Abstract
Purpose: To explore the contribution of flow cytometry immunophenotyping (FCI) in detecting leptomeningeal disease in patients with solid tumors.
Experimental design: Cerebrospinal fluid (CSF) samples from 78 patients who received a diagnosis of epithelial-cell solid tumors and had clinical data suggestive of leptomeningeal carcinomatosis (LC) were studied. A novel FCI protocol was used to identify cells expressing the epithelial cell antigen EpCAM and their DNA content. Accompanying inflammatory cells were also described. FCI results (positive or negative for malignancy) were compared with those from CSF cytology and with the diagnosis established by the clinicians: patients with LC (n = 49), without LC (n = 26), and undetermined (n = 3).
Results: FCI described a wide range of EpCAM-positive cells with a hyperdiploid DNA content in the CSF of patients with LC. Compared with cytology, FCI showed higher sensitivity (75.5 vs 65.3) and negative predictive value (67.6 vs 60.5), and similar specificity (96.1 vs 100) and positive predictive value (97.4 vs 100). Concordance between cytology and FCI was high (Kp = 0.83), although misdiagnosis of LC did not show differences between evaluating the CSF with 1 or 2 techniques (P = .06). Receiver-operator characteristic curve analyses showed that lymphocytes and monocytes had a different distribution between patients with and without LC.
Conclusion: FCI seems to be a promising new tool for improving the diagnostic examination of patients with suspicion of LC. Detection of epithelial cells with a higher DNA content is highly specific of LC, but evaluation of the nonepithelial cell compartment of the CSF might also be useful for supporting this diagnosis.
Keywords: flow cytometry, immunophenotype, leptomeningeal carcinomatosis
Leptomeningeal carcinomatosis (LC) is a devastating cancer complication developing in at least 5%–10% of patients with solid tumors. Its prognosis is poor, with a median survival of 3–6 months among patients receiving chemotherapy.1–3 Early diagnosis of LC and treatment initiation could offer the best chance of controlling symptoms and prevent the establishment of irreversible neurologic deficits that impair the patient's quality of life.3–5 However, LC diagnosis currently remains a challenge. Cytological identification of malignant cells in the cerebrospinal fluid (CSF) remains the gold standard for LC diagnosis, but up to 45% of patients have an initial negative result of cytological examination of the CSF. This sensitivity may increase up to 90% when a high number of lumbar punctures are performed.6,7 The implementation of imaging techniques and biochemical analysis of the CSF offers useful information for diagnosis; however, both of them should be considered in the right clinical context, because they lack specificity and their sensitivity is low.1,3 It is therefore imperative to improve diagnostic tools.
Multiparametric flow cytometry immunophenotyping (FCI) is an established and necessary laboratory instrument for diagnosis and follow-up of a wide range of hematological malignancies. In turn, FCI is not routinely used for the study of nonhematological tumors. Today, the number of monoclonal antibodies (mAbs) directed against nonhematopoietic antigens is progressively increasing, and authors have recently shown the potential role of FCI in distinguishing mesothelioma and adenocarcinoma,8 identifying epithelial neoplasms in body fluids9 and detecting micrometastases in sentinel lymph node.10,11 In all these situations, the main advantage of FCI over cytology is a rapid and early diagnosis in relatively urgent clinical situations.8,9,12
The aim of this prospective study was to explore the contribution of FCI to identify LC in a group of patients with solid tumors and clinical data suggesting LC. Previous studies on aggressive lymphoma13–15 showed a better sensitivity of FCI over classical cytology examination of the CSF for the diagnosis of meningeal dissemination. To the best of our knowledge, this is the first time that FCI was used to evaluate the CSF of patients with solid tumors in search of LC.
Material and Methods
Clinical Data
From March 2009 through July 2010, 99 patients who received a diagnosis of solid tumors were recruited for the study in 28 Spanish hospitals. Inclusion criteria were a previous diagnosis of epithelial-cell neoplasia and the development of clinical signs or symptoms suggestive of LC. Patients with no history of neoplasia but serious suspicion of LC were also considered. Those with hematological malignancies, melanoma, primary brain tumors, or those receiving intrathecal treatment for LC before sending a CSF sample for study were excluded.
A complete data collection and follow-up was performed in 78 patients (median age, 57 ± 12; 54 females) from 19 participating hospitals. The remaining patients with incomplete submitted information were excluded, and per protocol analyses were performed.
In 2 patients of the series, 2 neoplasms were simultaneously detected: breast and gastrointestinal adenocarcinoma in the first patient, and colon and lung adenocarcinoma in the second one. The primary location of the tumor was unknown in 4 patients. In the remaining 72, the distribution of tumors was the following: breast (n = 44), lung (n = 23), gastrointestinal (n = 4), and cavum (n = 1). Adenocarcinoma was the histology subtype in 69 patients, and the remaining were 1 oat-cell, 2 squamous cell lung carcinoma, 3 undifferentiated tumors, 2 carcinomas with unknown primary location, and 1 malignant radiological lung lesion without histology. Clinical features, primary tumor-related characteristics, and CSF findings were also registered.
Study Design
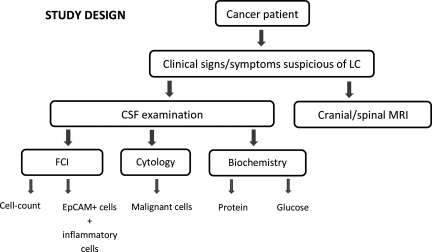
After neurological signs or symptoms appeared in a patient with cancer or suspicion of cancer, a neuraxis MRI was performed, and then a CSF sample was obtained for cellular studies (FCI and cytology) and biochemical tests (protein and glucose concentration; see Fig. 1). The clinician at the institution of origin was responsible for equally distributing the volume of CSF obtained for cellular studies into 2 tubes: the one not containing fixative agent was sent for cytology examination and the one with fixative agent was sent for FCI studies. FCI was blinded to cytology and MRI data.
Fig. 1.
Definitive LC diagnosis for every patient studied was verified by the clinicians at each hospital, based on either detection of malignant cells in CSF, on radiological findings consistent with LC on MRI, or biochemical abnormalities in the CSF.4 MRI studies and the cytological and biochemical examination of the CSF were done at the institution of origin using validated standard protocols and machines. Basic guidelines for cytological examination were dilution of CSF, location of an appropriate sample volume on a cytospin, and centrifugation. Films were dried and stained (Papanicolau and/or Giemsa) for light microscopy. No immunocytochemical analyses were performed for epithelial adhesion molecule (EpCAM).
CSF glucose levels were measured using enzymatic assays, and protein level measurements were performed using either turbidimetric or colorimetric procedures. FCI analyses were centralized at Fundación Jiménez Díaz (Madrid, Spain). FCI was only used as complementary information, and clinicians did not establish the diagnosis of LC based only on FCI data.
Local ethics committees of participating institutions approved the study, and informed consent was obtained from each patient before enrolment. All procedures were in accordance with the ethical standards of the Helsinki Declaration.
Samples and FCI Studies
CSF samples obtained from a lumbar puncture (n = 77) and from an Ommaya reservoir (n = 1) were collected in EDTA tubes containing 0.2 mL of an immunofixative reagent (Transfix, Immunostep SL), necessary to guarantee safe transportation. Volume and macroscopic characteristics (including blood contamination or not) of each CSF sample were recorded. All samples were processed within 5 days from extraction at the Fundación Jiménez Díaz in accordance with current FCI recommendations for processing CSF samples in search of hematological malignancies.16
An aliquot of the CSF sample received was used for cell count using the fluorescent dye DRAQ5 (Biostatus Limited) for DNA staining17,18 and Perfect-COUNT microspheres (Cytognos). The remaining sample was equally distributed into 2 different aliquots (tube 1 and tube 2). After centrifugation, a 2-step procedure was applied for the cell-pellet staining. Tube 1 was always stained with a 2-color (fluorescein isothyocyanate, FITC/phycoeritrin, PE) mAb combination directed against the epithelial cell antigen molecule. Ber-EP4 FITC (clone Ber-EP4) was purchased from DAKO Cytomation and EpCAM-PE (clone EBA-1) from BDB.
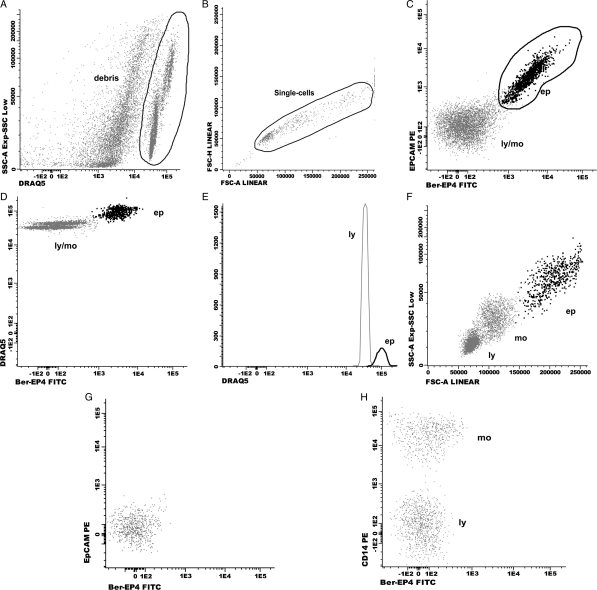
After incubation (30 min in the dark) and centrifugation (5 min at 540 g), the cell pellet was resuspended in PBS, and an appropriate dilution of DRAQ5 was added. The whole volume of sample was acquired on a FACSCantoII (Becton Dickinson Biosciences [BDB]) using the FACSDiva software (BDB). Analyses were performed using the INFINICYT software program (Cytognos). If the result of tube 1 was negative for malignancy or not conclusive, the same combination of mAb described for tube 1 was added to tube 2. If the result was positive for malignancy, tube 2 was stained with a 4–6-color mAb combination to confirm the epithelial origin of the malignant cells (intracellular cytokeratin) and label the inflammatory cell populations. FCI categorized a sample as positive for malignancy when at least 10 clustered events were positive for the 2 mAbs directed against EpCAM. The sequence of FCI analysis included identification of nucleated cells through DRAQ5 staining and then localization of epithelial cells using information from FITC and PE signals (Fig. 2). Calculation of DNA index was performed in those cases with sufficient number of events corresponding to epithelial cells. Ploidy was expressed as the ratio of the peak value of fluorescence intensity of epithelial cells with respect to that of lymphocytes (diploid cell compartment).19 Lymphocytes, monocytes, and polimorphonuclear cells were located on the basis of their forward and side scatter characteristics or information obtained from additional mAb.
Fig. 2.
Dot-plots showing an EpCAM positive CSF sample (A–F) and a negative sample for LC (G, H). Consecutive steps for data analysis: first step (A): identification of the CSF cell compartment based on the selection of DRAQ5 positive events. Second step (B): single-cell gate based on the selection of FSC-H vs FSC-A. Third step (C): identification of epithelial cells (ep) based on expression of EpCAM. Forth step (D, E): confirmation of a higher DNA content on epithelial cells with respect to lymphocytes DNA content. Fifth step (F): identification of inflammatory cells: lymphocytes (ly) and monocytes (mo). Epithelial cells (ep) have a heterogeneous size (FSC, forward scatter), and granularity (SSC, side scatter) as compared to lymphocytes and monocytes. No positive cells for the mAb Ber-EP4 and EpCAM are found in G and H. Positive staining for CD14 identifies monocytes. FITC: fluorescein isothiocyanate; PE: phycoerythrin.
Statistics
First, we determined the usefulness of cytology and FCI for diagnosing LC by calculating the sensitivity, specificity, and the positive and negative predictive values. Correlation between the 2 diagnostic methods was performed by κ index with 95% confidence interval (CI), and statistical difference between cytology and FCI was assessed by McNemar test. Second, we focused on the description of the FCI findings in every group identified by FCI, and the χ2 was used to identify differences among them. Quantitative variables were expressed as median and interquartile range. Third, we considered nonepithelial cells to determine whether they could also be helpful in predicting LC. The nonparametric tests used were Kruskal-Wallis test for multiple comparisons and Mann-Whitney test for comparisons between 2 groups. A 2-sided P value <.05 was considered as a statistically significant difference. Receiver-operator characteristics (ROC) analysis was used to compare the capability of the inflammatory subpopulations to discriminate between cases with and without LC. Calculations were performed using the SPSS software package, version 12.0 (SPSS).
Results
Accuracy Between FCI Examination and Current LC Diagnostic Criteria
LC was established in 49 of 78 patients. Supplementary Table 1 shows the results of the diagnostic tests performed on each patient. In brief (Table 1), CSF cytology was the basis for diagnosing LC in 32 patients, and for the remaining 17, diagnosis of LC required suitable clinical symptoms and signs and either MRI and biochemical CSF findings (n = 11), MRI only (n = 2), or only positive CSF biochemical data (n = 4).
Table 1.
Description of methods used for diagnosis of LC
| LC (n = 49) |
|||
|---|---|---|---|
| Negative CSF cytology | Positive CSF biochemistry | Negative CSF biochemistry | |
| Positive MRI | 11 | 2 | |
| Negative MRI | 4 | 0 | |
| Positive CSF cytology | 32 | ||
Abbreviations: LC, leptomeningeal carcinomatosis; CSF, cerebrospinal fluid; MRI, Magnetic resonance imaging.
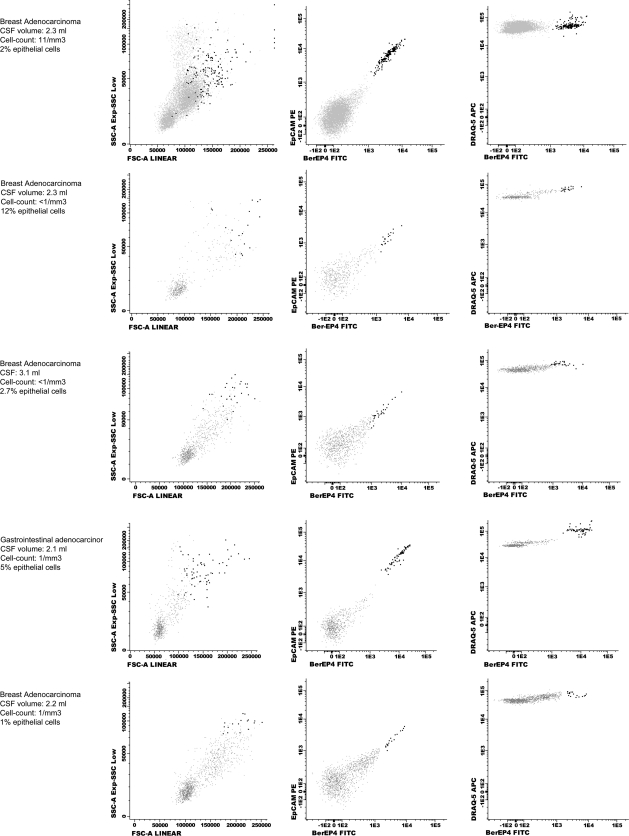
FCI detected a wide range of EpCAM-positive cells (0.1%–91%) in the CSF from patients with LC and positive CSF cytology. FCI also identified malignant epithelial cells in 5 additional cases in which CSF cytology was negative for malignancy (Figure 3). In these patients with negative cytology and positive FCI data (patients 45–49;Supplementary Table 1), the final diagnosis of LC was established by MRI and CSF biochemical data (n = 4) or biochemical data alone (n = 1) and was confirmed by clinical follow-up. Only one of these patients had brain metastases. In turn, the remaining 12 cases with LC (5 lung and 7 breast adenocarcinoma) had double negative CSF cytology and FCI findings (patients 33–44;Supplementary Table 1).
Fig. 3.
FCI data from patients in the TP FCI group. Epithelial cells are painted in dark black, and inflammatory cells in grey. In all cases, cytology was informed as no data of malignancy. Volume of CSF sample received, CSF cell-count and percentage of malignant cells detected by FCI in every patient are specified. FSC: forward scatter; SSC: side scatter; FITC: fluorescein isothiocyanate; PE: phycoerythrin.
LC was excluded in 26 patients, and after 6 months of follow-up, other diagnoses were determined in 23 of these 26 patients: B cell non-Hodgkin lymphoma (n = 1), infectious meningitis (n = 2), myasthenia gravis (n = 1), amyotrophic lateral sclerosis (n = 1), peripheral vestibular syndrome (n = 2), benign facial nerve palsy (n = 2), ischemic stroke (n = 1), primary central nervous system vasculitis (n = 1), paraneoplastic encephalitis (n = 1), intoxication due to administered therapy drugs (n = 2), adenoma hypophysis (n = 1), and metastases (brain [n = 3], dural [n = 2], and other locations [n = 3]). FCI was negative for malignancy in 25 cases. In the remaining patient, clinicians excluded the diagnosis of LC despite positive FCI findings. This CSF sample had a low volume (1.5 mL) and a low cell count (<1 cell/mm3). CSF cell populations showed 25% lymphocytes, 53% monocytes, and 8% diploid epithelial cells. This patient received a diagnosis of an undifferentiated tumor of the cavum and was studied for abrupt dementia. CSF cytology, MRI, and CSF biochemistry did not support malignancy. The patient died within a few days with a medical diagnosis of multifactorial dementia. A necropsy study was not performed.
Finally, the remaining 3 patients in our series had a very high clinical suspicion of LC, but MRI and repeated CSF studies were normal. FCI results were also negative for malignancy. Likewise, necropsy studies were not performed. In this context, a certain diagnosis of LC was not established; thus, these 3 patients were excluded from the analyses. Table 2 summarizes the relationship between CSF cytology, FCI findings, and final medical diagnoses.
Table 2.
CSF cytology and FCI findings in the 3 diagnostic groups
| Final diagnosis |
|||
|---|---|---|---|
| LC | Not-LC | Indeterminate | |
| Pos Cyt/Pos FCI | 32 | 0 | 0 |
| Neg Cyt/Neg FCI | 12 | 25 | 3 |
| Neg Cyt/Pos FCI | 5 | 1 | 0 |
| TOTAL | 49 | 26 | 3 |
Abbreviations: LC, leptomeningeal carcinomatosis. Cyt, cytology; FCI, flow cytometry immunophenotyping.
Sensitivity, specificity, and the positive and negative predictive values were calculated for every technique (Table 3). Correlation between cytology and FCI results was high (κ= 0.83; 95% CI, 0.70–0.96), but the rate of misdiagnosis of LC was 35% using only CSF cytology and 25% using both CSF cytology and FCI examination (P = .06).
Table 3.
Sensitivity, specificity, PPV and NPV for CSF cytology and FCI evaluation
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Cytology | 65.3 (52.0–78.6) | 100 (100–100) | 100 (100–100) | 60.5 (45.8–75.1) |
| FCI | 75.5 (63.5–87.6) | 96.1 (88.8–100) | 97.4 (92.3–100) | 67.6 (52.5–82.7) |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value. FCI, flow cytometry immunophenotyping.
Comparison of FCI Findings Among Patient Groups
Following established medical diagnosis, FCI data were classified as with or without cytology agreement. Cytology agreement included true-positive samples for malignancy (TP; n = 32), true-negative samples (TN; n = 25), and false-negative samples (FN; n = 12). Cases with discrepancies between the techniques included FN cytology but TP FCI (n = 5) and TN cytology but false-positive (FP) FCI (n = 1). The main CSF data of the 4 major groups are described in Table 4.
Table 4.
Statistical study of the 4 major groups identified by FCI. %: median percentage of CSF cells. Interquartile range is specified inside brackets
| CSF data | TP (n = 32) | TN (n = 25) | FN (n = 12) | TP FCI (n = 5) |
|---|---|---|---|---|
| Volume | 2.7 (2.1–3.5) | 2.6 (2.1–3.3) | 2.4 (1.4–3.2) | 2.3 (2.1–2.7) |
| Cells/mm3 | 8.0 (4.8–40.8)a | 1.0 (<1–7.0) | 1.0 (<1–4.7) | <1 |
| % epithelial cells | 14 (3.0–26.5)b | 0 | 0 | 3.0 (1.5–8.5)c |
| Epithelial cells/mm3 | <1 (<1–3.75)b | <1 | <1 | <1 (<1–3.0) |
| % lymphocytes | 55 (40–73)d | 73 (41–87)e | 49 (19–62) | 40 (30–65) |
| % monocytes | 35 (19–49) | 17 (9–31)f | 39 (18–57) | 48 (28–68) |
| % PMN | 4 (1–13) | 0 (0–15) | 2 (0–23) | 6 (0–13) |
Abbreviations: TP, true positive for malignancy cytology concordant; TN, true negative for malignancy cytology concordant; FN, false negative cytology/FCI; TP FCI, false negative cytology/true positive FCI; PMN, polimorphonuclear; FCI, flow cytometry immunophenotyping.
aP < .01 vs TN, FN and TP FCI groups.
bP < .001 vs TN and FN groups.
cP≤ .001 vs TN and FN groups.
dP < .05 vs TN group.
eP < .01 vs FN group.
fP < .01 vs TP, FN, and TP FCI groups.
Age, sex, and localization of the primary tumor showed no differences among groups. Median CSF sample volume was similar in each group, although median CSF value of cells/mm3 was higher in the TP group with regard to the other groups (P < .01). In the same way, a low cell count (≤1 cell/mm3) was found in 1 (3%) of 32 patients in the TP Group compared with 9 (36%) of 25 in the TN group (P < .01) and 4 (33%) of 12 in the FN group (P < .01). Of importance, the 3 patients in the TN group with ≥50 cells/mm3 had either infectious (n = 2) or lymphomatous meningitis (n = 1).
As expected, the median rate of epithelial cells showed differences between the 2 groups with epithelial cells in the CSF and the 2 groups without epithelial cells (based on either positive cytology and FCI or only positive FCI; P < .001). The median number of epithelial cells showed no differences among TN, FN, and TP FCI groups.
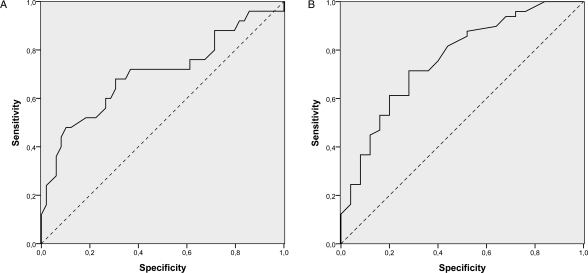
The distribution of the different inflammatory cell compartments was studied (Table 4) to determine whether any other parameter not related to the detection of epithelial cells in the CSF could be useful to suspect LC in the FN group. No differences among groups could be attributed to the rate of polimorphonuclear cells in the CSF, but the rate of lymphocytes had a tendency of increasing in patients without LC. Conversely, monocytes tended to increase in patients with LC, reaching values >30% in 8 of the 12 patients in the FN group. This association was observed after comparison of the TN group with the TP and FN groups (P < .01). Moreover, the TN and TP FCI groups only showed statistical differences in the distribution of the monocyte population (P < .01). This correlation did not change after excluding those CSF with blood contamination from the analyses (n = 10). An 84% sensitivity and 50% specificity for LC was described for a rate of 17.5% of monocytes in the CSF, but according to ROC curves, an exact cutoff point with enough sensitivity and specificity to discriminate between positive and negative cases was not found (Figure 4).
Fig. 4.
ROC analyses for the different percentage of lymphocytes (A) and monocytes (B) described in all cases included in the study.
Evaluation of DNA Ploidy
The DNA index could be accurately calculated in 25 patients: 24 were included in the TP group and showed a hyperdiploid DNA content (DNA index ranging from 1.6 to 4.24). The remaining patient was included in the FP FCI group and had a diploid DNA content (DNA index = 1.02). In the remaining patients in the TP and TP FCI groups, epithelial cells also had a higher DNA content compared with lymphocytes DNA content. However, the scarce number of cells was not enough to calculate the mathematical value for a DNA index.
Discussion
Cytology examination of the CSF remains as the gold standard for LC diagnosis. It identifies LC in 5%–10% of patients with solid tumors, but this incidence might be higher because all series with LC include patients who received a diagnosis only by MRI,1,3,20 and necropsy studies reveal LC in 19% of the evaluated cases.21 Continuous testing of new biomarkers22,23 indicates the strong necessity of improving diagnosis of LC. In this work, we introduce a novel FCI approach to look for malignant epithelial cells in the CSF based on the identification of the epithelial cell adhesion molecule, EpCAM, and description of their DNA content.
Overall, the results showed a good correlation between cytology and FCI CSF findings: FCI did not detect malignant epithelial cells in 25 of 26 patients for whom the diagnosis of LC was excluded, and all patients who received a diagnosis of LC according to CSF cytology also had a positive detection of malignant cells using FCI. Furthermore, FCI was also able to detect malignant cells in the CSF of 6 patients with LC and negative CSF cytology, and in 5 (83%) of them, a strong diagnosis of LC was established. Of note, comparison of both techniques might be biased because of the number of cytologists participating in the study and because the CSF volume analyzed was <10.5 mL, as recommended for obtaining the highest cytology sensitivity.6 However, our results show that FCI improves the sensitivity and negative predictive value of classical CSF cytology, even with the CSF volume obtained and studying only one CSF sample per patient.24
The key issue of the protocol that we propose here is the identification of EpCAM-positive cells in the CSF. EpCAM is a cell surface protein expressed on healthy human epithelia and corresponding malignant tumors.25–29 Immunohistochemical staining protocols have shown that most adenocarcinomas express EpCAM, with differences in intensity of expression among histology subtypes.25,26,28,29 In general, colon, ovarian, stomach, pancreas, lung, and breast show high EpCAM expression (>90%). A much lower expression (<20%) is exhibited on hepatocellular and clear cell kidney carcinoma, but our study does not include any of these. According to some reports,28–30 EpCAM expression on metastatic cancers may be close to 100%. From a technical point of view, EpCAM does not need cell permeabilization (and subsequent loss of cells) required for identification of cytoplasmic cytokeratin. EpCAM-positive cells can only be detected at very low frequencies circulating in peripheral blood;31,32 thus, in contrast to leukemia and lymphoma, they cannot interfere with the analysis of CSF samples contaminated with blood.13 On the other hand, focusing on the detection of epithelial cells means that the protocol will not be useful for detecting LC in patients with melanoma, sarcoma, or mesothelioma.24–26 Moreover, CSF samples where meningeal carcinomatosis was excluded and a very high number of lymphocytes were found should be carefully evaluated with a larger panel of reagents to exclude the malignant origin of the lymphoid compartment.
The design of this new FCI protocol, which uses 2 different mAb against different epitopes of the same molecule EpCAM, intended to solve the following issues. First, there was a need to recognize a criteria that helped to cluster the CSF malignant epithelial cells, because in contrast to most of lymphomas, they have a very disperse distribution in the forward and side scatter dot-plot. This probably reflects different size and granular cell content26,33,34 but makes it very difficult for FCI to localize them through their physical properties. Second, there was a need to increase the chances of detecting low intensity of EpCAM expression, as described for some histological subtypes of breast and lung cancers,26,27 because this might limit the accuracy of FCI. Third, there was a need to detect low numbers of malignant cells in the CSF because, in line with the observations by Strik et al;3 >50% of patients in our TP group had either <5% of CSF malignant cells or <1 malignant cell/mm3. The alignment of EpCAM positive cells showed a homogeneous image that helped distinguish epithelial cells from all the remaining acquired events. Finally, DNA content has also been useful to identify neoplastic epithelial cells, because all our patients with LC had a hyperdiploid population. Difficulties may arise when a diploid cell population is found, as happened in the only case classified as FP FCI result. In this patient, clinical and laboratory information did not support LC, but we lacked definitive information from a necropsy. Tumors in situ may have a diploid content;35 thus, despite our results, we still believe that LC diagnosis should not be rejected in diploid cases.
Even with FCI, 24% of patients in our series received a diagnosis of LC with use of techniques not related to the identification of pathological cells in the CSF. It might be a point of argument whether LC should only be established on clinical and biochemical CSF data, but most of the patients in our study had positive MRI findings. Different tumors were involved, but in all these cases, a very low CSF cell count was found. Whether this describes a low tumor burden or diminished free-floating neoplastic CSF cells with lower capability to metastatize along neuraxis and better survival prognosis remains to be established. In any case, because FCI and cytology analyze cells, some of the reasons described in the literature for explaining FN cytology could also explain FN FCI results.1,3 A CSF sample obtained far away from localized meningeal lesions, CSF flow obstruction, random shedding of malignant cells, or a diffuse distribution of LC might justify the absence of malignant cells in the CSF. The hypothesis of adherent types of LC might also be suggested,1 and strictly speaking, in all these situations, FCI and cytology findings should not be considered as FN results. In these cases with minimal or absent CSF involvement, searching for epithelial cells in the CSF would be ineffective for diagnosing LC, but according to our data, evaluation of the distribution of the inflammatory cell compartment might be helpful. When compared with the distribution described in normal CSF,36 monocytes tended to increase in the CSF of patients with LC in which no epithelial cells were detected. In contrast, TN CSF samples did not show this distribution, and lymphocytes were the subpopulation with a major increase. Little is known about the distribution of the inflammatory compartment of the CSF in patients with LC; thus, further studies are needed to determine which subpopulations of monocytes and lymphocytes are involved.
In summary, FCI seems to be a promising new tool for improving the diagnosis of LC in patients with adenocarcinoma. As a complement of cytology, FCI might be particularly useful to identify difficult LC cases with a very low rate of malignant cells in the CSF. Studies with many more patients are needed to determine whether inclusion of FCI to CSF study would increase accuracy of standard LC diagnostic tools.
Supplementary Material
Conflict of interest statement. None declared.
Funding
Mundipharma has collaborated with financial support.
Supplementary Material
Acknowledgments
The authors thank Dr. Santiago Escrivá from Hospital Mollet del Valles (Barcelona, Spain), Dr. Francesc Graus from Hospital Clinic (Barcelona, Spain), Dr. Carolina Méndez from Hospital Virgen de la Macarena (Sevilla, Spain), Dr. José Antonio García-Vela from Hospital Universitario de Getafe, (Getafe, Spain) Dr. Noelia Martínez from Hospital Ramón y Cajal (Madrid, Spain), and Dr. Ramón García-Arroyo from Complejo Hospitalario de Pontevedra (Pontevedra, Spain) for their collaboration in the study.
References
- 1.Chamberlain MC, Glantz M, Groves MD, Wilson WH. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol. 2009;36(4, Suppl 2):S35–S45. doi: 10.1053/j.seminoncol.2009.05.005. doi:10.1053/j.seminoncol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5(5):443–452. doi: 10.1016/S1474-4422(06)70443-4. doi:10.1016/S1474-4422(06)70443-4. [DOI] [PubMed] [Google Scholar]
- 3.Strik H, Prömmel P. Neoplastic meningitis. Diagnosis and individualized therapy. Expert Rev Anticancer Ther. 2010;10(7):1137–1148. doi: 10.1586/era.10.86. doi:10.1586/era.10.86. [DOI] [PubMed] [Google Scholar]
- 4.Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009;115(2):381–389. doi: 10.1002/cncr.24041. doi:10.1002/cncr.24041. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro RS, Johanson CE, Boogera W. Treatment modalities for leptomeningeal metastases. Sem Oncol. 2009;36(4 Suppl 2):S46–54.6. doi: 10.1053/j.seminoncol.2009.05.006. doi:10.1053/j.seminoncol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. doi:10.1002/(SICI)1097-0142(19980215)82:4<733::AID-CNCR17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626–632. doi: 10.1001/archneur.1996.00550070064013. [DOI] [PubMed] [Google Scholar]
- 8.Yaziji H, Battifora H, Barry TS, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol. 2006;19(4):514–523. doi: 10.1038/modpathol.3800534. doi:10.1038/modpathol.3800534. [DOI] [PubMed] [Google Scholar]
- 9.Krishan A, Ganjei-Azar P, Jorda M, Hamelik RM, Reis IM, Nadji M. Detection of tumor cells in body cavity fluids by flow cytometric and immunocytochemical analysis. Diagn Cytopathol. 2006;34(8):528–541. doi: 10.1002/dc.20496. doi:10.1002/dc.20496. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson M, Nilsson O, Thörn M, Winqvist O. Detection of metastatic colon cancer cells in sentinel nodes by flow cytometry. J Immunol Methods. 2008;334(1–2):122–133. doi: 10.1016/j.jim.2008.02.008. doi:10.1016/j.jim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Leers MP, Schoffelen RH, Hoop JG, et al. Multiparameter flow cytometry as a tool for the detection of micrometastatic tumour cells in the sentinel lymph node procedure of patients with breast cancer. J Clin Pathol. 2002;55(5):359–366. doi: 10.1136/jcp.55.5.359. doi:10.1136/jcp.55.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang A, Benda PM, Wood BL, Kussick SJ. Lineage-specific identification of nonhematopoietic neoplasms by flow cytometry. Am J Clin Pathol. 2003;119(5):643–655. doi: 10.1309/FU3F-DKYN-8AU0-891N. doi:10.1309/FU3FDKYN8AU0891N. [DOI] [PubMed] [Google Scholar]
- 13.Quijano S, López A, Manuel Sancho J, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin's lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27(9):1462–1469. doi: 10.1200/JCO.2008.17.7089. doi:10.1200/JCO.2008.17.7089. [DOI] [PubMed] [Google Scholar]
- 14.Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. doi: 10.1182/blood-2004-05-1982. doi:10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 15.Subirá D, Górgolas M, Castañón S, et al. Advantages of flow cytometry immunophenotyping for the diagnosis of central nervous system non-Hodgkin's lymphoma in AIDS patients. HIV Med. 2005;6(1):21–26. doi: 10.1111/j.1468-1293.2005.00260.x. doi:10.1111/j.1468-1293.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraan J, Gratama JW, Haioun C, et al. Flow cytometric immunophenotyping of cerebrospinal fluid. Curr Protoc Cytom. 2008 doi: 10.1002/0471142956.cy0625s45. Chapter 6:Unit 6.25:6.25.1–6.25.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin RM, Leonhardt H, Cardoso MC. DNA labeling in living cells. Cytometry A. 2005;67(1):45–52. doi: 10.1002/cyto.a.20172. [DOI] [PubMed] [Google Scholar]
- 18.Smith PJ, Wiltshire M, Errington RJ. DRAQ5 labeling of nuclear DNA in live and fixed cells. Curr Protoc Cytom. 2004 doi: 10.1002/0471142956.cy0725s28. Chapter 7:Unit 7.25:7.25.1–7.25.11. [DOI] [PubMed] [Google Scholar]
- 19.Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of cellular DNA content by flow and laser scanning cytometry. Adv Exp Med Biol. 2010;676:137–147. doi: 10.1007/978-1-4419-6199-0_9. doi:10.1007/978-1-4419-6199-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeiser R, Burger JA, Bley TA, Windfuhr-Blum M, Schulte-Mönting J, Behringer DM. Clinical follow-up indicates differential accuracy of magnetic resonance imaging and immunocytology of the cerebral spinal fluid for the diagnosis of neoplastic meningitis - a single centre experience. Br J Haematol. 2004;124(6):762–768. doi: 10.1111/j.1365-2141.2004.04853.x. doi:10.1111/j.1365-2141.2004.04853.x. [DOI] [PubMed] [Google Scholar]
- 21.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29(10):1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 22.Groves MD, Hess KR, Puduvalli VK, et al. Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol. 2009;94(2):229–234. doi: 10.1007/s11060-009-9819-2. doi:10.1007/s11060-009-9819-2. [DOI] [PubMed] [Google Scholar]
- 23.Stockhammer G, Poewe W, Burgstaller S, et al. Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology. 2000;54(8):1670–1676. doi: 10.1212/wnl.54.8.1670. [DOI] [PubMed] [Google Scholar]
- 24.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. doi:10.1002/1097-0142(19820215)49:4<759::AID-CNCR2820490427>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Winter MJ, Nagtegaal ID, van Krieken JH, Litvinov SV. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol. 2003;163(6):2139–2148. doi: 10.1016/S0002-9440(10)63570-5. doi:10.1016/S0002-9440(10)63570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Went P, Dirnhofer S, Schopf D, Moch H, Spizzo G. Expression and prognostic significance of EpCAM. J Cancer Mol. 2008;3(6):169–174. [Google Scholar]
- 27.Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat Rev. 2011 doi: 10.1016/j.ctrv.2011.04.002. doi:10.1016/j.ctrv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol. 2011;64(5):415–420. doi: 10.1136/jcp.2011.090274. doi:10.1136/jcp.2011.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimino A, Halushka M, Illei P, Wu X, Sukumar S, Argani P. Epithelial cell adhesion molecule (EpCAM) is overexpressed in breast cancer metastases. Breast Cancer Res Treat. 2010;123(3):701–708. doi: 10.1007/s10549-009-0671-z. doi:10.1007/s10549-009-0671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Went P, Vasei M, Bubendorf L, et al. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94(1):128–135. doi: 10.1038/sj.bjc.6602924. doi:10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao CG, Chianese D, Doyle GV, et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27(1):49–57. [PubMed] [Google Scholar]
- 32.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. doi:10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krantz BR. Detection of rare malignant cells and their apoptotic fragments in cerebrospinal fluid. The Lancet. 2000;356(7):1242–1243. doi: 10.1016/S0140-6736(00)02794-X. doi:10.1016/S0140-6736(00)02794-X. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Jia H, Yang Y, Dai W, Su X, Zhao G. Cerebrospinal fluid cytology and clinical analysis of 34 cases with leptomeningeal carcinomatosis. J Int Med Res. 2009;37(6):1913–1920. doi: 10.1177/147323000903700629. [DOI] [PubMed] [Google Scholar]
- 35.Fernández Val JF, Losada J, Arregui Murua MA, Sarría R. Cell proliferation, nuclear ploidy, and EGFr and HER2/neu tyrosine kinase oncoproteins in infiltrating ductal breast carcinoma. Cancer Genet Cytogenet. 2002;138(1):69–72. doi: 10.1016/s0165-4608(02)00567-8. doi:10.1016/S0165-4608(02)00567-8. [DOI] [PubMed] [Google Scholar]
- 36.Svenningsson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol. 1995;63(1):39–46. doi: 10.1016/0165-5728(95)00126-3. doi:10.1016/0165-5728(95)00126-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.