Abstract
The increased chemosensitivity of oligodendroglial tumors has been associated with loss of heterozygosity (LOH) on chromosomes 1p and 19q. Other clinical and molecular factors have also been identified as being prognostic and predictive for treatment outcome. Seventy-seven patients with anaplastic oligodendroglioma (AO) or anaplastic oligoastrocytoma (AOA), treated in Beijing Tiantan Hospital from 2006 through 2008, were reviewed. LOH 1p, LOH 19q, IDH1 mutation, O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation, and protein expression level of MGMT, P53, EGFR, and Ki-67 were evaluated. Age at diagnosis, LOH 1p and 19q, IDH1 mutation, P53 expression level, reoperation when progression, and adjuvant chemotherapy were statistically significant factors for overall survival (OS) in univariate analysis. Further multivariate analysis showed that age at diagnosis (P = .010), LOH 1p and 19q (P = .016), IDH1 mutation (P = .011), and reoperation after progression (P = .048) were independent predictors for longer survival in these patients. Nonrandom associations were found between LOH 1p and LOH 19q, MGMT promoter methylation and LOH 1p or 19q, IDH1 mutation and LOH 1p and 19q, IDH1 mutation and MGMT promoter methylation, whereas mutual exclusion was found between MGMT promoter methylation and MGMT expression level. The present study confirmed that age at diagnosis, LOH 1p and 19q, IDH1 mutation, and reoperation after progression were independent significant prognostic factors for patients with anaplastic oligodendroglial tumors. Inter-relationship between LOH 1p, LOH 19q, IDH1 mutation, MGMT promoter methylation, and MGMT expression level were also revealed. Future clinical trials for AO and AOA should consider the molecular alterations of patients.
Keywords: anaplastic oligodendroglial tumor, IDH1 mutation, LOH 1p and 19q, MGMT, prognostic factor
Anaplastic oligodendroglial tumors (anaplastic oligodendroglioma [AO] and anaplastic oligoastrocytoma [AOA] according to the World Health Organization [WHO] 2007 classification) constitute ∼25% of high-grade gliomas in adults.1 They generally occur during young to middle adulthood and frequently involve the frontal lobe. Standard treatment for AO and AOA consists of maximum surgical resection and radiotherapy (RT)/chemotherapy.2 Although seldom curative, long-term survival is relatively common after such treatment. Median survival time is also relatively long, ranging from 3 to 5 years.3,4
Combined allelic loss of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) is associated with higher chemosensitivity and radiosensitivity and longer survival for patients with anaplastic oligodendroglial tumors.5 Younger age, good performance status, total tumor resection, and postoperative radiotherapy are clinical or therapeutic factors that positively correlate with the patient's survival. Anaplastic oligodendroglial tumors are generally thought to be chemosensitive based on the high response rates to procarbazine, lomustine, and vincristine (PCV) in several studies.6,7 However, 2 large randomized trials investigating the use of sequential chemoradiotherapy in patients with AO and AOA failed to show any survival advantage over radiotherapy alone, with chemotherapy reserved for salvage treatment.3,4
In our study, in addition to loss of heterozygosity (LOH) of 1p and 19q, the prognostic value of IDH1 mutation, methylation of the O(6)-methylguanine-DNA methyltransferase (MGMT) gene promoter and protein expression level of MGMT, P53, epidermal growth factor receptor (EGFR), and Ki-67 are explored in a cohort of patients with AO or AOA from China. The relationships among these molecular biomarkers are discussed.
Materials and Methods
Clinical Material
We retrospectively identified all the patients with AO and AOA, who underwent surgical resection and radiation in the Glioma Treatment Center of Beijing Tiantan Hospital from 2006 through 2008. The histological diagnosis was reaffirmed by 2 independent neuropathologists and graded according to the WHO classification.8 Cases with discrepancies were re-reviewed by another pathologist until a consensus was reached. Clinical data, including patient's age at diagnosis, sex, preoperative Karnofsky performance status (KPS) score, and operation status were obtained from the medical records. Overall survival (OS) time, defined as the period from operation to death, was collected mainly when patients visited the clinics and during phone interview with patients and/or their relatives. Patients who died of nonprimary diseases were excluded. The study was approved by the ethics committee of Beijing Tiantan Hospital, and a written informed consent was obtained from all patients.
Treatment
Standard treatment consisted of surgery and postoperative radiotherapy, with or without adjuvant chemotherapy. Maximal tumor bulk resection while preserving the key eloquent cortex was the principle goal during surgery. Preoperative functional magnetic resonance image (MRI) and intraoperative awake brain mapping were used when necessary. Extent of resection was assessed on the postoperative enhanced MRI within 24 h and graded as total or subtotal resection. Postoperative limited-radiotherapy was routinely delivered to the patient within 1 month after surgery. The total dose was 60 Gy, which was divided into 30 daily fractions of 2 Gy each. For patients receiving adjuvant chemotherapy, the treatment was given 4 weeks after radiation, and at least 2 cycles of chemotherapy were administered. Adjuvant drugs were mainly lomustine (CCNU) or temozolomide (TMZ). A total of 6 cycles of chemotherapy were administered if no disease progression or irreversible hematological toxic effects were observed.
Assessment of 1p and 19q Status by Denaturing High-Performance Liquid Chromatography (DHPLC)
As described previously,9–12 DHPLC was used to assess the status of 1p and 19q. The microsatellite markers D1S548 (1p36.23), D1S1608 (1p36.32), and D1S1592 (1p36.13) were used to identify LOH 1p. To determine LOH 19q, the markers D19S431 (19q12), D19S433 (19q12), and D19S601 (19q13.41) were used. Each of the microsatellite markers was amplified seperately by polymerase chain reaction (PCR) using tumor genomic DNA and peripheral blood lymphocyte genomic DNA. PCR products were eluted from a DNASep column (Transgenomic) using a flow rate of 0.9 mL/min over a period of 11–13 min. Eluted products were detected by UV analysis at 260 nm.
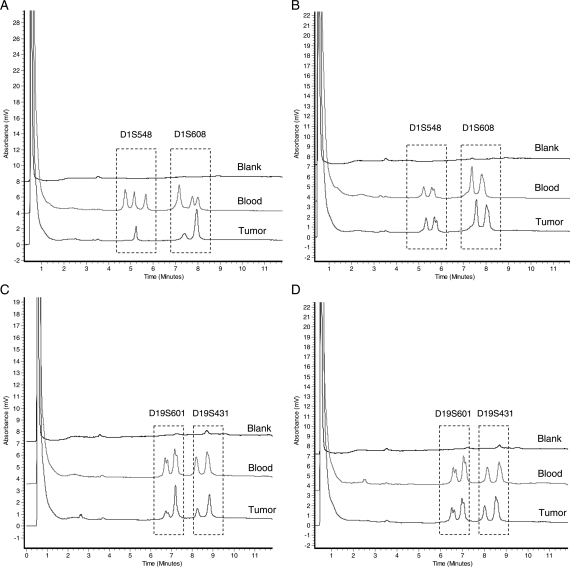
On the DHPLC chromatogram, the peak height or peak area of PCR products from normal tissue and tumor was measured by the WAVEMaker software; then, the height ratio (HR) or area ratio (AR) was calculated. To assess LOH, the tumor was compared with corresponding normal tissue. As illustrated in Fig. 1, the peak height or peak area reduced by >50% indicated that there was a LOH (ie, the HR or AR > 29,10) (see Fig. 1).
Fig. 1.
Assessment of LOH 1p and LOH 19q by DHPLC. (A) Markers D1S548 and D1S1608 (LOH); (B) Markers D1S548 and D1S1608 (no LOH); (C) Markers D19S601 and D19S431 (LOH); (D) Markers D19S601 and D19S431 (no LOH).
Assessment of IDH1 Mutation
IDH1 alterations were assessed by bidirectional cycle sequencing of PCR-amplified fragments. Primers used were IDH1-forward 5′-CTCCTGATGAGAAGAGGGTTG-3′ and IDH1-reverse 5′-TGGAAATTTCTGGGCCATG-3′. The sequencing was performed by Gene Tech (Shanghai) Company Limited.
Analysis of MGMT Promoter Methylation Status by Methylation-specific PCR (MSP)
DNA methylation patterns in the promoter region of MGMT were determined by MSP. This sensitive technique is based on the premise that unmethylated cytosines in bisulfite-modified genomic DNA are converted to uracil bases, whereas methylated cytosines are preserved. Subsequent PCR products with primers specific for either the methylated or the unmethylated DNA confirm the existence of methylation. Primer sequences for the unmethylated (U) reaction were: 5′-TTT GTG TTT TGA TGT TTG TAG GTT TTT GT-3′ forward and 5′-AAC TCC ACA CTC TTC CAA AAA CAA AAC A-3′ reverse; for the methylated (M) reaction were: 5′- TTT CGA CGT TCG TAG GTT TTC GC-3′ forward and 5′-GCA CTC TTC CGA AAA CGA AAC G-3′ reverse. DNA from peripheral blood lymphocytes from healthy donors was used as the unmethylated control, whereas CpGenomet Universal Methylated Control DNA (S7824; Chemicon) was used as the methylated control. A control experiment without DNA was performed for each set of PCR reaction. PCR products were separated on 2.0% agarose gels and visualized under UV illumination (see Fig. 2).
Fig. 2.
Methylation analysis of MGMT promoter. Product sizes: MGMT unmethylated, 93 bp; MGMT methylated, 81 bp. (PM: positive control of methylated PCR reaction; PU: positive control of unmethylated PCR reaction; N: negative control; 1, 3, 4: unmethylated cases; 2: methylated cases).
Evaluation of MGMT, P53, EGFR and Ki-67 Expression Level by Immunohistochemistry
Immunoperoxidase staining for MGMT, P53, EGFR, and Ki-67 (Invitrogen) was performed on formalin-fixed, paraffin-embedded tissue sections following the standard protocol recommended by the manufacturer. Each slide stained for MGMT, P53, EGFR, and Ki-67 was individually reviewed and scored by 2 independent observers. Discrepancies in scoring between the two observers were resolved by additional review of the specimens and discussion between the reviewers until a consensus was achieved. Approximately 15–20 fields at 400× magnification were analyzed per specimen. Scoring for MGMT, P53, EGFR, and Ki-67 was done on a 5-point scale from 0 to 4. A score of 0 indicated no or rare occurrence of stained nuclei, 1 indicated that <10% of cells had positive staining, 2 indicated that 10%–30% of cells stained positively, 3 indicated that 30%–60% of cells stained positively, and 4 indicated that >60% of cells had positive staining. For statistical analysis, the immunoreactivity of MGMT protein was evaluated semi-quantitatively by estimating the fraction of positive cells. High p53 expression was defined as strong nuclear staining in at least 30% (score 3/4) of the tumor cells. High EGFR expression was defined as >30% (score 3/4) of positive staining cells in the tumor. High Ki-67 labeling index (Ki-67 LI) was defined if at least 10% (score 2/3/4) of the tumor cells were positive.
Statistical Analysis
SPSS, version 13.0 (SPSS) was used for statistical analysis. Survivor function curves were calculated with the Kaplan–Meier method, and differences were evaluated with the log-rank test. Multivariate Cox models were used after univariate analysis. The χ2 test was applied for statistical analysis of the correlation for 2 independent variables. Statistical significance was defined as P <.05.
Results
Patient Population and Genetic Alteration
In total, 77 patients were reviewed in the study, including 36 with AO and 41 with AOA, who ranged in age from 16 to 71 years (median, 43 years). The preoperative KPS score ranged from 40 to 100 (median, 80). Twenty-eight cases had total tumor resection, and 49 cases had subtotal tumor resection. Fifty-one patients had surgery, radiation, and adjuvant chemotherapy. The other 26 patients had surgery and radiation only. No differences in age, sex, KPS score, tumor resection extent, reoperation after progression, and chemotherapy were observed between histological types (see details in Table 1). In a median follow-up of 38.2 months (range, 14.0–49.5 months), 14 patients had a second operation because of uncontrolled tumor progression, and 48 patients died. The patients’ median OS time was 33.5 months (95% confidence interval [CI], 24.2–50.4 months).
Table 1.
Characteristics of the patients
| Variable | AO (n =36) | AOA (n =41) | P value |
|---|---|---|---|
| Age | |||
| median | 39 | 45 | .113 |
| Gender | |||
| Male | 22 | 25 | |
| Female | 14 | 16 | .961 |
| Preoperative KPS score | |||
| ≥80 | 31 | 32 | |
| <80 | 5 | 9 | .755 |
| LOH 1p | |||
| Yes | 19 | 20 | |
| No | 17 | 21 | .570 |
| LOH 19q | |||
| Yes | 25 | 21 | |
| No | 11 | 20 | .393 |
| LOH 1p or 19q | |||
| Yes | 28 | 27 | |
| No | 8 | 14 | .665 |
| LOH 1p and 19q | |||
| Yes | 14 | 14 | |
| No | 22 | 27 | .615 |
| MGMT promoter gene | |||
| Methylated | 19 | 19 | |
| Unmethylated | 17 | 22 | .726 |
| IDH1 | |||
| Not mutated | 9 | 13 | |
| Mutated | 14 | 11 | .385 |
| MGMT expression | |||
| Low | 16 | 20 | |
| High | 20 | 21 | .529 |
| EGFR expression | |||
| Low | 26 | 20 | |
| High | 10 | 21 | .225 |
| P53 expression | |||
| Low | 11 | 11 | |
| High | 25 | 30 | .593 |
| Ki-67 expression | |||
| Low | 21 | 28 | |
| High | 15 | 13 | .395 |
| Reoperation after progression | |||
| Yes | 6 | 8 | |
| No | 30 | 33 | .879 |
| Extent of resection | |||
| Total | 14 | 14 | |
| Subtotal | 22 | 27 | .880 |
| Adjuvant chemotherapy | |||
| Yes | 23 | 28 | |
| No | 13 | 13 | .244 |
| Mean OS time (months) | 40.6 | 30.8 | .44 |
Abbreviations: AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; KPS, Karnofsky performance status; LOH, loss of heterozygosity; OS, overall survival.
All of the patients were successfully analyzed for LOH 1p, LOH 19q, and MGMT promoter methylation. In brief, 39 tumors showed LOH 1p (19 AO, 20 AOA), and 46 cases had LOH 19q (25 AO, 21 AOA). The combination of LOH 1p and LOH 19q was detected in 28 cases (14 AO, 14 AOA). MGMT promoter methylation was found in 38 (19 AO, 19 AOA) cases. From 47 patients, enough material was present to assess the mutational status of IDH1. Twenty-five cases (14 AO, 11 AOA) contained an IDH1 mutation. All mutations were located at amino acid residue 132, and all were G → A mutations (Arg → His) except one CGT → AGT mutation (Arg → Ser). The details of MGMT, p53, EGFR, and Ki-67 protein expression level are summarized in Table 1. No statistically significant differences were found in these genetic alterations between AO and AOA (Table 1).
Survival Analysis
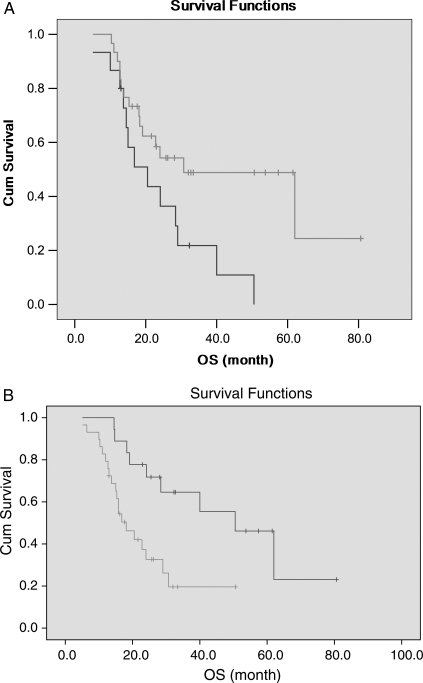
Age at diagnosis, LOH 1p and 19q, IDH1 mutation, P53 expression level, reoperation after progression, and adjuvant chemotherapy were statistically significant factors for OS in univariate analysis (see details in Table 2). They were further introduced into the multivariate model. It was shown that age at diagnosis (P = .010), LOH 1p and 19q (P = .016), IDH1 mutation (P = .011), and reoperation after progression (P = .048) were independent factors for OS. Among them, age ≤45 years, existence of LOH 1p and 19q, IDH1 gene mutation, and receiving second operation because of uncontrolled tumor progression were favorable factors. (Table 3, Fig. 3).
Table 2.
Factors associated with OS in the univariate analysis for anaplastic oligodendroglial tumors
| Variable | Cases | Median OS (month) | P value |
|---|---|---|---|
| Age | |||
| ≤45 | 46 | 50.5 | |
| >45 | 31 | 15.3 | .007 |
| Gender | |||
| Male | 47 | 19.1 | |
| Female | 30 | 28.4 | .188 |
| Preoperative KPS score | |||
| ≥80 | 63 | 28.4 | |
| <80 | 14 | 16.8 | .109 |
| Pathology | |||
| AO | 36 | 40.0 | |
| AOA | 41 | 31.0 | .511 |
| LOH 1p | |||
| Yes | 39 | 29.0 | |
| No | 38 | 22.8 | .130 |
| LOH 19q | |||
| Yes | 46 | 29.0 | |
| No | 31 | 22.8 | .696 |
| LOH 1p or 19q | |||
| Yes | 55 | 29.0 | |
| No | 22 | 24.0 | .373 |
| LOH 1p and 19q | |||
| Yes | 28 | 29.0 | |
| No | 49 | 19.1 | .038 |
| MGMT promoter gene | |||
| Methylated | 38 | 30.7 | |
| Unmethylated | 39 | 24.1 | .495 |
| IDH1 | |||
| Not mutated | 22 | 18.1 | |
| Mutated | 25 | 50.5 | .005 |
| MGMT expression | |||
| Low | 36 | 40.0 | |
| High | 41 | 19.1 | .094 |
| EGFR expression | |||
| Low | 41 | 18.3 | |
| High | 36 | 28.4 | .191 |
| P53 expression | |||
| Low | 22 | 40.0 | |
| High | 55 | 19.1 | .048 |
| Ki-67 expression | |||
| Low | 49 | 29.0 | |
| High | 28 | 22.8 | .366 |
| Reoperation after progression | |||
| Yes | 14 | 62.0 | |
| No | 63 | 19.1 | .000 |
| Extent of resection | |||
| Total | 28 | 29.0 | |
| Subtotal | 49 | 24.0 | .636 |
| Adjuvant chemotherapy | |||
| Yes | 51 | 30.7 | |
| No | 26 | 20.5 | .028 |
Abbreviations: OS, overall survival; KPS, Karnofsky performance status; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; LOH, loss of heterozygosity.
Table 3.
Prognostic factors associated with OS in the multivariate analysis for anaplastic oligodendroglial tumors
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 4.299 | 1.411–13.096 | .010 |
| LOH 1p and 19q | 0.256 | 0.089–0.735 | .016 |
| IDH1 mutation | 0.153 | 0.037–0.663 | .011 |
| P53 expression | 2.508 | 0.843–5.430 | .097 |
| Reoperation after progression | 0.099 | 0.013–1.010 | .048 |
| Adjuvant chemotherapy | 0.312 | 0.029–3.290 | .334 |
Abbreviations: OS, overall survival; LOH, loss of heterozygosity.
Fig. 3.
Kaplan–Meier estimates of overall survival time according to (A) LOH 1p and 19q and (B) IDH1 mutation by the log-rank test.
Correlation Between Genetic Alterations
Association and exclusion between the molecular alterations in 77 patients with anaplastic oligodendroglial tumor are shown in Table 4. Nonrandom associations were found between LOH 1p and LOH 19q, MGMT promoter methylation and LOH 1p or 19q, IDH1 mutation and LOH 1p and 19q, and IDH1 mutation and MGMT promoter methylation, whereas mutual exclusion was found between MGMT promoter methylation and MGMT expression level.
Table 4.
Correlation between genetic moleculars in anaplastic oligodendroglial tumors
| LOH 1p | LOH 19q | LOH 1p or 19q | LOH 1p and 19q | MGMT promoter methylation | MGMT | EGFR | P53 | Ki-67 | |
|---|---|---|---|---|---|---|---|---|---|
| LOH 19q | 0.286 (0.041) | 1 | |||||||
| LOH 1p or 19q | 0.605 (0.000) | 0.751 (0.000) | 1 | ||||||
| LOH 1p and 19q | 0.794 (0.000) | 0.620 (0.000) | 0.465 (0.001) | 1 | |||||
| MGMT promoter methylation | NS | NS | 0.320 (0.028) | NS | 1 | ||||
| MGMT | NS | NS | NS | NS | −0.464 (0.001) | 1 | |||
| EGFR | NS | NS | NS | NS | NS | NS | 1 | ||
| P53 | NS | NS | NS | NS | NS | NS | NS | 1 | |
| Ki-67 | NS | NS | NS | NS | NS | NS | NS | NS | 1 |
| IDH1 mutation | 0.367 (0.011) | NS | NS | 0.409 (0.004) | 0.367 (0.011) | NS | NS | NS | NS |
Abbreviations: LOH, loss of heterozygosity.
Discussion
In recent years, the histological criteria for diagnosis of AO and AOA have been well documented, and as a result, the rate of diagnosis has increased. Because of their heightened response to chemotherapy and ability to be divided into prognostic subgroups based on molecular biology,13,14 they have attracted great attention.
Age at diagnosis and preoperative KPS score have been the most well-documented predictors for survival in not only anaplastic oligodendroglial tumors but also other gliomas.15–17 In our study, patients aged <45 years were found to experience a longer survival; the median survival time of patients >45 years of age is 15.3 months and that of patients aged <45 years is 50.5 months. In multivariate analysis, age was also an independent prognostic factor. We think that the more malignant biological nature of tumors in older patients and less tolerance to surgery and other adjuvant therapies may contribute to the difference. The investigation did not reveal favorable survival association with preoperative KPS score. Population bias with more good-performance patients may be the reason.
Although an interobserver variability in recognizing AO and AOA existed among pathologists, no differences have been found in demographic factors and genetic alterations between AO and AOA in this cohort of patients. The cohorts do not have much difference in OS time either. All of these findings support the idea that although astrocytic component dose exists in AOA, it may be mainly from the pathological reactive course; the natural histories of AO and AOA do not differ significantly. Second operation because of uncontrolled tumor progression has been identified as an independent significant prognostic factor in the study, but the P value is very close to .05. Trials including more patients with second operations are needed to further confirm these results.
The survival benefit of surgery for patients with anaplastic oligodendroglial tumors is less robust than that with low-grade tumors.18 Dehgani et al.19 reported improved survival for patients treated with total (73% 1-year survival) versus subtotal (25% 1-year survival) resection. We did not find that extent of resection was a predictor of survival in the analysis. In view of the clinical benefits and improved biopathological characterization possible with larger tumor samples, a maximum safe surgical resection seems advisable for these patients.
The recognition that anaplastic oligodendroglial tumors are chemosensitive has been one of the most significant developments in neuro-oncology in recent years. To our disappointment, like the prospective trial finished recently,3,4 we failed to show chemotherapy had any survival advantage in multivariate analysis. The European Organization for Research and Treatment of Cancer (EORTC) 26951 randomized 368 patients with newly diagnosed AO or AOA to receive RT (59.4 Gy) alone or RT followed by 6 cycles of standard dose adjuvant PCV chemotherapy. Radiation Therapy Oncology Group (RTOG) 9402 (with 298 patients) was designed slightly differently with the control arm receiving RT alone and the experimental arm receiving 4 cycles of intensive-dose PCV prior to RT (59.4 Gy). Both studies only showed that PFS was significantly longer in the arms receiving PCV chemotherapy. The investigators acknowledged that these studies might have effectively compared RT and upfront PCV with RT and delayed chemotherapy at point of tumor progression. The similarity in OS time in all treatment arms suggesting that effective salvage RT can be administered when tumors progressed. Results of temozolomide (TMZ) to these patients have been recently reported.20,21 RTOG 013121 evaluated the efficacy of pre-RT TMZ and the toxicity of concurrent RT and TMZ in patients with newly diagnosed AO/AOA. It was noted that 6.3% of the patients had a complete response, 28.1% experienced a partial response, and 50% of the patients were disease stable, whereas only 28.1% had progression. The upcoming international phase III trial of EORTC protocol 26081 will compare TMZ chemotherapy alone, RT alone, and a chemoradiation regimen to better define the optimal frontline treatment for these diseases.
As determined in the studies of EORTC 26951 and RTOG 9402, our results reaffirmed that the presence of LOH 1p and 19q was an independent prognostic factor for AO and AOA that was associated with significantly longer OS regardless of treatment received. Patients with an IDH1 mutation were confirmed to have longer survival in the multivariate analysis too. However, the incidence of LOH 1p and 19q and an IDH1 mutation was a little lower in comparison to the reports from Europe or America.3,4,22 Ethnic differences might partly explain it. In regard to the prognostic value of methylation status of the MGMT promoter, Hegi et al.23 first confirmed methylation of the MGMT promoter as an independent prognostic and predictive factor for patients with GBM in a phase III clinical trial. We failed to show MGMT promoter methylation was a survival predictor in these anaplastic oligodendroglial tumor patients. The prognostic value of MGMT, P53, EGFR, and Ki-67 expression levels was not verified either.
For the internal relationship of molecular alterations, correlation was reaffirmed between LOH 1p and LOH 19q in these anaplastic oligodendroglial tumors from China. Mollemann et al.24 reported that MGMT promoter methylation and reduced expression was commonly seen in oligodendroglial tumors, in particular in those with combined 1p/19q deletion. In our study, association was observed between MGMT promoter methylation and LOH 1p or 19q; a strong mutual exclusion was found between MGMT promoter methylation and MGMT expression. In addition, correlation (P < .0001) was found between IDH1 mutation and MGMT promoter methylation. IDH1 mutations were observed in 57% of the MGMT promoter–methylated tumors, as opposed to only 21% of the MGMT-unmethylated tumors. LOH 1p and 19q was also highly correlated with IDH1 mutation. All of them were in accordance with the results from the European Organization for Research and Treatment of Cancer.13 A mutually exclusive relationship between TP53 mutation and LOH 1p and 19q25,26 has been reported for oligodendroglial tumors. They are regarded as mutually exclusive pathways in the pathogenesis of glioma and considered to represent astrocytic and oligodendroglial lineages, respectively.27–29 We did not find such correlation between them. The examination method of p53 here was not compelling enough to address it. Further tests by Western blot or sequencing may well explain it.
In conclusion, the present study showed age at diagnosis, LOH 1p and 19q, IDH1 mutation, and second operation because of tumor progression were independent significant prognostic factors for patients with anaplastic oligodendroglial tumors. Inter-relationships between LOH 1p, LOH 19q, IDH1 mutation, MGMT promoter methylation, and MGMT expression were revealed. Future clinical trials for AO and AOA should take consideration of the molecular alterations of patients.
Conflict of interest statement. None declared.
Funding
This study was supported by the funds from the National Key Project of Science and Technology Supporting Programs of China (No. 2007BAI05B08).
Acknowledgments
We thank Yuling Yang for tissue sample collection and clinical data retrieval.
References
- 1.Bromberg JE, van den Bent MJ. Oligodendrogliomas: molecular biology and treatment. Oncologist. 2009;14:155–163. doi: 10.1634/theoncologist.2008-0248. doi:10.1634/theoncologist.2008-0248. [DOI] [PubMed] [Google Scholar]
- 2.Blakeley J, Grossman S. Anaplastic oligodendroglioma. Curr Treat Options Neurol. 2008;10:295–307. doi: 10.1007/s11940-008-0032-y. doi:10.1007/s11940-008-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. doi:10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 4.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. doi:10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 5.Kros JM, Gorlia T, Kouwenhoven MC, et al. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol. 2007;66:545–551. doi: 10.1097/01.jnen.0000263869.84188.72. doi:10.1097/01.jnen.0000263869.84188.72. [DOI] [PubMed] [Google Scholar]
- 6.Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23:360–364. doi: 10.1002/ana.410230408. doi:10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- 7.Kim L, Hochberg FH, Thornton AF, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for grade III and grade IV oligoastrocytomas. J Neurosurg. 1996;85:602–607. doi: 10.3171/jns.1996.85.4.0602. doi:10.3171/jns.1996.85.4.0602. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chernova OB, Barnett GH, Cowell JK. Rapid detection of allelic losses in brain tumours using microsatellite repeat markers and high-performance liquid chromatography. Br J Cancer. 2003;88:1889–1893. doi: 10.1038/sj.bjc.6601025. doi:10.1038/sj.bjc.6601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleymenova E, Walker CL. Determination of loss of heterozygosity in frozen and paraffin embedded tumors by denaturating high-performance liquid chromatography (DHPLC) J Biochem Biophys Methods. 2001;47:83–90. doi: 10.1016/s0165-022x(00)00154-8. doi:10.1016/S0165-022X(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Jiang T, Yuan F, et al. Correlations between molecular profile and tumor location in Chinese patients with oligodendroglial tumors. Clin Neurol Neurosurg. 2008;110:1020–1024. doi: 10.1016/j.clineuro.2008.06.020. doi:10.1016/j.clineuro.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Jiang T, Yuan F, et al. Correlation of chromosomes 1p and 19q status and expressions of O6-methylguanine DNA methyltransferase (MGMT), p53 and Ki-67 in diffuse gliomas of World Health Organization (WHO) grades II and III: a clinicopathological study. Neuropathol Appl Neurobiol. 2009;35:367–379. doi: 10.1111/j.1365-2990.2008.01002.x. doi:10.1111/j.1365-2990.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- 13.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. doi:10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 14.Kouwenhoven MC, Gorlia T, Kros JM, et al. Molecular analysis of anaplastic oligodendroglial tumors in a prospective randomized study: A report from EORTC study 26951. Neuro Oncol. 2009;11:737–746. doi: 10.1215/15228517-2009-011. doi:10.1215/15228517-2009-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson JF, Afaghi V, Payne CA, et al. The impact of molecular and clinical factors on patient outcome in oligodendroglioma from 20 years’ experience at a single centre. J Clin Neurosci. 2011;18:329–333. doi: 10.1016/j.jocn.2010.07.101. doi:10.1016/j.jocn.2010.07.101. [DOI] [PubMed] [Google Scholar]
- 16.Shirai K, Suzuki Y, Okamoto M, et al. Influence of histological subtype on survival after combined therapy of surgery and radiation in WHO grade 3 glioma. J Radiat Res (Tokyo). 2010;51:589–594. doi: 10.1269/jrr.10055. doi:10.1269/jrr.10055. [DOI] [PubMed] [Google Scholar]
- 17.Yang LS, Huang FP, Zheng K, et al. Factors affecting prognosis of patients with intracranial anaplastic oligodendrogliomas: a single institutional review of 70 patients. J Neurooncol. 2010;100:113–120. doi: 10.1007/s11060-010-0146-4. doi:10.1007/s11060-010-0146-4. [DOI] [PubMed] [Google Scholar]
- 18.Soffietti R. Chemotherapy of anaplastic oligodendroglial tumours. Expert Opin Pharmacother. 2004;5:295–306. doi: 10.1517/14656566.5.2.295. doi:10.1517/14656566.5.2.295. [DOI] [PubMed] [Google Scholar]
- 19.Dehghani F, Schachenmayr W, Laun A, et al. Prognostic implication of histopathological, immunohistochemical and clinical features of oligodendrogliomas: a study of 89 cases. Acta Neuropathol. 1998;95:493–504. doi: 10.1007/s004010050830. doi:10.1007/s004010050830. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen T, Doyle T, Anderson J, et al. Temozolomide single-agent chemotherapy for newly diagnosed anaplastic oligodendroglioma. J Neurooncol. 2009;92:57–63. doi: 10.1007/s11060-008-9735-x. doi:10.1007/s11060-008-9735-x. [DOI] [PubMed] [Google Scholar]
- 21.Vogelbaum MA, Berkey B, Peereboom D, et al. Phase II trial of preirradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: RTOG BR0131. Neuro Oncol. 2009;11:167–175. doi: 10.1215/15228517-2008-073. doi:10.1215/15228517-2008-073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. doi:10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. doi:10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 24.Mollemann M, Wolter M, Felsberg J, et al. Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer. 2005;113:379–385. doi: 10.1002/ijc.20575. doi:10.1002/ijc.20575. [DOI] [PubMed] [Google Scholar]
- 25.Ueki K, Nishikawa R, Nakazato Y, et al. Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors. Clin Cancer Res. 2002;8:196–201. [PubMed] [Google Scholar]
- 26.Okamoto Y, Di Patre PL, Burkhard C, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108:49–56. doi: 10.1007/s00401-004-0861-z. doi:10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- 27.Jeon YK, Park K, Park CK, et al. Chromosome 1p and 19q status and p53 and p16 expression patterns as prognostic indicators of oligodendroglial tumors: a clinicopathological study using fluorescence in situ hybridization. Neuropathology. 2007;27:10–20. doi: 10.1111/j.1440-1789.2006.00735.x. doi:10.1111/j.1440-1789.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Kujas M, Lejeune J, Benouaich-Amiel A, et al. Chromosome 1p loss: a favorable prognostic factor in low-grade gliomas. Ann Neurol. 2005;58:322–326. doi: 10.1002/ana.20543. doi:10.1002/ana.20543. [DOI] [PubMed] [Google Scholar]
- 29.Yakut T, Gutenberg A, Bekar A, et al. Correlation of chromosomal imbalances by comparative genomic hybridization and expression of EGFR, PTEN, p53, and MIB-1 in diffuse gliomas. Oncol Rep. 2007;17:1037–1043. [PubMed] [Google Scholar]