Abstract
Background
Elevated visceral adiposity is strongly predictive of cardiometabolic disease, but, due to the high cost of biomedical imaging, assessment of factors contributing to normal variation in visceral (VAT) and subcutaneous (SAT) adipose tissue partitioning in large cohorts of healthy individuals are few, particularly in ethnic and racial minority populations.
Objective
To describe age, menopausal status, smoking and physical activity differences in VAT and abdominal subcutaneous adipose tissue (ASAT) mass in African-American (AA) and European-American (EA) women.
Methods
Magnetic resonance imaging measures of VAT and ASAT mass and VAT% (VAT/VAT + ASAT, %) were obtained from a cross-sectional sample of 617 EA and 111 AA non-diabetic women aged 18–80 years. Multivariate linear regression was used to test independent effects of the covariates.
Results
VAT and VAT% were higher in EA than AA women (p < 0.01). Differences in VAT, ASAT and VAT% across age groups began in early adulthood in both ethnic groups, but the association of age with VAT% was stronger in EA women (p for interaction = 0.03). Current smokers had higher VAT and VAT% (p < 0.01) and lower TBF than non-smokers. Frequent participation in sports activities was associated with ~ 30% lower VAT in older (> 55 years) as well as younger (< 40 years) women (p < 0.0001).
Conclusion
Greater allocation of abdominal adipose tissue into the visceral compartment occurs in EA than AA women and in older than younger women. Avoidance of cigarette smoking and frequent participation in sports activities may partially counteract this deleterious phenomenon of ageing.
Keywords: Menopause, obesity, visceral adipose tissue, adiposity, magnetic resonance imaging, race, ethnicity, ageing, African-American, women
INTRODUCTION
Visceral adipose tissue (VAT), although small in absolute quantity, is more strongly associated with cardiometabolic risk (Boyko et al. 2000; Montague and O’Rahilly 2000; Miyazaki et al. 2002; Misra and Vikram 2003; Despres and Lemieux 2006; Kuk et al. 2006) than is the subcutaneous adipose tissue mass, which is 5–10-times larger. In women, the menopausal transition is known to be associated with increases in VAT (Lanska et al. 1985; Ley et al. 1992; Kotani et al. 1994; Svendsen et al. 1995; Panotopoulos et al. 1996; Toth et al. 2000; Genazzani and Gambacciani 2006; Lovejoy et al. 2008) and changes in FSH, testosterone and other aspects of ageing in ovarian function are likely to be key mediators (Sowers et al. 2007; Janssen et al. 2010). However, the focus on changes during mid-life and the development of overt cardiometabolic disease in women, as well as men, has meant that there are relatively few comparative studies of age trends in VAT that include data on early adulthood when metabolic and cardiovascular risk factors are developing and clinical outcomes may be prevented.
The fragmentary nature of published data describing normal age variation in adipose tissue partitioning was noted by Shen et al. (2009). This is likely due to the relatively high cost of intra-abdominal imaging for large cohort studies, the justifiable focus on obese, diabetic, and mid-life individuals at greatest immediate risk of diabetes and cardiovascular diseases, and the rarity of large studies of ethnic and racial minority populations. In addition, there remains debate as to whether increases in visceral adipose tissue in women are primarily due to hormonal shifts during the menopausal transition (Sowers et al. 2007; Lovejoy et al. 2008; Janssen et al. 2010), and whether they are entirely attributable to the increase in total adiposity, as has been suggested (Lara-Castro et al. 2002). Further, single axial image protocols that measure VAT area at the L4–L5 inter-vertebral space predominate in the literature, but while VAT area at L4–L5 is highly correlated with total VAT mass, it over-estimates the visceral adiposity of women compared to men, because in men, deposition of VAT tissue is principally above (cranial to) L4–L5, whereas in women, VAT deposition is greater at or below L4–L5 (Demerath et al. 2008).
The aim of the present study is to use data from one of the larger magnetic resonance imaging studies conducted to date to describe age differences in visceral, abdominal subcutaneous and total adipose tissue mass in healthy non-diabetic, African American and European American women measured over a wide range of adult ages. Objectives were to determine whether age differences vary by self-reported race/ethnicity, to assess the extent to which age-related and ethnicity-related differences are accounted for by menopausal status, cigarette smoking and physical activity level and to test whether or not older women reporting frequent physical activity display benefits of lower visceral and total adiposity similar to those seen in younger women.
METHODS
Subjects
The sample included 728 apparently healthy women (111 African-Americans, 617 European-Americans) aged 18–80 years, none of whom had Type 1 or Type 2 diabetes mellitus and who were not selected on the basis of having any health condition. All are enrolled in one of three longitudinal studies of body composition and cardiovascular disease risk at the Lifespan Health Research Center (Dayton, OH) in 2003–2006. These women comprised > 75% of all adult women currently being followed in those studies and they did not differ from those who did not participate in the imaging study in age, BMI or activity level. The Fels Longitudinal Study, Southwest Ohio Family Heart Study and the Miami Valley Family Ageing Study are community-dwelling individuals in families from the southwestern Ohio region sampled in different ways. The Fels Longitudinal Study is a convenience sample of predominantly European American families living in Yellow-Springs, OH and being followed on a semi-annual basis for growth and health information. The Southwest Ohio Family Heart Study participants were enrolled from African-American and European-American families identified from the MRFIT study as having a male family member with essential hypertension and the Miami Valley Family Ageing Study participants were individuals over 40 years of age from large sibships and were recruited from a general medical practice in Springfield, OH, in local churches and by local advertisement. Race/ethnicity (hereafter referred to as ‘ethnicity’) was self-reported by the subjects as non-Hispanic African-American (AA) or non-Hispanic European American (EA). Subjects were pre-screened to insure they were free of any contraindications for MRI. The study protocols and informed consent documents were approved by the Wright State University Institutional Review Board and all subjects provided written consent prior to participation.
Anthropometry and total body composition
Subjects wore light clothing (shorts, sleeveless shirts) during measurement. Weight was measured to the nearest 0.01 kg and stature to the nearest 0.01 cm using a digital scale and digital stadiometer. DXA, using a Hologic QDR 4500 Elite x-ray densitometer (Bedford, MA) was used in the fast whole-body scanning mode to estimate total body fat mass (TBF) in kilograms (kg). Percent body fat (%BF) was calculated by dividing TBF by weight and multiplying by 100%.
Abdominal MRI was conducted at the Good Samaritan Hospital Greater Dayton MRI Consortium in Dayton, OH. Axial images were obtained every 10 mm with a Siemens Magnetom Vision 1.5 Tesla whole body scanner (Siemens Canada, Ltd., Mississauga, Canada) using a T1-weighted fast-spin echo pulse sequence as previously described (Demerath et al. 2007) and segmentation of the axial images into VAT areas was performed by two trained observers using image analysis software (slice-O-matic™, version 4.2, Tomovision, Inc., Montreal, Quebec, Canada). VAT and SAT areas were summed across all images to obtain VAT and SAT volumes and then these volumes were multiplied by 0.9 g/cm3, the approximate density of adipose tissue to obtain total VAT mass (kg) and total ASAT mass (kg). We created a derived variable to further characterize abdominal adiposity: percentage visceral adipose tissue (%VAT = (VAT mass/(VATmass + ASAT mass))* 100).
Physical activity and smoking
Habitual physical activity (PA) was self-reported using the sports activity index of the Baecke questionnaire of physical activity (Baecke et al. 1982). This sports activity index (Sport PA) has a stronger association with body composition (including VAT) than the leisure or work activity indices (Choh et al. 2008). Sport PA is coded on a scale of 1–5. Current smoking was self-reported (yes/no) and confirmed in a sub-set using a cotinine assay (Laboratory Corporation of America® Holdings, Burlington, NC).
Menopausal status and hormone therapy
Women who reported regular menstrual cycles over the previous 12 months were classified as pre-menopausal, women who reported missing between 3–11 menstrual cycles in the last year, menopausal symptoms (e.g. hot flashes, vaginal dryness) and/or changes in cycle length or duration of menses were classified as peri-menopausal and women reporting having a uterus and at least one ovary, but experiencing 12 or more continuous months without a menstrual cycle were classified as having natural menopause (Gracia et al. 2005). Women who reported a hysterectomy or a bilateral oophorectomy, regardless of menstrual cycle information, were classified as having surgical menopause, Women with natural menopause (n = 209) did not differ from those with surgical menopause (n = 81) in mean VAT, ASAT, %AF, %VAT or age in either race (p > 0.20 for all). When we restricted the analysis to only include post-menopausal women with natural menopause, this did not alter the results, and therefore we included all post-menopausal women in the same category. Current hormone replacement therapy with oestrogen/progesterone medications) was documented at the time of examination and recorded as ‘yes’ or ‘no’. Information on hormone use was available for 488 women (442 EA, 46 AA women). This sub-set was not significantly different from the entire sample in BMI, %BF, VAT, ASAT or age (p > 0.10 for all).
Statistical analysis
Descriptive statistics were generated and analysed for deviations from normality and variables with significant skewness were log-transformed prior to analysis. Two-sample chi-squared and t-tests were used to assess differences by race. Multivariate linear regression models were tested to assess the independent effects of age, menopausal status, ethnicity, TBF, physical activity and current smoking on the dependent variables (VAT, ASAT, %VAT and TBF). The interactions of age and menopausal status, age and ethnicity and age and sport PA were tested. Differences in body composition in women using exogenous hormones and women not using hormones were also tested using multivariate linear regression in the sub-set with these data. Finally, we compared least squares means for VAT and TBF (adjusted for all covariates above, except TBF) across tertiles of Sport PA, stratified by age tertiles, in order to test whether the beneficial effect of higher levels of Sport PA on body composition was modified by age. Age ranges within the lowest to highest age tertiles were 18–39 years, 40–54 years and 55–80 years, respectively. All analyses were conducted using SAS version 9.2 (SAS institute, Cary, NC).
RESULTS
Women in the study sample were overweight or obese on average and were relatively sedentary, with a mean Baecke sport activity score of 2 out of 5 (Table I). AA women had significantly greater BMI and ASAT and had lower Sport PA and %VAT than EA women (all p < 0.05). Significantly fewer AA women reported taking exogenous hormone replacement medications. In these unadjusted comparisons, we found no significant differences in age, VAT, %BF or %AF between AA and EA women.
Table I.
Description of study sample.
| European-American women (n = 617) | African-American women (n = 111) | |
|---|---|---|
| Age | 48.1 ± 15.5 | 46.3 ± 14.9 |
| Weight (kg) | 74.2 ±17.1 | 83.2 ± 20.8* |
| BMI (kg/m2) | 27.6 ± 6.1 | 31.2 ± 7.7* |
| %BF | 35.7 ± 7.1 | 36.9 ± 7.7 |
| TBF (kg) | 26.8 ± 9.8 | 30.4 ± 11.0 |
| Sport PA (score, 1–5) | 2.1 ± 0.7 | 1.9 ± 0.6* |
| VAT (kg) | 1.7 ± 1.2 | 1.6 ± 1.0 |
| ASAT (kg) | 4.9 ± 3.0 | 6.8 ± 4.2* |
| %VAT | 24.7 ± 8.3 | 19.7 ± 7.3* |
| Menopausal status (n, %) | ||
| Pre-menopausal | 251 (40.7) | 39 (35.1) |
| Peri-menopausal | 144 (23.3) | 36 (32.4) |
| Post-menopausal | 222 (36.0) | 36 (32.4) |
| Smoking (% yes) | 24 | 15 |
| Current hormone replacement (% yes), n = 488 | 21 | 7** |
BMI, body mass index; %BF, percentage body fat; Sport PA, sport index score from the Baecke physical activity questionnaire, range 1–5; VAT, visceral adipose tissue mass; ASAT, abdominal subcutaneous adipose tissue mass; %VAT, percentage visceral adipose tissue, VAT/VAT + ASAT, %; %AF, percentage abdominal adipose tissue, VAT + ASAT/TBF, %; Smoking, current cigarette smoking.
Significant race difference, t-test, p < 0.01;
Significant race difference, χ2 = 5.43, p = 0.0198.
Ethnicity differences in abdominal and total adipose tissue mass by age group
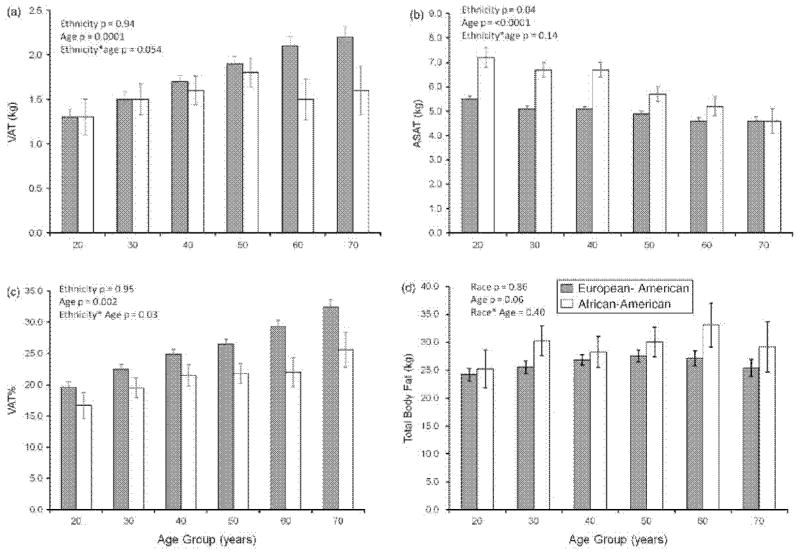
VAT, ASAT, VAT% and TBF were compared between EA and AA women by decade of age, adjusting for menopausal status, sports activity level, current smoking and total adiposity (Figure 1). VAT was higher in older age groups than younger age groups (p < 0.0001), although this was less clear in AA women who had smaller n per age group, especially at the upper ages. There was a marginally significant age group *ethnicity interaction on VAT (p = 0.054), suggesting a greater dependence of VAT on age in EA than AA women. ASAT was higher in AA than EA women (p = 0.04) and negatively associated with age group in both groups (p < 0.0001). As a result of the opposite associations between age and VAT mass (increased with age) and ASAT mass (decreased with age), older women had greater relative visceral adiposity (VAT%) than younger women in both ethnicity groups. This relationship was stronger in EA than AA women (interaction of race and age group, p = 0.03). TBF was marginally higher among older than younger women (p for age = 0.06) and did not differ significantly by ethnicity Post-hoc comparisons from the fully adjusted models indicated significant differences in VAT% between each successive 10-year age group in EA women (all p < 0.05). Only the VAT% difference between 30–39 years and 40–49 years was significant in African-American women.
Figure 1.
Multivariate-adjusted estimates of visceral and abdominal subcutaneous adipose tissue mass, percentage abdominal visceral adiposity and total body fat by age group and ethnicity (mean, standard error). VAT, visceral adipose tissue mass; ASAT, abdominal subcutaneous adipose tissue mass; %VAT, percentage visceral adipose tissue, VAT/VAT + ASAT, %; TBF, total body fat. In European American women (gray bars), n = 94, 105, 143, 132, 85, 58. In African-American women (white bars), n = 17, 28, 19, 26, 12, 9. p-values are from an ethnic-group-combined linear regression model including ethnicity, age group, ethnicity*age group, menopausal status, total adipose tissue mass (TBF), current smoking (yes/no) and sport activity level.
Variance explained by individual predictors of VAT, ASAT, VAT% and TBF
The variance in body composition explained by different independent variables was compared with a series of linear regression models where covariates were added sequentially (Table II). In Model 1, age explained 14% of the total variance in VAT mass, 28% of the variance in VAT% and 2% of the variance in TBF. After considering age, menopausal status (Model 2) explained 1% of the variance in VAT mass and after covariate adjustment (Model 5) post-menopausal women had ~ 0.23 kg higher VAT mass than pre-menopausal women (p = 0.04). Menopausal status group differences in ASAT, VAT% and TBF were also very small and usually non-significant, depending on the model. Exogenous hormone use was not associated with any of the body composition variables examined (data not shown).
Table II.
Multivariate analysis of abdominal and total adiposity in 617 White and 111 African American women: Parameter estimate (standard error)
| Model | Independent variable
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (per decade) | Menopausal status | Ethnicity (EA) | Total body fat (per kg) | Sport activity (per unit) | Current smoking (yes) | Model R2 | |||
| VAT (kg) | 1 | 0.30 (0.02)*** | – | – | – | – | – | – | 0.14 |
| 2 | 0.22 (0.04)*** | Post | 0.27 (0.14) | – | – | – | – | 0.15 | |
| Peri | 0.28 (0.11)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 3 | 0.22 (0.04)*** | Post | 0.28 (0.13) | 0.10 (0.11) | – | – | – | 0.15 | |
| Peri | 0.29 (0.09)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 4 | 0.22 (0.03)** | Post | 0.26 (0.09)** | 0.44 (0.08)*** | 0.08 (0.003)*** | – | – | 0.60 | |
| Peri | 0.20 (0.08)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 5 | 0.24 (0.02)*** | Post | 0.23 (0.10)* | 0.51 (0.08)*** | 0.08 (0.002)*** | −0.06 (0.05) | 0.25 (0.08)** | 0.63 | |
| Peri | 0.15 (0.08) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| ASAT (kg) | 1 | 0.06 (0.07) | – | – | – | – | – | <0.01 | |
| 2 | 0.03 (0.10) | Post | 0.23 (0.43) | – | – | – | – | <0.01 | |
| Peri | 0.84 (0.35) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 3 | 0.06 (0.12) | Post | 0.16 (0.42) | −1.76 (0.34)*** | – | – | – | 0.05 | |
| Peri | 0.70 (0.34) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 4 | −0.23 (0.04)** | Post | 0.09 (0.14) | −0.28 (0.004)** | 0.26 (0.004)*** | – | – | 0.86 | |
| Peri | 0.23 (0.11)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 5 | −0.32 (0.08)*** | Post | 0.13 (0.14) | −0.27 (0.12)* | 0.27 (0.004)*** | 0.06 (0.08) | 0.39 (0.11)** | 0.86 | |
| Peri | 0.20 (0.12) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| %VAT | 1 | 2.9 (0.24)*** | – | – | – | – | – | 0.28 | |
| 2 | 2.4 (0.32)*** | Post | 1.99 (0.92)* | – | – | – | – | 0.28 | |
| Peri | 1.0 (0.74)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 3 | 2.4 (0.32)*** | Post | 2.2 (0.9)* | 4.6 (0.7)*** | – | – | – | 0.32 | |
| Peri | 1.4 (0.7) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 4 | 2.4 (0.31)*** | Post | 2.1 (0.9)* | 4.3 (0.7)*** | 0.002 (0.03) | – | – | 0.32 | |
| Peri | 1.5 (0.7)* | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 5 | 2.5 (0.32)*** | Post | 1.7 (0.9) | 5.0 (0.76)*** | −0.00 (0.03) | −1.1 (0.4)* | 2.2 (0.7)** | 0.35 | |
| Peri | 1.1 (0.8) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| TBF (kg) | 1 | 1.0 (0.24)*** | – | – | – | – | – | 0.02 | |
| 2 | 0.9 (0.42)* | Post | 0.45 (1.32) | – | – | – | – | 0.02 | |
| Peri | 0.58 (1.08) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 3 | 1.0 (0.44)** | Post | 0.31 (1.3) | −3.8 (1.1)*** | – | – | – | 0.04 | |
| Peri | 0.28 (1.1) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
| 5 | 0.6 (0.43) | Post | 0.36 (1.2) | −3.4 (1.1)** | – | −4.6 (0.6)*** | −2.3 (1.0)* | 0.14 | |
| Peri | −0.05 (0.9) | ||||||||
| Pre (Ref.) | 0.0 | ||||||||
Sport PA, sport index score from the Baecke physical activity questionnaire, range 1–5; VAT, visceral adipose tissue mass, kg; ASAT, abdominal subcutaneous adipose tissue mass, kg; %VAT, percentage visceral adipose tissue, VAT/VAT + ASAT, %; TBF, total body fat mass (kg).
Model: Model 1 includes age only; Model 2 includes Model 1 + menopausal status; Model 3 = Model 2 + Ethnicity; Model 4 = Model 3 + TBF; Model 5 = Model 4 + Current smoking and Sports PA. An age*ethnicity interaction term was also included for VAT% in Model 5.
p < 0.05,
p < 0.01;
p < 0.0001.
Self-reported ethnicity explained 5% of the variance in ASAT, 4% of the variance in VAT% and 2% of the variance in TBF. TBF explained most of the variance in abdominal adipose tissues, as expected (e.g. partial R2 = 0.45 for VAT mass and partial R2 = 0.80 for ASAT mass, Model 4). After adjustment for TBF and other covariates, EA women had, on average, lower total adiposity and slightly lower ASAT mass, but higher VAT (0.51 kg higher) and 5% higher %VAT than AA women (all p < 0.05, Model 5). Cigarette smoking had a negative association with TBF, but a positive association with VAT and VAT%. Sport PA and cigarette smoking together explained 3% of variance in VAT mass and VAT% and 10% in TBF, but did not contribute any additional variance in ASAT (Model 5).
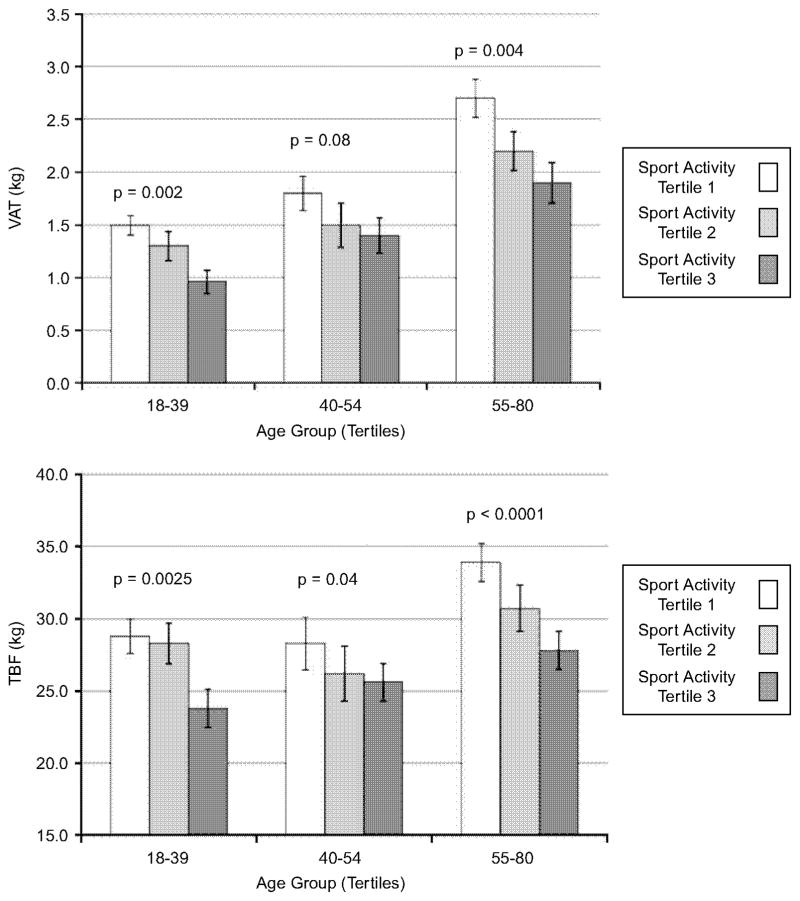
To test whether or not the association of physical activity level with VAT and TBF was similar in younger and older women, we tested the interaction effect between Sport PA (categorized in tertiles for purpose of illustration) and age (also in tertiles) on VAT and TBF, adjusting for all covariates discussed above, other than TBF (Figure 2). There was no evidence of effect modification; in each age group, women in the top tertile for Sport PA had ~30–33% lower VAT and 20–30% lower TBF compared to those in the bottom tertile.
Figure 2.
Multivariate-adjusted estimates of visceral and total adipose tissue mass by tertiles of age group and sport activity level. VAT, visceral adipose tissue mass; TBF, total body fat mass. Tertile 1 had the lowest frequency of sport activity participation and Tertile 3 had the highest, p-values correspond to the F statistic for the effect of sport activity tertile, within age group strata, in linear regression models with all covariates listed in Figure 1 except TBF.
DISCUSSION
Associations of age with VAT and ASAT
Numerous cross-sectional and longitudinal studies have shown age-related increases in visceral adiposity (e.g. Enzi et al. 1986; Seidell et al. 1988; Couillard et al. 2000; Despres et al. 2000); and recently reviewed by Kuk et al. (2009), which may be the single most important contributor to the metabolic syndrome (Carr et al. 2004). The relationship of visceral adiposity to disease is likely causal; visceral adipose tissue responds to increased nutrient intake with a greater increase in expression of pro-inflammatory and insulin desensitizing genes such as TNF-alpha, resistin and plasminogen activator inhibitor-1 (PAI-1) (Einstein et al. 2005) and in rodent models, removal of VAT delayed the onset of diabetes (Barzilai et al. 1999) and increased median and maximal lifespan, despite no changes in total adipose tissue mass (Muzumdar et al. 2008). As previously noted (Shen et al. 2009), existing data on normal human variation in VAT across the lifespan are somewhat fragmentary.
In the current study, we used data from a large cross-sectional abdominal MRI study of healthy non-diabetic women to examine age differences in visceral adipose tissue mass and to determine whether VAT differences with age exceed those expected given the established increases in total body fat and are accompanied by simultaneous differences in abdominal subcutaneous tissue mass. We also sought to test whether these differences remained when adjusting for menopausal status, differed by self-reported ethnicity and remained in women with relatively high self-reported physical activity. VAT was positively associated with age and this was apparent both before and after adjustment for TBF, menopausal status, ethnicity, sports activity level and smoking. Simultaneously, ASAT was negatively associated with age. This resulted in a greater proportion of VAT in the abdomen in older than younger women; specifically, mean VAT% was 17% for women in their 20’s and 27% for women in their 70’s. Thus, this study, in addition to the previously reported significant positive association of age on VAT, suggests a re-allocation of adipose tissue within the abdominal region from the subcutaneous to the visceral compartment, and that this pattern appears prior to the menopausal period in both EA and AA women.
This conclusion confirms recent findings in a number of smaller cross-sectional and longitudinal studies. After adjustment for total body fat, ethnicity and menopause status in 188 adult women, cross-sectional age differences in VAT decreased up to age 26 and then increased thereafter (Shen et al. 2009), with the greatest differences occurring in women in their 40’s compared to those in their 50’s, independent of menopausal status. Pre-menopausal age-related increases in VAT were also demonstrated by Pascot et al. (1999). They compared VAT and related cardiometabolic risk factors in 122 younger (average age 27.7 years) and 52 older (average age 49.5 years) women, all of whom were pre-rnenopausal and demonstrated significant age-related differences in both VAT and its associated cardiometabolic risk factors that preceded menopause. In the longitudinal IRAS (Insulin Resistance Atherosclerosis Study) in Hispanic and AA adults, the young adult age-group (20–29 years) had the largest 5-year increase in measured VAT area of any age group (18 and 12 cm2 among AA and Hispanic women, respectively) (Hairston et al. 2009). The preferential growth of the visceral adipose tissue compartment in pre-menopausal AA and EA women was also shown longitudinally by Lara-Castro et al. (2002); in 65 women followed for up to 4 years, VAT, adjusted for TBF, nearly doubled. However, in the small number of women among whom TBF remained stable, VAT did not appreciably increase (Lara-Castro et al. 2002), suggesting that preferential expansion of the intra-abdominal fat mass is not an essential characteristic of ageing, but rather depends on the sustained energy imbalance typical of most adults.
Lower mean ASAT in older AA and EA women is a concern because subcutaneous adipose tissue compartments have protective effects against the metabolic syndrome, particularly among individuals carrying a high load of visceral adipose tissue such as older adults (Goodpaster et al. 2005; Demerath et al. 2008; Porter et al. 2009). It is not clear why a higher absolute quantity of subcutaneous adipose tissue is beneficial in the presence of high VAT, but it is in accord with the ectopic fat paradigm, in which increased metabolic risk stems from exceeding the capacity of the subcutaneous adipocytes to differentiate and accommodate excess circulating lipids, which results in ectopic deposition of lipids—that is, deposition in the liver, muscles, pericardium and visceral compartments (Danforth 2000). In keeping with this paradigm, lipectomy of subcutaneous adipose tissue in rodents induces the metabolic syndrome (Weber et al. 2000). Subcutaneous and total adipose tissue loss declines in older adults after ~60–70 years of age in Whites (Guo et al. 1999; Chumlea et al. 2002); however, the apparent gradual redistribution of adipose tissue within the abdomen away from the subcutaneous fat depots during early and mid-adulthood in AA as well as EA women has not to our knowledge been noted previously.
Differences in African-American and European American women in VAT and ASAT
Cross-sectional data from the Pennington Center Longitudinal Study, the largest imaging study to date of race/ethnicity differences in abdominal adiposity, recently found that VAT area was greater in White than AA women after adjusting for total adiposity and similarly (Katzmarzyk et al. 2010). Findings in the present study were similar; after statistical adjustment for TBF and other covariates, VAT mass was ~ 0.5 kg higher in EA than AA women. As noted by Katzmarzyk et al. (2010) evidence is fairly evenly mixed on whether absolute VAT is on average higher or similar in White compared to African women, with no significant differences found in the CARDIA, Healthy Transitions, HERITAGE and Rancho Bernardo studies (Hill et al. 1999; Despres et al. 2000; Lovejoy et al. 2001; Araneta and Barrett-Connor 2005) but significantly higher levels in White women in at least as many other studies (e.g. Kanaley et al. 2001; Hoffman et al. 2005; Carroll et al. 2008).
A possible explanation of this difference of findings is that we also found a significant ethnicity*age interaction on VAT% (p = 0.03) and VAT (p = 0.05), such that the greater visceral adiposity of EA women compared to AA women was strongest in older women. To our knowledge, this finding is novel. Lara-Castro et al. (2002) found no difference in the longitudinal increase in VAT or ASAT over 4 years in a smaller sample of African-American and white women restricted to be pre-menopausal at baseline and of normal BMI. Katzmarzyk et al. (2010) did examine this issue by stratifying on age and found no difference in the effect of race on VAT or ASAT in women greater than compared to less than 45 years of age. However, most published studies in multi-racial/ethnic samples have not specifically tested the interaction between ethnicity and age and thus our finding requires further replication in large data sets with adequate power to detect interaction effects and preferably in longitudinal studies. If the finding is replicated, it may explain some of the differences among the studies above, wherein samples among older or exclusively post-menopausal women will be more likely to find excess VAT in EA compared to AA women than will studies conducted in younger women. The apparent paradox that cardiometabolic disease rates are higher in individuals of African as compared to those of European ethnicity, despite their lower relative VAT levels, may be explained by the higher circulating concentrations of pro-inflammatory peptides IL-6, CRP and fibrinogen in AA women than White women at the same levels of visceral and subcutaneous adiposity (Carroll et al. 2008).
Menopausal status, physical activity and smoking status
Relatively little of the variance in VAT (or VAT%) was attributable to the independent statistical effect of menopausal status. This is at odds with the widely quoted cross-sectional data on 96 women from Kotani et al. (1994), in which the estimated rate of VAT increase in post-menopausal women was approximately twice that of pre-menopausal women, and with the results of numerous other small (n < 100) cross-sectional investigations (reviewed in Kuk et al. 2009). The difference in the results between cross-sectional analyses specifically focused on menopause and ours is most likely due to differences in the age ranges examined; it is a common statistical observation that the correlation between x and y will tend to attenuate with restriction of the sample to a narrower range of x than is observed in the population. In studies aimed at understanding shifts during the menopausal transition, age is typically restricted to the 10 years surrounding menopause. Only two longitudinal studies have been published to directly examine whether VAT or VAT% indeed increase during the menopausal transition independent of age. One of these used mixed-longitudinal data from 156 pre-menopausal African-American and white women followed for 4 years and suggested faster VAT gain in those that became post-menopausal during the follow-up compared to those that did not (Lovejoy et al. 2008), although effects of time (age) appeared to be similar regardless of whether women entered or did not enter menopause over the course of the study. A recent study of eight pre-menopausal women followed longitudinally for 8 years to post-menopause concluded that increases in VAT were entirely dependent on increases in ASAT and TBF and relative visceral abdominal adiposity did not change (Franklin et al. 2009). Our cross-sectional study was not designed to disentangle the effects of time, age and menopausal status and larger longitudinal studies are needed to do so. Our lack of HRT effects on adiposity traits may relate to the fact that our questionnaire did not include many detailed questions on the duration or type of HRT exposure. There exists some variation in findings on this matter, where for example significant differences in VAT by HRT status were found by Munoz et al. (2002) but not by Ryan et al. (2002).
If visceral adiposity is predominantly affected by non-modifiable factors (age, ethnicity) and TBF, to what extent will counselling women to be physically active or refrain from smoking diminish central and visceral adiposity in particular? Some cross-sectional studies suggest that the increased abdominal fat characteristic of later female life is primarily attributable to decreases in physical activity (Hunter et al. 1996; Crawford et al. 2000; Kanaley et al. 2001; Simkin-Silverman et al. 2003; Sternfeld et al. 2004; 2005). Randomized exercise and weight loss intervention studies also show that abdominal adipose tissue declines at a faster rate than does TBF (e.g. Ross et al. 2004) and declines most rapidly in those whose baseline TBF and the ratio of VAT to TBF are the greatest (Smith and Zachwieja 1999; Hallgreen and Hall 2008). This would suggest that older women will see an important benefit to physical activity on central and total adiposity. Here, Sport PA did not diminish the effect of age on body composition variables, but was similarly and independently associated with lower TBF and VAT in all age groups. Thus, TBF in habitually active older women was as low as that in low-to moderately active younger women and they also demonstrated a 33% reduction in VAT compared to their less active older peers. The magnitude of the effect of habitual exercise on visceral adipose tissue is in line with previous studies, including intervention studies (Abe et al. 1996; Ross et al. 2004) and suggest that frequent participation in sports activities may reduce women’s adiposity at any age.
Compared with non-smokers, current smokers in this study had both higher VAT and higher ASAT, but lower TBF. Surprisingly, few studies to date have presented evidence on differences in visceral and abdominal subcutaneous adipose tissue volumes or areas in smokers and non-smokers. In the Framingham Heart Study, both former and current smokers had higher VAT than non-smokers, but the association with ASAT was less clear (Molenaar et al. 2009). A study in Japan found a non-significant trend toward increased VAT in male smokers (Komiya et al. 2006) while another found a lower VAT area in women in Turkey who were smokers compared to non-smokers at baseline (Onat et al. 2009). It is possible that the decrement found in Turkish women was due to lower TBF, because the VAT/TBF ratio was not different in current and non-smokers. Further work is required to understand the differing cellular responses to smoking in peripheral and abdominal adipocytes.
Limitations of our study include the cross-sectional design, which conflates age and cohort effects. Given the obesity epidemic of the past 20 years, women born more recently in the century (i.e. younger women here) would be expected to have higher body fat at a given age than the cohorts of women born earlier in the century (i.e. older women here). Thus, the age-related differences in VAT shown here likely under-estimate those that would be seen in a longitudinal study. Further, a cross-sectional investigation cannot effectively partition chronological ageing from ovarian function changes; however, these data do place findings from the few existing longitudinal studies into the broader context of trends occurring in women from young adulthood to older age. The strengths of this study include a large sample of healthy women with multiple-image MRI, allowing the measurement of visceral and subcutaneous tissues not possible with DXA and examination of differences in AA and EA women by age. Our future work will follow these women longitudinally to assess genetic and environmental factors that slow early adulthood increases in VAT in men and women.
The main conclusion of this cross-sectional study is that visceral adipose tissue mass in women is incrementally higher across advancing age groups, while abdominal subcutaneous adipose tissue mass is incrementally lower across advancing age groups, beginning as early as the 20’s and 30’s, and that these associations hold in both AA and EA women. Novel findings include a stronger association of age with relative visceral adiposity in EA women than AA women and greater abdominal adiposity despite lower total adiposity in current female smokers. Women with the most frequent participation in sports activities had lower visceral adipose tissue mass, compared to women with the least frequent participation, regardless of age. The findings from this study encourage further examination of specific behavioural factors that can mitigate the accrual of VAT in young adulthood, when chronic disease intervention efforts will likely have their greatest effectiveness.
Acknowledgments
We acknowledge the valuable contributions of the Fels Longitudinal Study, Miami Valley Family Aging Study, the Southwest Ohio Family Study and the dedicated research staff at the Lifespan Health Research Center, Wright State University Boonshoft School of Medicine for their technical assistance.
Footnotes
Declaration of interest: The study was supported by National Institutes of Health Grants DK064870, DK 064391, HD12252, and HL69995. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abe T, Sakurai T, Kurata J, Kawakami Y, Fukunaga T. Subcutaneous and visceral fat distribution and daily physical activity: comparison between young and middle aged women. Br J Sports Med. 1996;30:297–300. doi: 10.1136/bjsm.30.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intraabdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- Choh A, Demerath E, Lee M, Williams K, Towne B, Siervogel R, Cole S, Czerwinski S. Genetic analysis of self-reported physical activity and adiposity: The Southwest Ohio Family Study. Public Health Nutr. 2009;12:1052–1060. doi: 10.1017/S1368980008003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Despres JP, Bouchard C. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab. 2000;85:1026–1031. doi: 10.1210/jcem.85.3.6427. [DOI] [PubMed] [Google Scholar]
- Crawford SL, Casey VA, Avis NE, McKinlay SM. A longitudinal study of weight and the menopause transition: results from the Massachusetts Women’s Health Study. Menopause (New York, NY) 2000;7:96–104. doi: 10.1097/00042192-200007020-00005. [DOI] [PubMed] [Google Scholar]
- Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88:1263–1271. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Shen W, Lee M, Choh AC, Czerwinski SA, Siervogel RM, Towne B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85:362–368. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, Muzumdar R, Barzilai N. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–678. doi: 10.2337/diabetes.54.3.672. [DOI] [PubMed] [Google Scholar]
- Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Ploutz-Snyder L, Kanaley JA. Longitudinal changes in abdominal fat distribution with menopause. Metabolism. 2009;58:311–315. doi: 10.1016/j.metabol.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Gambacciani M. Effect of climacteric transition and hormone replacement therapy on body weight and body fat distribution. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2006;22:145–150. doi: 10.1080/09513590600629092. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Hairston KG, Scherzinger A, Foy C, Hanley AJ, McCorkle O, Haffner S, Norris JM, Bryer-Ash M, Wagenknecht LE. Five-year change in visceral adipose tissue quantity in a minority cohort: the Insulin Resistance Atherosclerosis Study (IRAS) family study. Diabetes Care. 2009;32:1553–1555. doi: 10.2337/dc09-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgreen CE, Hall KD. Allometric relationship between changes of visceral fat and total fat mass. Int J Obes (Lond) 2008;32:845–852. doi: 10.1038/sj.ijo.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74. doi: 10.1038/oby.2005.9. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Kekes-Szabo T, Treuth MS, Williams MJ, Goran M, Pichon C. Intra-abdominal adipose tissue, physical activity and cardiovascular risk in pre- and post-menopausal women. Int J Obesity Rel Metab Dis J Int Ass Study Obesity. 1996;20:860–865. [PubMed] [Google Scholar]
- Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010;18:604–610. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA, Weinstock RS. Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age, and menopausal status. Metab Clin Exp. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya H, Mori Y, Yokose T, Tajima N. Smoking as a risk factor for visceral fat accumulation in Japanese men. Tohoku J Exp Med. 2006;208:123–132. doi: 10.1620/tjem.208.123. [DOI] [PubMed] [Google Scholar]
- Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–212. [PubMed] [Google Scholar]
- Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lanska DJ, Lanska MJ, Hartz AJ, Rimm AA. Factors influencing anatomic location of fat tissue in 52,953 women. Int J Obes. 1985;9:29–38. [PubMed] [Google Scholar]
- Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19:457–466. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:1135–1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- Molenaar EA, Massaro JM, Jacques PF, Pou KM, Ellison RC, Hoffmann U, Pencina K, Shadwick SD, Vasan RS, O’Donnell CJ, Fox CS. Association of lifestyle factors with abdominal subcutaneous and visceral adiposity: the Framingham Heart Study. Diabetes Care. 2009;32:505–510. doi: 10.2337/dc08-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- Munoz J, Derstine A, Gower BA. Fat distribution and insulin sensitivity in postmenopausal women: influence of hormone replacement. Obes Res. 2002;10:424–431. doi: 10.1038/oby.2002.59. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat A, Ayhan E, Hergenc G, Can G, Barlan MM. Smoking inhibits visceral fat accumulation in Turkish women: relation of visceral fat and body fat mass to atherogenic dyslipidemia, inflammatory markers, insulin resistance, and blood pressure. Metabolism. 2009;58:963–970. doi: 10.1016/j.metabol.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Panotopoulos G, Ruiz JC, Raison J, Guy-Grand B, Basdevant A. Menopause, fat and lean distribution in obese women. Maturitas. 1996;25:11–19. doi: 10.1016/0378-5122(96)01119-x. [DOI] [PubMed] [Google Scholar]
- Pascot A, Lemieux S, Lemieux I, Prud’homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, Despres JP. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Nicklas BJ, Berman DM. Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care. 2002;25:127–133. doi: 10.2337/diacare.25.1.127. [DOI] [PubMed] [Google Scholar]
- Seidell JC, Oosterlee A, Deurenberg P, Hautvast JG, Ruijs JH. Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. Eur J Clin Nutr. 1988;42:805–815. [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, Allison DB, Heymsfield SB. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med Pub Soc Behav Med. 2003;26:212–220. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- Smith SR, Zachwieja JJ. Visceral adipose tissue: a critical review of intervention strategies. Int J Obes Relat Metab Disord. 1999;23:329–335. doi: 10.1038/sj.ijo.0800834. [DOI] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, Yosef M, Symons J. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP., Jr Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37:1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Wang H, Quesenberry CP, Abrams B, Everson-Rose S, Greendale G, Matthews K, Torrens J, Sowers M. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metab Clin Exp. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci. 2000:502–506. doi: 10.1111/j.1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am J Physiol Regul Integr Comp Physiol. 2000;279:936–943. doi: 10.1152/ajpregu.2000.279.3.R936. [DOI] [PubMed] [Google Scholar]