Abstract
We explored the feasibility of concurrent palliative chemotherapy and low-dose fractionated radiotherapy (LD-FRT) in glioblastoma multiforme (GBM). Patients with recurrent/progressive GBM at least 3 months after the end of primary radiotherapy received 0.3 Gy twice daily with cisplatin and fotemustine if progressing on temozolomide, or 0.4 Gy twice daily with temozolomide if recurrent 4–6 months later (retreatment group). Newly diagnosed GBM with gross residual mass received 30 Gy with concomitant and adjuvant temozolomide and 0.4 Gy twice daily from the second adjuvant cycle (naive group) for 2–4 cycles. Twenty-six patients were enrolled. In the retreatment group (n = 17; median LD-FRT total dose 7.2 Gy [range 2.4–11.6]), grade 3 or 4 hematological toxicity was observed in 5.9% of patients. Median follow-up time was 20 months (range 4–35). Median progression-free survival (PFS) and overall survival (OS) from the time of recurrence or progression were 4 and 8 months, respectively (OS at 6 months, 69%; at 12 months, 16.7%). In the naive group (n = 9; median LD-FRT total dose 8 Gy [range 3.2–16]), grade 3 or 4 hematological toxicity was observed in 11.1% of patients. Median follow-up time was 17 months (range 8–20)—median PFS was 9 months, with PFS at 6 months and at 1 year of 66.7% and 26.7%, respectively; and median OS was 12 months, with OS at 6 months and at 1 year of 77.8% and 34.6%, respectively. LD-FRT with concurrent chemotherapy was well tolerated.
Keywords: glioblastoma, hyper-radiosensitivity, low-dose fractionated radiotherapy, palliative treatment, radiochemotherapy
Glioblastoma multiforme (GBM) accounts for 75% of primary adult brain tumors.1 The standard of care for newly diagnosed GBM is surgical resection, followed by 3-dimensional conformal radiotherapy with concomitant and adjuvant chemotherapy, resulting in a median survival of 12–15 months.2 Patients submitted to only biopsy and patients with gross residual tumor after surgery are included in recursive partitioning analysis (RPA) classes V and VI, characterized by a far worse prognosis, with a median survival of 5–9 months.3,4
Treatment for recurrent disease generally includes surgery, stereotactic radiation therapy, chemotherapy, or supportive care, and the choice is usually based on size and location of the tumor, on previous treatment, and on patients' performance status.5–7
Different approaches have been investigated in order to improve this poor outcome without enhancing toxicity, both in treatment-naive patients and in those with recurrent or progressive tumor.
Many molecular mechanisms may contribute to the clinical resistance of GBM to alkylating drugs and to radiation with standard fractionation levels.8–11 Some studies suggest that the induction of apoptosis in cancer cells has an important role in the efficacy of chemotherapy and radiotherapy, and some evidence points to a determinant role for p53 in apoptosis induction by anticancer agents12,13 and radiotherapy.14 The majority of GBM cases are characterized by expression of mutant forms of p53 or by functional inactivation of the p53 protein,15–18 and it could be argued that the high degree of GBM resistance to high doses of radiation may be due to the impairment of p53 activity.19 Several in vitro studies20–22 have shown hyper-radiosensitivity (HRS) of some human malignant glioma cell lines to low radiation doses (<1 Gy). Nevertheless, Krause et al.23 did not observe any improvement in outcomes when ultrafractionated irradiation was translated in vivo in some human glioblastoma cell lines. On the contrary, increased resistance to radiation was observed for higher doses, a phenomenon termed increased radioresistance.24–30 In order to increase cell killing at low doses of radiation, systemic chemotherapy could be added. Preclinical studies12,22 demonstrated a synergistic effect between low doses of radiotherapy and chemotherapy, showing that low-dose fractionated radiotherapy (LD-FRT) improves the effectiveness of multiple chemotherapeutic agents such as carboplatin, cisplatin, docetaxel, etoposide, gemcitabine, and paclitaxel.31–37
Temozolomide (TMZ) is an oral alkylating agent with blood–brain barrier penetration, and its efficacy in treating newly diagnosed glioma has been largely demonstrated in combination with radiotherapy. However, there are limited data for recurrent disease.2 Novel chemotherapy agents and altered TMZ schedules are under evaluation as second-line treatments for recurrent or progressive disease; therefore, even if clear data are not yet available, nitrosourea-based chemotherapy should be considered a good option,38,39 as should TMZ rechallenge for patients without disease progression during TMZ treatment.40 Fotemustine (FTM) is a third-generation nitrosourea with an alkylating cytotoxic activity, characterized by a phosphoalanine carrier group grafted on the nitrosourea radical, which gives it high lipophilicity that allows it to cross the blood–brain barrier.41 FTM showed both in vitro and in vivo marked antineoplastic activity on human GBM and medulloblastoma cell lines.42
Considering this clinical and molecular background, in this prospective study we evaluated the feasibility and efficacy of a novel palliative radiochemotherapy schedule of low-dose radiation therapy combined with TMZ or FTM plus cisplatin in GBM patients. Two groups of patients were included: (1) patients who experienced disease recurrence or progression during or after TMZ treatment (retreatment group) and (2) newly diagnosed GBM patients with a poor prognosis because they were treated by only biopsy or because they had gross residual tumor mass after surgery and therefore were included in RPA classes V–VI (naive group).
Materials and Methods
Eligibility
In the retreatment group we enrolled patients with recurrent or progressive GBM after radiotherapy/TMZ treatment, observed on MRI and occurring at least 3 months after completion of radiotherapy,43 while patients with gross residual mass after surgery or treated by only biopsy were included in the naive group. The extent of residual disease was defined on postoperative MRI, performed 20–30 days after surgery, according to the surgeon’s assessment. We defined a “gross residual mass” as a residual tumor of more than 50% compared with initial disease, and a “partial resection” as surgery leaving 25%–50% of the initial disease. Further entry criteria were age ≥18 years; KPS > 70; white blood cell count ≥3.5 × 109/L; platelet count ≥150 × 109/L; bilirubin and creatinine levels 1.5 times the upper limit of normal; and aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase ≥2.5 times the upper limit of normal. All patients provided written informed consent before receiving treatment.
Radiotherapy and Concomitant Chemotherapy
Pretreatment gadolinium-enhanced MRI was performed in all cases. Patients were immobilized with a thermoplastic mask, and image fusion with MRI scans was performed for contouring. The clinical target volume, defined by a radiation oncologist and a neuroradiologist, included surgical cavity contrast enhancement plus the tumor or gross residual tumor or recurrent/progressive disease related to the particular clinical situation, plus a 30-mm margin. Treatment planning was carried out with Eclipse Treatment Planning Systems (Varian Medical System), and 3 or 4 (coplanar or not) multileaf-conformed radiation fields were used.
The organ-at-risk constraints were those described by Emami et al.44 and by the recent update by the QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) initiative.45 Previous radiotherapy treatments were taken into account and cumulative dose volume histograms were calculated in case of retreatment.
Retreatment patients, who were previously treated according to the study schedule of the European Organisation for Research and Treatment of Cancer and the National Cancer Institute of Canada trial,2 received 2 different schedules of LD-FRT depending on the time of observation on MRI of recurrent or progressive disease:
Cisplatin 30 mg/m2 on days 1, 8, and 15 for patients with disease progressing during TMZ therapy, and FTM 40 mg/m2 on days 2, 9, and 1646 concomitant with 0.3 Gy twice daily, with at least a 6-h interfraction interval24,31 on days 1–2, 8–9, and 15–16, every 42 days, depending on the chemotherapy schedule (2 cycles: total dose of 7.2 Gy with an overall treatment time of 58 days).
TMZ rechallenge (150/200 mg/m2) combined with LD-FRT 0.4 Gy twice daily for patients with recurrent disease 4–6 months after the end of adjuvant TMZ treatment,24 with at least a 6-h interfraction interval, over 5 consecutive days, every 28 days, always depending on chemotherapy schedule (2 cycles: total dose of 8 Gy with an overall treatment time of 33 days).
A dose per fraction <0.5 Gy was chosen because such doses proved to be more effective than higher doses per unit dose in killing clonogenic cells of many tumor cell lines.12,22 In our study, 2 different fractionations (0.3 Gy and 0.4 Gy) were chosen in order to reach the similar total dose of 7–8 Gy after 2 cycles, given the different schedules of concomitant chemotherapy used in the 2 groups. Chemotherapy was delivered between the 2 daily fractions of radiotherapy.
Naive patients with newly diagnosed GBM received 30 Gy47 (3 Gy/day over 2 weeks) with concomitant and adjuvant TMZ (75 mg/m2 from start to end of radiotherapy), followed by adjuvant TMZ; LD-FRT (0.4 Gy twice daily, over 5 consecutive days, every 28 days) was combined with TMZ (200 mg/m2) starting from the second adjuvant cycle. This short palliative regime was selected to reduce overall treatment time, the standard approach of 6 weeks being probably inappropriate for patients with a life expectancy lower than 6 months.
Patients were weekly evaluated for tolerance and toxicity during radiation therapy, with a baseline examination including cranial MRI, physical and neurological examination, and a Mini-Mental State Examination.
MRI scans were performed after every second radiochemotherapy cycle, and another 2 treatment cycles were scheduled if neither disease progression nor unacceptable toxicity was recorded.
Toxicity
The primary endpoints of the study were the evaluation of treatment-related toxicity and compliance of patients. Toxicity was graded using the Common Terminology Criteria for Adverse Events version 4.0.48 Patients’ compliance with treatment was evaluated as the percentage of patients who completed at least 2 cycles of concomitant radiochemotherapy with LD-FRT.
Efficacy Outcomes
Clinical response was evaluated by MRI, performed after every second cycle of radiochemotherapy, according to the modified World Health Organization (Macdonald) criteria.49 Progression-free survival (PFS) and overall survival (OS) were also evaluated.
Statistical Analysis
Survival analysis was performed by the Kaplan–Meier model, using MedCalc software (www.medcalc.be).
For patients in the naive group, OS was calculated from the time of diagnosis until death or last follow-up; in the retreatment group, it was calculated from the time of diagnosis of recurrence or progression until death or last follow-up. PFS was calculated for the retreatment group from the first fraction of radiotherapy delivered after recurrent/progressive disease, while for the naive group it was calculated from the time of biopsy or surgery.
Results
Study Population
From February 2008 to June 2010, 26 eligible patients underwent LD-FRT and concurrent chemotherapy. Patient characteristics are shown in Table 1. There were 17 patients enrolled in the retreatment group, with a median age of 64 years (range 21–71) and a male/female ratio of 2.4. The median total dose of LD-FRT delivered was 7.2 Gy (range 2.4–11.6); LD-FRT was combined with TMZ treatment in 59% of patients and with cisplatin and FTM in 41% of patients. There were 9 patients enrolled in the naive group, with a median age of 65 years (range 35–74) and a male/female ratio of 0.28. They received a median total dose of LD-FRT of 8 Gy (range 3.2–19.2).
Table 1.
Patient characteristics
| Variable | Retreatment group (n = 17) | Naive group (n = 9) | Total (n = 26) |
|---|---|---|---|
| Median age, years (range) | 64 (21–71) | 65 (35–74) | 63 (21–74) |
| Number of males/females | 12/5 | 2/7 | 14/12 |
| KPS | |||
| 100% | 0 | 1 | 1 |
| 90% | 5 | 2 | 7 |
| 80% | 9 | 2 | 11 |
| 70% | 3 | 4 | 7 |
| RPA class | |||
| V | – | 3 | 3 |
| VI | – | 6 | 6 |
| Type of surgery | |||
| Gross total resection | 3 | 3 | 6 |
| Biopsy | 14 | 6 | 20 |
| Tumor volume, cc (range) | 110 (13.52–372) | 41.5 (14.5–368.55) | 85.2 (13.52–372) |
| 0.4 Gy + temozolomide, n (%) | 10 (58.8) | 9 (100) | 19 (73) |
Toxicity
Although the twice-daily fractionation regimen could be difficult for patients, it was always well accepted. Patients’ compliance with treatment was 70.5% for the retreatment group and 88.8% for the naive group. All toxicity was reversible and no treatment-related deaths were observed. No late toxicity was observed in patients who underwent retreatment. The main adverse effects observed were fatigue grade 2 in 18 patients; alopecia grade 2 in 22 patients; skin reaction grade 1 in 7 patients; and headache grade 1 in 3 patients. In the retreatment group, we observed hematological toxicity (leukopenia) in 7 out of 17 patients (41.2%, with grades 1–2 in 35.3%, grades 3–4 in 5.9%). In the naive group, 3 out of 9 patients (33.3%) had acute toxicity (leukopenia and thrombocytopenia, grades 1–2 in 22.2%, grades 3–4 in 11.1%) (Table 2).
Table 2.
Acute toxicities (according to Common Toxicity Criteria v.4)
| Variable | Retreatment group (n = 17) | Naive group (n = 9) | Total (n = 26) |
|---|---|---|---|
| Leukopenia | |||
| Grades 1/2 | 2 | – | 2 |
| Grades 3/4 | 1 | 1 | 2 |
| Thrombocytopenia | |||
| Grades 1/2 | 4 | 2 | 6 |
| Grades 3/4 | – | 1 | 1 |
| Fatigue (grade 2) | 13 | 5 | 18 |
| Alopecia (grade 2) | 15 | 7 | 22 |
| Skin reaction (grade 1) | 5 | 2 | 7 |
| Headache (grade 1) | 1 | 2 | 3 |
Clinical Response
In the retreatment group, 1 patient (5.9%) had a partial response, 3 patients (17.6%) experienced stable disease for at least 2 months, and 13 (76.5%) had progressive disease observed. In the naive group, 3 patients (33.3%) experienced a partial response, 2 patients (22.2%) had stable disease for at least 2 months, and 4 patients (44.4%) were documented with disease progression.
Survival
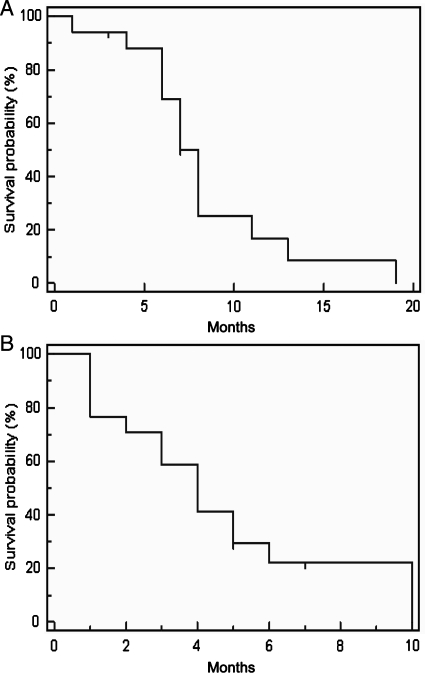
For the retreatment group, the median follow-up time from diagnosis of progression or recurrence was 20 months (range 4–36). The median PFS was 4 months, with PFS of 6 months for 22.1% (Fig. 1A). Median OS was 8 months, with OS of 6 and 12 months for 69% and 16.7%, respectively (Fig. 1B). At the time of our analysis, 3 patients were alive.
Fig. 1.
Progression-free survival (A) and overall survival (B) in the retreatment group
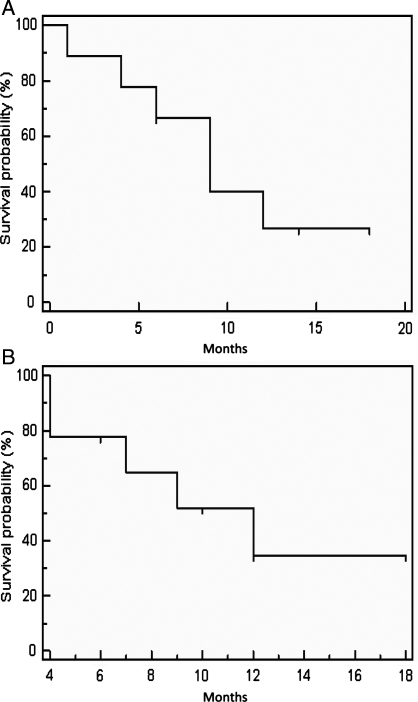
For the naive group, the median follow-up time was 17 months (range 8–20). The median PFS was 9 months, with PFS of 6 months for 66.7% and of 1 year for 26.7% (Fig. 2A). Median OS was 12 months, with OS of 6 months for 77.8% and of 12 months for 34.6% (Fig. 2B). At the time of analysis, 4 patients were alive with disease, while 5 patients had died.
Fig. 2.
Progression-free survival (A) and overall survival (B) in the naive group.
Discussion
Glioblastoma is one of the most resistant brain tumors.50 Radiation therapy, even with the addition of TMZ, does not significantly improve the outcome of this disease. Alternative fractionation regimens, such as hyperfractionation and accelerated fractionation, failed to ameliorate survival rates;51–53 therefore, research for new therapeutic strategies is open.
A novel fractionation regimen is by low-dose radiation; it has been studied in vitro for a variety of tumor cell lines.50,54–56 Several studies20,22,31 have recently shown a response to low (<1 Gy) radiation doses with an increased cell kill per unit dose.57,58 Ultrafractionated irradiation at low doses can increase radiosensitivity of radioresistant glioma cell lines.20,22,31,59 In fact, Krause23 and Short31 noted an increased cell kill in radioresistant T98G glioma cell lines when very low (0.4 Gy) doses were given 3 times daily, 4 h apart, for 5 or 7 days compared with 1.2 Gy or 1.68 Gy given once daily for 30 days. The molecular mechanisms underlying the HRS/increased radioresistance transition are still unclear. Nevertheless, it is well known that the radiosensitivity of human tumor cells may be correlated with their different capacities to correctly repair radiation damage. At higher doses, there is sufficient damage to trigger or induce radioprotective mechanisms so that cells show greater radioresistance, whereas at doses <1 Gy/fraction, DNA repair may not be induced; thus cells may remain radiosensitive and lower doses may be more effective than higher doses.60,61 Several authors have recently studied the correlation between low-dose HRS and the cell cycle;62–64 they observed that in rapidly proliferating tissues, ultrafractionated irradiation might be relevant because cells quickly progress into the cell cycle. The first low-dose fraction may eliminate subpopulations of cells that failed to undergo early G2-phase checkpoint arrest (evasion of the G2 checkpoint). During the interfraction interval, a small number of cells may progress through the cell cycle; a second fraction may then eliminate this small subpopulation, resulting in a larger therapeutic gain in rapidly proliferating tissue. In this context, Short et al.31 suggested an ultrafractionated treatment schema that requires a 4-h interfraction interval in order to have a cumulative effect on cell survival. Recently Beauchesne et al.66 published a phase II trial proving the feasibility and efficacy of the ultrafractionation radiation regimen in patients with newly diagnosed inoperable glioblastoma: they compared OS and PFS with the findings of the European Organisation for Research and Treatment of Cancer-National Cancer Institute of Canada trial65 demonstrating ultrafractionation superiority over radiotherapy alone but not over radiotherapy and TMZ.
Combined radiochemotherapy strategies that enrich the G2-phase fraction before radiotherapy have been developed, showing promising results in experimental settings. In preclinical studies, LD-FRT has been demonstrated to enhance the effectiveness of multiple chemotherapeutic agents, including carboplatin, cisplatin, docetaxel, etoposide, gemcitabine, and paclitaxel, in a variety of tumor cell lines.32,33,37 Preliminary results of clinical trials combining chemotherapy with low-dose radiation have shown recently that this treatment can be both feasible and effective.33,37 Phase I trials have indicated that LD-FRT combined with gemcitabine for pancreatic cancer, concomitant with paclitaxel and carboplatin in locally advanced squamous cell carcinoma of the head and neck or combined with other drugs in patients with relapse or metastases of epithelial tumors, demonstrates acceptable toxicity and interesting response rates.67
Patients affected by GBM with unfavorable prognostic factors, such as recurrent or progressive disease or newly diagnosed disease, who were only biopsied or have gross residual mass after surgery have the worst survival. In fact, the former have a median survival ranging from 3 to 6 months, based on the retreatment option and selection criteria,7,68–70 while the latter (RPA classes V–VI) generally survive from 4.6 to 9.4 months.3,65 In these patients, not all therapies may be indicated. Aggressive treatment, with potential toxicity, may worsen their quality of life without prolonging predicted survival.
In patients with recurrence, surgical morbidity and mortality are 15% and 5%, respectively.7 Reirradiation is complex; it can be used only in selected cases69 and is known to induce radionecrosis in 22%–36% of patients.7 Concomitant radiochemotherapy, tested in selected patients, has a mild toxicity profile.7 Schedules of prolonged TMZ administration determine a grade 3 or 4 hematological toxicity in up to 24.4% of patients.7,70 Other chemotherapy agents have been tested alone or in combined treatments with even a higher toxicity (grade 3 or 4 hematological toxicity 4.1%–42%).7 In newly diagnosed GBM, radiotherapy plus temozolomide resulted in grade 3 or 4 hematological toxicities in 7% of patients.2
To our knowledge, this is the first study using LD-FRT with concomitant chemotherapy in patients affected by GBM with unfavorable prognostic factors (only biopsied or with gross residual tumor, recurrent/progressive disease, age >70 years, RPA class V–VI). Since the safety and the tolerance of this new radiation regimen was unknown, we carried out this study to assess toxicity and patient compliance.
Of course, there are limitations to our study. We treated a negatively selected patient population, and the sample size is relatively small. However, our data showed good patient compliance (70.5% and 88.8% for the retreatment and the naive group, respectively) when LD-FRT was administered concurrently with TMZ or FTM and cisplatin. An acceptable toxicity profile was recorded. In the retreatment group, we observed a very low toxicity profile, with a grade 3 or 4 hematological toxicity of 5.9%, represented mainly by leukopenia; and neither acute nor late neurological toxicity was recorded. Response rate and median OS were similar to published data.7 In the naive group, we observed a transient grade 3 or 4 hematological toxicity in only 1 patient (11.1%), without acute or late neurological toxicity. A clinical benefit (partial response + stable disease lasting more than 9 weeks) was observed in 55.5% of patients, and the median survival of 12 months was quite satisfying, given the prognosis of these patients.
These data suggest that LD-FRT combined with chemotherapy might represent a new well-tolerated palliative schedule, both for patients with recurrent/progressive disease and for patients with gross residual tumors after surgery. Although far from being conclusive, the data are promising and support the development of further studies exploring LD-FRT as a new treatment modality for GBM patients with poor prognoses.
Conflict of interest statement. None declared.
References
- 1.American Cancer Society. Cancer Facts & Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):690–691. doi: 10.1093/jnci/85.9.704. doi:10.1093/jnci/85.9.690. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms: an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. doi:10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 5.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trial. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 6.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CC1–779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. doi:10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 7.Niyazi M, Siefert A, Schwarz SB, et al. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98(1):1–14. doi: 10.1016/j.radonc.2010.11.006. doi:10.1016/j.radonc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Taghian A, Ramsay J, Allalunis-Turner J, et al. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1993;25(2):243–249. doi: 10.1016/0360-3016(93)90345-v. doi:10.1016/0360-3016(93)90345-V. [DOI] [PubMed] [Google Scholar]
- 9.Johannessen TC, Bjerkvig R, Tysnes BB. DNA repair and cancer stem-like cells—potential partners in glioma drug resistance? Cancer Treat Rev. 2008;34(6):558–567. doi: 10.1016/j.ctrv.2008.03.125. doi:10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 10.Lowe SW, Ruley HE, Jacks T, Housman DE, et al. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. doi:10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin B, David C, Dieter L, et al. Defective p53antiangiogenic signaling in glioblastoma. Neuro-Oncol. 2010;12(9):894–907. doi: 10.1093/neuonc/noq051. doi:10.1093/neuonc/noq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chendil D, Oakes R, Alcock RA, et al. Low dose fractionated radiation enhances the radiosensitization effect of paclitaxel in colorectal tumor cells with mutant p53. Cancer. 2000;89:1893–1900. doi: 10.1002/1097-0142(20001101)89:9<1893::aid-cncr4>3.3.co;2-2. doi:10.1002/1097-0142(20001101)89:9<1893::AID-CNCR4>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Ella LK, Robin W, Anne R. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncol. 2010;12(4):389–400. doi: 10.1093/neuonc/nop046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen P, Iavarone A, Fick J, et al. Constitutional p53 mutations associated with brain tumors in young adults. Cancer Genet Cytogenet. 1995;82:106–115. doi: 10.1016/0165-4608(94)00213-u. doi:10.1016/0165-4608(94)00213-U. [DOI] [PubMed] [Google Scholar]
- 15.Wu JK, Ye Z, Darras BT, et al. Frequency of p53 tumor suppressor gene mutations in human primary brain tumors. Neurosurg. 1993;33:824–830. doi: 10.1227/00006123-199311000-00006. doi:10.1227/00006123-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Van Meir EG, Polverini PJ, Chazin VR, et al. Analysis of the p53 gene and its expression in human glioblastoma cells. Cancer Res. 1994;54:649–652. [PubMed] [Google Scholar]
- 17.Sidransky D, Mikkelsen T, Schwechheimer K, et al. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992;355:846–847. doi: 10.1038/355846a0. doi:10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- 18.Frankel RH, Bayona W, Koslow M, Newcomb EW. P53 mutations in human malignant gliomas: comparison of loss of heterozygosity with mutation frequency. Cancer Res. 1992;52:1427–1433. [PubMed] [Google Scholar]
- 19.D'Avenia P, Porrello A, Berardo M, et al. p53-gene transfer induces hypersensitivity to low doses of X-rays in glioblastoma cells: a strategy to convert a radio-resistant phenotype into a radiosensitive one. Cancer Lett. 2006;231:102–112. doi: 10.1016/j.canlet.2005.01.033. doi:10.1016/j.canlet.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Short SC, Mitchell SA, Boulton P, Woodcock M, Joiner MC. The response of human glioma cell lines to low-dose radiation exposure. Int J Radiat Biol. 1999;75:1341–1348. doi: 10.1080/095530099139214. doi:10.1080/095530099139214. [DOI] [PubMed] [Google Scholar]
- 21.Short S, Mayes C, Woodcock M, Johns H, Joiner MC. Low-dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol. 1999;75:847–855. doi: 10.1080/095530099139908. doi:10.1080/095530099139908. [DOI] [PubMed] [Google Scholar]
- 22.Beauchesne PD, Bertrand S, Branche R, et al. Human malignant glioma cell lines are sensitive to low radiation doses. Int J Cancer. 2003;105:33–40. doi: 10.1002/ijc.11033. doi:10.1002/ijc.11033. [DOI] [PubMed] [Google Scholar]
- 23.Krause M, Wohlfarth J, Georgi B, et al. Low-dose hyperradiosensitivity of human glioblastoma cell lines in vitro does not translate into improved outcome of ultrafractionated radiotherapy in vivo. Int J Radiat Biol. 2005;81(10):751–758. doi: 10.1080/09553000500491537. doi:10.1080/09553000500491537. [DOI] [PubMed] [Google Scholar]
- 24.Lambin P, Malaise EP, Joiner MC. The effect of very low radiation doses on the human bludder cell line RT112. Radiother Oncol. 1994;32:63–72. doi: 10.1016/0167-8140(94)90450-2. doi:10.1016/0167-8140(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 25.Lambin P, Marples B, Fertil B, Malaise EP, Joiner MC. Hypersensitivity of a human tumour cell line to very low radiation doses. Int J Radiat Biol. 1993;63:639–650. doi: 10.1080/09553009314450831. doi:10.1080/09553009314450831. [DOI] [PubMed] [Google Scholar]
- 26.Joiner MC. Induced radioresistance: an overview and historical perspective. Int J Radiat Biol. 1994;65:79–84. doi: 10.1080/09553009414550111. doi:10.1080/09553009414550111. [DOI] [PubMed] [Google Scholar]
- 27.Singh B, Arrand JE, Joiner MC. Hypersensitive response of normal human lung epithelial cells at low radiation doses. Int J Radiat Biol. 1994;65:457–464. doi: 10.1080/09553009414550531. doi:10.1080/09553009414550531. [DOI] [PubMed] [Google Scholar]
- 28.Wouters BG, Skarsgard LD. The response of a human tumor cell line to low radiation doses: evidence of enhanced sensitivity. Radiat Res. 1994;138:S76–S80. doi:10.2307/3578767. [PubMed] [Google Scholar]
- 29.Wouters BG, Sy AM, Skarsgard LD. Low dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res. 1996;146:399–413. doi:10.2307/3579302. [PubMed] [Google Scholar]
- 30.Marples B, Lambin P, Skov KA, Joiner MC. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int J Radiat Biol. 1997;71:721–735. doi: 10.1080/095530097143725. doi:10.1080/095530097143725. [DOI] [PubMed] [Google Scholar]
- 31.Short SC, Kelly J, Mayes CR, Woodcock M, Joiner MC. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int J Radiat Biol. 2001;77:655–664. doi: 10.1080/09553000110041326. doi:10.1080/09553000110041326. [DOI] [PubMed] [Google Scholar]
- 32.Dey S, Spring PM, Arnold S, et al. Low-dose fractionated radiation potentiates the effects of Paclitaxel in wild-type and mutant p53 head and neck tumor cell lines. Clin Cancer Res. 2003;9:1557–1565. [PubMed] [Google Scholar]
- 33.Gupta S, Arnold AM, Spring P, et al. Low-dose fractionated radiation potentiates the effect of cisplatin independent of the hyper-radiation sensitivity phenomenon in human lung cancer cell lines [Abstract] Proc Am Assoc Cancer Res. 2004;45:1369a. [Google Scholar]
- 34.Arnold SM, Regine WF, Ahmed MM, et al. Low-dose fractionated radiation as a chemopotentiator of neoadjuvant paclitaxel and carboplatin for locally advanced squamous cell carcinoma of the head and neck: Results of a new treatment paradigm. Int J Radiat Oncol Biol Phys. 2004;58:1411–1417. doi: 10.1016/j.ijrobp.2003.09.019. doi:10.1016/j.ijrobp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Spring PM, Arnold SM, Shajahan S, et al. Low dose fractionated radiation potentiates the effects of taxotere in nude mice xenografts of squamous cell carcinoma of head and neck. Cell Cycle. 2004;3:479–485. [PubMed] [Google Scholar]
- 36.Garofalo MC, Bacler-Kubiczek EK, Ahmed MM, et al. Chemopotentiation with low-dose fractionated radiation therapy: A promising novel treatment paradigm. Lancet Oncol. In press. [Google Scholar]
- 37.Regine WF, Hanna N, Garofalo MC, et al. Low-dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: A phase I study exploring a new treatment paradigm. Int J Radiat Oncol Biol Phys. 2007;68(1):172–177. doi: 10.1016/j.ijrobp.2006.11.045. doi:10.1016/j.ijrobp.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 38.Simpson L, Galanis E. Recurrent glioblastoma multiforme: advances in treatment and promising drug candidates. Expert Rev Anticancer Ther. 2006;6(11):1593–1607. doi: 10.1586/14737140.6.11.1593. doi:10.1586/14737140.6.11.1593. [DOI] [PubMed] [Google Scholar]
- 39.Brandes AA, Tosoni A, Basso U, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) J Clin Oncol. 2004;22(23):4779–4786. doi: 10.1200/JCO.2004.06.181. doi:10.1200/JCO.2004.06.181. [DOI] [PubMed] [Google Scholar]
- 40.Franceschi E, Omuro AM, Lassman AB, et al. Salvage temozolomide for prior temozolomide responders. Cancer. 2005;104(11):2473–2476. doi: 10.1002/cncr.21564. doi:10.1002/cncr.21564. [DOI] [PubMed] [Google Scholar]
- 41.Meulemans A, Giroux B, Hannoun P, Robine D, Henzel D. Comparative diffusion study of two nitrosoureas: carmustine and fotemustine in normal rat brain, human and rat brain biopsies. Chemotherapy. 1991;37:86–92. doi: 10.1159/000238838. doi:10.1159/000238838. [DOI] [PubMed] [Google Scholar]
- 42.Fischel JL, Formento P, Etienne MC, Gioanni J, et al. In vitro chemosensitivity testing of fotemustine (S 10036), a new antitumor nitrosourea. Cancer Chemother Pharmacol. 1990;25:337–341. doi: 10.1007/BF00686233. doi:10.1007/BF00686233. [DOI] [PubMed] [Google Scholar]
- 43.De Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 44.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. doi:10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodier JM, Da Costa L, Adams D, et al. Fotemustine and cisplatin association in patients with recurrent malignant glioma [Abstract] Proc Am Sol Clin. 1996:292. [Google Scholar]
- 47.Phillips C, Guiney M, Smith JA. Randomized trial comparing 35Gy in ten fractions with 60Gy in 30 fractions of cerebral irradiation for glioblastoma multiforme and older patients with anaplastic astrocytoma. Radiother Oncol. 2003;68(1):23–26. doi: 10.1016/s0167-8140(03)00206-8. doi:10.1016/S0167-8140(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 48.Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC) v4.0. ctep.cancer.gov/reporting/ctc.html.
- 49.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 50.Joiner MC, Denekamp J, Maughan RL. The use of “top-up” experiments to investigate the effect of very small doses per fraction in mouse skin. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:565–580. doi: 10.1080/09553008514552811. doi:10.1080/09553008514552811. [DOI] [PubMed] [Google Scholar]
- 51.Deutsch M, Green SB, Strike TA, et al. Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidazole plus radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys. 1989;16:1389–1396. doi: 10.1016/0360-3016(89)90939-5. doi:10.1016/0360-3016(89)90939-5. [DOI] [PubMed] [Google Scholar]
- 52.Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83–02. Cancer. 1996;15:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. doi:10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Brada M, Baumert B. Focal fractionated conformal stereotactic boost following conventional radiotherapy of high-grade gliomas: a randomized phase III study. A joint study of the EORTC (22972) and the MRC (BR10) Front Radiat Ther Oncol. 1999;33:241–243. doi: 10.1159/000061233. doi:10.1159/000061233. [DOI] [PubMed] [Google Scholar]
- 54.Joiner MC, Johns H. Renal damage in the mouse: the response to very small doses per fraction. Radiation Research. 1988;114:385–398. doi:10.2307/3577233. [PubMed] [Google Scholar]
- 55.Turesson I, Nyman J, Qvarnström F, et al. A low-dose hypersensitive keratinocyte loss in response to fractionated radiotherapy is associated with growth arrest and apoptosis. Radiother Oncol. 2010;94(1):90–101. doi: 10.1016/j.radonc.2009.10.007. doi:10.1016/j.radonc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Rachidi W, Harfourche G, Lemaitre G, Amiot F, Vaigot P, Martin MT. Sensing radiosensitivity of human epidermal stem cells. Radiother Oncol. 2007;83(3):267–276. doi: 10.1016/j.radonc.2007.05.007. doi:10.1016/j.radonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Gay H. Role of the linear-quadratic model in high doses per fraction. Radiother Oncol. 2010;94(1):122–123. doi: 10.1016/j.radonc.2009.08.019. doi:10.1016/j.radonc.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 58.Courdi A. High doses per fraction and the linear-quadratic model. Radiother Oncol. 2010;94(1):121–122. doi: 10.1016/j.radonc.2009.08.019. doi:10.1016/j.radonc.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Short SC, Woodcock M, Marples B, Joiner MC, et al. Effects of cell cycle phase on low-dose hyper-radiosensitivity. Int J Radiat Biol. 2003;79(2):99–105. [PubMed] [Google Scholar]
- 60.Lambin P, Malaise EP, Joiner MC. Might intrinsic radioresistance of human tumor cells be induced by radiation. Int J Radiat Biol. 1996;69:279–290. doi: 10.1080/095530096145832. doi:10.1080/095530096145832. [DOI] [PubMed] [Google Scholar]
- 61.Joiner MC, Marples B, Lambin P, Short SC, Turesson I, et al. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:37989. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 62.Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC, et al. Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiationdamaged G2-phase cells. Radiat Res. 2004;161:247–255. doi: 10.1667/rr3130. doi:10.1667/RR3130. [DOI] [PubMed] [Google Scholar]
- 63.Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M, et al. Low-dose radiation hypersensitivity is associated with p53-dependent apoptosis. Mol Cancer Res. 2004;2(10):557–566. [PubMed] [Google Scholar]
- 64.Short SC, Woodcock M, Marples B, Joiner MC. Effects of cell cycle phase on low-dose hyper-radiosensitivity. Int J Radiat Biol. 2003;79(2):99–105. [PubMed] [Google Scholar]
- 65.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide vs radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 66.Beauchesne P, Bernier V, Carnin C, et al. Prolonged survival for patients with newly diagnosed, inoperable glioblastoma with 3-times daily ultrafractionated radiation therapy. Neuro-Oncol. 2010;12(6):595–602. doi: 10.1093/neuonc/noq008. doi:10.1093/neuonc/noq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valentini V, Massaccesi M, Balducci M, et al. Low-dose hyperradiosensitivity: is there a place for future investigation in clinical settings? Int J Radiat Oncol Biol Phys. 2010;76(2):535–539. doi: 10.1016/j.ijrobp.2009.02.075. doi:10.1016/j.ijrobp.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 68.Franceschi E, Tosoni A, Bartolini S, Mazzocchi V, Fioravanti A, Brandes AA. Treatment options for recurrent glioblastoma: pitfalls and future trends. Expert Rev Anticancer Ther. 2009;9(5):613–619. doi: 10.1586/era.09.23. doi:10.1586/era.09.23. [DOI] [PubMed] [Google Scholar]
- 69.Nieder C, Adam M, Molls M, Grosu AL. Therapeutic options for recurrent high-grade glioma in adult patients: recent advances. Crit Rev Oncol Hematol. 2006;60(3):181–193. doi: 10.1016/j.critrevonc.2006.06.007. doi:10.1016/j.critrevonc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Wick A, Pascher C, Wick W, et al. Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol. 2009;256(5):734–741. doi: 10.1007/s00415-009-5006-9. doi:10.1007/s00415-009-5006-9. [DOI] [PubMed] [Google Scholar]