Abstract
Introduction
In clinical trials, fixed-dose enoxaparin (40 mg once daily) reduces the risk of venous thromboembolism (VTE) in medically-ill patients. However, morbidly obese patients were under-represented in these trials and using fixed-dose enoxaparin in obese patients may be inadequate. We completed a pharmacokinetic study in morbidly obese, medically-ill patients to determine if weight-based dosing of enoxaparin for VTE prophylaxis was feasible, without excessive levels of anticoagulation, as determined by peak anti-Xa levels.
Materials and Methods
Twenty eight morbidly obese (BMI≥35 kg/m2) patients were enrolled and completed the study protocol. Enoxaparin 0.5 mg/kg was administered once daily subcutaneously and peak anti-Xa levels were measured approximately 4–6 hours after the enoxaparin dose.
Results and Conclusions
Overall, 46% of patients were female, the average age (±SD) was 54 (±11) years, and the average weight and BMI were 135.6 kg (±25.3) and 48.1 kg/m2 (±11.1), respectively. The average daily dose of enoxaparin was 67 mg (±12). The average peak anti-Xa level was 0.25 (SD±0.11, range 0.08 to 0.59) units/mL. Peak anti-Xa levels did not significantly correlate with weight or BMI. There were no bleeding events, symptomatic VTE, or significant thrombocytopenia.
In morbidly obese, medically-ill patients, use of weight-based enoxaparin dosed at 0.5 mg/kg once daily is feasible and results in peak anti-Xa levels within or near recommended range for thromboprophylaxis, without any evidence of excessive anti-Xa activity. These data suggest that this weight-based regimen may be more effective than standard fixed-dose enoxaparin. Clinical outcome studies are warranted to determine the clinical safety and efficacy of this regimen.
Keywords: Low-molecular weight heparin, Medically-Ill, Obesity, Pharmacokinetics, Prophylaxis, Venous Thromboembolism
1. Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant cause of morbidity and mortality in hospitalized patients. Anticoagulant-based prophylaxis, including low molecular weight heparins, (LMWHs), unfractionated heparin, and factor Xa inhibitors reduce the relative risk of VTE by 45% to 63% in medically-ill patients [1–3].
Thromboprophylaxis dosing with LMWHs is well characterized for normal weight patients. However, obesity affects drug distribution and pharmacokinetics. Obese individuals have an increased percentage of fat per kilogram of total bodyweight and blood flow in adipose tissue is lower than lean body mass [4]. These differences in lean body mass and vascularization may affect drug kinetics. As the incidence of morbid obesity is increasing [5], understanding the pharmacokinetics and optimal prophylactic dosing of LMWH in morbidly obese patients is paramount.
In thromboprophylaxis studies, including those investigating fixed-dose enoxaparin, morbidly obese patients (BMI≥35 kg/m2) are under-represented [1], creating several challenges. Federal Drug Administration (FDA) approved doses for thromboprophylaxis (e.g. enoxaparin 40 mg once daily or dalteparin 5000 International Units once daily) are fixed doses that do not take into account actual body weight and, since the drug distribution of LMWHs is weight dependent, anticoagulant levels may differ in obese patients when fixed-doses are prescribed [6–9]. In support of this, a negative correlation between anti-Xa activity and actual body weight was demonstrated in obese patients given enoxaparin 40 mg once a day [10].
Obese patients are also at higher risk for VTE, even when thromboprophylaxis is prescribed [11,12]. Although pharmacokinetic studies of therapeutic LMWHs in obese patients suggest that dosing by actual body weight, without capping the dose, is appropriate [13–15] there are limited data describing optimal use of prophylactic dose LMWHs in morbidly obese, medically-ill patients. When used in prophylactic doses in healthy, obese volunteers, weight adjusted tinzaparin (e.g. 75 IU/kg), a LMWH, has been shown to result in predictable anti-Xa levels up to body weights of 170 kilograms [16]. However, tinzaparin is not widely utilized in the U.S. and uncertainties remain about whether predictable anti-Xa levels can be achieved with a weight-based prophylactic regimen using other LMWHs more commonly employed in the U.S., such as enoxaparin. In addition, it is not yet known if pharmacokinetics differ above weights of 170 kg. In the current study, we investigated the feasibility of weight-based prophylactic dosing of enoxaparin in morbidly obese, medically-ill patients.
2. Materials and Methods
Eligible patients were medically-ill patients>18 years of age with a body mass index (BMI)≥35 kg/m2 and at risk for VTE, as determined by the patient’s admitting physician, thus warranting thrombophrophylaxis. Patients were excluded if they were pregnant, on therapeutic anticoagulation, had a bleeding disorder, platelet count of less than 100,000/mL, coagulopathy, active bleeding, estimated creatinine clearance less than 30 mL/min (based on both the MDRD, modification of diet in renal disease, and Cockcroft-Gault equation using adjusted body weight), or stroke, surgery or trauma within 14 days. This study was approved by the institutional Ethics Committee on human research.
Once informed consent was obtained from each patient and baseline clinical and demographic information were collected, study patients were given enoxaparin 0.5 mg/kg subcutaneously once daily for prophylaxis for two consecutive days. Weight was determined upon hospital admission with a standardized, calibrated scale. The dose of enoxaparin was not capped and was rounded to the nearest 5 mg unit.
Peak anti-Xa levels were obtained using a local, national reference laboratory (ARUP, www.aruplab.com) through venipuncture approximately 4–6 hours after the first or second dose of enoxaparin was given, in the following manner. A pilot tube was drawn first. An exact ratio of 9 volumes of blood to 1 volume of anticoagulant (32 g/L citrate) was maintained. Blood samples were transported directly to the laboratory, where they were immediately centrifuged and then assayed according to standard protocols using chromogenic inhibition of factor Xa. The Rotachrom® assay using the STA-Compact instrument (Diagnostica Stago, Parsippany, NJ) was used to quantitate anti-Xa (LMWH) activity for enoxaparin. The sensitivity of this assay is 0.2 U/mL and within run imprecision is 5.5 (% CV) at 1 U/mL. The assay is linear between 0.2–2.0 U/mL. Serial measurements of anti-Xa levels for area under the curve calculation were not feasible due to the frequency of peak values around 0.2 IU/mL or less, as the precision of the assay below 0.2 IU/mL has not been established.
After the second dose of enoxaparin was given, patients continued VTE prophylaxis at a dose deemed necessary by their attending physician through the remainder of their hospital stay. Most patients were switched to fixed-dose enoxaparin at FDA approved doses (i.e. 40 mg SC once daily). Adverse events such as symptomatic VTE, any bleeding events, and significant thrombocytopenia (≥50% decrease from baseline) were recorded during the hospital stay. Patients were followed until hospital discharge for the development of any adverse events.
Data were analyzed with regards to peak anti-Xa levels and variance between subjects using descriptive statistics and linear regression analyses. The sample size chosen was based on prior literature [16].
3. Results
28 patients met eligibility criteria and completed the protocol. Demographics are illustrated in Table 1. Overall, 46% of the patients were female, the average age (±SD) was 54 (±11) years, and the average weight and BMI were 135.6 kg (±25.3) and 48.1 kg/m2 (±11.1) respectively. Common risk factors for VTE and their frequency in our population are listed in Table 2. The average daily dose of enoxaparin administered was 67 mg (±12). The average peak anti-Xa level was 0.25 (SD±0.11, range 0.08 to 0.59) units/mL, when measured an average 277 minutes after the dose of enoxaparin (range 236–407 minutes). There were no bleeding events, symptomatic DVT or PE, or significant thrombocytopenia.
Table 1.
Demographic of the study population
| Demographic | Number (±SD, range) |
|---|---|
| Female | 13 (46%) |
| Caucasian | 27 (96%) |
| Age (years) | 54.7 (±11.2, 32–80) |
| Weight (kg) | 135.6 (±25.3, 100–210) |
| Height (cm) | 168.7 (±10.2, 149–188) |
| BMI (kg/m2) | 48.1 (±11.1, 35.8–85.2) |
| Serum Creatinine (mg/dL) | 0.99 (±0.34, 0.50–1.80) |
| Calculated Creatinine Clearance† (ml/min) | 117 (±56, 51–342) |
| Mean Enoxaparin Dose (mg) | 67 (±13, 50–105) |
Creatinine clearance was calculated using adjusted body weight (ABW), BMI: Body Mass Index.
Table 2.
VTE risk factors in our study population
| Risk Factors | Number (%) |
|---|---|
| Acute Respiratory Failure | 4 (14%) |
| CHF as Admit Diagnosis | 1 (4%) |
| Age>70 | 2 (7%) |
| ICU Admission | 1 (4%) |
| Prior VTE | 3 (11%) |
| Acute CVA/AMI/Paresis | 1 (4%) |
| Obesity (weight>120 kg) | 21 (75%) |
| Acute Infection as Admit Diagnosis | 17 (61%) |
| Estrogen/OCP Use | 1 (4%) |
| CHF | 2 (7%) |
| Age 40–70 | 22 (79%) |
| Acute Rheumatologic Disorder | 2 (7%) |
| Chronic Lung Disease | 7 (25%) |
| Cancer | 0 (0%) |
CHF: Congestive heart failure; OCP: Oral contraceptives; ICU: Intensive Care Unit; VTE: venous thromboembolism.
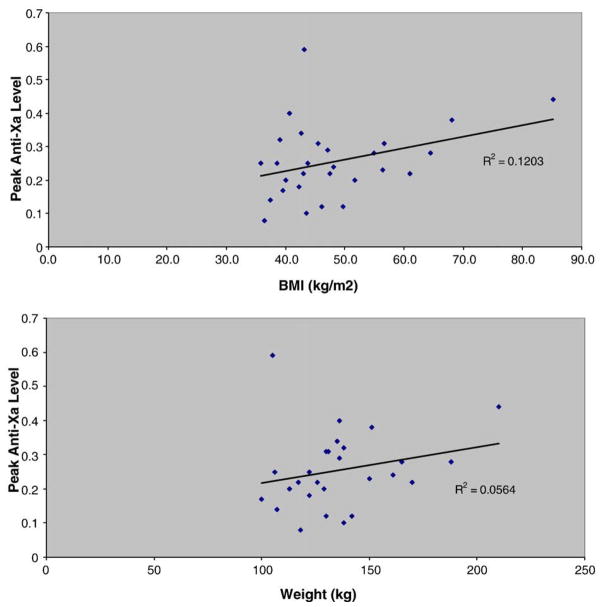
Using linear regression analyses, peak anti-Xa levels did not significantly correlate with either total body weight or BMI (Fig. 1). Based upon dosing in these patients with total body weights up to 210 kg (BMI 85.2 kg/m2), there did not appear to be a weight above which the dose of enoxaparin should be capped.
Fig. 1.
Peak anti-Xa levels, drawn 4–6 hours after the dose of enoxaparin, did not correlate with weight or BMI.
4. Discussion
Although large, randomized clinical trials have demonstrated the efficacy and safety of LMWHs in preventing VTE, medically-ill, morbidly obese patients have been under-represented [1–3]. Obese patients have a higher risk of VTE than normal-weight patients, making optimal VTE prophylaxis paramount in this population. Prior data have demonstrated that peak anti-Xa activity correlates negatively with total body weight [17] and, in severely obese surgical patients, standard, fixed-dose LMWH is associated with higher failure rates of VTE prophylaxis [18].
The results of this study demonstrate that in medically-ill, morbidly obese patients weight-based dosing of enoxaparin (0.5 mg/kg once daily SQ) for VTE prophylaxis leads to anti-Xa levels within or near recommended ranges for prophylaxis (peak 0.25, range 0.08 to 0.59 units/mL). Importantly, there were no patients who had a therapeutic level of anticoagulation (anti-Xa level 0.6 – 2.0 IU/ml). We also found that neither weight nor BMI correlated with peak anti-Xa levels when a weight-based dosing approach was used, confirming that this method resulted in acceptable levels of anti-coagulation.
Our findings are consistent with prior reports and extend these published data to higher weights and to medically-ill patients. A recent study compared peak anti-Xa levels among obese, bariatric surgery patients randomized to receive one of two thromboprophylaxis regimens (enoxaparin 30 mg SQ BID or enoxaparin 40 mg SQ BID). In the group who received the higher enoxaparin dose, peak anti-Xa levels were 0.14 and 0.15 units/mL after first and third doses respectively [19] but only 30.8% of subjects had an appropriate level (defined as 0.18–0.44 IU/ml) after their first dose. These results suggest that, based upon anti-Xa levels, the dosing scheme of 40 mg twice daily may be inadequate.
Our study extends published data on prophylactic LMWH dosing in obese patients in several ways. First, our data builds on prior reports by enrolling patients weighing above 170 kg. In addition, prior data was published in healthy obese outpatient controls [16]. Our data set demonstrates that weight-based dosing is feasible and does not lead to excessive anti-Xa levels in hospitalized, morbidly obese, medically-ill patients. Finally, our study employed the more widely utilized LMWH in the United States, enoxaparin.
The weight-based dosing regimen of enoxaparin used in our study appeared to be safe from both a pharmacologic and clinical standpoint. There were no bleeding events, symptomatic VTE, or cases of heparin-induced thrombocytopenia (HIT) among our study population, although the study was not powered to determine clinical efficacy or safety. Importantly, we did not enroll patients with a creatinine clearance <30 mL/min. Enoxaparin, like other LMWHs, undergoes renal elimination and may accumulate with repeated dosing [20]. If enoxaparin is used in patients with a creatinine clearance<30 mL/min, the dose should be adjusted as indicated in the package insert.
This study was limited by a relatively small sample size, lack of long-term clinical outcome data, and the use of only a single anti-Xa level, rather than repeat measures. In healthy volunteers given repeat doses of enoxaparin 40 mg once a day, steady state appears to be achieved on day 2 [21]. However, peak anti-Xa levels are fairly well predicted by single dose pharmacokinetics [8,9]. Although we sought to measure anti-Xa levels after both the first and second dose, due to an average length of stay of only 3 days, this was often not feasible.
Moreover, this study was designed to determine if a weight-based dosing algorithm led to predictable peak anti-Xa levels in morbidly obese patients and was not powered for clinical outcomes. Furthermore, because we were using prophylactic dose LMWHs in morbidly obese patients, anti-Xa levels were low and precise calculations of the AUC were not possible. We did find that peak anti-Xa levels were within the range recommended by some authors (0.2–0.6 units/mL) when prophylactic doses are administered. As the regular, clinical measurement of anti-Xa levels may not be possible in a timely fashion at all centers, our results provide justification for unmonitored weight-based prophylactic dosing using this regimen.
Although this study extends our understanding of how to optimally prescribe VTE prophylaxis in morbidly obese, medically-ill patients, additional clinical outcomes studies are needed as an optimal approach remains unproven. Some authors have advocated empirically increasing the dose of prophylactic LMWH in morbidly obese patients [10,16,18,22] while others, including the authors of the 2008 American College of Chest Physicians (ACCP), highlight the available data, including the data in bariatric surgery patients, and recommend weight-based dosing of LMWHs in obese patients based on actual body weight [23]. Unfortunately, these guidelines do not provide specific dosing regimens. Our study suggests that a dose of enoxaparin 0.5 mg/kg once daily is reasonable. Importantly, a larger, clinical outcomes study is needed to compare the efficacy and safety of this dosing regimen to standard, fixed-dose regimen of enoxaparin 40 mg subcutaneously once daily in morbidly obese patients.
5. Conclusions
Obese patients are at an increased risk of developing VTE while hospitalized and fixed-dose LMWHs may be sub-optimal in this population. Current practice guidelines recommend utilizing weight-based dosing of LMWH in obese patients, but do not provide specific dosing guidance [23,24]. We found that a specific weight-based dose of enoxaparin (0.5 mg/kg subcutaneously once daily without capping the dose) is feasible and results in peak anti-Xa levels within or near recommended range for thromboprophylaxis, without any evidence of excessive anti-Xa activity. Although this data is encouraging, additional clinical outcome studies evaluating this regimen are necessary to determine its reproducibility, safety, and efficacy. In the interim, clinical judgment and application of pharmacodynamic-based information is prudent.
Acknowledgments
Funding Source: This work was supported by an investigator-initiated grant from sanofi-aventis. Sanofi-aventis had no role in the study design, data collection, analysis, or interpretation of this study nor did sanofi-aventis have any role in manuscript preparation or the decision to submit the manuscript for publication.
Abbreviations
- VTE
venous thromboembolism
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- LMWH
low molecular weight heparin
- BMI
body mass index
- FDA
federal drug administration
- MDRD
modification of diet in renal disease
- IRB
institutional review board
- IU
international units
- HIT
heparin-induced thrombocytopenia
- BID
twice daily
- AUC
area under the curve
- ACCP
American College of Chest Physicians
- CHF
congestive heart failure
- OCP
oral contraceptives
- ICU
intensive care unit
- SD
standard deviation
Footnotes
Conflict of Interest Statement: Dr. Pendleton is on the speaker’s bureau for Sanofi-Aventis and has received grant support from Sanofi-Aventis. Dr. Rondina is on the speaker’s bureau for Sanofi-Aventis, Pfizer, and GSK and has received grant support from Sanofi-Aventis and Pfizer. Dr. Rodgers is on the speaker’s bureau for GSK. Drs. Draper and Wheeler have no conflicts of interest to report.
References
- 1.Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, et al. A comparison of enoxaparinwith placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 2.Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–9. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 3.Spyropoulos AC. Emerging strategies in the prevention of venous thromboembolism in hospitalized medical patients. Chest. 2005;128:958–69. doi: 10.1378/chest.128.2.958. [DOI] [PubMed] [Google Scholar]
- 4.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93:S1–8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 6.Duplaga BA, Rivers CW, Nutescu E. Dosing and monitoring of low-molecular-weight heparins in special populations. Pharmacotherapy. 2001;21:218–34. doi: 10.1592/phco.21.2.218.34112. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs MJ, Weir K, MacKinnon K, Keeney M, Brien WF, Cruickshank MK. Body weight does not predict for anti-Xa levels after fixed dose prophylaxis with enoxaparin after orthopedic surgery. Thromb Res. 1998;91:137–42. doi: 10.1016/s0049-3848(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 8.Matzsch T, Bergqvist D, Hedner U, Ostergaard P. Effects of an enzymatically depolymerized heparin as compared with conventional heparin in healthy volunteers. Thromb Haemost. 1987;57:97–101. [PubMed] [Google Scholar]
- 9.Vitoux JF, Aiach M, Roncato M, Fiessinger JN. Should thromboprophylactic dosage of low molecular weight heparin be adapted to patient’s weight? Thromb Haemost. 1988;59:120. [PubMed] [Google Scholar]
- 10.Frederiksen SG, Hedenbro JL, Norgren L. Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg. 2003;90:547–8. doi: 10.1002/bjs.4068. [DOI] [PubMed] [Google Scholar]
- 11.Clagett GP, Anderson FA, Jr, Geerts W, Heit JA, Knudson M, Lieberman JR, et al. Prevention of venous thromboembolism. Chest. 1998;114:531S–60S. doi: 10.1378/chest.114.5_supplement.531s. [DOI] [PubMed] [Google Scholar]
- 12.Rocha AT, de Vasconcellos AG, da Luz Neto ER, Araujo DM, Alves ES, Lopes AA. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg. 2006;16:1645–55. doi: 10.1381/096089206779319383. [DOI] [PubMed] [Google Scholar]
- 13.Sanderink GJ, Le Liboux A, Jariwala N, Harding N, Ozoux ML, Shukla U, et al. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72:308–18. doi: 10.1067/mcp.2002.127114. [DOI] [PubMed] [Google Scholar]
- 14.Bazinet A, Almanric K, Brunet C, Turcotte I, Martineau J, Caron S, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116:41–50. doi: 10.1016/j.thromres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SJ, Wilbur K, Burton E, Anderson DR. Effect of patient weight on the anticoagulant response to adjusted therapeutic dosage of low-molecular-weight heparin for the treatment of venous thromboembolism. Haemostasis. 2001;31:42–8. doi: 10.1159/000048043. [DOI] [PubMed] [Google Scholar]
- 16.Hainer JW, Barrett JS, Assaid CA, Fossler MJ, Cox DS, Leathers T, et al. Dosing in heavy-weight/obese patients with the LMWH, tinzaparin: a pharmacodynamic study. Thromb Haemost. 2002;87:817–23. [PubMed] [Google Scholar]
- 17.Samama MM, CV, LC Relation between weight, obesity, and frequency of deep vein thrombosis after enoxaparin in orthopedic surgery [abstr] Thromb Haemost. 1995;73:977. [Google Scholar]
- 18.Hamad GG, Choban PS. Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing bariatric surgery: findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obes Surg. 2005;15:1368–74. doi: 10.1381/096089205774859245. [DOI] [PubMed] [Google Scholar]
- 19.Rowan BO, Kuhl DA, Lee MD, Tichansky DS, Madan AK. Anti-Xa levels in bariatric surgery patients receiving prophylactic enoxaparin. Obes Surg. 2008;18:162–6. doi: 10.1007/s11695-007-9381-y. [DOI] [PubMed] [Google Scholar]
- 20.Crowther M, Lim W. Low molecular weight heparin and bleeding in patients with chronic renal failure. Curr Opin Pulm Med. 2007;13:409–13. doi: 10.1097/MCP.0b013e328216430d. [DOI] [PubMed] [Google Scholar]
- 21.Frydman A. Low-molecular-weight heparins: an overview of their pharmacodynamics, pharmacokinetics and metabolism in humans. Haemostasis. 1996;26 (Suppl 2):24–38. doi: 10.1159/000217270. [DOI] [PubMed] [Google Scholar]
- 22.Forestieri P, Quarto G, De Caterina M, Cuocolo A, Pilone V, Formato A, et al. Prophylaxis of thromboembolism in bariatric surgery with parnaparin. Obes Surg. 2007;17:1558–62. doi: 10.1007/s11695-007-9259-z. [DOI] [PubMed] [Google Scholar]
- 23.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:141S–59S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 24.Geerts W, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism. Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]