Abstract
Introduction
A pharmacogenomic approach was used to further localize the genetic region responsible for previously observed enhanced cardiovascular sensitivity to propofol in Dahl Salt Sensitive (SS) vs. control Brown Norway (BN) rats.
Methods
Propofol infusion levels that decreased blood pressure by 50% were measured in BN.13SS rats (substitution of SS chromosome 13 into BN) and in 5 congenic (partial substitution) strains of SS.13BN. The effect of superfused 2,6 diisopropylphenol on small mesenteric arterial vascular smooth muscle transmembrane potential was measured in congenic strains before and during superfusion with Rp-cAMPS and Rp-8-pCPT-cGMPS, inhibitors of protein kinase A and G respectively. The genetic locus and potential role of the renin gene in mediating VSM sensitivity to propofol were determined in three selected sub-congenic SS.BN13 strains.
Results
A 30 – 32% smaller propofol infusion rate reduced blood pressure by 50% in BN.13SS compared to BN and the SS.13BN congenic containing a 80 BN gene substitution. Compared to the latter, SS exhibited greater protein kinase A dependent vascular smooth muscle hyperpolarization in response to propofol. Using sub-congenics, the increased propofol-induced cardiovascular sensitivity and hyperpolarization was further localized to an 8-gene region (containing the BN renin gene). Blockade of angiotensin (AT1) receptors with losartan in this sub-congenic, elevated propofol-induced hyperpolarization by 3 fold, to that observed in SS.
Conclusions
Enhanced cardiovascular sensitivity to propofol in SS (compared to BN) is caused by an altered renin gene. Through modified second messenger function, this differentially regulates VSM contractile state and reduces vascular tone exacerbating cardiovascular depression by propofol.
INTRODUCTION
Hemodynamic depression is a side effect of general anesthetics that has been managed by anesthesiologists for decades but clinicians also recognize significant variations in cardiovascular sensitivity to these agents 1. Such variation is often attributed to altered pharmacokinetic uptake, distribution, and/or elimination of drug. However, significant differences in susceptibility to the same concentration of anesthetic at a particular effector site may also result from differential genetic expression leading to variation in the response of certain regulatory mechanisms to drugs1. The present study utilized a functional genomics approach to identify the regions of the genome that are responsible for different physiological responses to various anesthetic agents 2. We examined the genetic basis for differences in propofol-induced cardiovascular depression by comparing different strains of rats with distinct phenotypic sensitivity to anesthetics. These strains were part of a unique genetic animal model of chromosomal substitution 3.
Previously, we observed that Dahl Salt Sensitive rats (SS/JrHsdMcwi, abbreviated as SS) are anesthetized at much lower doses of several different anesthetics than Brown Norway rats (BN/NhsdMcwi abbreviated as BN) 4. This difference also includes enhanced cardiovascular sensitivity. Specifically, SS exhibits a fall in arterial blood pressure and cardiovascular collapse at much lower doses of sodium pentobarbital 5 and propofol 6 than BN rats. Concomitant enhancement in in situ arterial vascular smooth muscle (VSM) hyperpolarization, reflects a greater reduction in VSM tone in small resistance-regulating arteries. The basis for this hyperpolarization difference appears to be a genetic alteration in normal vascular control mechanisms, particularly calcium-sensitive potassium channel function and expression 7. Such greater VSM sensitivity to anesthetics predisposes the animal to exaggerated depressor responses during anesthetic exposure. Moreover, all of these observed differences in response to propofol appear to be linked to a gene or genes on chromosome 13, since transfer of BN chromosome 13 into the SS genetic background (to produce a SS.13BN consomic strain) completely prevents the enhanced VSM sensitivity to propofol both in vivo and in isolated vessels in situ 5,6. Of particular interest is the renin gene (known to be carried on chromosome 13 in the rat). Although cardiovascular regulation involves a multitude of genetic factors, the renin gene is recognized as a “master regulatory gene” of the circulatory system 8. Transfer of chromosomal segments containing the BN renin gene to an otherwise unchanged SS background has conferred the BN blood pressure regulation phenotype to the SS 9. The renin-angiotensin-aldosterone system is well established as a major mechanism in the maintenance of circulatory stability. Genetic polymorphisms of the renin gene and of genes coding for other components of the renin-angiotensin-aldosterone system have been associated with different cardiovascular disorders 10,11. However, while pharmacogenomic differences have been identified for anesthetic responses to different anesthetics (including propofol), 12 none to date have been associated with cardiovascular sensitivity to anesthetics and/or the renin gene. The hypothesis for this study was that an alteration in renin gene function in the SS contributes to the propofol-induced enhancement of cardiovascular sensitivity and VSM hyperpolarization (reflecting a reduction of VSM tone).
MATERIALS AND METHODS
Animals
The animals used in this study were 8 to 12 week-old male rats from the SS, BN, BN.13SS, and SS.13BN consomic and congenic strains, ranging in weight from 250 – 400 g. All protocols were reviewed and approved by the Animal Care and Use Committee at the Medical College of Wisconsin. The BN.13SS and SS.13BN consomic strains and the SS.13BN congenic strains were obtained from The Medical College of Wisconsin’s PhysGen Program of Genomics Applications and were derived as previously described 3. All rats were maintained on a low (0.4%) sodium chloride diet to minimize the development of hypertension and associated end organ damage in SS 13.
Characterization of the hypotensive response to propofol in the BN.13SS strain
We have previously reported that introgression of BN chromosome 13 into the SS genetic background (SS.13BN strain) reduces the enhanced sensitivity of the SS to the cardiovascular depressive effects of propofol to the same level seen in BN 6. This suggested that the strain differences are due to a gene or genes on this chromosome. In the present study, we first used a reciprocal BN.13SS strain 7 to determine whether introgression of SS chromosome 13 into the BN genetic background would enhance cardiovascular sensitivity to propofol.
Ten BN.13SS consomic rats were used to measure the infusion rate of propofol required to reduce mean arterial blood pressure by 50%. The animals were anesthetized with isoflurane in a 30% oxygen - 70% nitrogen carrier mixture (Abbott Laboratories, North Chicago, IL) via an Ohio Medical Products vaporizer (Airco Inc., Madison, WI). End tidal carbon dioxide was maintained between 35 and 40 mmHg. Both end tidal carbon dioxide and isoflurane concentrations were measured with a POET 2 Infrared Capnograph and End Tidal Agent Monitor (Criticare Systems, Inc., Waukesha, WI). A tracheotomy was performed and cannulas were placed in the femoral artery and vein for measurement of arterial pressure and intravenous infusions respectively. After catheter placement, isoflurane was discontinued and the animal was allowed to breathe room air until the end tidal isoflurane concentration reached 0 - 0.2%. Then IV propofol (Diprivan®, AstraZeneca Pharmaceuticals LP, Wilmington, DE) was administered at a dose of 0.55 mg/Kg/min, the lowest rate to reliably prevent spontaneous movement. The rate was sequentially increased in 0.03 mg/Kg/min steps every 3 min until the animal no longer demonstrated extremity or tail movement in response to tactile stimulation. At this time point, the rate of propofol infusion was increased in steps up to 0.30 mg/Kg/min or until the mean arterial blood pressure was reduced by 50% relative to the measurement immediately prior to initiation of the propofol infusion. Ventilation was controlled throughout this protocol.
Phenotyping of congenic strains of the SS.13BN
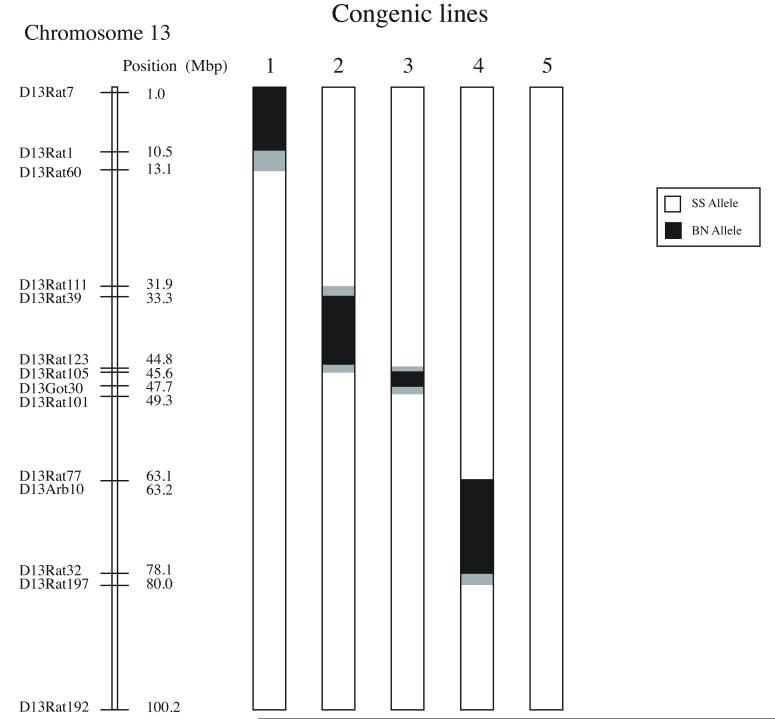
Based on the previous finding that established a causal relationship between the presence of SS chromosome 13 and the elevated depressor response to propofol, 6,7 attention was focused on further defining the genetic regions of interest on SS chromosome 13. This was accomplished by measuring the cardiovascular responses to propofol in five congenic strains of the SS.13BN. These strains designated as congenic lines 1,2,3,4 and 5, were developed in a fashion similar to that described for the SS.13BN, except that each contained only a segment of BN chromosome 13 rather than the whole chromosome as in the SS.13BN consomic. They were created by backcrossing the SS.13BN consomic strain with SS rats and intercrossing the F1 progeny 14. A genetic map presenting the regions of BN chromosome 13 introgressed into the SS.13BN congenic strains is presented in figure 1. Line 1 contained approximately 13 million base pairs (Mbp) ranging from markers D13Rat7 to D13Rat60. Line 2 contained approximately 13 Mbp ranging from markers D13Rat111 to D13Rat105. Line 3 contained approximately 4.5 Mbp ranging from markers D13Rat123 to D13Rat101. Line 4 contained approximately 16 Mbp ranging from markers D13Rat77 to D13Rat197 and Line 5 contained no BN genomic segments on Chromosomse 13 and served as a control for the backcrossing protocol. It was rederived from the SS.13BN to be identical to the SS. Five rats in each SS.13BN congenic group were phenotyped to determine the infusion rate of propofol required to reduce mean arterial blood pressure by 50%.
Figure 1.
Map of the five congenic substrains of the SS.13BN consomic rat strain that were used in the current study. Genome substitutions for the Brown Norway rats (BN/NhsdMcwi abbreviated as BN) are indicated by the black segments. Confidence intervals are indicated by grey shading. The remaining background genome is from the Dahl Salt Sensitive rat strain (SS/JrHsdMcwi, abbreviated as SS). Line 5 contains no BN substitution and is identical to the SS. It was re-derrived from the SS.13BN as an internal control.
Effects of 2,6-diisopropylphenol on VSM Em responses and second messenger pathways in mesenteric arteries obtained from SS and BN rats and in the SS.13BN congenic strains
Previous observations indicated that 2,6-diisopropylphenol (the active anesthetic agent in the intralipid emulsion known as propofol) hyperpolarizes VSM in small mesenteric arteries to a greater extent in SS rats than in SS.13BN consomic rats in which BN chromosome 13 is substituted into the SS genetic background. Moreover, this enhanced hyperpolarization was attributed to increased (big) calcium sensitive potassium channel activity 7. An enhanced propofol-induced hyperpolarization of arterial VSM in SS resistance arteries correlates with earlier loss of VSM tone and consequent vascular instability. This protocol was used to determine if such differences in the regulation of VSM tone in response to 2,6-diisopropylphenol also correlated with observed differences in vascular stability during propofol administration in the congenic (i.e., partial chromosome substitution) strains. An additional objective of the protocol was also intended to determine if differences in second messenger-mediated regulation of (big) calcium sensitive potassium channels could contribute to the observed altered anesthetic sensitivity. The activity of these channels in VSM is regulated by phosphorylation via second messenger pathways that include cyclic 3′, 5′ guanosine monophosphate (cGMP), acting through protein kinase G (PKG), and cyclic 3′, 5′ adenosine monophosphate (cAMP), acting through protein kinase A (PKA) 15. To investigate 2,6-diisopropylphenol’s effects on VSM Em in the consomic strains and whether these effects are mediated through the cGMP and/or cAMP second messenger pathways, the previously established VSM transmembrane potential (Em) preparation was used 7. Two protocols were followed, both of which initially used six SS and six BN rats.
Animals were anesthetized with 2.5% inhaled isoflurane followed by tracheotomy and femoral arterial and venous cannulation. A midline laparotomy was also performed through which a loop of terminal ileum was externalized and superfused with physiologic salt solution containing (in mM) 1.17 MgSO4, 1.60 CaCl2, 24.0 NaHCO3 and 0.03 EDTA. The solution was continuously aerated with 90% nitrogen, 5% oxygen and 5% carbon dioxide and maintained at a pH of 7.4. Next, a segment of a small mesenteric artery (approximately 200 μm o.d.) was dissected free of perivascular fat and stabilized between 50 μm o.d. stainless steel pins, which were anchored in the silastic chamber floor. Inhaled isoflurane was adjusted to 1% and in situ VSM Em’s were recorded from the adventitial side of the mesenteric artery by means of 3M KCl – filled glass microelectrodes (tip diameter of 0.1 μm with an impedance of approximately 40 MΩ) connected to a biological amplifier 16. In both protocols VSM Em’s were measured sequentially: 1) before, during and after superfusion of the vessels with 5.0 μM 2,6-diisopropylphenol, 2) during superfusion with one of the second messenger inhibitors, 3) during superfusion with the second messenger inhibitor plus 5.0 μM 2,6-diisopropylphenol. In one group, 2.5 μM (Rp)-8-(para--chlorophenylthio)guanosine-3′,5′-cyclic monophosphorothioate (Rp-8-pCPT-cGMPS) was added to the superfusate to inhibit cGMP-mediated activation of PKG 17. In the other protocol 24.5 μM Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS) was superfusate to inhibit cAMP-mediated activation of PKA 18. At least five Em measurements (each 6 s or longer in duration) were obtained for each of three experimental conditions in every animal. Based on initial results in the Rp-8-pCPT-cGMPS protocol with the SS and BN, these experiments were extended to include the study of three animals from the line 2 and line 3 SS.13BN congenic strains. Line 3 was chosen because its systemic cardiovascular responses to propofol (in the phenotyping protocol) were similar to those of the SS.13BN. The SS chromosome 13 in this line contained the smallest segment of BN chromosome 13 (less than 5 million base pairs) that was able to revert propofol-induced cardiovascular responses back to the level observed in BN. Line 2 was chosen as a representative strain for all of the other congenic strains (Lines 1, 2, 4, and 5) since (unlike Line 3) none of these other congenic strains altered the cardiovascular responses to propofol (relative to SS rats).
2,6-diisopropylphenol and physiologic salt solution reagents were purchased from Sigma Aldrich Chemical Co., (St. Louis, MO). Rp-8-pCPT-cGMPS and Rp-cAMPS were purchased from BIOMOL International L.P. (Plymouth Meeting, PA).
Effect of systemic propofol and in situ 2,6-diisopropylphenol on sub congenic strains of Line 3
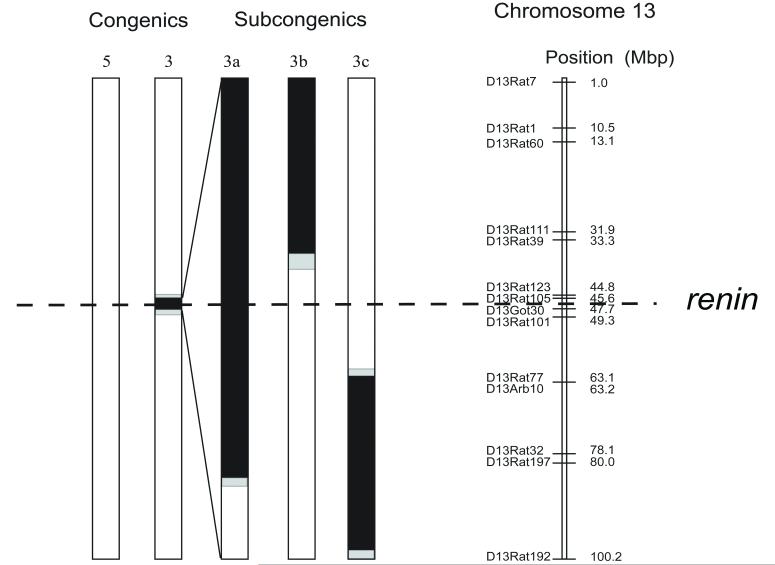
Once it was determined that the normalization of the phenotypic response for cardiovascular sensitivity to propofol as well as the enhanced VSM hyperpolarization response to superfused 2,6-diisopropylphenol were localized to the chromosomal segment contained in Line 3, three additional subcongenic strains derived from line 3 were studied to further isolate the region of interest using the identical methodologies. These additional strains were termed Lines 3a, 3b, and 3c. Figure 2 illustrates the genetic map for the region of BN chromosome 13 that was transferred into these strains.
Figure 2.
Maps of congenic substrains Lines 5 and 3 are identical to those depicted in Figure 1. Subcongenic maps of Lines 3a, b and c are “blow ups” of the small genome substitution from the Brown Norway strain (BN/NhsdMcwi abbreviated as BN) contained within the Line 3 congenic. All BN genome substitutions are indicated by the black segments. Confidnce intervals are indicated by the grey shading. The dashed line indicates the location of the renin gene.
Six rats of each subcongenic strain were phenotyped to determine the infusion rate of propofol required to reduce mean arterial blood pressure by 50%. Following this phenotyping protocol the response of in situ VSM Em to superfused 2,6-diisopropylphenol was measured in Lines 3a, b and c. Again, six rats from each subcongenic group were studied. Measurements were made before, during, and after superfusion of the vessel with physiologic salt solution and 5 μM 2,6-diisopropylphenol (Sigma Aldrich Chemical Co.).
Cardiovascular sensitivity to propofol and effect of 2,6-diisopropylphenol on in situ transmembrane potential of arterial VSM in animals treated with losartan
This protocol determined whether differences in cardiovascular sensitivity to propofol between SS and BN are related to genetic variation in the expression of the renin gene. Two groups of rats were studied (n = 6 each). One group consisted of SS rats and the other consisted of Line 3a SS.13BN subcongenic strains. The rats were treated with a 40 mg/Kg/day dose of losartan in the drinking water for 7 days. We then determined the infusion rate of propofol required to reduce mean arterial blood pressure by 50% and measured the effect of 2,6-diisopropylphenol on in situ VSM Em. The results obtained were compared with corresponding results obtained in Line 3a animals and in previously studied SS animals.
Data analysis
Mean ± standard deviation (SD) are presented. For characterization of the hypotensive response of a particular strain to systemically administered propofol, the infusion rate of propofol that was being delivered when mean arterial blood pressure was half of baseline (i.e., prepropofol) pressure was compared with that of other strains. A one-way analysis of variance was used for evaluation of the hypotensive response to propofol in the BN.13SS strain in which the independent variable was the strain of the animal and the dependent variable was the infusion rate of propofol that reduced the mean arterial blood pressure by 50%. For the second messenger studies (fig. 3) and the sub congenic comparison (fig. 4), the interaction between strain and pharmacologic intervention (i.e., 2,6-diisopropylphenol treatment, second messenger treatment etc.) was evaluated using a two-way analysis of variance for repeated measures. The “between” factor was “animal strain” and the “within” (repeated) factor was pharmacologic intervention with 4 levels (fig. 3) or 3 levels (fig. 4). In each, the dependent variable was VSM Em. Once a significant interactive effect was established, a one-way analysis of variance was performed using the Bonferroni/Dunn post hoc test for planned comparisons of cells that contributed to the major effect. A one-way analysis of variance was also used for evaluation of the losartan data. In these analyses, the independent variable was the animal group (treated or untreated SS and treated or untreated Line 3a) and the dependent variable was the infusion rate of propofol that reduced the mean arterial blood pressure by 50% or the change in VSM Em (fig. 5). For all comparisons in the present study, two-tailed testing was performed and p ≤ 0.05 was considered to be significant and n values for each comparison are reported in the figures. All statistical analyses were performed using the SPSS program (IBM Corporation, Somers, NY).
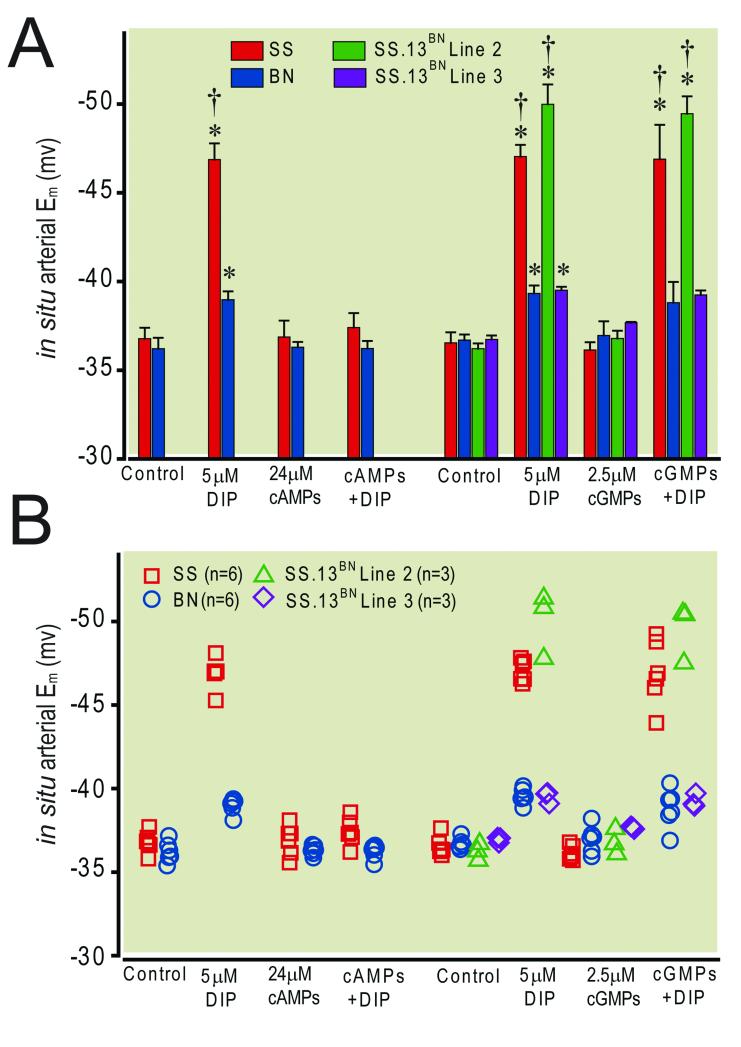
Figure 3.
Panel A illustrates the differential effect of 24.5 μM Rp-adenosine-3′,5′-cyclic monophosphorothioate (cAMPs) and 2.5 μM (Rp)-8-(para--chlorophenylthio)guanosine-3′,5′-cyclic monophosphorothioate (cGMPs) on the hyperpolarization response to 5.0 μM 2,6-diisopropylphenol. cAMPs abolished the response in both the Dahl Salt Sensitive rat strain (SS/JrHsdMcwi, abbreviated as SS) and the Brown Norway rat strain (BN/NhsdMcwi abbreviated as BN) , whereas cGMPs abolished the response in BN but not in SS. cGMPs also abolished the response in Line 3 but not in Line 2 of SS.13BN consomic rat strain. Bars represent mean ± standard deviation. Panel B is a scatter plot of all individual data points comprising the means illustrated in panel A. * = p ≤ 0.05 vs respective control, † = p ≤ 0.05 vs BN, n = 3 - 6 animals.
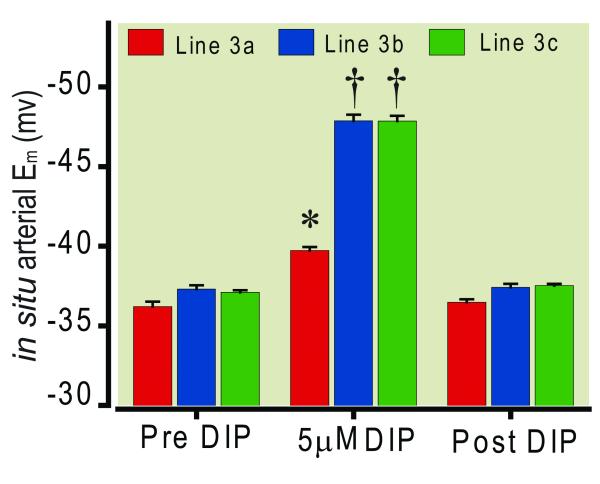
Figure 4.
Significantly less hyperpolarization of small mesenteric arterial vascular smooth muscle cells (VSM) by 5.0 μM 2,6-diisopropylphenol in sub congenic Line 3a of the SS.13BN consomic rat strain compared with sub congenic Lines 3b and c of the SS.13BN consomic rat strain. Bars represent mean ± standard deviation, * = p ≤ 0.05 vs corresponding Pre and Post DIP. † = p ≤ 0.05 vs corresponding Pre and Post DIP and vs Line 3a DIP, n = 6 animals per sub congenic.
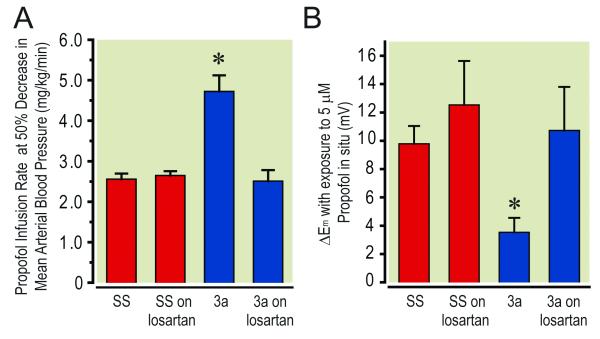
Figure 5.
Significant effect of losartan treatment on sub congenic Line 3a of the SS.13BN consomic rat strain compared with the Dahl Salt Sensitive parental rat strain (SS/JrHsdMcwi, abbreviated as SS). Panel A illustrates the greater mean ± standard deviation infusion rate of propofol required to reduce mean arterial blood pressure by 50% in untreated Line 3a animals compared to losartan treated Line 3a and treated as well as untreated SS animals. Panel B indicates the significantly attenuated change in Em of small mesenteric arterial VSM in untreated Line 3a animals compared to losartan treated Line 3a and treated as well as untreated SS animals. * = p ≤ 0.05 vs all other groups, n = 6 animals per group.
RESULTS
Hypotensive response to propofol in the BN.13SS strain
Systemic infusion of various concentrations of propofol in the BN.13SS consomic strain produced a stepwise reduction in arterial blood pressure. The mean infusion rate required to reduce mean arterial blood pressure by 50% was 2.40 ± 0.88 mg/Kg/min in this consomic strain (n = 10). This infusion rate (for reduction of blood pressure) is similar to that observed in SS rats (2.40 ± 0.60 mg/Kg/min, n = 10) and is significantly less than values previously reported for BN and SS.13BN (3.40 ± 1.10 mg/Kg/min, n - 11 and 3.50 ± 0.60 mg/Kg/min, n = 10 respectively)6. Of note, in previously unreported preliminary studies, we observed no significant differences in pooled baseline (i.e., prepropofol infusion) mean ± SD arterial pressure among the SS, BN, and SS.13BN strains (142 ± 16, 133 ± 21 and 139 ± 18 mm Hg for SS, BN and SS.13BN respectively.
Narrowing the region of interest by phenotyping SS.13BN congenic strains
The mean infusion rates of propofol required to reduce mean arterial blood pressure by 50% in the SS.13BN congenic Lines 1, 2, 4, and 5 were similar and averaged 2.60 ± 0.95 mg/Kg/min. In contrast, the rate for congenic Line 3 was 4.30 ± 0.60 mg/Kg/min. This rate is similar to the rate measured in previously studied BN and in the SS.13BN. It is also significantly higher than the rate for the SS and the other SS.13BN congenic lines studied.
Effects of 2,6-diisopropylphenol and inhibitors of PKA and PKG activity on in situ VSM Em in the SS and BN strains and in the SS.13BN congenic strains
The results of these studies are presented in figure 3. In situ superfusion of small mesenteric arteries with 5.0 μM DIP hyperpolarized VSM in all four of the strains that were studied. However, the DIP-induced hyperpolarization responses in SS and in congenic Line 2 were significantly greater than those measured previously in BN rats and in SS.13BN congenic Line 3. Superfusion of small mesenteric arteries with an inhibitor of either PKA or PKG activity alone did not significantly alter pretreatment baseline Em (i.e., prior to superfusion with 2,6-diisopropylphenol) in either SS or BN animals. However, the PKA inhibitor abolished the 2,6-diisopropylphenol – induced hyperpolarization in both (SS and BN) parental strains. In contrast, the PKG inhibitor abolished 2,6-diisopropylphenol – induced hyperpolarization in the BN but not in the SS parental strain. In the presence of the PKG inhibitor, 2,6-diisopropylphenol – induced hyperpolarization in SS was 10.0 ± 0.5 mV, which is similar to that observed for 2,6-diisopropylphenol alone. The PKG inhibitor abolished the 2,6-diisopropylphenol – induced hyperpolarization, in the Line 3 congenic strain (similar to its effect in BN). However, the PKG inhibitor did not affect the 2,6-diisopropylphenol – induced hyperpolarization in the Line 2 substrain (similar to its lack of effect in SS).
Mean arterial blood pressure response to systemic propofol and VSM Em response to in situ superfusion with 2,6-diisopropylphenol in Line 3 SS.13BN sub congenic strains
The results of these studies are illustrated in figure 4. The rate of propofol infusion required to reduce mean arterial blood pressure by 50% for line 3a was 4.72 ± 0.40 mg/Kg/min. This rate was similar to the value for BN and the SS.13BN. However, in line 3b and 3c the rates were 3.04 ± 0.20 and 2.96 ± 0.20 mg/Kg/min respectively. These rates were significantly lower than for Line 3a and similar to those for SS rats and congenic Lines 1, 2, 4, and 5. Superfusion of in situ small mesenteric arteries with 2,6-diisopropylphenol hyperpolarized Em in all three subcongenic strains (Lines 3a, 3b, and 3c). However the magnitude of the hyperpolarization response in Line 3a (2.1 mV) was significantly less than that seen in Lines 3b (11.0 mV) and 3c (11.6 mV).
Effect of losartan on mean arterial blood pressure response to systemic propofol and on VSM Em response to in situ superfusion with 2,6-diisopropylphenol
The results of these studies are presented in figure 5. The infusion rate of propofol required to reduce mean arterial blood pressure by 50% in losartan treated SS was 2.66 ± 0.08 mg/Kg/min and 2.51 ± 0.17 mg/Kg/min in the Line 3a strain. These rates were not significantly different from each other and from the corresponding rate previously observed for untreated SS (2.40 ± 0.60 mg/Kg/min) 6. They were also significantly less than the 4.72 ± 0.40 mg/Kg/min rate observed for the untreated Line 3a animals. Likewise, the 2,6-diisopropylphenol -induced hyperpolarization in losartan-treated Line 3a (11.6 ± 2.7 mV) was significantly greater than the 3.5 ± 1.0 mV we observed in untreated Line 3a animals and similar to the 12.5 ± 1.3 mV we observed for losartan treated and untreated (10.5 ± 0.5 mV) SS that were reported previously 7.
DISCUSSION
Relatively little is known about the genetic determinants of cardiovascular responses to propofol. This study provided evidence for involvement of an altered function of the renin gene on chromosome 13 and the renin angiotensin system in the development of this enhanced cardiovascular response. Initial evidence was the observation that transfer of the entire SS chromosome 13 into the BN genetic background (to produce a BN.13SS consomic strain) increased systemic cardiovascular sensitivity to propofol to a level almost identical to that seen in the SS 6. This further supported our observations that the loss of in situ VSM tone in the SS in response to 2,6-diisopropylphenol is linked to genes found on rat chromosome 13 7. Other studies also demonstrated that the transfer of chromosome 13 from BN to SS rats (to produce the SS.13BN strain) has related cardiovascular effects. Specifically, such transfer opposes the development of hypertension 14,19. It has also been shown to restore endothelium-mediated vasodilator responses to acetylcholine in cerebral vessels 20,21. Collectively these findings support the existence of gene(s) on chromosome 13 that also can alter vascular reactivity to propofol and other vasoactive substances in SS rats.
To further isolate genes involved we next studied SS.13BN congenic strains in which smaller segments of BN chromosome 13 were introgressed into the SS chromosome. Transfer of a region from 45.3 to 47.3 Mbp of BN chromosome 13 fully eliminated the elevated cardiovascular suppression caused by propofol in the SS and BN.13SS (when compared to the level of suppression observed in the full SS.13BN consomic and the BN). The latter two strains are resistant to the propofol-induced cardiovascular suppression. In contrast, the other congenic strains also exhibited the same increased sensitivity to propofol as seen in the SS. The same pattern was observed for the in situ VSM Em responses to propofol for the SS.13BN containing the segment of BN chromosome 13 between 45.3 to 47.3 Mbp. This suggests that altered vascular regulation at the level of the VSM Em contributes to the reduced vascular tone produced by propofol. Thus the BN phenotype for circulatory response to propofol is transferred to the SS via the small portion of chromosome 13 contained in the Line 3 congenic. However, since the region of interest in Line 3 was still too large (approximately 4.5 million base pairs Mbp) and contained 85 known and predicted genes, it was premature to draw any conclusions regarding a causal relation between a specific gene in this region and the altered response to anesthetics in SS.
A further narrowing of the region of interest to still smaller segments was accomplished with the 3a, 3b and 3c subcongenic lines. The fact that Line 3a exhibited a cardiovascular sensitivity to propofol that was similar to the SS.13BN and to the BN, whereas Lines 3b and 3c duplicated the sensitivity of the SS parental strain is evidence that the gene(s) responsible for the difference is/are contained within the small region representing the non overlapping portions between Line 3a and Lines 3b and 3c. This region, which contains the renin gene was further evaluated as the region containing a candidate gene for the altered vascular response to propofol. The SS rats are a low renin model of hypertension and the renin-angiotensin system has been linked to altered cardiovascular responses in SS rats 3. In particular, transfer of the same region of chromosome 13 from BN to SS rats in the line 3a congenic has been shown recently to restore the impaired angiogenic response to nerve stimulation that exists in SS rats. Such restoration is linked to an intact renin-angiotensin system 9,22. Moreover, in mesenteric arteries similar to those used in this study, angiotensin has been shown to influence PKA – mediated regulation of outward K+ current channels 23,24. Thus, an altered renin gene function could lead to a reduced renin and angiotensin II level in SS rats that in turn, could lead to an increase in K channel activity resulting in an increased VSM hyperpolarization and a coupled loss of arterial VSM tone.
The link between an elevated vascular sensitivity, VSM hyperpolarization to propofol and a mechanism of enhanced cAMP - mediated opening of (big) calcium sensitive potassium channels in SS is supported by the results reported in the present study. Also, in skeletal muscle angiogenesis studies using consomic models similar to those used in the studies reported here, the renin gene from SS animals has been shown to function differently from the BN renin gene 9. We suspect that the same is true in the present study and that the relatively low levels of renin and angiotensin II in SS rats, allows a greater expression of PKA. Greater PKA – induced potassium current in VSM would result in more hyperpolarization in response to (vasodilator) drugs such as propofol resulting in less vascular tone and possibly an earlier circulatory instability (consistent with what we observed).
The renin gene is one of the “master” regulatory genes in the cardiovascular system. 8 The fact that it was also one of the 8 genes that remained in the segment of BN introgressed into line 3a strongly supports its causal role in our observed differential cardiovascular response to propofol. Additional studies using losartan, a blocker of the AT1 receptor were performed to obtain direct pharmacologic evidence for involvement of strain-releated differences in the action of the renin-angiotensin system in mediating the differential response to propofol in SS versus the 3a congenic strain. Pretreatment with systemic losartan had little effect on 2,6-diisopropylphenol-induced VSM hyperpolarization or the enhanced circulatory instability in response to systemic propofol in SS. However, it had a far greater effect in the 3a subcongenic line. These results provide further evidence that transfer of the BN renin gene into the SS (to produce the SS.13BN) increases in the activity of the renin-angiotensin system in this strain and that activation of this system reduces the vasodilator response to propofol. We believe that pretreatment with losartan did not alter normal function of the renin-angiotensin system in SS, because this function was already depressed due to inherently different activity of the renin gene. Thus, propofol had no additional hemodynamic instability effect in the SS after losartan treatment. We further postulate that the renin–angiotensin system function is at a higher level in the Line 3a strain. Thus pretreatment with losartan in this strain reduced its renin-angiotensin system function to a level similar to that observed in SS. Consequently, this functional alteration leads to an increased propofol-induced VSM hyperpolarization and circulatory instability in the losartan-treated Line 3a, to the level observed in SS. The fact that the Line 3a strain differs from SS only by the small 8 gene substitution from the BN genome and that losartan causes both the propofol-induced VSM hyperpolarization and the circulatory instability responses in the Line 3a strain to become similar to that in an SS provides additional evidence that an altered renin function is most likely responsible for mediating the increased cardiovascular depression to propofol and the hyperpolarization responses to 2,6-diisopropylphenol in the SS compared to BN. Finally, the VSM hyperpolarization data in the present study strongly suggests that the enhanced propofol sensitivity of the SS involves an effect on peripheral vasculature but it does not preclude the possibility of altered myocardial sensitivity to propofol as well. Regardless, altered renin gene function does seem to be a common feature in the present model.
Examples of pharmacogenomic differences between strains of animals related to anesthetic administration have been reported previously 12,25 although the genetic basis of the differences in these responses has not been identified. In lower animals, specific quantitative trait loci have been associated with mechanisms of altered mitochondrial function in response to volatile anesthetics and these findings have been correlated with observed differences in and among patients 26. Also, human pharmacogenomic variation has been identified in analgesic responses to opioids 27,28 and it is likely that similar variation exists for other anesthetic responses in humans. It should be recognized that in the present study, the animals used were all age-matched males. Moreover, (by design) the congenic and sub congenic strains studied, were genetically identical to the SS, except for the small portion of the BN genome that was transferred. For these reasons, factors such as age, sex difference, and comorbidity (which are present in the clinical setting) were intentionally excluded to study the effects of the genetic substitution separately. Such other factors could potentially enhance or mask underlying pharmacogenomic differences. This (along with the fact that it is an animal study) limits the direct applicability to patient care. However, it does demonstrate that pharmacogenomic differences clearly exist for this phenotype. Although there is no specifically known correlate for enhanced human sensitivity to propofol (or other general anesthetics at this time) variation in the renin gene in humans has already been demonstrated to account for altered blood pressure regulation 11. Accordingly, it may well (at least partially) account for the altered circulatory responses to anesthesia, that are not infrequently encountered in the clinical setting.
SUMMARY STATEMENT.
There is a pharmacogenomic difference in Dahl Salt Sensitive rats compared to Brown Norway control rats manifesting as enhanced cardiovascular sensitivity to propofol. This is attributable to altered renin gene function in the Dahl strain.
MS #201011058 – Final Boxed Summary Statement.
What We Already Know about This Topic
Significant differences in the susceptibility to the same concentration of anesthetic at a particular effector site may result from differential genetic expression The present study investigated the genetic basis for differences in propofol-induced cardiovascular depression by comparing different strains of rats with distinct phenotypic sensitivity to anesthetics.
What This Article Tells Us That Is New
There are pharmacogenomic differences in Dahl Salt Sensitive rats compared to Brown Norway control rats manifesting as enhanced cardiovascular sensitivity to propofol. This is attributable in part due to altered renin gene function in the Dahl strai
ACKNOWLGEMENTS
We gratefully acknowledge the support of Dr. Allen W. Cowley Jr., Ph.D., Professor and Chairman of the Department of Physiology, The Medical College of Wisconsin, Milwaukee, Wisconsin and Dr. Howard J. Jacob, Ph.D., Warren P. Knowles Professor in Human and Molecular Genetics, Professor of Physiology and Director of the Human and Molecular Genetics Center at The Medical College of Wisconsin, Milwaukee, Wisconsin for making available the parental and chromosomal substitution strains of rats used in this study. We also thank Merck & Co., Inc. (Rahway, New Jersey) for generously providing the losartan used in this study.
FUNDING This work was supported in part by the General Medicine Institute grant number 068725-02 and by the PhysGen Program for Genomic Applications, National Heart, Lung, and Blood Institute grant number U01 HL66579, both of which are through the National Institutes of Health, Bethesda, Maryland,. Development of the congenic lines was supported by National Institutes of Health- National Heart Lung Blood Institute grants HL54998, HL36279 and HL82798.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schwinn DA, Booth JV. Genetics infuses new life into human physiology: Implications of the human genome project for anesthesiology and perioperative medicine. Anesthesiology. 2002;96:261–3. doi: 10.1097/00000542-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SN, Giesecke NM, Body SC, Shernan SK, Fox AA, Collard CD. Pharmacogenetics of anesthetic and analgesic agents. Anesthesiology. 2005;102:663–71. doi: 10.1097/00000542-200503000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand. 2004;181:585–92. doi: 10.1111/j.1365-201X.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 4.Stekiel TA, S JC, Bosnjak ZJ, Kampine JP, Roman RJ, Stekiel WJ. Reversal of minimum alveolar concentrations of volatile anesthetics by chromosomal substitution. Anesthesiology. 2004;101:796–8. doi: 10.1097/00000542-200409000-00032. [DOI] [PubMed] [Google Scholar]
- 5.Stekiel TA, Contney SJ, Bosnjak ZJ, Kampine JP, Roman RJ, Stekiel WJ. Chromosomal substitution-dependent differences in cardiovascular responses to sodium pentobarbital. Anesth Analg. 2006;102:799–805. doi: 10.1213/01.ane.0000195582.22822.e7. [DOI] [PubMed] [Google Scholar]
- 6.Stekiel TA, Weber CA, Contney SJ, Bosnjak ZJ. Differences in cardiovascular sensitivity to propofol in a chromosome substitution rat model. Croat Med J. 2007;48:312–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Stadnicka A, Contney SJ, Moreno C, Weihrauch D, Bosnjak ZJ, Roman RJ, Stekiel TA. Mechanism of differential cardiovascular response to propofol in Dahl salt-sensitive, Brown Norway, and chromosome 13-substituted consomic rat strains: Role of large conductance Ca2+ and voltage-activated potassium channels. J Pharmacol Exp Ther. 2009;330:727–35. doi: 10.1124/jpet.109.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley SD, Coffman TM. In hypertension, the kidney breaks your heart. Curr Cardiol Rep. 2008;10:470–6. doi: 10.1007/s11886-008-0074-5. [DOI] [PubMed] [Google Scholar]
- 9.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics. 2008;33:33–40. doi: 10.1152/physiolgenomics.00150.2007. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby DS, Rader DJ. Renin-angiotensin system and atherothrombotic disease: From genes to treatment. Arch Intern Med. 2003;163:1155–64. doi: 10.1001/archinte.163.10.1155. [DOI] [PubMed] [Google Scholar]
- 11.Chiang FT, Hsu KL, Tseng CD, Lo HM, Chern TH, Tseng YZ. Association of the renin gene polymorphism with essential hypertension in a Chinese population. Clin Genet. 1997;51:370–4. doi: 10.1111/j.1399-0004.1997.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 12.Sonner JM, Gong D, Eger EI., 2nd Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91:720–6. doi: 10.1097/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 13.Iwai J, Knudsen KD, Dahl LK, Tassinari L. Effects of adrenalectomy on blood pressure in salt-fed, hypertension- prone rats. Failure of hypertension to develop in absence of evidence of adrenal cortical tissue. J Exp Med. 1969;129:663–78. doi: 10.1084/jem.129.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW., Jr Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics. 2007;31:228–35. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 15.Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–12. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 16.Stekiel TA, Contney SJ, Kokita N, Bosnjak ZJ, Kampine JP, Stekiel WJ. Mechanisms of isoflurane-mediated hyperpolarization of vascular smooth muscle in chronically hypertensive and normotensive conditions. Anesthesiology. 2001;94:496–506. doi: 10.1097/00000542-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Fouty B, Komalavilas P, Muramatsu M, Cohen A, McMurtry IF, Lincoln TM, Rodman DM. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol. 1998;274:H672–8. doi: 10.1152/ajpheart.1998.274.2.H672. [DOI] [PubMed] [Google Scholar]
- 18.Shi WX, Bunney BS. Roles of intracellular cAMP and protein kinase A in the actions of dopamine and neurotensin on midbrain dopamine neurons. J Neurosci. 1992;12:2433–8. doi: 10.1523/JNEUROSCI.12-06-02433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–61. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 20.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol. 2004;287:H957–62. doi: 10.1152/ajpheart.01087.2003. [DOI] [PubMed] [Google Scholar]
- 21.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–91. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 22.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension. 2001;37:386–90. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 23.Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol. 2001;530:193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K(+) channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;281:H2480–9. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- 25.Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–62. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk MJ, Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function modulates volatile anesthetic sensitivity in C. elegans. Curr Biol. 2006;16:1641–5. doi: 10.1016/j.cub.2006.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosek E, Jensen KB, Lonsdorf TB, Schalling M, Ingvar M. Genetic variation in the serotonin transporter gene (5-HTTLPR, rs25531) influences the analgesic response to the short acting opioid Remifentanil in humans. Mol Pain. 2009;5:37. doi: 10.1186/1744-8069-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda K, Hayashida M, Ide S, Saita N, Kokita Y, Kasai S, Nishizawa D, Ogai Y, Hasegawa J, Nagashima M, Tagami M, Komatsu H, Sora I, Koga H, Kaneko Y, Ikeda K. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. 2009;147:194–201. doi: 10.1016/j.pain.2009.09.004. [DOI] [PubMed] [Google Scholar]