Abstract
A phase I study was conducted to determine the dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) for the combination of vorinostat with bevacizumab and CPT-11 in recurrent glioblastoma. Vorinostat was combined with bevacizumab and CPT-11 and was escalated using a standard 3 + 3 design. Vorinostat was escalated up to 2 actively investigated doses of this compound or until the MTD was identified on the basis of DLTs. Correlative science involving proteomic profiling of serial patient plasma samples was performed. Nineteen patients were treated. The MTD of vorinostat was established at 400 mg on days 1–7 and 15–21 every 28 days when combined with bevacizumab and CPT-11. Common toxicities were fatigue and diarrhea. DLTs included fatigue, hypertension/hypotension, and central nervous system ischemia. Although the MTD was established, CPT-11 dose reductions were common early in therapy. High-dose vorinostat had an improved progression-free survival and overall survival when compared with low-dose vorinostat. Serum proteomic profiling identified IGFBP-5 and PDGF-AA as markers for improved PFS and recurrence, respectively. A MTD for the combination of vorinostat with bevacizumab and CPT-11 has been established, although it has poor long-term tolerability. With the increased toxicities associated with CPT-11 coupled with its unclear clinical significance, investigating the efficacy of vorinostat combined with bevacizumab alone may represent a more promising strategy to evaluate in the context of a phase II clinical trial.
Keywords: bevacizumab, CPT-11, recurrent glioblastoma, vorinostat
Each year, >14 000 new cases of malignant gliomas are diagnosed in the United States, with the most aggressive form, glioblastoma, being the most common.1 Despite comprehensive, multimodality treatment consisting of surgery, radiation therapy, and chemotherapy, prognosis remains poor, with a majority of patients developing disease recurrence within months after definitive therapy.2 In the context of recurrent disease, standard cytotoxic chemotherapies have historically been ineffective, with median progression-free survival after recurrence being ∼9 weeks.3 Therefore, identifying novel approaches to improve clinical gains in recurrent glioblastoma is of high priority.
The rich vascular network typically associated with glioblastoma has made tumor angiogenesis an attractive target in this malignancy. Two recent phase II studies using the monoclonal antibody bevacizumab, which binds to and inhibits the activity of vascular endothelial growth factor (VEGF) combined with irinotecan, have demonstrated striking response rates in recurrent glioblastoma.4,5 Vredenburgh et al published the first prospective study using this regimen in 35 patients with recurrent glioblastoma, resulting in both response and 6-month progression-free survival (PFS-6) rates of ∼50%.5 These favorable findings were validated in both a single institution6 and a large multicenter trial,4 supporting the recent US Food and Drug Administration approval of bevacizumab in recurrent glioblastoma. Despite representing progress, limitations to this regimen have since been recognized, with the most notable being its limited capacity in improving overall survival in this patient population.7,8 Therefore, identifying and combining agents with the capacity of enhancing the antitumor activity of bevacizumab and irinotecan holds promise in furthering the clinical gains of this regimen.
Vorinostat is a small molecule inhibitor of class I and II histone deacetylases (HDACs) that was recently approved for the treatment of cutaneous T-cell lymphoma and is currently being investigated in a variety of solid tumors, including glioblastoma.9,10 Although the independent activity of vorinostat in recurrent glioblastoma has been modest,10 preclinical data suggest the capacity of this class of agents to both target tumor angiogenesis independently11 and have the capacity of augmenting the antitumor activity of both angiogenesis12 and topoisomerase inhibitors13–16 in a variety of model systems, including glioblastoma.16 On the basis of this preclinical rationale, a phase I trial was performed to study the effect of combining vorinostat with the established bevacizumab and irinotecan platform in patients with recurrent glioblastoma. The primary objectives of this study were to determine the toxicity and maximum tolerated doses of vorinostat in this combination. Secondary end points were to determine the efficacy of this strategy and to explore the potential of plasma-based biomarkers to be used in both defining patient populations likely to respond to an angiogenesis inhibitor-based regimen and evaluating proteomic dynamics after recurrence.
Patients and Methods
Patient Eligibility and Selection
Patients were required to have a histologically confirmed diagnosis of intracranial glioblastoma or gliosarcoma with pathologic or radiographic confirmation of tumor progression following standard front-line therapy consisting of external beam radiation therapy and temozolomide. Patients were also required to be ≥18 years of age, have a Karnofsky performance status (KPS) of ≥60, have satisfactory hematologic (hemoglobin level, ≥10 g/dL; absolute neutrophil count, ≥1500 cells/µL; platelet count, ≥100 000 cells/µL) and biochemical results (serum creatinine level, ≤1.5 mg/dL; aspartate aminotransferase and alanine transaminase levels, <2.5 times the upper limit of normal; bilirubin level, ≤1.6 mg/dL). For patients not receiving stable anticoagulation, prothrombin time must have been within normal limits, whereas patients receiving full-dose anticoagulants (eg, warfarin or low molecular weight heparin) must have had no active bleeding or pathological condition that carried a high risk of bleeding and in-range international normalized ratio on a stable dose of oral anticoagulant or low molecular weight heparin. Patients receiving cytochrome P450 enzyme-inducing anti-epileptics were not included; patients previously receiving these agents must have discontinued their use at least 2 weeks prior to registration. Patient could not have undergone >3 prior therapies, and any prior therapy consisting of bevacizumab or irinotecan were not allowed. Patients could not have been previously treated with any other HDAC inhibitors (other than valproic acid for management of seizures). If previously treated with valproic acid as treatment for seizures, the drug was stopped at least 30 days before exposure to vorinostat. A minimum of 28 days had to elapse from any previous surgery or cytotoxic therapy. Patients were excluded from this study if they demonstrated evidence of acute intratumoral hemorrhage on imaging or any severe, active comorbidities, including transmural myocardial infarction, unstable angina, or stroke within 6 months. Pregnant or nursing women were ineligible. All patients gave written informed consents with the approval of our institutional review board.
Treatment Design
The planned vorinostat dose-escalation schema is depicted in Table 1, with patients receiving vorinostat combined with bevacizumab (10 mg/kg intravenously [IV] on days 1 and 15 every 28 days) and CPT-11 (125 mg/m2 IV on days 1 and 15 every28 days). A 3 + 3 dose-escalation design was used. If a dose-limiting toxicity (DLT) occurred in 1 of 3 patients, the group was expanded to 6 patients. If no further toxicity was seen, 3 patients were enrolled at the next dose level. Maximum tolerated dose (MTD) was defined as the dose level below the level in which ≥2 DLTs occurred. A minimum of 6 patients were treated at the MTD. In the initial protocol, the highest planned dose level of vorinostat to be examined was 400 mg daily on days 1–7 and 15–21 every 28 days (dose level 3A). However, on the basis of the poor long-term patient tolerability of the initial arms of this trial, which involved a 7 day-on/7 day-off vorinostat schedule, the protocol was amended to include an alternate 3-day, twice daily vorinostat dosing regimen that is also actively being investigated in ongoing clinical trials (dose level 3B; 300 mg twice daily on days 1–3 and 15–17 every 28 days) in an effort to improve long-term tolerability.
Table 1.
Dose-escalation schema
| Dose Level | Vorinostat + CPT-11 (125 mg/m2 IV days 1 and 15 q28 days) + Bevacizumab (10 mg/kg IV) days 1 and 15 q28 days) |
|---|---|
| 1 | 200 mg d 1–7 and 15–21 q28d |
| 2 | 300 mg d 1–7 and 15–21 q28d |
| 3A | 400 mg d 1–7 and 15–21 q28d |
| 3B | 300 mg BID d 1–3 and 15–17 q28d |
Assessment of Toxicity
Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, over each 28-day cycle. A DLT was defined as any drug-related CTCAE grade 3 or 4 nonhematologic event except alopecia and manageable nausea/vomiting and fatigue and any CTCAE grade 4 hematologic event excluding neutropenia and thrombocytopenia other than (1) febrile neutropenia defined as grade 3–4 neutropenia with fever (temperature, ≥38.5°C) and/or infection, (2) any grade 4 neutropenia lasting ≥5 days, (3) grade 4 thrombocytopenia or platelet count <25 000 cells/μL of any duration, and (4) failure of absolute neutrophil count to recover to ≥1000 cells/μL or platelets to recover to ≥50 000 cells/μL within 14 days of holding therapy. Because venous thromboembolic disease is a common complication occurring in a large proportion of patients with glioblastoma, grade 3 deep vein thrombosis was not considered a DLT. Observation for DLT was performed during cycle 1 for a minimum period of 28 days.
Plasma Biomarkers
Plasma was isolated from whole blood from 10 patients enrolled in dose levels 3A and 3B. Samples were obtained pretreatment and serially every other cycle until tumor progression. Expression profiles of serum were analyzed using a customized Human Antibody Array (RayBiotech). Plasma samples were collected and stored at −80°C. For the experiment, samples were diluted 2-fold with the 1X blocking buffer. The glass chip was assembled into an incubation chamber to create a containment well for each subarray in the slide. Blocking was done at 4°C overnight prior to the addition of plasma. After overnight incubation, the slides were washed, incubated in biotin conjugated antibodies overnight, washed, and stained with Alexa Flour 555 conjugated streptavidin. The microarray slide was then disassembled, washed, centrifuged, and allowed to air dry. Scanning was done with an Axon GenePix scanner. Mean signal intensities obtained from the laser scanner were background subtracted and normalized with positive, negative, and internal controls. Signal intensities of the prestudy samples were compared with the median signal values of all the prestudy samples, and the log2 ratio was used to draw the heat map. Posttreatment sample signal intensities were compared with the respective prestudy signal values (Supplementary material, Fig. S1), and the log2 ratio was used to draw the heat map.
Statistics
Clinical, demographic, and treatment characteristics were summarized using descriptive statistics. For continuous variables, such as age and survival months, mean, median and standard deviation were calculated. For sex, histology, and treatment information, frequency and percentage were presented. Estimates of overall and progression-free survival were evaluated using the Kaplan-Meier product limit method and compared using a Wilcoxon log-rank test. All analyses were performed using SAS, version 9.1.3 (SAS Institute). The statistical analysis for the proteomic profiles was done using a Student's t test.
Results
Twenty patients consented to this study, of which 19 were treated, with 1 patient being deemed ineligible because of rapid clinical progression prior to starting therapy. Patient demographic characteristics are shown in Table 2. Of the 19 enrolled patients, 2 had gliosarcoma. The majority of patients had a good performance status, a majority of whom had KPS ≥90. Of the enrolled patients, 5 of 19 had a complete gross total resection for their recurrent disease prior to enrollment, and a majority of patients received additional chemotherapy beyond temozolomide. As of May 6, 2011, 3 patients continued to receive treatment in the study, currently in cycles 7 and 10 (dose level 3B) and cycle 22 (dose level 3A).
Table 2.
Patient characteristics
| Parameter | Dose Level 1–2 | Dose Level 3A and 3B | All |
|---|---|---|---|
| No. of patients | 7 | 12 | 19 |
| Median age ± Standard Deviation (range) | 55.43 ± 7.96 (42–66) | 49.42 ± 14.11 (21–74) | 51.63 ± 12.32 (21–74) |
| Gender | |||
| Male | 4 (57%) | 8 (67%) | 12 (63%) |
| Female | 3 (43%) | 4 (33%) | 7 (37%) |
| Histology | |||
| Glioblastoma | 7 (100%) | 10 (83%) | 17 (89%) |
| Gliosarcoma | 0 (0%) | 2 (17%) | 2 (11%) |
| KPS | |||
| 90–100 | 2 (29%) | 8 (67%) | 10 (53%) |
| 70–80 | 5 (71%) | 4 (33%) | 9 (47%) |
| 60 | 0 (0%) | 0 (0%) | 0 (0%) |
| Surgery | |||
| GTR | 2 (33%) | 3 (25%) | 5 (28%) |
| None | 4 (67%) | 9 (75%) | 13 (72%) |
| Prior chemotherapeutic agents | |||
| 1 | 2 (29%) | 6 (50%) | 8 (42%) |
| 2 | 5 (71%) | 3 (25%) | 8 (42%) |
| 3 | 0 (0%) | 3 (25%) | 3 (16%) |
Toxicities
A summary of grade 3 and 4 toxicities potentially related to the treatment is shown in Table 3. The MTD of vorinostat, when combined with bevacizumab and irinotecan, was defined at 400 mg daily on days 1–7 and 15–21 every 28 days (dose level 3A). No patients experienced DLTs in dose levels 1 and 2. However, 2 patients in dose level 2 exited the study because of toxicities soon after evaluation for DLTs (during cycles 2 and 3) for severe mucositis and fatigue/diarrhea, respectively. One of the 6 patients enrolled in dose level 3A experienced a DLT. This was a patient who experienced unresolved, alternating hypertension with orthostatic hypotension during his treatment course. His symptoms resolved after trial discontinuation, although reoccurred when he continued treatment with bevacizumab alone, making the contribution of vorinostat and/or CPT-11 with these symptoms unclear. Similar to dose level 2, 3 of the 5 remaining patients in dose level 3A required considerable CPT-11 dose reductions, with no patient tolerating >5 cycles without a dose reduction. One patient with recurrent gliosarcoma had an initial CPT-11 dose reduction during his third cycle and was eventually discontinued after 8 cycles because of persistent nausea, vomiting, and diarrhea. All of these symptoms had resolved following discontinuation of CPT-11, and this patient remained in study cycle 22. Two patients experienced a DLT in dose level 3B. One patient had intractable stomach pain and diarrhea, which improved following discontinuation of CPT-11. This patient also required a dose reduction of vorinostat after 5 cycles for a rash that developed in his hands bilaterally, lower abdomen, and groin; the rash improved after dose reduction. He remained in the study, having completed 10 cycles. The remaining DLT was observed in a patient who developed an acute infarct in the left basal ganglia ∼1 week following his first cycle. As observed in dose levels 2 and 3A, significant dose reductions of CPT-11 were also required in 3 of the 4 remaining patients in dose level 3B, with no patient tolerating this regimen without a dose reduction for >3 cycles.
Table 3.
Hematologic and nonhematologic toxicities occurring during any treatment coursea
| Nature | Hematologic toxicities (events/patients) |
|
|---|---|---|
| Grade 3 | Grade 4 | |
| Neutropenia | 1/1 | |
| Nature | Nonhematologic toxicities (events/patients) | |
| Grade 3 | Grade 4 | |
| Diarrhea | 3/3 | |
| Mucositis | 1/1 | |
| Stomach pain | 1/1b | |
| Fatigue | 4/4 | 1/1 |
| CNS ischemia | 1/1 | 1/1b |
| Hypertension/hypotension | 1/1b | |
| Sensory neuropathy | 1/1 | |
aOnly those toxicities deemed possibly, probably, or definitely related to the treatment are included in the table.
bDose-limiting toxicity.
Outcome
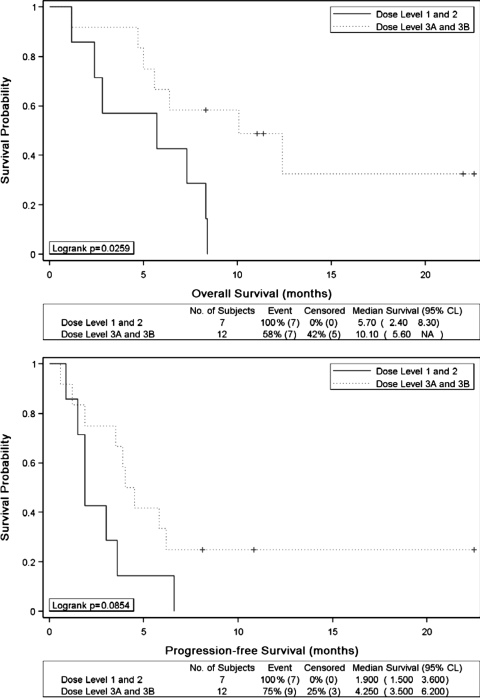
The median PFS and overall survival in all patients were 3.6 months and 7.3 months, respectively. When analyzed by dose levels, patients receiving lower doses of vorinostat (dose levels 1 and 2) had a median PFS of 1.9 months (range, 0.9–6.6 months) and OS of 5.7 months (range, 1.2–8.4 months). Patients who were still alive and/or free from disease progression at the time of data analysis were considered as censored; there were no patients lost to follow-up. Kaplan-Meier analysis showed that patients who received higher doses of vorinostat (dose levels 3A and 3B) had a significantly better OS when compared with patients who received lower doses (median survival, 10.1 vs 5.7 months; log rank P = .026) (Fig. 1A). The median PFS among patients receiving higher dose vorinostat had an improved response, although not statistically significant, when compared with that among patients receiving lower doses (4.25 vs 1.9 months; log rank P = .085) (Fig. 1B). Of note, the 2 patients with recurrent gliosarcoma enrolled in this trial had a PFS ≥6 months with this regimen (dose levels 3A and 3B). In addition, of the 12 patients enrolled in dose levels 3A and 3B, the 3 patients who had a GTR or near GTR prior to trial entry remained alive at the time of this submission, with 2 patients being 22 months and the third at 10 months from trial entry.
Fig. 1.
Kaplan–Meier estimates for overall survival (A) and progression-free survival (B) stratified by vorinostat dose levels.
Plasma Biomarkers
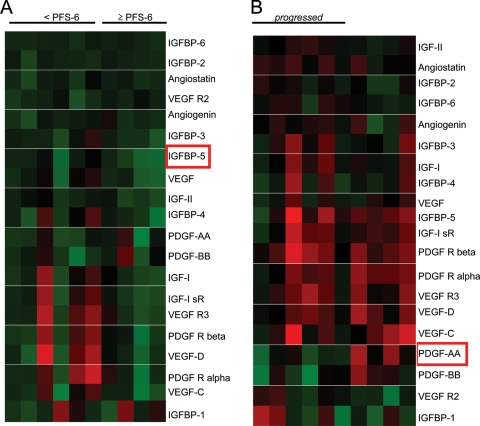
Because obtaining tissue for correlative studies is often limited in the context of recurrent glioblastoma, we performed an initial investigation to apply a proteomics-based platform to determine the potential of serial plasma to serve as a biomarker for an angiogenesis-based regimen in this malignancy. With use of a customized antibody array evaluating proteins involved in glioblastoma recurrence and angiogenesis, serial plasma samples from 10 patients treated at dose levels 3A and 3B were evaluated. We initially evaluated the capacity of the pretreatment plasma expression of these proteins in predicting response to this regimen, as measured by PFS-6. Of the 20 proteins tested, decreased pretreatment expression IGFBP-5 was statistically significant in predicting improved PFS (range, 1.00–2.55-fold reduction; P = .022) (Fig. 2A). We then explored the potential of using serial plasma samples as a surrogate for changes in tumor biology associated with radiographic recurrence. Of the 20 proteins tested by comparing pretreatment proteomic profiles with those obtained after recurrence, decreasing plasma levels of PDGF-AA was statistically significant in predicting tumor recurrence (P = .015) (Fig. 2B). Specifically, of the 7 patients demonstrating progression, 5 exhibited decreases in plasma PDGF-AA levels (range, 1.47–2.94-fold reduction). Of interest, of the 2 patients who did not demonstrate a decrease in plasma expression of PDGF-AA, 1 patient discontinued the trial because of clinical progression (an MRI confirming progression was not obtained) and the second patient continues to be a long-term survivor, almost 2 years since trial enrollment. The 3 patients who remained in the study with stable disease did not demonstrate a decrease in plasma PDGF-AA through serial evaluation.
Fig. 2.
Plasma Proteomic Profiles. (A) A heat map was generated to represent relative expression of plasma proteins from pretreatment samples. Ratios were normalized to the median value of the entire cohort and color intensity was assigned to ratios of protein expression; shades of red, proteins that are upregulated; shades of green, proteins that are downregulated; black, proteins that are unchanged. (B) Ratios of plasma protein expression following recurrence (or last available plasma sample for patients currently on study) were normalized to their pretreatment values.
Discussion
Bevacizumab alone or in combination with irinotecan has been adopted as a standard therapy for recurrent glioblastoma. Despite dramatic response rates, the capacity of this regimen to improve survival in this patient population remains unclear, and salvage therapies after recurrence are generally ineffective.8 The HDAC inhibitor vorinostat has been recently approved for the treatment of cutaneous T-cell lymphoma9 and is actively being investigated in solid tumors, with demonstrated activity in glioblastoma.10 In addition to independent activity, preclinical data have demonstrated the capacity of this class of agents to enhance the antitumor activity of both topoisomerase and angiogenesis inhibitors.11–16 Therefore, based on this underlying rationale, we conducted a phase I trial to determine the safety of combining the HDAC inhibitor vorinostat with bevacizumab and CPT-11 to further clinical gains in recurrent glioblastoma. During the defined evaluation period for DLTs, this regimen was well tolerated in dose levels 1–3, with patients experiencing toxicities typically attributed to bevacizumab and CPT-11; however, frequent CPT-11 dose reductions were required in subsequent cycles. In addition, all of the patients who had a durable response to therapy discontinued CPT-11 early in the course of their treatment. Therefore, based on both the cumulative toxicity associated with CPT-11 and its unclear efficacy in glioblastoma,4 a more promising strategy for future investigation would be to combine vorinostat with bevacizumab alone in recurrent glioblastoma.
Although any conclusions involving the efficacy of this combination are clearly limited by the small sample size and the dose escalation design, when compared with patients receiving low-dose vorinostat (dose levels 1 and 2), patients receiving more biologically relevant doses of vorinostat (dose levels 3A and 3B)9 demonstrated both improved survival and PFS, with 2 patients remaining alive nearly 2 years after enrollment, suggesting the activity of this agent. However, of note, dose levels 3A and 3B had a higher proportion of patients with a KPS of 90–100, which is a clear prognostic factor in this disease. Of interest, the 2 patients with recurrent gliosarcoma did relatively well, 1 of whom being a long-term survivor, free of disease recurrence for nearly 2 years since enrollment and still in the study. In addition, patients undergoing a surgical resection (GTR or near GTR) appeared to respond particularly well to this regimen. It is tempting to speculate that, rather than the direct inhibition of tumor-associated neoangiogenesis typically associated with these bulky, highly vascular tumors, a specific patient population that may render benefit from an angiogenesis inhibitor-based regimen are those with minimal tumor burden, where these agents may disrupt the residual glioma stem cell niche critical for the maintenance of this population of these cells purported to be involved in therapeutic resistance.17 Clearly, more work is required to better understand and identify predictive factors for this target-based regimen.
Based on the difficulty in obtaining tissue for correlative studies in recurrent glioblastoma, we explored the potential of using a proteomics-based approach to evaluate serial plasma samples from patients undergoing treatment to provide insight into factors predicting response to therapy and potential changes in tumor biology associated with disease recurrence. As an initial investigation, we generated a customized array focused on proteins involved in tumor angiogenesis and insulin growth factor signaling. Insulin growth factor signaling was chosen on the basis of both its central role in glioblastoma biology18–23 and recent reports demonstrating its prognostic value when analyzed in plasma samples from patients with both glioblastoma19,24,25 and prostate cancer.26 Specific to glioblastoma, plasma IGF-related proteins have been shown to be predictive of grade, local recurrence, disease-free survival,24 and PTEN-null tumors.19 Because this was a pilot study involving only 10 patients, limiting any rigorous statistical analyses, our results could clearly be observational in nature rather than describing a specific biologic phenomenon; therefore, these findings warrant further investigation. Acknowledging these inherent limitations, we identified that decreased pretreatment levels of plasma IGFBP-5 predicted patients with a PFS ≥6 months (P = .02). Although clearly further studies are required in larger patient samples to determine its relevance as a predictive biomarker or prognostic factor, of note, IGFBP-5 has been demonstrated to predict responsiveness in breast and ovarian cancer27,28 and demonstrated to play a role in tumor angiogenesis,29 making IGFBP-5 an interesting determinant of response for a regimen targeting tumor vasculature.
In addition to biomarker development, we profiled serial plasma samples obtained from patients enrolled on this study as an initial investigation into providing insight into changes in tumor biology underlying therapeutic resistance. By comparing pretreatment proteomic profiles with profiles obtained following recurrence, decreasing plasma levels of PDGF-AA emerged as a statistically significant predictor of tumor recurrence (P = .015). The central role the PDGF pathway plays in both glioblastoma biology30–32 and angiogenesis33–36 has been extensively studied. Because this pathway has typically been associated with proliferative pathways and VEGF-independent angiogenesis, it remains unclear why decreased expression of PDGF-AA was associated with tumor progression. However, recent studies have identified that, unlike β-PDGFR signaling, α-PDGFR, which represent the specific receptor of PDGF-AA, has the unique capacity of both agonistic and antagonistic activities for cell growth and motility.32,37 Therefore, on the basis of the intimate cross-talk and coregulation between VEGF and PDGF signaling,35,36 it can be hypothesized that continued VEGF inhibition may modulate PDGF-AA expression through regulatory feedback inhibition, thereby attenuating inhibitory signaling contributing towards VEGF-independent progression. Further work is required to support these observations by expanding to a larger patient cohort and validating at the cellular level; however, these findings may offer insight into the underlying biology of acquired resistance to bevacizumab-based regimens in glioblastoma and direction for future therapeutic regimens.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Conflict of interest statement. P. C. is currently funded by Merck Research Laboratories, The Ben and Catherine Ivy Foundation, The American Cancer Society (RSG-11-029-01-CSM), and the US Army (W81XWH-08-2-0101). All other authors: no conflicts.
Funding
This work was supported by the Merck Investigator-Initiated Studies Program.
Supplementary Material
Acknowledgments
We would like to thank Pam Smith, with the Clinical Research Unit at the H. Lee Moffitt Cancer Center, and John Schriner, with Pharm D, for their continued efforts contributing toward the successful completion of this trial.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England Journal of Medicine. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. doi:10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 6.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. Journal of Neuro-oncology. 2009;92(2):149–155. doi: 10.1007/s11060-008-9745-8. doi:10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 8.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5(11):610–620. doi: 10.1038/nrneurol.2009.159. doi:10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 9.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109(1):31–39. doi: 10.1182/blood-2006-06-025999. doi:10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. doi:10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12(2):634–642. doi: 10.1158/1078-0432.CCR-05-1132. doi:10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 12.Qian DZ, Wang X, Kachhap SK, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Research. 2004;64(18):6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. doi:10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 13.Bevins RL, Zimmer SG. It's about time: scheduling alters effect of histone deacetylase inhibitors on camptothecin-treated cells. Cancer Research. 2005;65(15):6957–6966. doi: 10.1158/0008-5472.CAN-05-0836. doi:10.1158/0008-5472.CAN-05-0836. [DOI] [PubMed] [Google Scholar]
- 14.Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. Valproic acid alters chromatin structure by regulation of chromatin modulation proteins. Cancer Research. 2005;65(9):3815–3822. doi: 10.1158/0008-5472.CAN-04-2478. doi:10.1158/0008-5472.CAN-04-2478. [DOI] [PubMed] [Google Scholar]
- 15.Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. In vivo synergy between topoisomerase II and histone deacetylase inhibitors: predictive correlates. Molecular Cancer Therapeutics. 2005;4(12):1993–2000. doi: 10.1158/1535-7163.MCT-05-0194. doi:10.1158/1535-7163.MCT-05-0194. [DOI] [PubMed] [Google Scholar]
- 16.Sarcar B, Kahali S, Chinnaiyan P. Vorinostat enhances the cytotoxic effects of the topoisomerase I inhibitor SN38 in glioblastoma cell lines. Journal of Neuro-oncology. 2010;99(2):201–207. doi: 10.1007/s11060-010-0127-7. doi:10.1007/s11060-010-0127-7. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. doi:10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Dunlap SM, Celestino J, Wang H, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11736–11741. doi: 10.1073/pnas.0703145104. doi:10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrian-Shai R, Chen CD, Shi T, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5563–5568. doi: 10.1073/pnas.0609139104. doi:10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore LM, Holmes KM, Smith SM, et al. IGFBP2 is a candidate biomarker for Ink4a-Arf status and a therapeutic target for high-grade gliomas. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16675–16679. doi: 10.1073/pnas.0900807106. doi:10.1073/pnas.0900807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santosh V, Arivazhagan A, Sreekanthreddy P, et al. Grade-specific expression of insulin-like growth factor-binding proteins-2, -3, and -5 in astrocytomas: IGFBP-3 emerges as a strong predictor of survival in patients with newly diagnosed glioblastoma. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1399–1408. doi: 10.1158/1055-9965.EPI-09-1213. doi:10.1158/1055-9965.EPI-09-1213. [DOI] [PubMed] [Google Scholar]
- 22.Soroceanu L, Kharbanda S, Chen R, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3466–3471. doi: 10.1073/pnas.0611271104. doi:10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Wang H, Shen W, et al. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Research. 2003;63(15):4315–4321. [PubMed] [Google Scholar]
- 24.Lin Y, Jiang T, Zhou K, et al. Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro-oncology. 2009;11(5):468–476. doi: 10.1215/15228517-2008-114. doi:10.1215/15228517-2008-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreekanthreddy P, Srinivasan H, Kumar DM, et al. Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1409–1422. doi: 10.1158/1055-9965.EPI-09-1077. doi:10.1158/1055-9965.EPI-09-1077. [DOI] [PubMed] [Google Scholar]
- 26.Shariat SF, Lamb DJ, Kattan MW, et al. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20(3):833–841. doi: 10.1200/JCO.2002.20.3.833. doi:10.1200/JCO.20.3.833. [DOI] [PubMed] [Google Scholar]
- 27.Ahn BY, Elwi AN, Lee B, et al. Genetic screen identifies insulin-like growth factor binding protein 5 as a modulator of tamoxifen resistance in breast cancer. Cancer Research. 2010;70(8):3013–3019. doi: 10.1158/0008-5472.CAN-09-3108. doi:10.1158/0008-5472.CAN-09-3108. [DOI] [PubMed] [Google Scholar]
- 28.Walker G, MacLeod K, Williams AR, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res. 2007;13(5):1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. doi:10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- 29.Rho SB, Dong SM, Kang S, et al. Insulin-like growth factor-binding protein-5 (IGFBP-5) acts as a tumor suppressor by inhibiting angiogenesis. Carcinogenesis. 2008;29(11):2106–2111. doi: 10.1093/carcin/bgn206. doi:10.1093/carcin/bgn206. [DOI] [PubMed] [Google Scholar]
- 30.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. Journal of Neuro-oncology. 2000;50(1–2):121–137. doi: 10.1023/a:1006436624862. doi:10.1023/A:1006436624862. [DOI] [PubMed] [Google Scholar]
- 31.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Research. 2004;64(14):4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. doi:10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. Journal of Biochemistry and Molecular Biology. 2003;36(1):49–59. doi: 10.5483/bmbrep.2003.36.1.049. doi:10.5483/BMBRep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 33.Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. doi:10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. The EMBO Journal. 2004;23(14):2800–2810. doi: 10.1038/sj.emboj.7600289. doi:10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg JI, Shields DJ, Barillas SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456(7223):809–813. doi: 10.1038/nature07424. doi:10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shikada Y, Yonemitsu Y, Koga T, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Research. 2005;65(16):7241–7248. doi: 10.1158/0008-5472.CAN-04-4171. doi:10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 37.Faraone D, Aguzzi MS, Toietta G, et al. Platelet-derived growth factor-receptor alpha strongly inhibits melanoma growth in vitro and in vivo. Neoplasia. 2009;11(8):732–742. doi: 10.1593/neo.09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.