells (Figure 1A and C). Overall, these results indicate that in F9 Wt cells, but not in RARα−/− cells, the levels of several transcripts, including Mest, Tex13, Slc38a4 and Stmn2, are maintained in an RA-independent manner.
The epigenetic signatures of Mest, Tex13, Slc38a4, and Stmn2 promoters are altered in RARα knockout cells
The largest differences in transcript levels between the Wt and RARα−/− cells were observed for Slc38a4 and Mest (>10-fold difference in RARα−/− versus Wt cells). These genes are both paternally expressed (36,43), which suggests that transcription of Slc38a4 and Mest, and possibly also of Stmn2 and Tex13 are regulated by differential CpG promoter methylation in Wt versus RARα−/− cells. We identified CpG-rich promoter regions and assessed the promoter-specific methylation status of Slc38a4, Mest, Stmn2 and Tex13 (the regions amplified are specified in Figure 2C (Supplementary Data)). Because the Mest, Tex13, Slc38a4 and Stmn2 transcript levels are not affected by RA treatment (Figure 1A and C), we assessed the methylation status of each of these genes in untreated F9 Wt and RARα−/− cells.
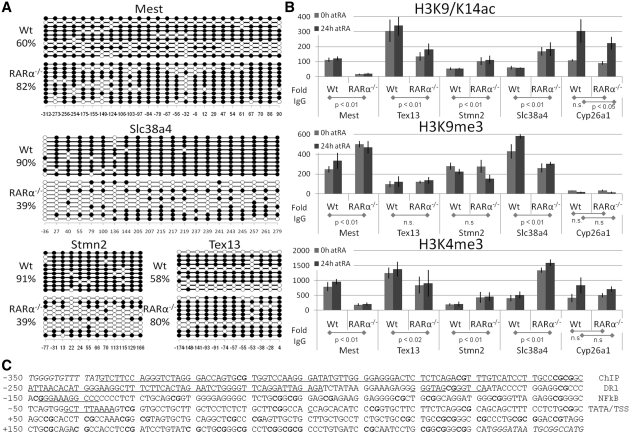
Figure 2.
The Epigenetic Signatures of Mest, Tex13, Stmn2 and Slc38a4 promoter regions. (A) Mest and Tex13 promoters displayed increased methylation in RARα−/− relative to Wt cells. In contrast, Slc38a4 and Stmn2 displayed decreased methylation in RARα−/− relative to Wt cells. Each horizontal line represents the methylation status of an independent allele. The numbers below the figures indicate the CpG position relative to the transcriptional start site (+1). (B–D) Promoter specific ChIP were quantified by real-time PCR on chromatin IPed from Wt and from RARα knockout cells treated with vehicle or RA (vehicle—0 h; gray and RA—24 h; dark gray bars). (B) Histone modification in F9 Wt and RARα−/− cells. H3K9/14ac modifications (upper panel). In RARα−/− cells H3K9/K14ac levels at the Mest and Tex13 promoters is decreased, while levels at the Stmn2 and Slc38a4 promoters is increased relative to Wt. (C) H3K9me3 modifications (middle panel). In RARα−/− cells the H3K9me3 level at the Mest promoter is increased, while the level at the Slc38a4 promoter is decreased. H3K9me3 levels at the Tex13 and Stmn2 promoters are not significantly changed in RARα−/− cells. A low signal (15- to 30-fold above the IgG) for H3K9me3 is seen at the Cyp26a1 promoter. (D) H3K4me3 modifications (lower panel). In RARα−/− cells H3K4me3 levels at the Mest and Tex13 promoters are decreased, whereas the levels at the Stmn2 and Slc38a4 promoters are increased relative to Wt. The signal from the IgG IP was set to 1 for each PCR. The data represent three independent IPs for each Ab, harvesting new chromatin for each IP. Statistical significance is demonstrated by P-values below 0.05 for the indicated comparisons. (C) Mest proximal promoter region. The promoter (excluding the sequences in italics) was evaluated for CpG methylation (bold). Underlined sequences indicate putative elements: RARE (DR1), NFkB-binding site, TATA box (TATA), transcriptional start site (TSS) and the exact region targeted by ChIP primers (labels to the right).
The methylation analysis revealed increased CpG methylation of the Mest and Tex13 promoter regions in F9 RARα−/− cells, from ~60% for either gene in Wt cells to 80% in RARα−/− cells (Figure 2A). Notably, two CpGs of the Mest promoter region (−32 and −149) persistently show low levels of methylation, even in RARα−/− cells. The increased promoter methylation correlates with the decreased Mest and Tex13 transcript levels in RARα−/− cells (Figure 1C). The increased promoter methylation in the RARα−/− cells supports the idea of a cellular memory, through increased DNA methylation, by which de novo methylation is maintained through subsequent cell divisions. Conversely, methylation of the Slc38a4 and Stmn2 promoters is decreased in F9 RARα−/− cells from ~90% for both genes in Wt to 40% in RARα−/− cells (Figure 2A). In summary, the knockout of F9 RARα results in increased Mest and Tex13 promoter methylation and decreased Slc38a4 and Stmn2 promoter methylation, each of which is correlated with altered transcript levels in RARα−/− relative to F9 Wt cells (Figures 1A, C and 2A). In contrast, the Cyp26a1 promoter region was unmethylated even when transcriptionally silent, i.e. in the absence of RA [data not shown and (31)]
We wanted to elucidate the underlying mechanisms of RA-independent transcriptional regulation by RARα by measuring key histone marks that characterize the epigenetic signatures of the Mest, Tex13, Slc38a4 and Stmn2 promoter regions. Specifically, we assessed the H3K9/K14ac, H3K9me3 and H3K4me3 marks on the Mest, Tex13, Slc38a4 and Stmn2 promoters in the Wt and RARα−/− cell lines by chromatin immunoprecipitation (ChIP). In addition, the histone marks on the RA inducible Cyp26a1 promoter (Figure 1E) were assessed as a control (Mest, Tex13, Stmn2, Slc28a4 and Cyp26a1 ChIP target regions (Supplementary Data)).
The H3K9/K14ac levels at the Mest and Tex13 promoters decreased, while levels at the Stmn2 and Slc38a4 promoters increased in RARα−/− relative to Wt (Figure 2B, upper panel). Increased H3K9/K14ac levels at the Cyp26a1 promoter in RA-treated cells corroborate transcriptional activation by RA in Wt and in RARα−/− cells (Figures 1D and 2B). We also found that the H3K9me3 levels at the Mest promoter increased, while levels at the Slc38a4 promoter decreased in RARα−/− relative to Wt cells (Figure 2B, middle panel). H3K9me3 levels displayed no changes at the Tex13 and Stmn2 promoters in F9 RARα−/− relative to Wt cells (Figure 2B, middle panel). The H3K4me3 levels at the Mest and Tex13 promoters decreased in RARα−/− relative to Wt cells (Figure 2B, lower panel), while levels at the Stmn2 and Slc38a4 promoters increased relative to Wt cells (Figure 2B, lower panel). Increased H3K4me3 levels at the Cyp26a1 promoter in RA-treated cells further corroborate transcriptional activation by RA (Figure 2B, lower panel). Consequently, the changes in DNA methylation in the Mest, Tex13, Slc38a4 and Stmn2 promoter regions upon knockout of RARα correlate with changes in histone marks associated with the respective promoters. Importantly, these specific changes in epigenetic signatures also correlate with specific changes in Mest, Tex13, Slc38a4 and Stmn2 transcript levels in RARα−/− cells relative to Wt cells.
RARα and RXRα associate with the Mest, but not the Tex13, Slc38a4, and Stmn2 promoters
We next wanted to determine if Mest, Tex13, Slc38a4 and Stmn2 are direct targets of RARα, we performed ChIP using an RARα antibody and measured the enrichment of Mest, Tex13, Slc38a4 and Stmn2 promoters in chromatin immunoprecipitated from Wt relative to that from RARα−/− cells. In order to assess RARα heterodimerization with RXRα we performed parallel ChIP assays using an RXRα antibody (Supplementary Data).
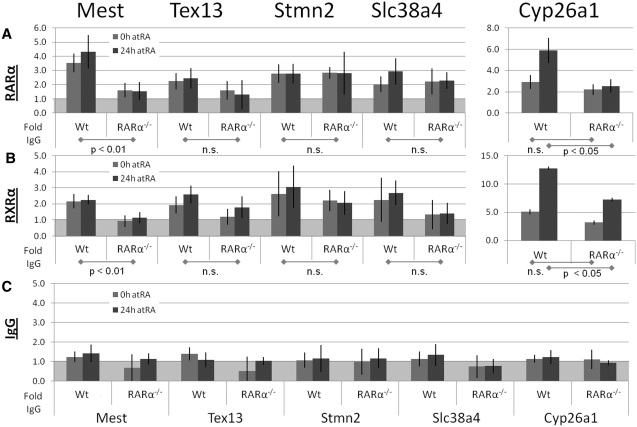
We identified RARα at the Mest promoter (>2-fold in both untreated and RA-treated Wt cells relative to RARα−/− cells, P < 0.05), but not at the Tex13, Stmn2 and Slc38a4 promoter regions (each <2-fold enrichment, P > 0.05). This suggests that only the Mest promoter is a direct target of RARα (Figure 3A). In contrast, RARα levels at the Cyp26a1 promoter increased upon RA treatment in Wt cells (>2-fold, P < 0.05), but not in RARα−/− cells (P > 0.05). However, the transcription of Cyp26a1 is not dependent on RARα since similar Cyp26a1 transcript levels are observed in Wt and RARα−/− cells after RA addition (Figure 1E), which is consistent also with transcription of Cyp26a1 relying primarily RARγ (29). We conclude that RARα is associated with the Mest promoter, but not with Tex13, Stmn2, and Slc38a4 promoters. In support of our findings a genome-wide mapping of RARα-binding sites in MCF-7 breast cancer cells demonstrated binding of RARα proximal to the Mest promoter, but not to the Tex13, Stmn2 and Slc38a4 promoters (12).
Figure 3.
RARα and RXRα binding to target promoters. Promoter specific ChIP were quantified by real-time PCR on chromatin IPed from Wt and from RARα−/− cells treated with vehicle or RA (vehicle–0 h; gray and RA–24 h; dark gray bars). The chromatin was IPed using (A) an RARα Ab, (B) an RXRα Ab or (C) IgG negative control. The western blot in Figure 1f demonstrates antibody specificity toward RARα. The average signal from IgG IP was set to 1 (marked by the light gray background in A, B and C). The data represent four independent IPs for each Ab, harvesting new chromatin for each IP. Statistical significance is demonstrated by P < 0.05 for the indicated comparisons.
We identified RXRα at the Mest promoter only in Wt cells (>2-fold relative to RARα−/− cells, P < 0.05), but not at the Tex13, Stmn2 and Slc38a4 promoters (each <2-fold enrichment, P > 0.05). This suggests that only the Mest promoter is a direct target of RXRα (Figure 3B). In contrast, we detected an RA dependent increase in RXRα at the Cyp26a1 promoter in Wt and in RARα−/− cells (evident by a >2-fold higher signal in Wt and RARα−/− cells after RA, P = 0.01 and 0.03, respectively). The minor RA dependent increase in RXRα levels at the Cyp26a1 promoter even in RARα−/− (~2-fold) suggests that RARβ and/or RARγ are partners of RXRα at the Cyp26a1 promoter, thus corroborating the role of RARγ in the regulation of Cyp26a1 (30). The assays demonstrate that RXRα is associated with the Mest promoter only in Wt cells, e.g. only in the presence of RARα. The reported heterodimerization of RARα and RXRα (56) corroborates the requirement of RXRα on RARα for association with the Mest promoter. We conclude that direct binding of RARα/RXRα heterodimers is required to maintain RA-independent transcription of the Mest gene (Figure 5B).
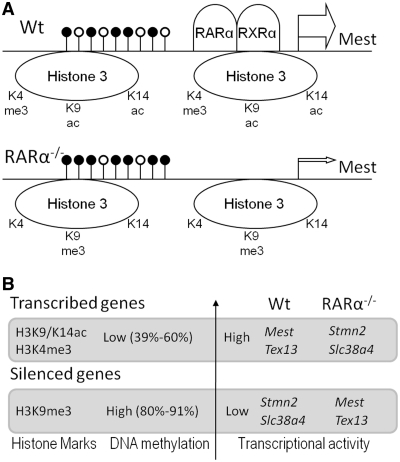
Figure 5.
Model for RARα dependent epigenetic regulation. (A) Ligand-independent binding of RARα/RXRα heterodimers to the Mest promoter region is required to maintain transcriptionally permissive histone modifications (H3K4me3 and H3K9/K14ac) and relatively low methylation levels of promoter CpG islands (upper panel). Knockout of RARα results in higher levels of Mest promoter methylation, loss of permissive histone modifications, and gain of repressive histone modifications (lower panel). (B) Actively transcribed genes display relative low levels of promoter methylation and high levels of H3K9/K14ac and H3K4me3 (Mest and Tex13 in Wt, Stmn2 and Slc38a4 in RARα−/−), whereas silenced genes display high levels of promoter methylation and, if paternally expressed, high H3K9me3 levels (Slc38a4 in Wt, Mest in RARα−/−). Transcriptional start sites (TSS) and relative transcriptional activities are indicated by the arrows and arrow sizes, respectively. The relative levels of CpG promoter methylation are denoted by black lollipops (the CpG-rich regions assessed each span the TSS but for clarity are drawn upstream of the TSS).
Ectopic expression of RARα2 in F9 RARα−/− cells partially restores Mest transcript levels
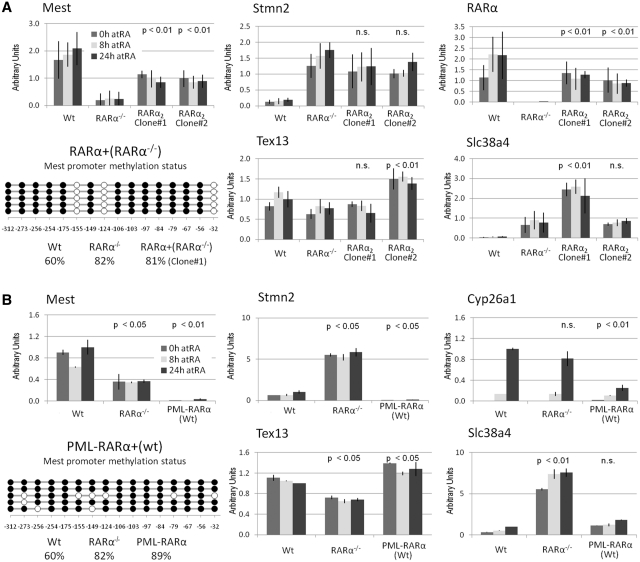
To confirm that the altered transcriptional activity observed in RARα−/− cells relative to Wt cells is indeed caused by the loss of RARα we re-introduced RARα2, which in F9 cells is the predominantly expressed isoform originating from the RARα gene. We derived two independent cell lines from the F9 RARα−/− cell line (RARα−/− :RARα2, clone #1 and clone #2), which stably express murine RARα2 in the RARα null background (Supplementary Data)). We then assessed Mest, Stmn2, Tex13 and Slc38a4 transcript levels in each of the two RARα2 restoration cell lines.
We observed increased Mest transcript levels in both RARα2 restoration cell lines relative to the parent F9 RARα−/− cell line (Figure 4A, upper left, P < 0.01). The RARα2 transcript levels in each of the RARα2 restoration cell lines were below the RARα transcript levels seen in Wt (67% and 50%, respectively), but still significantly higher than in the RARα−/− cells (Figure 4A, upper right, P < 0.01). The overall Mest promoter methylation was not reversed by restoring RARα2 expression (Figure 4A, lower right), but in contrast to both Wt and RARα knockout cells, specific CpG sites (−155, −124 and −32) displayed extremely low levels of methylation. Stmn2 transcript levels were reduced in both RARα2 restoration cell lines relative to the parent F9 RARα−/− cell line (Figure 4A, upper middle). The Tex13 and Slc38a4 transcript levels were inconsistent between the two RARα2 restoration cell lines (Figure 4A, lower).
Figure 4.
Ectopic RARα expression affects Mest transcript levels in F9 cells. (A) Full-length RARα2 was stably expressed in F9 RARα−/− cells (upper right). Transcript levels assessed by real-time PCR demonstrate partial restoration of Mest transcript levels (upper left), but no reversal of the overall promoter methylation (lower left). Stmn2 transcript levels were not affected (middle panel). Tex13 and Slc38a4 transcript levels were inconsistent between the two independent RARα2 restoration lines (lower middle and right panel). The P-values show a comparison of RARα2 restoration cell lines to the RARα knockout cell line. The data represent three independent assays, harvesting new RNA for each experiment. (B) The PML–RARα oncogene was stably expressed in F9 Wt cells. Transcript levels assessed by real-time PCR (upper left panel) suggest a dominant negative function of the PML–RARα protein for Mest associated with increased levels of promoter methylation (lower left panel), while Tex13, Slc38a4 and Stmn2 transcript levels were not affected (Wt, 24 h RA was set to 1). RA induced transcription of Cyp26a1 was impaired in PML–RARα expressing cells (upper right panel). The duration of the RA treatment is indicated by the bar color (0 h; gray, 8 h; light gray, and 24 h; dark gray bars). The P-values show a comparison of the PML–RARα cell line to the F9 Wt cells. The data represent three independent assays, harvesting new RNA for each experiment.
These data indicate that the altered Mest and Stmn2 transcript levels in the RARα−/− cell lines are related to the lack of RARα. Furthermore, our observation that Mest and Stmn2 transcript levels are only partially restored upon RARα2 restoration in RARα−/− cells (Figure 4A, upper left), suggests that this RARα−/− phenotype is not fully reversible.
Expression of the PML–RARα oncogene alters transcript levels of Mest, a direct RARα target gene
APL is frequently caused by a t(15;17) (q22;q12–21) translocation which introduces the expression of an oncogenic PML–RARα fusion protein (2,57). Several reports suggest that oncogenic RARα fusion proteins cause APL in part by functioning as a dominant negative RAR (4,58,59). We hypothesized that the oncogenic PML–RARα fusion protein would function as a dominant negative regulatory protein, effectively preventing RARα (and possibly RARβ and RARγ) from functioning in F9 Wt cells. If our hypothesis is correct, genes such as Mest, whose expression is maintained by RARα, would be silenced upon expression of the PML–RARα oncoprotein in F9 Wt cells. In addition, if the phenotype from overexpression of the dominant negative PML–RARα in F9 Wt cells mimics the phenotype of RARα knockout cells, this would be validation of the role of RARα in RA-independent transcriptional regulation.
In order to test this hypothesis, we generated an F9 cell line, PML–RARα+(Wt), which stably expresses a murine PML–RARα fusion protein in the wild-type background (the 562 amino-terminal residues of PML fused to 400 carboxy-terminal residues of RARα (Supplementary Data)). We then assessed Mest, Tex13, Slc38a4 and Stmn2 transcript levels in the PML–RARα+(Wt) cells. Since oncogenic RARα fusion proteins attenuate the RA responsiveness of HL-60 and U937 cells (4,59), the transcriptional induction of Cyp26a1 by RA treatment of the F9 PML–RARα+(Wt) cells was assessed in parallel.
We observed reduced levels of Mest transcript and increased levels of promoter methylation in the F9 PML–RARα+(Wt) relative to F9 Wt cells (Figure 4B). In contrast, Tex13 expression was elevated by ~20% in PML–RARα+(Wt) compared to Wt cells (Figure 4B). Only minor changes in Slc38a4 and Stmn2 transcript levels were observed in PML–RARα+(Wt) relative to Wt cells (Figure 4B). Consequently, expression of the PML–RARα fusion protein in F9 Wt cells recapitulated the RARα−/− phenotype with respect to Mest, but not with respect to Tex13, Slc38a4 and Stmn2 transcript levels. In addition, with Cyp26a1 mRNA levels reduced to 25% in the PML–RARα+(Wt) cells relative to Wt cells after 24 h of RA (Figure 4B), the RA responsiveness of the Cyp26a1 gene was greatly attenuated upon overexpression of PML–RARα. In summary, whereas the knockout of RARα was associated with decreased Mest and Tex13 transcript levels and increased Slc38a4 and Stmn2 transcript levels, only Mest transcript levels were decreased upon overexpression of a PML–RARα fusion protein in F9 Wt cells. Consequently, stably expressing the PML–RARα protein in F9 Wt cell recapitulate the RARα knockout phenotype with respect to the Mest transcript levels and promoter methylation.
DISCUSSION
Transcriptional silencing through DNA methylation has been extensively studied, yet the reverse process of transcriptional activation through DNA demethylation has started to receive attention only in recent years. We have demonstrated that knockout of RARα is accompanied by increased methylation of the Mest and Tex13 promoter regions and decreased methylation of the Slc38a4 and Stmn2 promoter regions, an effect that, with respect to Mest and Stmn2 transcript levels, is partially reverted upon restoration of RARα2 expression in RARα−/− cells. Furthermore, with respect to Mest the RARα−/− phenotype is recapitulated by overexpression of PML–RARα in F9 Wt cells.
In RARα−/− cells both Mest and Tex13 overall promoter methylation was increased from 60% to 80%. However, whereas Mest transcript levels and H3K9/K14ac levels were severely reduced (to 13% and 15%, −/+ RA, respectively), the Tex13 transcript levels and H3K9/K14ac levels were only moderately reduced (to 49% and 48%, −/+ RA, respectively). Consequently, promoter methylation only partially reflects the transcriptional activity. The H3K9me3 repressive histone mark displays a much stronger association with the Mest promoter region than with the Tex13 promoter region, which may account for the more pronounced repression of Mest relative to Tex13 in RARα−/− cells. Overall, the altered levels of promoter methylation correlate with specific changes in histone modifications (H3K9me3, H3K9/K14ac and H3K4me3). The RA-independent association of RARα and RXRα with the Mest promoter region contrasts with the RA dependent enrichment of RXRα at the Cyp26a1 promoter region and points to a direct role of RARα/RXRα heterodimers in preventing DNA methylation of the Mest promoter region of Wt cells. Our data also suggest that the lower level of DNA methylation of the Tex13 promoter region and the highly methylated states of the Slc38a4 and Stmn2 promoter regions are indirectly maintained by RARα in F9 Wt cells (Figure 5B). However, we cannot exclude the possibility that distant RARα-binding sites regulate Tex13, Stmn2 and Slc38a4 expression, as these would elude detection in the promoter specific ChIP assay.
A novel ligand-independent function of RARα
Retinoid receptors and other nuclear hormone receptors has been reported to regulate transcription mainly by their ability to activate and repress transcriptional activities reversibly in the presence and absence of ligands, respectively (30,31,60–62). We identified several transcripts, including Mest, Tex13, Slc38a4 and Stmn2, which are differentially expressed between F9 Wt and RARα−/− cells in a RA-independent manner, e.g. the exogenous addition of RA had no effect on the transcriptional activities measured by transcript levels (Figure 1), promoter methylation and histone modifications (Figure 2). We cannot rule out that these differences are caused by endogenously produced ligands, but a number of findings argue for an unliganded function of RARα. First, several known RA responsive genes, including Hoxb5, Hoxa5, Cyp26a1 and Hoxb2, were potently induced in F9 Wt and in RARα−/− cells upon RA addition (Figure 1E), thus demonstrating a positive transcriptional effect of RA in the RARα−/− cells. Second, RARα has been shown by us and others to mediate RA-dependent transcription of reporter plasmids (63,64), and thus to have the capability to mediate ligand-dependent transcriptional activation. Third, using a non-biased, genome-wide approach, RARα-binding sites have been identified proximal to both RA responsive and RA non-responsive genes (11,12), and it was suggested that RA responsiveness may be dictated by the specific configuration of the RARE. In this respect, it is particularly intriguing that the Mest promoter contains a degenerate RARE immediately downstream of the ChIP target region (Figure 2C). Our findings demonstrate that in F9 cells the levels of RARα and RXRα at the Mest promoter region were not affected by the presence of RA, since the IP signals displayed no statistically significant change upon RA treatment of Wt cells [p(RARα) = 0.28 and p(RXRα) = 0.72, respectively, Figure 3]. This suggests that RARα/RXRα heterodimers can maintain transcription of Mest independently of RA. In contrast, the levels of RARα and RXRα at the Cyp26a1 promoter region increased upon RA treatment of Wt cells [p(RARα) = 0.04 and p(RXRα) = 0.01, respectively, Figure 3]. These results suggest that RA treatment results in increased levels of RARα (and RXRα) at RA responsive elements (as observed for the promoter proximal RARE of Cyp26a1), whereas RARα targets whose transcriptional activities are independent of ligand binding (e.g. Mest) do not display changes in the levels of RARα (and RXRα) upon RA treatment (Figure 5A). Intriguingly, the model of RARs and RXRs constitutively associated with RAREs even in the absence of ligand was recently challenged by a study which demonstrated a highly dynamic and RA-dependent association of RARs and RXRs with endogenous RAREs (65). It is worth noting that the Mest promoter contains a degenerate putative RARE (DR1), whereas the Cyp26a1 promoter contains two proximal RAREs (DR5), thus potentially explaining why RA induces transcription of Cyp26a1 but not of Mest. Alternatively, cis-bound transcription factors may modulate the function of RARα. In this respect, the relatively low levels of methylation of the two CpGs in the Mest promoter, even in the RARα−/− cells, may indicate the presence of constitutively bound transcription factors. A putative NFkB-binding site (GGRNNYYCC) located next to CpG(−149) and a potential TATA-box positioned proximal to CpG (−32) may indicate coordinated action of several transcription factors. Upon restoration of RARα2 expression it is interesting that CpG (−32) becomes unmethylated. In contrast, the CpG (−149) is highly methylated, but the two neighboring sites to CpG (−149) exhibit low levels of DNA methylation. In summary, the Mest transcript levels are partially rescued by restored RARα2 expression. However, the promoter methylation is reversed only for specific sites. This suggests that these sites may be key in regulating the transcriptional activity of Mest.
Expression of PML–RARα in F9 Wt cells mimics the aberrant Mest transcription in RARα knockout cells
The Mest and Cyp26a1 transcript levels were differentially affected by the expression of PML–RARα in F9 Wt cells (Figure 4B). The transcriptional induction of Cyp26a1 by RA was attenuated in PML–RARα+(Wt) but not in RARα−/− cells. In contrast, Mest expression was reduced in both PML–RARα+(Wt) and in RARα−/− cells relative to Wt cells (Figure 4B). Whereas transcription of Cyp26a1 is regulated by ligand binding to RARβ and/or RARγ (29), the transcription of Mest shows a strong correlation with promoter methylation and presence of RARα/RXRα dimers in the proximal promoter region. Consequently, the expression of PML–RARα and the knockout of RARα have similar effects on Mest expression, but different effects on Cyp26a1 expression. The ligand-independent association of RARα only with the Mest promoter (Figure 3) may explain why the introduction of PML–RARα mimicked the loss of RARα only for the Mest gene, and not for Tex13, Slc38a4 and Stmn2. We suggest that PML–RARα expression attenuates transcription from RA-inducible promoters (e.g. Cyp26a1) as previously reported, but functionally mimics the lack of RARα at RARα target promoters, which are not RA responsive (e.g. Mest).
Association with RARα maintains high transcriptional activity and low levels of promoter methylation
When comparing the set of genes with aberrant transcript levels in RARα knockout cells with the genomic RARα targets identified in a genome-wide study evaluating DNA binding of RARα in MCF-7 cells (12), an intriguing pattern emerges. The RARα-binding sites identified by Hua et al. mapped exclusively to promoter regions of genes whose transcript levels were decreased in the F9 RARα knockout cells (Mest, Tcfap2a, Cux1, HMGcs1 and Gab1, Figure 1A and B (Supplementary Data)). None of the more than 7000 RARα-binding sites identified (12) mapped to genes with transcript levels elevated in the F9 RARα knockout cells (relative to F9 Wt cells), which indicates that target genes directly regulated by RARα display decreased expression in the absence of RARα. We observed direct association of RARα with the Mest promoter in Wt cells, and increased promoter methylation of Mest in RARα knockout cells, which suggests that RARα through direct association can hinder CpG methylation of specific promoters. Mixed-lineage leukemia (MLL) which is a H3K4 methyltransferase of the Trithorax family maintains transcription by preventing of CpG methylation in the Hoxa9 promoter regions of murine embryonic fibroblasts (66). The decreased H3K4me3 association with the Mest promoter region in RARα−/− cells (Figure 2B, lower panel) argues that the H3K4 methyltransferase activity of MLL plays an important role in the epigenetic regulation of Mest and possibly other genes whose expression is reduced in RARα−/− cells. Intriguingly, RARα has been reported to interact directly with MLL5, a key MLL in hematopoiesis (67,68). This provides a biologically relevant, potential mechanism for the methylation inhibitory function of RARα.
The function of RARα may be modulated by TIF1β
The notion that Mest transcriptional activity is maintained by RARα/RXRα heterodimers poses the intriguing question of why Mest is not induced by RA treatment. It has been suggested that binding of RAR/RXR heterodimers is required, but not sufficient for RA responsiveness (11). Indeed, this model is consistent with the observed RARα binding to genomic elements devoid of RA responsive genes (12). Alternatively, the lack of RA responsiveness could result from the activities of co-repressors (e.g. Sin3a, N-CoR2 or TIF1β) which modulate the activity of RARα. In manipulated F9 cells, heterozygotic for TIF1β, the expression of Mest was RA inducible (69), suggesting that the activity of RARα can be modulated by TIF1β. Another candidate is the PRAME co-repressor, which can bind to RARs in the presence of RA and prevent ligand-induced transcriptional activation (70), potentially by inhibiting RARα. However, ectopically expressed PRAME and RARα both caused resistance to growth arrest and apoptosis induced by HDAC inhibitors (71), which indicate similar, not opposing functions of ectopically expressed PRAME and RARα.
Alternatively, RARE configuration was suggested to determine RA responsiveness (11), but may be further modulated by promoter specific epigenetic signatures. Indeed, the increased levels of RARα at the RA inducible Cyp26a1 promoter versus the unchanged levels of RARα at the RA non-responsive Mest promoter (Figure 3) indicate that the promoter epigenetic signature play a role in determining the RAR association and thus RA responsiveness. This is supported by histone modifications and transcript levels, which demonstrate that the Mest gene is transcriptionally active in the absence of RA (Figure 1C), whereas Cyp26a1 is silent in the absence of RA (Figure 1E).
The biological relevance of RARα regulated imprinting
The majority of genes whose transcript levels differ between the Wt and RARα−/− cell lines are expressed in the placenta, the testis and the brain (Supplementary Data). These tissues are all sites where imprinting plays a major role in transcriptional regulation (54,55), and importantly, RARα is expressed in these specific tissues (72–74). We predict that RARα plays a key role in regulating genetic imprinting in animals, but this has yet to be investigated.
It was recently demonstrated that in the growth plate, a cartilage structure located between the epiphysis and metaphysis of long bones, differentiation from stem-like to hypertrophic chondrocytes is associated with decreased Mest expression and increased Slc38a4 expression (75). This inverse correlation corroborates our findings and provides a physiological framework for the molecular mechanisms identified here. Based on the key role of RARα in hematopoiesis (1) and the interaction of RARα with MLL5 (67), we speculate that similar changes in Mest and Slc38a4 expression occur during the differentiation of hematopoietic stem cells and that these changes in transcriptional activities are associated with the same epigenenetic changes in DNA and histones as here reported. The ligand-independent mechanism of RARα identified here sheds new light on the mechanisms of RA receptor action, and suggests that in addition to ligand-induced transcriptional regulation, ligand-independent regulation should be considered when investigating the epigenetic actions of RA receptors.
ACCESSION NUMBER
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online
FUNDING
Funding for open access charge: National Institutes of Health (R01CA043796 to L.J.G.); Weill Cornell Medical College funds.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr Pierre Chambon for providing RARα1, RARβ2 and RARγ1 expression vectors, and Dr Nigel Mongan for keen and insightful scientific discussions.
REFERENCES
- 1.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 2.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 3.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Guidez F, Rousselot P, Agadir A, Chen SJ, Wang ZY, Degos L, Zelent A, Waxman S, Chomienne C. PLZF-RAR alpha fusion proteins generated from the variant t(11;17)(q23;q21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid receptors. Proc. Natl Acad. Sci. USA. 1994;91:1178–1182. doi: 10.1073/pnas.91.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu J, Huang Y, Chen G, Chen Z, Tweardy DJ, Dong S. Aberrant chromatin remodeling by retinoic acid receptor alpha fusion proteins assessed at the single-cell level. Mol. Biol. Cell. 2007;18:3941–3951. doi: 10.1091/mbc.E07-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He LZ, Merghoub T, Pandolfi PP. In vivo analysis of the molecular pathogenesis of acute promyelocytic leukemia in the mouse and its therapeutic implications. Oncogene. 1999;18:5278–5292. doi: 10.1038/sj.onc.1203088. [DOI] [PubMed] [Google Scholar]
- 7.Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, Atwater S, Bishop JM. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Croce L, Raker VA, Corsaro M, Fazi F, Fanelli M, Faretta M, Fuks F, Lo Coco F, Kouzarides T, Nervi C, et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002;295:1079–1082. doi: 10.1126/science.1065173. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, Schifano E, Booth J, van Putten W, Skrabanek L, et al. DNA methylation signatures identify biologically distinct subtypes in acute myeloid leukemia. Cancer Cell. 2010;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens JH, Brinkman AB, Simmer F, Francoijs KJ, Nebbioso A, Ferrara F, Altucci L, Stunnenberg HG. PML-RARalpha/RXR alters the epigenetic landscape in acute promyelocytic leukemia. Cancer Cell. 2010;17:173–185. doi: 10.1016/j.ccr.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B, Davidson I. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol. Cell. Biol. 2010;30:231–244. doi: 10.1128/MCB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khetchoumian K, Teletin M, Tisserand J, Mark M, Herquel B, Ignat M, Zucman-Rossi J, Cammas F, Lerouge T, Thibault C, et al. Loss of Trim24 (Tif1alpha) gene function confers oncogenic activity to retinoic acid receptor alpha. Nat. Genet. 2007;39:1500–1506. doi: 10.1038/ng.2007.15. [DOI] [PubMed] [Google Scholar]
- 14.Cohn E, Ossowski L, Bertran S, Marzan C, Farias EF. RARalpha1 control of mammary gland ductal morphogenesis and wnt1-tumorigenesis. Breast Cancer Res. 2010;12:R79. doi: 10.1186/bcr2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J. Cell Physiol. 2010;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Koch CM, Andrews RM, Flicek P, Dillon SC, Karaoz U, Clelland GK, Wilcox S, Beare DM, Fowler JC, Couttet P, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 19.Wiencke JK, Zheng S, Morrison Z, Yeh RF. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene. 2008;27:2412–2421. doi: 10.1038/sj.onc.1210895. [DOI] [PubMed] [Google Scholar]
- 20.Ooi SK, O'Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. J. Cell. Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biliya S, Bulla LA., Jr Genomic imprinting: the influence of differential methylation in the two sexes. Exp. Biol. Med. 2010;235:139–147. doi: 10.1258/ebm.2009.009251. [DOI] [PubMed] [Google Scholar]
- 22.Butler MG. Genomic imprinting disorders in humans: a mini-review. J. Assist. Reprod. Genet. 2009;26:477–486. doi: 10.1007/s10815-009-9353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klug M, Heinz S, Gebhard C, Schwarzfischer L, Krause SW, Andreesen R, Rehli M. Active DNA demethylation in human postmitotic cells correlates with activating histone modifications, but not transcription levels. Genome Biol. 2010;11:R63. doi: 10.1186/gb-2010-11-6-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudas LJ. Retinoids, retinoid-responsive genes, cell differentiation, and cancer. Cell Growth Differ. 1992;3:655–662. [PubMed] [Google Scholar]
- 26.Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- 27.Strickland S, Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978;15:393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- 28.Boylan JF, Lufkin T, Achkar CC, Taneja R, Chambon P, Gudas LJ. Targeted disruption of retinoic acid receptor alpha (RAR alpha) and RAR gamma results in receptor-specific alterations in retinoic acid-mediated differentiation and retinoic acid metabolism. Mol. Cell. Biol. 1995;15:843–851. doi: 10.1128/mcb.15.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie RF, Gudas LJ. Retinoic acid receptor isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic response elements. J. Biol. Chem. 2007;282:33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells. J. Mol. Biol. 2007;372:298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid mediated transcriptional responses in stem cells and fibroblasts. J. Biol. Chem. 2010;285:14534–14548. doi: 10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 33.Laursen KB, Mielke E, Iannaccone P, Fuchtbauer EM. Mechanism of transcriptional activation by the proto-oncogene Twist1. J. Biol. Chem. 2007;282:34623–34633. doi: 10.1074/jbc.M707085200. [DOI] [PubMed] [Google Scholar]
- 34.Mortensen RM, Zubiaur M, Neer EJ, Seidman JG. Embryonic stem cells lacking a functional inhibitory G-protein subunit (alpha i2) produced by gene targeting of both alleles. Proc. Natl Acad. Sci. USA. 1991;88:7036–7040. doi: 10.1073/pnas.88.16.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowling T, Desler M, Kuszynski C, Rizzino A. Transfection of embryonal carcinoma cells at high efficiency using liposome-mediated transfection. Mol. Reprod. Dev. 2002;63:309–317. doi: 10.1002/mrd.90014. [DOI] [PubMed] [Google Scholar]
- 36.Smith RJ, Dean W, Konfortova G, Kelsey G. Identification of novel imprinted genes in a genome-wide screen for maternal methylation. Genome Res. 2003;13:558–569. doi: 10.1101/gr.781503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundberg BE, Waag E, Jacobsson JA, Stephansson O, Rumaks J, Svirskis S, Alsio J, Roman E, Ebendal T, Klusa V, et al. The evolutionary history and tissue mapping of amino acid transporters belonging to solute carrier families SLC32, SLC36, and SLC38. J. Mol. Neurosci. 2008;35:179–193. doi: 10.1007/s12031-008-9046-x. [DOI] [PubMed] [Google Scholar]
- 38.Prasad SC, Thraves PJ, Soldatenkov VA, Varghese S, Dritschilo A. Differential expression of stathmin during neoplastic conversion of human prostate epithelial cells is reversed by hypomethylating agent, 5-azacytidine. Int. J. Oncol. 1999;14:529–534. doi: 10.3892/ijo.14.3.529. [DOI] [PubMed] [Google Scholar]
- 39.Bruhn L, Munnerlyn A, Grosschedl R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRalpha enhancer function. Genes Dev. 1997;11:640–653. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 40.Nadano D, Notsu T, Matsuda T, Sato T. A human gene encoding a protein homologous to ribosomal protein L39 is normally expressed in the testis and derepressed in multiple cancer cells. Biochim. Biophys. Acta. 2002;1577:430–436. doi: 10.1016/s0167-4781(02)00445-1. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Mizuno R, Nishimura T, Ogawa Y, Yoshikawa H, Fujimura H, Adachi E, Kishimoto T, Yanagihara T, Sakoda S. Cloning and expression of myelin-associated oligodendrocytic basic protein. A novel basic protein constituting the central nervous system myelin. J. Biol. Chem. 1994;269:31725–31730. [PubMed] [Google Scholar]
- 42.MacLean-Hunter S, Makela TP, Grzeschiczek A, Alitalo K, Moroy T. Expression of a rlf/L-myc minigene inhibits differentiation of embryonic stem cells and embroid body formation. Oncogene. 1994;9:3509–3517. [PubMed] [Google Scholar]
- 43.Kobayashi S, Kohda T, Miyoshi N, Kuroiwa Y, Aisaka K, Tsutsumi O, Kaneko-Ishino T, Ishino F. Human PEG1/MEST, an imprinted gene on chromosome 7. Hum. Mol. Genet. 1997;6:781–786. doi: 10.1093/hmg/6.5.781. [DOI] [PubMed] [Google Scholar]
- 44.Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- 45.Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G, Esteller M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–264. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen IS, Dervan P, McGoldrick A, Harrison M, Ponchel F, Speirs V, Isaacs JD, Gorey T, McCann A. Promoter switch: a novel mechanism causing biallelic PEG1/MEST expression in invasive breast cancer. Hum. Mol. Genet. 2002;11:1449–1453. doi: 10.1093/hmg/11.12.1449. [DOI] [PubMed] [Google Scholar]
- 47.Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat. Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 48.Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 2006;5:1059–1064. doi: 10.4161/cbt.5.9.3151. [DOI] [PubMed] [Google Scholar]
- 49.Philipp J, Mitchell PJ, Malipiero U, Fontana A. Cell type-specific regulation of expression of transcription factor AP-2 in neuroectodermal cells. Dev. Biol. 1994;165:602–614. doi: 10.1006/dbio.1994.1279. [DOI] [PubMed] [Google Scholar]
- 50.Pellikainen JM, Kosma VM. Activator protein-2 in carcinogenesis with a special reference to breast cancer–a mini review. Int. J. Cancer. 2007;120:2061–2067. doi: 10.1002/ijc.22648. [DOI] [PubMed] [Google Scholar]
- 51.Dunwell T, Hesson L, Rauch TA, Wang L, Clark RE, Dallol A, Gentle D, Catchpoole D, Maher ER, Pfeifer GP, et al. A genome-wide screen identifies frequently methylated genes in haematological and epithelial cancers. Mol. Cancer. 2010;9:44. doi: 10.1186/1476-4598-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett KL, Romigh T, Eng C. AP-2alpha induces epigenetic silencing of tumor suppressive genes and microsatellite instability in head and neck squamous cell carcinoma. PLoS One. 2009;4:e6931. doi: 10.1371/journal.pone.0006931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolt KS, Karar J, Mishra MK, Salim J, Kumar R, Grover SK, Qadar Pasha MA. Transcriptional downregulation of sterol metabolism genes in murine liver exposed to acute hypobaric hypoxia. Biochem. Biophys. Res. Commun. 2007;354:148–153. doi: 10.1016/j.bbrc.2006.12.159. [DOI] [PubMed] [Google Scholar]
- 54.Trasler JM. Epigenetics in spermatogenesis. Mol. Cell. Endocrinol. 2009;306:33–36. doi: 10.1016/j.mce.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum. Reprod. Update. 2010;17:397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- 56.Nagpal S, Friant S, Nakshatri H, Chambon P. RARs and RXRs: evidence for two autonomous transactivation functions (AF-1 and AF-2) and heterodimerization in vivo. EMBO J. 1993;12:2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goddard AD, Borrow J, Freemont PS, Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991;254:1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 58.Lin RJ, Evans RM. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol. Cell. 2000;5:821–830. doi: 10.1016/s1097-2765(00)80322-6. [DOI] [PubMed] [Google Scholar]
- 59.Pandolfi PP, Grignani F, Alcalay M, Mencarelli A, Biondi A, LoCoco F, Pelicci PG. Structure and origin of the acute promyelocytic leukemia myl/RAR alpha cDNA and characterization of its retinoid-binding and transactivation properties. Oncogene. 1991;6:1285–1292. [PubMed] [Google Scholar]
- 60.Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 61.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 62.Kashyap V, Gudas LJ, Brenet F, Funk P, Viale A, Scandura JM. Epigenomic reorganization of the clustered Hox genes in embryonic stem cells induced by retinoic acid. J. Biol. Chem. 2011;286:3250–3260. doi: 10.1074/jbc.M110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc. Natl Acad. Sci. USA. 1989;86:9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruyt FA, van den Brink CE, Defize LH, Donath MJ, Kastner P, Kruijer W, Chambon P, van der Saag PT. Transcriptional regulation of retinoic acid receptor beta in retinoic acid-sensitive and -resistant P19 embryocarcinoma cells. Mech. Dev. 1991;33:171–178. doi: 10.1016/0925-4773(91)90025-2. [DOI] [PubMed] [Google Scholar]
- 65.Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, Gifford DK. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erfurth FE, Popovic R, Grembecka J, Cierpicki T, Theisler C, Xia ZB, Stuart T, Diaz MO, Bushweller JH, Zeleznik-Le NJ. MLL protects CpG clusters from methylation within the Hoxa9 gene, maintaining transcript expression. Proc. Natl Acad. Sci. USA. 2008;105:7517–7522. doi: 10.1073/pnas.0800090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujiki R, Chikanishi T, Hashiba W, Ito H, Takada I, Roeder RG, Kitagawa H, Kato S. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature. 2009;459:455–459. doi: 10.1038/nature07954. [DOI] [PubMed] [Google Scholar]
- 68.Liu H, Westergard TD, Hsieh JJ. MLL5 governs hematopoiesis: a step closer. Blood. 2009;113:1395–1396. doi: 10.1182/blood-2008-11-185801. [DOI] [PubMed] [Google Scholar]
- 69.Riclet R, Chendeb M, Vonesch JL, Koczan D, Thiesen HJ, Losson R, Cammas F. Disruption of the interaction between transcriptional intermediary factor 1{beta} and heterochromatin protein 1 leads to a switch from DNA hyper- to hypomethylation and H3K9 to H3K27 trimethylation on the MEST promoter correlating with gene reactivation. Mol. Biol. Cell. 2009;20:296–305. doi: 10.1091/mbc.E08-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Epping MT, Wang L, Plumb JA, Lieb M, Gronemeyer H, Brown R, Bernards R. A functional genetic screen identifies retinoic acid signaling as a target of histone deacetylase inhibitors. Proc. Natl Acad. Sci. USA. 2007;104:17777–17782. doi: 10.1073/pnas.0702518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 73.Mollard R, Viville S, Ward SJ, Decimo D, Chambon P, Dolle P. Tissue-specific expression of retinoic acid receptor isoform transcripts in the mouse embryo. Mech. Dev. 2000;94:223–232. doi: 10.1016/s0925-4773(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 74.Sapin V, Ward SJ, Bronner S, Chambon P, Dolle P. Differential expression of transcripts encoding retinoid binding proteins and retinoic acid receptors during placentation of the mouse. Dev. Dyn. 1997;208:199–210. doi: 10.1002/(SICI)1097-0177(199702)208:2<199::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 75.Andrade AC, Lui JC, Nilsson O. Temporal and spatial expression of a growth-regulated network of imprinted genes in growth plate. Pediatr. Nephrol. 2010;25:617–623. doi: 10.1007/s00467-009-1339-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.