Abstract
A site-specific isotope labeling technique of long RNA molecules was established. This technique is comprised of two simple enzymatic reactions, namely a guanosine transfer reaction of group I self-splicing introns and a ligation with T4 DNA ligase. The trans-acting group I self-splicing intron with its external cofactor, ‘isotopically labeled guanosine 5′-monophosphate’ (5′-GMP), steadily gave a 5′-residue-labeled RNA fragment. This key reaction, in combination with a ligation of 5′-remainder non-labeled sequence, allowed us to prepare a site-specifically labeled RNA molecule in a high yield, and its production was confirmed with 15N NMR spectroscopy. Such a site-specifically labeled RNA molecule can be used to detect a molecular interaction and to probe chemical features of catalytically/structurally important residues with NMR spectroscopy and possibly Raman spectroscopy and mass spectrometry.
INTRODUCTION
In mechanistic studies of functional RNA, the signals from the functional residues (pinpointed studies) are important for characterizing their chemical nature and for elucidating the mechanism of action of the RNA (1). For this purpose, isotope labeling of functionally/structurally important residues enables us to extract pinpoint information from the functional RNA, by using NMR/Raman spectroscopy and mass spectrometry. Currently, site-specific isotope labeling of RNA molecules has only been achieved with chemical syntheses (2–11), since an alternative enzymatic RNA synthesis is not compatible with site-specific modifications. However, the chemical synthesis is applicable only to ‘short’ RNA/DNA oligomers (typically <20 nt) (2–17), due to low yields of long RNA/DNA chains. Therefore, to directly access spectral data from important residues in ‘long’ functional RNAs and to skip the time-consuming assignment step, we established a site-specific isotope labeling technique of RNA molecules without any limitation of the RNA length (Figures 1 and 2).
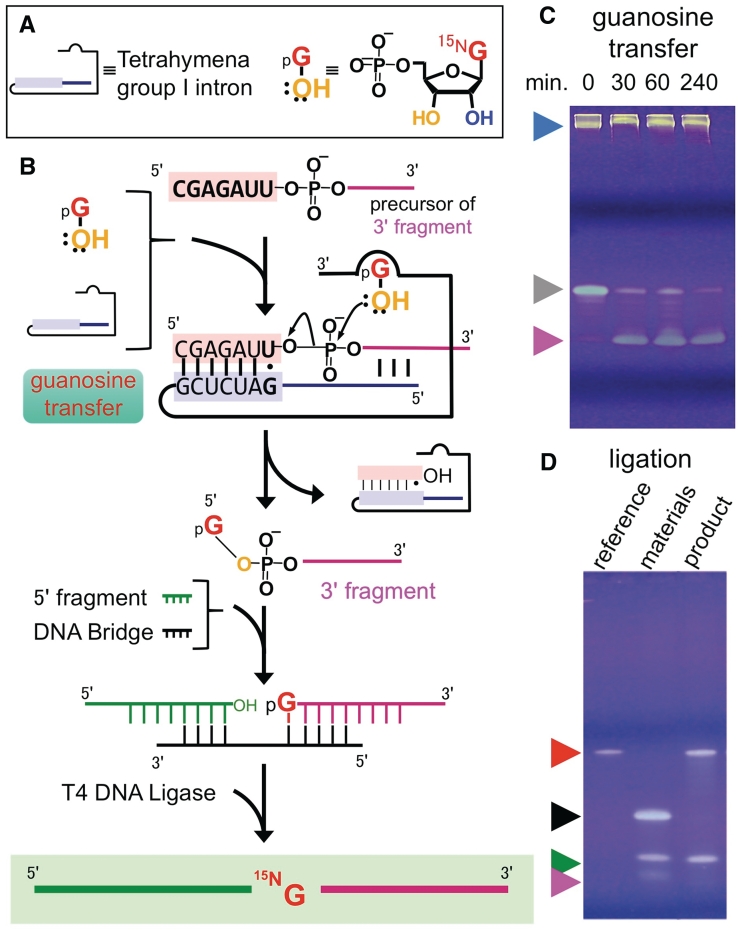
Figure 1.
The preparation of single-residue labeled RNA. (A) Definitions of symbols. (B) Reaction scheme. (C) Guanosine transfer reaction (D) Ligation. Because the reactant (3′-fragment) for ligases must be mono-phosphorylated at its 5′-end, we employed guanosine 5′-monophosphate (5′-GMP) as an external cofactor for the guanosine transfer reaction. Arrowheads: (blue) group I intron; (gray) the 3′-fragment precursor; (pink) 3′-fragment with labeled guanosine; (green) 5′ fragment; (black) DNA bridge; (red) labeled RNA (final product) and its reference.
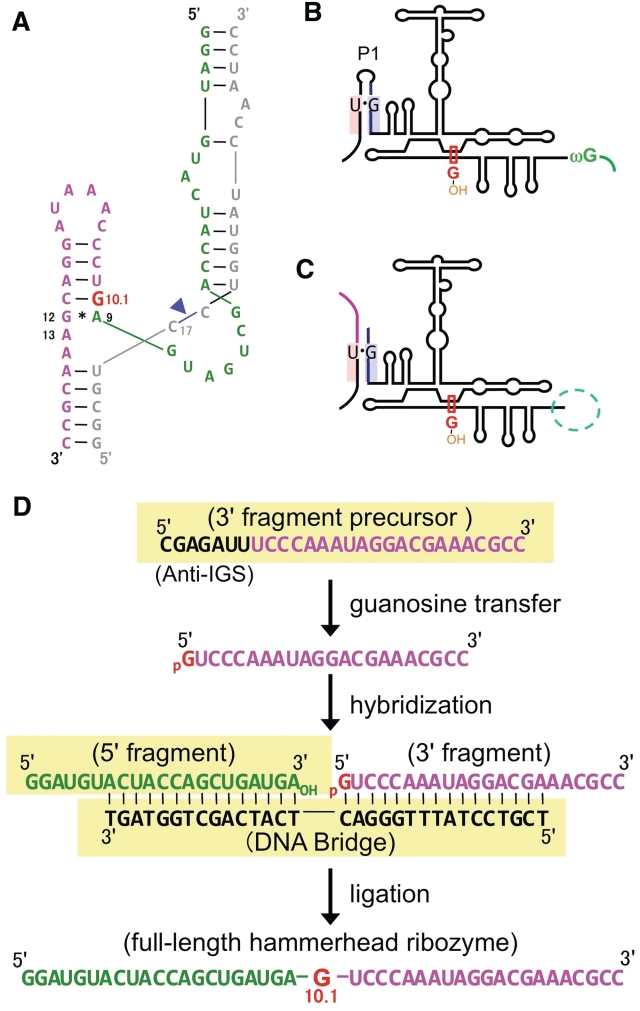
Figure 2.
Secondary structures of a hammerhead ribozyme and Tetrahymena group I intron, and the synthetic plan. (A) Secondary structure of a hammerhead ribozyme (53). Nitrogen-15-labeled guanosine (G10.1) is colored in red. The cleavage site is indicated by a blue arrowhead. Secondary structure of cis- (B) or trans- (C) acting Tetrahymena group I intron (36,39,41,44) with a guanosine-binding site (red rectangle). Reaction pathways of the cis- and trans-acting group I introns are illustrated in Supplementary Figures S1 and Figure 1b, respectively. (D) Simulation of the RNA and DNA strands, required for the preparation of single residue-labeled hammerhead ribozyme. The starting materials are highlighted with yellow background.
In NMR spectroscopy of RNAs, the isotope labeling technique [i.e. uniform (18,19) and nucleotide-specific (20–23) ones] expanded the applicable molecular size limit. Recently, the segmental isotope labeling technique of RNA (24–26) further enlarged this size limit. Although these techniques were oriented to 3D structure determination, a site-specific labeling technique of DNA/RNA oligomers is oriented to their chemical characterizations, such as the identification of the hydrogen bonding (2,5,12–15,27,28) and metalation site (3,4,6–8,11,16,17) in a pinpoint manner. Specifically, direct evidence of the 15N–15N J-coupling across the hydrogen bond (h2JNN) (12) and the HgII-mediated 15N–15N J-coupling (2JNN) in T-HgII-T base pairs (8,16,17), were obtained by using appropriate site-specifically labeled DNA/RNA oligomers (for 2JNN, 15N–HgII–15N and 15N–HgII–14N, respectively). More importantly, the derived fine spectral data were utilized to evaluate the strength of the hydrogen bond (5,13–15,27,28) and N-metal bond (8,17). Therefore, in order to apply such fine NMR spectra to any functional RNA molecule, site-specific labeling techniques without any applicable size-limit are becoming indispensable day by day.
MATERIALS AND METHODS
Synthesis of RNA oligomers for site-specific labeling of the hammerhead ribozyme
The sequences of the used RNA/DNA oligomers are highlighted with yellow background in Figure 2d. RNA oligomers (5′-fragment, 3′-fragment precursor and substrate strand for the hammerhead ribozyme) were synthesized by a DNA/RNA synthesizer (ABI model 392, CA, USA). The C17 residue at the cleavage site of the hammerhead ribozyme substrate (inhibitor) was substituted with 2′-O-methylcytidine to prevent the cleavage reaction with the hammerhead ribozyme (Figures 2a and 3a). The DNA bridge for the ligation reaction was purchased from TSUKUBA OLIGO SERVICE Co., Ltd. (Tsukuba, Japan). RNA oligomers were purified on an anion-exchange column (mono-Q; GE Healthcare UK, Ltd., Buckinghamshire, England) with a linear NaCl gradient (0–2 M) under denaturing conditions (8 M urea). Excess NaCl and urea were washed out using an ultrafiltration device (Amicon Ultra-15 3000 MWCO; Millipore, MA, USA).
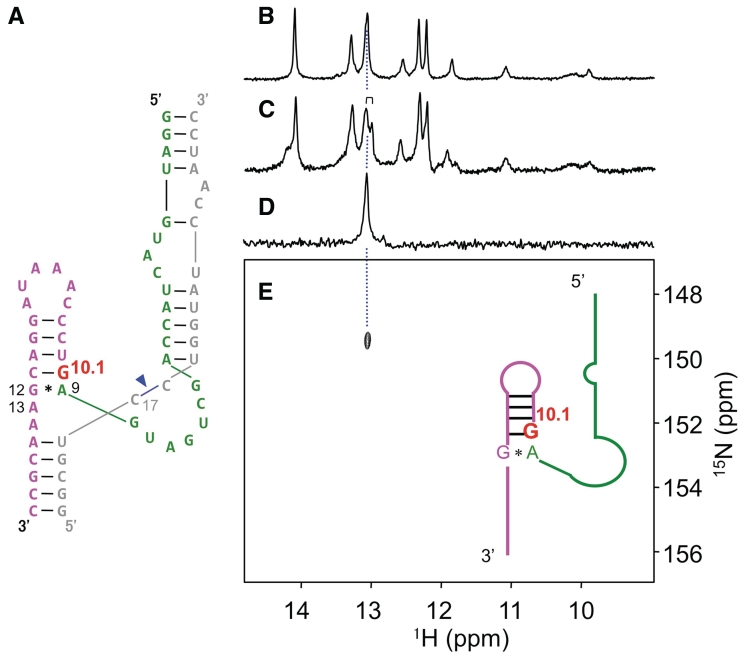
Figure 3.
Labeled RNA (hammerhead ribozyme) and its NMR spectra. (A) Hammerhead ribozyme (magenta, red and green) and substrate (grey) with its cleavage site (blue arrowhead). (B) One-dimensional 1H NMR spectrum of the authentic non-labeled sample. (C) One-dimensional 1H NMR spectrum of the labeled sample. (D) Nitrogen-15-edited 1D 1H NMR spectrum of the labeled sample. (E) 1H-15N HSQC spectrum of the labeled sample. The doublet imino proton signal of G10.1 is highlighted in the spectrum (c).
Preparation of non-labeled hammerhead ribozyme
The non-labeled hammerhead ribozyme was prepared by in vitro transcription. The template gene of the full-length hammerhead ribozyme was constructed in a pCR®2.1-TOPO® vector (TOPO® TA Cloning kit; Invitrogen, CA, USA), using synthetic DNA oligomers containing the T7 promoter and the coding sequence. Using this plasmid, PCR amplification was performed with the following primers. Forward primer: 5′-GCGTAATACGACTCACTATAGGATGTACTACCAGCTGATGAG-3′ and reverse primer: 5′-mGmGCGTTTCGTCCTATTTGGGACTC-3′. To prevent an overrun of T7 RNA polymerase, two 2′-O-methylguanosine (mG) residues were added to the 5′ end of the reverse primer (29,30). The RNA oligomer was directly transcribed from the PCR product using MEGA shortscript™ kit (Applied Biosystems, CA, USA). The transcript was purified on an anion-exchange column (mono-Q), with a linear NaCl gradient (0–2 M) in denaturing conditions (8 M urea), and desalted by a gel filtration column (TSK-GEL G3000PW; TOSOH, Tokyo, Japan).
Preparation of Tetrahymena group I intron and the general procedure for the optimization of the guanosine transfer
Trans-acting Tetrahymena group I intron (31–51) (the processing enzyme for the guanosine transfer reaction) was transcribed in vitro, using T7 RNA polymerase. For PCR amplification of the template DNA for in vitro transcription, we used a plasmid (pTZIVSU) (42,47) containing wild-type Tetrahymena group I intron (Figure 2b and Supplementary Figure S1), together with the following primers. By using the following primers, we obtained the trans-acting Tetrahymena group I intron (Figure 2c).
Forward primer:
5′-GAAGAGGCGTAATACGACTCACTATAGGGATCGGAGATCTCAAAAGTTATCAGGCATGCACCTGGTAGC-3′
Reverse primer:
5′-GTACTCCAAAACTAATCAATATACTTTCGCATACAAATTAG-3′
The Tetrahymena group I intron was transcribed from the PCR product using the script MAX™ Thermo T7 Transcription kit (TOYOBO, Osaka, Japan). To remove RNA polymerase, rNTPs and pyrophosphate, the transcript was cleaned by phenol–chloroform extraction and dialysis using an ultrafiltration device (Amicon Ultra-15 3000 MWCO) or a cellulose dialysis tube (Spectra/Por® Dialysis Membrane MWCO: 3500, Spectrum Laboratory Inc., Rancho Dominguez, USA). Without this treatment, residual guanosine 5′-triphosphate (5′-GTP) promoted the guanosine transfer reaction without adding any external guanosine 5′-monophosphate (5′-GMP; Supplementary Figure S2, lanes 4–6). In contrast, when including the dialysis step this reaction did not occur (Supplementary Figure S2, lanes 7–9). Experiments using a cellulose dialysis tube resulted in better exclusion of residual non-labeled 5′-GTP and higher enzymatic activity, compared with ultrafiltration dialysis. Hence, we were able to reduce the concentration of group I intron to 1.4 µM, and this result is presented in Supplementary Figure S2.
Guanosine transfer reaction for the NMR sample
Prior to the guanosine transfer reaction, the Tetrahymena group I intron was refolded using the following procedure. Dialyzed Tetrahymena group I intron (14 µM) was heated to 80°C and slowly cooled down to 58°C (reaction temperature) in a 3-ml solution (30 mM Tris–HCl pH 7.2, 10 mM NH4Cl and 3 mM MgCl2). Following Tetrahymena group I intron refolding, 50 µM 3′-fragment precursor and 2 mM uniformly 15N-labeled 5′-GMP (Cambridge Isotope Laboratories, MA, USA) were added to the solution and incubated for 4 h (Figure 1c). The derived product (labeled 3′-fragment) was purified on an anion-exchange column (mono-Q) with a linear NaCl gradient (0–2 M) under denaturing conditions (8 M urea). This was followed by ethanol precipitation and resuspension in TE buffer (10 mM Tris–HCl pH 7.5 and 1 mM EDTA).
Ligation reaction for the NMR sample
The 3′- and 5′-fragments were ligated with T4 DNA ligase (Fermentas, Ontario, Canada), using a DNA bridge (Figure 2d) as a complementary strand for the ligation site. The ligation reaction of the hammerhead ribozyme was basically performed as described in the literature (24), although the optimization of the RNA/DNA strand ratio is possible {see ‘Preparation of site-specifically 15N-labeled 76-nt RNA molecule [hXBP1 mRNA (516–591)]’ for modified conditions}. The three fragments were annealed in 2.5 ml of the pre-reaction mixture (100 µM 5′-fragment, 50 µM 3′-fragment, 75 µM DNA bridge, 40 mM Tris–HCl pH 7.6, 10 mM DTT, 10 mM MgCl2 and 0.5 mM 5′-ATP) that was heated to 95°C and slowly cooled down to 37°C (24). The T4 DNA ligase (850 units per 2.5 ml of pre-reaction mixture) was then added and incubated at 37°C for 12 h (Figure 1d). This was followed by a DNase I (TURBO DNase; Applied Biosystems, CA, USA) digestion of the DNA bridge. The ligated product was purified on an anion-exchange column (mono-Q) as described above. The resulting product was desalted by a gel filtration column (TSK-GEL G3000PW).
NMR measurements
We recorded NMR spectra of substrate-free hammerhead ribozymes, with and without isotope labeling, as well as substrate-bound labeled ribozyme (Figure 3, Supplementary Figures S3 and S4). NMR spectra of substrate-free labeled/non-labeled ribozymes were recorded in a solution containing 0.5 mM ribozyme, 10 mM sodium phosphate pH 7.0 and 50 mM NaCl in 5% D2O (Figure 3; Supplementary Figures S3 and S4). The NMR spectrum of the substrate-bound ribozyme was recorded in the same solution, but with 0.4 mM of the 1:1 ribozyme–substrate complex (Supplementary Figure S3). All NMR samples were heated to 58°C for 3 min and cooled to room temperature prior to NMR measurements. One-dimensional 1H NMR spectra were recorded on Bruker DRX800 spectrometer and 1H–15N HSQC spectra were acquired on Bruker Avance500 spectrometer, using cryo-cooled probes. All spectra were recorded at 293 K. Spectra were processed with the program NMRPipe (version 3.0) (52).
Optimizations of the guanosine transfer and ligation reactions
Optimizations of the guanosine transfer reaction with trans-acting Tetrahymena group I intron were performed in terms of concentrations of Mg2+, group I intron and 5′-GMP (Supplementary Figures S5 and S6), temperature (Supplementary Figure S7) and reaction volume (Supplementary Figure S8). The dialyzed group I intron (0.5–25 µM) was heated to 80°C and slowly cooled down to the reaction temperature (48–68°C) in a solution containing 30 mM Tris–HCl pH 7.2, 10 mM NH4Cl and MgCl2 (3–30 mM). After the refolding of Tetrahymena group I intron, 50 µM 3′-fragment precursor and 5′-GMP (0.05–2 mM) were added to the solution, and the reaction mixture was incubated at the temperature indicated in each experiment. All optimization experiments were carried out in 12 µl (Supplementary Figures S5–S7) except for the scale-up experiment (Supplementary Figure S8). Examinations of ligases were also performed as shown in Supplementary Figure S9. The reactions were monitored by polyacrylamide gel electrophoresis (PAGE). Optimizations of reaction volume and the total concentration of the substrates (under the fixed ratio of DNA/RNA fragments) were performed under the solution conditions indicated in the figure legend (Supplementary Figure S10). Reaction components were resolved by PAGE, containing 8 M urea, and stained with SYBR Gold (Invitrogen, CA, USA).
Preparation of site-specifically 15N-labeled 76-nt RNA molecule [hXBP1 mRNA (516–591)]
As an example of a site-specifically 15N-labeled long RNA molecule, hXBP1 mRNA (516–591) was prepared (Supplementary Figure S11). Its sequence and labeled position (G544) are shown in Supplementary Figure S11a. The 3′-fragment precursor (Supplementary Figure S11b) was processed with a group I intron in the solution containing 50 µM 3′-fragment precursor, 2.8 µM group I intron, 30 mM Tris–HCl pH 7.2, 10 mM NH4Cl and 3 mM MgCl2 (Supplementary Figure S11e). The resulting solution containing guanosine-transferred 3′-fragment was directly subjected to a ligation reaction without purification (Supplementary Figure S11f) in combination with the 5′-fragment (Supplementary Figure S11c) and the complementary DNA bridge (Supplementary Figure S11d). The solution conditions for the ligation were 52 µM 5′-fragment, 50 µM 3′-fragment, 75 µM DNA bridge, 40 mM Tris–HCl pH 7.6, 10 mM DTT, 10 mM MgCl2, 0.5 mM 5′-ATP and 0.5 unit/µl T4 DNA ligase. The derived full-length 76-nt RNA was isolated with PAGE and electro-elution. 1H-15N HSQC and 1D 1H NMR spectra were recorded with Bruker Avance-I 800 spectrometer, using cryo-cooled probe (Supplementary Figures S12 and S13). The solution for NMR measurements contains 0.2 mM 76-nt RNA molecule, 10 mM sodium phosphate pH 7.0 and 50 mM NaCl, in 10% D2O. All spectra were recorded at 303 K. Spectra were processed with the program NMRPipe (version 3.0) (52).
RESULTS
To produce site-specifically labeled RNA molecules, we employed an enzymatic activity of group I introns (31–51), specifically, the cleavage reaction of the 5′ exon–intron junction with an external guanosine cofactor (the first step of the self-splicing reaction; Figures 1b, 2d and Supplementary Figure S1). If this reaction takes place with isotopically labeled guanosine or its 5′-mono/di/tri-phosphate form, one could introduce a labeled guanosine residue at the 5′-end of the intron itself (Supplementary Figure S1). To make this reaction into an intermolecular reaction and obtain labeled RNA at its 5′-end, we employed a trans-acting Tetrahymena group I intron (36,39,41,44) (Figure 2c) instead of a wild-type cis-acting enzyme (Figures 2b and Supplementary Figure S1).
We prepared this trans-acting group I intron by in vitro transcription. The derived group I intron was dialyzed to eliminate co-existing non-labeled guanosine 5′-triphosphate and subjected to the guanosine transfer reaction (Supplementary Figure S2). Finally, we postulated that the remainder of the non-labeled 5′-fragment could be enzymatically ligated (10,24–26) (Figures 1b and 2d) to obtain a single-residue labeled RNA molecule.
We chose a hammerhead ribozyme from Schistosoma mansoni (53) as an example for functional RNA, and selected G10.1 [metal ion-binding site (3,6–8,11,54,55)] as the labeled site (Figure 2a). The starting materials (RNA fragments) were prepared according to the synthetic plan (Figure 2d). We then optimized the conditions for the guanosine transfer in terms of concentrations of Mg2+, group I intron and 5′-GMP (Supplementary Figures S5 and S6), temperature (Supplementary Figure S7) and reaction volume (Supplementary Figure S8). After the optimizations, we obtained a high yield of the 3′-fragment with 15N-labeled guanosine at the 5′-end (Figure 1c) (gel-based yield: ~80%, isolated yield after purification: ~50%). This labeled fragment was ligated with a non-labeled 5′-fragment, using T4 DNA ligase (Figure 1d), resulting in a site-specifically labeled RNA molecule with a 34% overall yield, including sample loss due to purification steps.
In order to confirm the incorporation of the labeled guanosine residue, we recorded NMR spectra of the labeled RNA (Figure 3; Supplementary Figures S3 and S4). In the 1H–15N HSQC and 15N-edited 1D 1H NMR spectra, we observed a unique H1–N1 cross peak and an imino proton resonance, respectively (Figure 3d and e), demonstrating that labeled guanosine was definitely incorporated in the RNA strand. Importantly, the observed unique cross peak unambiguously indicated that a single guanosine residue was labeled (Figure 3). Furthermore, following the addition of a substrate (inhibitor) strand against the labeled strand (hammerhead ribozyme) under low ionic conditions, we observed twin peaks of H8–N7 correlation (Supplementary Figure S3). Because only a single guanosine was labeled, we concluded that these peaks arose from G10.1 (Supplementary Figure S3), possibly due to the difference in substrate-binding states (substrate-free and bound forms) or a structural polymorphism.
In order to examine if this method is compatible with other sequences and much longer RNA, we produced a site-specifically labeled 76-nt RNA [hXBP1 mRNA (516–591)]. By using our labeling method, this 76-nt RNA molecule with labeled guanosine at the G544 site was prepared in a total yield of 19% (Supplementary Figure S11). In 1H–15N HSQC spectrum, a single cross peak was observed for this labeled RNA, which again indicates that a single residue labeled RNA was successfully produced (Supplementary Figure S12). Here, we demonstrated that our labeling method is compatible with long RNA and robust for sequence variation.
DISCUSSION
In this study, we established for the first time a site-specific labeling technique of RNA molecules without any applicable size-limit. We emphasize that our method is compatible with T4 RNA ligases I and II, as ligating enzymes (Supplementary Figure S9). In addition, an adenosine residue can also be labeled with our method, given that a mutant group I intron has been shown to promote an adenosine transfer reaction (46). Actually, we confirmed that this adenosine transfer reaction proceeds (Supplementary Figure S14).
Furthermore, our simple, two-step enzymatic reaction protocol gives a high yield of the hammerhead ribozyme, namely, total yield: 34%; guanosine transfer: ~80% (a conversion rate based on a gel image) and ~50% (isolated yield including an HPLC purification loss); ligation: ~70% (isolated yield including an HPLC purification loss). In the case of 76-nt RNA molecule, we found that the ligation with T4 DNA ligase can be performed successively after the guanosine transfer reaction without any purification of the guanosine-transferred RNA fragment. This simpler protocol gave 19% total yield even for such a long 76-nt RNA molecule. In any case, it was demonstrated that our enzymatic method can be applied for RNA molecules with different sequences and chain lengths. Thus, our labeling method is robust and adaptable to any size of RNA molecules. However, caution must be taken to the selection of Anti-Internal Guide Sequence (Anti-IGS) so as to avoid a secondary structure formation of 3′-fragment precursor (substrate for guanosine transfer reaction) which inhibits the reaction (Supplementary Figure S15). For the reaction scale, we confirmed that the reactions proceeded at 0.15 µmol scale in 3 ml reaction volume for guanosine transfer (Supplementary Figure S8) and at 0.15 µmol scale in 3 ml reaction volume for ligation (Supplementary Figure S10) without affecting the yield.
Next, we compared our enzymatic method with chemical synthesis. Technically, it is possible to obtain site-specifically labeled long RNA by chemical synthesis and ligation technique, where a site-specifically labeled short RNA oligomer is chemically synthesized and ligated with (a) non-labeled RNA oligomer(s). However, for the syntheses of site-specifically labeled oligonucleotides, one should have techniques of organic synthesis (2–6,9,12–17) that biochemists and structural biologists don't usually have. In contrast, our enzymatic pathway can be performed with commercially available sources (15N-labeled NMP, an RNA polymerase and a ligase), as well as fundamental biochemical techniques. Thus, this methodology has opened a new avenue to the preparation of site-specifically labeled long RNA molecule.
From a spectral point of view, the assignments of long RNA molecules are very time-consuming. However, with site-specifically labeled RNA molecules, one can directly access the spectral data of the desired site, e.g. catalytic residues and interfaces of intermolecular complexes. This progress will provide us with rapid assessment for mechanistic studies of functional, large RNA molecules.
In conclusion, we established a ribozyme-assisted single-guanosine-labeling technique of RNA molecules. Using this labeled RNA molecule, we were able to rapidly extract an NMR signal from a specific residue to explore complexation states (substrate-free and bound forms) and possibly electronic states of functional residues. It should also be mentioned that the derived site-specifically labeled RNA can be applicable to Raman spectroscopy and mass spectrometry. Thus, our method opened the door to analyze the mechanism of the biologically important RNA molecules.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–15.
FUNDING
Funding for open access charge: Human Frontier Science Program (HFSP) from HFSPO, France, and a Grant-in-Aid for Scientific Research (C) (20550145) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Chiiki-Innovation and Senryakuteki-Kibangijutu-Koudoka-Shienjigyo from Ministry of Economy, Trade and Industry, Japan.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
This work was performed using the NMR spectrometer with the ultra-high magnetic field under the Cooperative Research Program of Institute for Protein Research, Osaka University.
REFERENCES
- 1.Lippard SJ, Berg JM, editors. Principles of Bioinorganic Chemistry. CA: University Science Books; 1996. [Google Scholar]
- 2.Zhang X, Gaffney BL, Jones RA. 15N NMR of RNA fragments containing specifically labeled GU and GC pairs. J. Am. Chem. Soc. 1998;120:615–618. [Google Scholar]
- 3.Wang G, Gaffney BL, Jones RA. Differential binding of Mg2+, Zn2+, and Cd2+ at two sites in a hammerhead ribozyme motif, determined by 15N NMR. J. Am. Chem. Soc. 2004;126:8908–8909. doi: 10.1021/ja049804v. [DOI] [PubMed] [Google Scholar]
- 4.Fan Y, Gaffney BL, Jones RA. RNA GG•UU motif binds K+ but not Mg2+ J. Am. Chem. Soc. 2005;127:17588–17589. doi: 10.1021/ja0555522. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Kojima C, Yamazaki T, Kodama TS, Yasuno K, Miyashita S, Ono A, Ono A, Kainosho M, Kyogoku Y. Solution structure of an RNA duplex including a C-U base pair. Biochemistry. 2000;39:7074–7080. doi: 10.1021/bi000018m. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Kojima C, Morita EH, Kasai Y, Yamasaki K, Ono A, Kainosho M, Taira K. Identification of the metal ion binding site on an RNA motif from hammerhead ribozymes using 15N NMR spectroscopy. J. Am. Chem. Soc. 2002;124:4595–4601. doi: 10.1021/ja011520c. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Taira K. Detection of RNA nucleobase metalation by NMR spectroscopy. Chem. Commun. 2005:2069–2079. doi: 10.1039/b415137m. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Ono A. Nitrogen-15 NMR spectroscopy of N-metallated nucleic acids: Insights into 15N NMR parameters and N-metal bonds. Dalton. Trans. 2008:4965–4974. doi: 10.1039/b803510p. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Muto Y, Inoue M, Watanabe S, Kitamura A, Yokoyama S, Hosono K, Takaku H, Ono A, Kainosho M, et al. NMR analysis of the hydrogen bonding interactions of the RNA-binding domains of the Drosophila sex-lethal protein with target RNA fragments with site-specific [3-15N]uridine substitutions. Nucleic Acids Res. 1997;25:1565–1569. doi: 10.1093/nar/25.8.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtsuki T, Kawai G, Watanabe K. The minimal tRNA: unique structure of Ascaris suum mitochondrial tRNASerUCU having a short T arm and lacking the entire D arm. FEBS Lett. 2002;514:37–43. doi: 10.1016/s0014-5793(02)02328-1. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 11.Vogt M, Lahiri S, Hoogstraten CG, Britt RD, DeRose VJ. Coordination environment of a site-bound metal ion in the hammerhead ribozyme determined by 15N and 2H ESEEM spectroscopy. J. Am. Chem. Soc. 2006;128:16764–16770. doi: 10.1021/ja057035p. and the references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pervushin K, Ono A, Fernández C, Szyperski T, Kainosho M, Wüthrich K. NMR scalar couplings across Watson-Crick base pair hydrogen bonds in DNA observed by transverse relaxation-optimized spectroscopy. Proc. Natl Acad. Sci. USA. 1998;95:14147–14151. doi: 10.1073/pnas.95.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima C, Ono A, Kainosho M. Studies of physicochemical properties of N-H•••N hydrogen bonds in DNA, using selective 15N-labeling and direct 15N 1D NMR. J. Biomol. NMR. 2000;18:269–277. doi: 10.1023/a:1026717101063. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Ono A, Kainosho M, Bax A. H•••N hydrogen bond lengths in double stranded DNA from internucleotide dipolar couplings. J. Biomol. NMR. 2001;4:361–365. doi: 10.1023/a:1011250219293. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa R, Kojima C, Ono A, Kainosho M. Developing model systems for the NMR study of substituent effects on the N-H•••N hydrogen bond in duplex DNA. Magn. Reson. Chem. 2001;39:S159–S165. doi: 10.1093/nass/1.1.9. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Oda S, Yamaguchi H, Kondo Y, Kojima C, Ono A. 15N-15N J-coupling across HgII: Direct observation of HgII-mediated T-T base pairs in a DNA duplex. J. Am. Chem. Soc. 2007;129:244–245. doi: 10.1021/ja065552h. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Ono A. Structural Studies on MercuryII-mediated T-T Base-pair with NMR Spectroscopy. In: Hadjiliadis N, Sletten E, editors. Metal Complexes - DNA Interactions. UK: John Wiley & Sons, West Sussex; 2009. pp. 439–462. [Google Scholar]
- 18.Nikonowicz EP, Pardi A. Three-dimensional heteronuclear NMR studies of RNA. Nature. 1992;355:184–186. doi: 10.1038/355184a0. [DOI] [PubMed] [Google Scholar]
- 19.Batey RT, Inada M, Kujawinski E, Puglisi JD, Williamson JR. Preparation of isotopically labeled ribonucleotides for multidimensional NMR spectroscopy of RNA. Nucleic Acids Res. 1992;20:4515–4523. doi: 10.1093/nar/20.17.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polson AG, Crain PF, Pomerantz SC, McCloskey JA, Bass BL. The mechanism of adenosine to inosine conversion by the double-stranded RNA unwinding/modifying activity: a high-performance liquid chromatography-mass spectrometry analysis. Biochemistry. 1991;30:11507–11514. doi: 10.1021/bi00113a004. [DOI] [PubMed] [Google Scholar]
- 21.Nikonowicz EP, Sirr A, Legault P, Jucker FM, Baer LM, Pardi A. Preparation of 13C and 15N labelled RNAs for heteronuclear multi-dimensional NMR studies. Nucleic Acids Res. 1992;20:4507–4513. doi: 10.1093/nar/20.17.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michnicka MJ, Harper JW, King GC. Selective isotopic enrichment of synthetic RNA: application to the HIV-1 TAR element. Biochemistry. 1993;32:395–400. doi: 10.1021/bi00053a002. [DOI] [PubMed] [Google Scholar]
- 23.Batey RT, Battiste JL, Williamson JR. Preparation of isotopically enriched RNAs for heteronuclear NMR. Methods Enzymol. 1995;261:300–322. doi: 10.1016/s0076-6879(95)61015-4. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Lapham J, Crothers DM. Determining RNA solution structure by segmental isotopic labeling and NMR: application to Caenorhabditis elegans spliced leader RNA 1. Proc. Natl Acad. Sci. USA. 1996;93:44–48. doi: 10.1073/pnas.93.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I, Lukavsky PJ, Puglisi JD. NMR study of 100 kDa HCV IRES RNA using segmental isotope labeling. J. Am. Chem. Soc. 2002;124:9338–9339. doi: 10.1021/ja026647w. [DOI] [PubMed] [Google Scholar]
- 26.Tzakos AG, Easton LE, Lukavsky PJ. Complementary segmental labeling of large RNAs: economic preparation and simplified NMR spectra for measurement of more RDCs. J. Am. Chem. Soc. 2006;128:13344–13345. doi: 10.1021/ja064807o. [DOI] [PubMed] [Google Scholar]
- 27.Dingley AJ, Peterson RD, Grzesiek S, Feigon J. Characterization of the cation and temperature dependence of DNA quadruplex hydrogen bond properties using high-resolution NMR. J. Am. Chem. Soc. 2005;127:14466–14472. doi: 10.1021/ja0540369. [DOI] [PubMed] [Google Scholar]
- 28.Barfield M, Dingley AJ, Feigon J, Grzesiek S. A DFT study of the interresidue dependencies of scalar J-coupling and magnetic shielding in the hydrogen-bonding regions of a DNA triplex. J. Am. Chem. Soc. 2001;123:4014–4022. doi: 10.1021/ja003781c. [DOI] [PubMed] [Google Scholar]
- 29.Lu K, Miyazaki Y, Summers MF. Isotope labeling strategies for NMR studies of RNA. J. Biomol. NMR. 2010;46:113–125. doi: 10.1007/s10858-009-9375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao C, Zheng M, Rüdisser S. A simple and efficient method to reduce non templated nucleotide addition at the 3 terminus of RNAs transcribed by T7 RNA polymerase. RNA. 1999;5:1268–1272. doi: 10.1017/s1355838299991033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cech TR, Zaug AJ, Grabowski PJ. In vitro splicing of the ribosomal RNA precursor of tetehymena: involvement of guanosine nucleotide in the excision of the intervening sequence. Cell. 1981;27:487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- 32.Kruger K, Grabowski PJ, Zang AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 33.Davies RW, Waring RB, Ray JA, Brown TA, Scazzocchio C. Making ends meet: a model for RNA splicing in fungal mitochondria. Nature. 1982;300:719–724. doi: 10.1038/300719a0. [DOI] [PubMed] [Google Scholar]
- 34.Bass BL, Cech TR. Specific interaction between the self-splicing RNA of Tetrahymena and its guanosine substrate: implications for biological catalysis by RNA. Nature. 1984;308:820–826. doi: 10.1038/308820a0. [DOI] [PubMed] [Google Scholar]
- 35.Szostak JW. Enzymatic activity of the conservered core of a group I self-splicing intron. Nature. 1986;322:83–86. doi: 10.1038/322083a0. [DOI] [PubMed] [Google Scholar]
- 36.Zaug J, Been MD, Cech TR. The Tetrahymena ribozyme acts like an RNA restriction endonuclease. Nature. 1986;324:429–433. doi: 10.1038/324429a0. [DOI] [PubMed] [Google Scholar]
- 37.Burke JM, Irvine KD, Kaneko KJ, Kerker BJ, Oettgen AB, Tierney WM, Williamson CL, Zaug AJ, Cech TR. Role of conserved sequence elements 9L and 2 in self-splicing of the Tetrahymena ribosomal RNA precursor. Cell. 1986;45:167–176. doi: 10.1016/0092-8674(86)90380-6. [DOI] [PubMed] [Google Scholar]
- 38.Been MD, Cech TR. One binding site determines sequence specificity of Tetrahymena pre-rRNA self-splicing, trans-splicing, and RNA enzyme activity. Cell. 1986;47:207–216. doi: 10.1016/0092-8674(86)90443-5. [DOI] [PubMed] [Google Scholar]
- 39.Burke JM. Molecular genetics of group I introns: RNA structures and protein factors required for splicing–a review. Gene. 1988;73:273–294. doi: 10.1016/0378-1119(88)90493-3. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 40.Doudna JA, Cormack BP, Szostak JW. RNA structure, not sequence, determines the 5' splice-site specificity of a group I intron. Proc. Natl Acad. Sci. USA. 1989;86:7402–7406. doi: 10.1073/pnas.86.19.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy FL, Cech TR. Alteration of substrate specificity for the endoribonucleolytic cleavage of RNA by the Tetrahymena ribozyme. Proc. Natl Acad. Sci. USA. 1989;86:9218–9222. doi: 10.1073/pnas.86.23.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williamson CL, Desai NM, Burke JM. Compensatory mutations demonstrate that P8 and P6 are RNA secondary structure elements important for processing of a group I intron. Nucleic Acids Res. 1989;17:675–689. doi: 10.1093/nar/17.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michel F, Hanna M, Green R, Bartel DP, Szostak JW. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989;342:391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- 44.Cech TR. Self-splicing of group I introns. Annu. Rev. Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 45.Young B, Herschlag D, Cech TR. Mutations in a nonconserved sequence of the Tetrahymena ribozyme increase activity and specificity. Cell. 1991;67:1007–1019. doi: 10.1016/0092-8674(91)90373-7. [DOI] [PubMed] [Google Scholar]
- 46.Been MD, Perrotta AT. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991;252:434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- 47.Williams KP, Fujimoto DN, Inoue T. A region of group I introns that contains universally conserved residues but is not essential for self-splicing. Proc. Natl. Acad. Sci. USA. 1992;89:10400–10404. doi: 10.1073/pnas.89.21.10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strobel SA, Cech TR. Exocyclic amine of the conserved G·U pair at the cleavage site of the Tetrahymena ribozyme contributes to 5'-splice site selection and transition state stabilization. Biochemistry. 1996;35:1201–1211. doi: 10.1021/bi952244f. [DOI] [PubMed] [Google Scholar]
- 49.Shan S, Yoshida A, Sun S, Piccirilli JA, Herschlag D. Three metal ions at the active site of the Tetrahymena group I ribozyme. Proc. Natl Acad. Sci. USA. 1999;96:12299–12304. doi: 10.1073/pnas.96.22.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikawa Y, Shiraishi H, Inoue T. Minimal catalytic domain of a group I self-splicing intron RNA. Nat. Struct. Biol. 2000;7:1032–1035. doi: 10.1038/80947. [DOI] [PubMed] [Google Scholar]
- 51.Ikawa Y, Inoue T. Designed structural-rearrangement of an active group I ribozyme. J. Biochem. 2003;133:189–195. doi: 10.1093/jb/mvg023. [DOI] [PubMed] [Google Scholar]
- 52.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 53.Martick M, Scott WG. Tertiary contacts distant from the active site prime a ribozyme for catalysis. Cell. 2006;126:309–320. doi: 10.1016/j.cell.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pley HW, Flaherty KM, McKay DB. Three-dimensional structure of a hammerhead ribozyme. Nature. 1994;372:68–74. doi: 10.1038/372068a0. [DOI] [PubMed] [Google Scholar]
- 55.Scott WG. Biophysical and biochemical investigations of RNA catalysis in the hammerhead ribozyme. Q. Rev. Biophys. 1999;32:241–284. doi: 10.1017/s003358350000353x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.