Abstract
In this contribution, we present a system for efficient preconcentration of pathogens without affecting their viability. Development of miniaturized molecular diagnostic kits requires concentration of the sample, molecule extraction, amplification, and detection. In consequence of low analyte concentrations in real-world samples, preconcentration is a critical step within this workflow. Bacteria and viruses exhibit a negative surface charge and thus can be electrophoretically captured from a continuous flow. The concept of phaseguides was applied to define gel membranes, which enable effective and reversible collection of the target species. E. coli of the strains XL1-blue and K12 were used to evaluate the performance of the device. By suppression of the electroosmotic flow both strains were captured with efficiencies of up to 99%. At a continuous flow of 15 μl/min concentration factors of 50.17 ± 2.23 and 47.36 ± 1.72 were achieved in less than 27 min for XL1-blue and K12, respectively. These results indicate that free flow electrophoresis enables efficient concentration of bacteria and the presented device can contribute to rapid analyses of swab-derived samples.
INTRODUCTION
Miniaturized diagnostic systems for analysis of infectious pathogens have been widely investigated over the last two decades.1, 2 Amplification-based systems such as real-time Polymerase chain reaction (PCR) have been in the focus of this research. For applications at point of care or resource poor environments automated and easy to handle sample preparation techniques have to be developed. Sample preparation for nucleic acid testing includes concentration of the pathogens and extraction of nucleic acids. Commonly applied procedures for nucleic acid extraction require a large number of reagents and preparation steps, limiting applications in rapid pathogen testing.3 Recently, our group presented a microfluidic chip for lysis of target species and isolation of RNA.4 Due to the small volumes of such devices and the low analyte concentration of real-world samples, a preconcentration step is required. Conventional concentration techniques are based on centrifugation, membrane filtering, or capturing by functionalized magnetic beads.5 For the success of microfluidic diagnositic platforms automated, easy to handle and readily combinable functional units have to be developed.6 The three main concepts for chip-based pathogen concentration are physical trapping, functionalized particles, and electrokinetic techniques.2 Physical traps for bacteria are fabricated by shallow channels or arrays of microbeads.7, 8 Although these devices are simple, clogging, and capturing of small pathogens such as viruses are major difficulties. Antibody-coated particles have been used to selectively bind to the target species. These particles are trapped in microchannels by physical barrieres9 or magnetic fields.10, 11, 12 The capture efficiency strongly depends on the quality of the coatings and proper mixing of particles and analytes. For a successful integration and an increased capture efficiency, further advances in bead modification and controllability of the magnetic field have to be made.13, 14
The electrokinetic principles, dielectrophoresis (DEP) and electrophoresis have the advantage to be electrically controllable and easy to integrate.6 Both methods depend on the conductivity of the liquid medium. Hence, analysis of swab-derived pathogens, transferred to the medium is a preferable application to direct use of physiological samples. Swabs are the most common method for identification of wound infections in clinical diagnostics.15 Other applications of swabs are the detection of respiratory infections,16 and contaminated food and environmental surfaces.17
Dielectrophoresis has widely been used for preconcentration and separation of cells and bacteria.18 Many of them direct cells to certain positions within a prefilled chip19, 20, 21, 22, 23 rather than reducing the volume. A number of DEP traps and separators have been optimized for continuous operation by careful scaling of the device and electrode dimensions to the analyte, as the dielectrophoretic force depends on the volume of the biological particles.24, 25, 26, 27, 28, 29, 30 As analyzed by Kuczenski et al.26 the high field strengths required to manipulate bacteria can strongly affect their viability. In addition, high fields induce disturbing effects such as electrothermal flow and AC electroosmosis.22, 31, 32 Bacteria and viruses exhibit a negative charge at physiological pH values. Therefore, electrophoretic concentration has the advantage to be universally applicable for a wide range of pathogens. Free flow electrophoresis (FFE) including isoelectric focusing (IEF) and zone electrophoresis (ZE) has been used to separate biological particles33, 34, 35, 36 but has received considerably less attention for the concentration of bacteria or viruses. To date, only two groups have reported on devices for electrophoretic concentration: Yager et al.37, 38 presented microfluidic devices for continuous concentration and capture of bacteria. Balasubramanian et al.39 demonstrated the on-chip capture of bacteria and viruses from water.
Previously, our group developed a device for the concentration of bacteria. A factor of 17.76 for gram positive bacteria was achieved within 30 min. Besides the throughput also the capture efficiency was still unsatisfyingly at around 80%.40 In this contribution, we analyze the influencing factors and present an optimized device and method for concentration of bacteria by means of FFE. A highly efficient but gentle concentration of gram negative bacteria, which are far more sensitive to lysis is demonstrated. Together with the earlier developed chip for lysis and nucleic acid isolation4 the presented method can lead to rapid analysis of swab-derived pathogens.
THEORY
Bacterial cells exhibit a negative surface charge due to ionized carboxylate and phosphoryl groups and therefore experience a force in an electric field. The resulting migration velocity is proportional to the electric field,
| (1) |
where μ is defined as the electrophoretic mobility (EM). Electrophoretic mobilities of bacteria are typically in the range of −1 to −4 × 10−8 m2/Vs and are highest at low ionic strength of the medium.41, 42, 43 The pH has a minor influence above a value of 6 where mobilities are almost constant.44
The major side-effect, influencing the motion of bacteria is due to electroosmotic flow (EOF). The electroosmotic flow is caused by the interaction of an electric field and the double layer formed at the liquid/glass interface. The high surface-to-volume ratio in microfluidic channels causes EOF to have a major influence on micro-electrophoretic techniques. The electric field moves the liquid boundary layer towards the cathode and drags the bulk fluid along. Since the channel is closed in the direction of the electric field a circular flow develops that interferes the electrophoretic migration of bacteria. Cationic surfactants have been used to suppress EOF.37 As these surfactants are known biocides they have to be avoided to ensure high bacterial yield.
Polymeric additives, such as hydroxypropylmethylcellulose (HPMC) can be used to suppress electroosmotic flow. HPMC acts as a dynamic coating, increasing the viscosity within the double layer and reducing the surface charge density at the channel wall. By addition of 0.1% w/w methylcellulose Cui et al. have shown a reduction of EOF by a factor of 10.45
DESIGN
Formation of bubbles at the electrodes due to electrolysis, and as a consequence the unstable performance, is one of the main limiting factors of μFFE. To overcome this problem a variety of strategies have been developed, including different buffer additives, membrane barriers, or multiple depths designs.46, 47 In the present device, the concept of phaseguides is used to prevent bubbles from blocking the current paths and to enable controlled filling of the chip. Phaseguides are stripes of resist within the channel to control an advancing liquid-air meniscus.48 Differences in geometry or material cause a change in capillary pressure. A liquid meniscus reaching a phaseguide is pinned on the phaseguide and tends to advance along the boundary.49 A controlled overflow can be achieved by introducing angles smaller than the critical angle according to the Concus-Finn condition, αcrit = π − 2θ, where θ is the contact angle of the advancing liquid.50
The phaseguides at the electrodes act as a pressure barrier, preventing gas bubbles to cross (Fig. 1). Furthermore, for a bubble that develops between two triangles, the pressure drop across the larger liquid/gas interface is lower than across the smaller surface. Thus, the bubble tends to grow towards the open end of the triangles and leaves the channel through the venting hole.
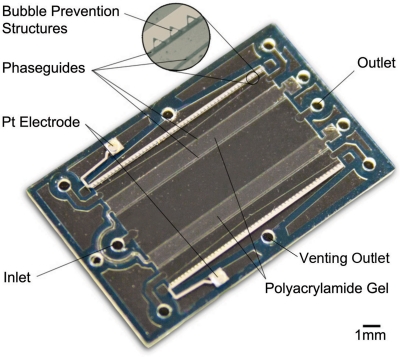
Figure 1.
Photograph of the microfluidic chip: Phaseguides are used to ensure controlled filling of polyarcylamide gel. Triangular structures are placed on top of the electrodes to expulse bubbles to the venting outlet. Device dimensions are 14 mm × 22 mm with 120μm channel height. The concentration channel, defined by gel barriers measures 3.5 mm × 15 mm.
Polyacrylamide gel is introduced via phaseguides to define the concentration channel. The nanoporous gel polymerizes without shrinking. It is used to collect the target species but allow electrical current to flow.
MATERIAL AND METHODS
Finite element simulations
To optimize the parameters of the concentration method, bacterial trajectories were calculated by finite element simulations in COMSOL Multiphysics and matlab. The stationary velocity of the pressure driven flow was evaluated by a 3-dimensional model of the microfluidic channel. For the calculation of the electric field distribution and the corresponding electroosmotic flow a 2D model was sufficient because the field is uniform along the channel. The simulation results were used in a matlab script to calculate the resulting bacteria motion by summing up the pressure driven flow, EOF and the electrophoretic migration at discrete time steps. Simulation parameters were matched to the experimental settings. The zeta potential of glass with 1 mM sodium borate, ζ = −75 mV was taken from Lee et al.51
Chip fabrication
Two 4-in., 500 μm thick glass wafers were used as the base material of the devices. Platinum electrodes were structured on the bottom substrate by a standard lift-off process. Holes for fluidic access were drilled through the top substrate. The microfluidic structures of the device were formed within a dry film resist layer (Ordyl SY330). The resist with a thickness of 30 μm was laminated onto one substrate in multiple layers at 95 °C and photolithographically patterned. Two layers were laminated and exposed to obtain phaseguides of 60 μm height. Another two layers were added to form the channels and the bubble expulsion structures of a total height of 120 μm (Fig. 1). After resist development, the top substrate was bonded at a pressure of 60 N/cm2 and a temperature of 95 °C without the need of further adhesives. For details of dry film resist chip fabrication refer to Vulto et al.52 Finally, the bonded wafers were diced into single devices of 14 mm × 22 mm dimensions.
After fabrication, a 16% polyacrylamide gel (40% Acrylamide/Bisacrylamide 19:1, sodium borate) was filled into the chips. The gel was crosslinked in a nitrogen-flooded chamber and the devices were filled with sodium borate (SB) medium to avoid dehydration of the gel until use.
Sample preparation
Two different strains of E.coli, namely, K12 and XL1-blue were used as model organisms. K12 cultures were grown overnight for 14 h to 16 h in lysogeny browth (LB) medium at 37 °C in a shaking incubator. To receive log-phase bacteria, 5 μl of the culture were transferred to 5 ml of fresh LB medium and incubated for 2.5 h. For better insight into the concentration and resuspension process green fluorescent bacteria were utilized. XL1-blue cells were transformed with pBAD vector harbouring genes for expression of green fluorescent protein (GFP) and ampicillin resistance. Since GFP expression induced by arabinose takes between 8 and 24 h, it is not possible to receive log-phase fluorescent bacteria. Therefore, the XL1-blue cells were grown on LB agar with 0.5% w/w arabinose and 50 μg/ml ampicillin.
The samples were suspended in SB, which is proposed by Brody and Kern53 to be a superior electrophoresis medium. Gram-negative bacteria exposed to Ethylenediaminetetraacetic acid (EDTA) shows an enhanced membrane leakage giving rise to bacterial death rate.54 Therefore, SB medium appears to be more suitable for bacterial concentration experiments. The solution consisted of 1 mM sodium tetraborate. The pH was adjusted to 8.5 by addition of boric acid. The final conductivity was adjusted to σSB = 250 μS/cm. In addition, hydroxypropyl methylcellulose was added in a concentration of 0.1% w/w to effectively suppress electroosmotic flow. Prior the experiments, the bacteria were washed in SB and diluted to a final concentration of about 5 × 104 CFU/ml. This concentration was the minimum to have sufficient bacteria in the waste for enumeration.
Experimental setup
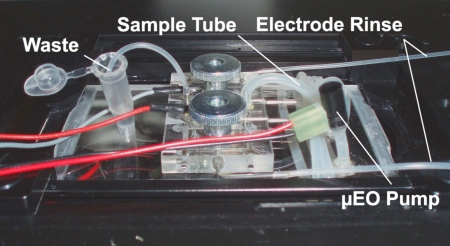
For fluidic and electrical access, the chip was placed into an acrylic holder. The setup was placed on a microscope table to follow the experiments (Fig. 2). As part of the planned construction of a portable system a miniaturized electroosmotic pump (Nano Fusion Technologies) was used in this study. Since the working liquid of the pump had to be de-ionized water, the sample was introduced into a tube and pumped indirectly through the chip. The concentration experiments were conducted at the maximum flow rate of the electroosmotic pump of 15 μl/min.
Figure 2.
Experimental Setup: Chip in acrylic holder, sealed by two knurled screws. An electroosmotic pump (Nano Fusion Technologies) drives the sample into the chip. The electrode chambers are hydrostatically flushed with medium.
To avoid electrolyte exhaustion, the electrode chambers were rinsed with fresh buffer. A constant current was applied to the electrodes to maintain a stable electric field and a constant migration velocity throughout the experiments. The maximum concentration factor of 61.54 was determined by the used sample volume of 400 μl and the chip volume of 6.5 μl. With the flow rate of 15 μl/min, the experiments lasted approximately 27 min. The drop plate method was used for enumeration because of less time and material needed compared with the spread plate method.55 The sample reference was plated prior the experiments to avoid overestimation of the concentration factor due to lysis during the experiments. 100 μl of the sample (=Volref) were 10-fold serially diluted in LB medium and 16 drops of 10 μl of each dilution step were plated for enumeration. After collecting the bacteria at the gel, a negative current was applied to resuspend them to the medium. The chip volume was emptied with a pipette, transferred to 993.5μl LB, serially diluted and plated. The waste was plated without dilution to receive sufficient colony forming units (CFU). After incubating the agar plates overnight, the CFU were counted and the capture efficiency C, concentration factor fc, and recovery rate R were calculated as follows:
| (2a) |
| (2b) |
| (2c) |
RESULTS AND DISCUSSION
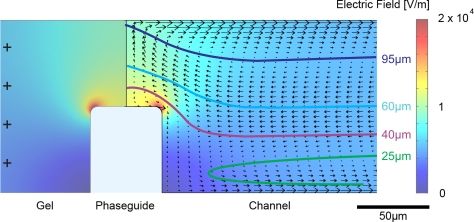
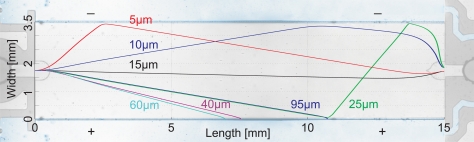
Results of the finite element simulations of the concentration process are shown in Figs. 34. The surface plot in Fig. 3 shows the electric field distribution at the anodic gel/channel interface. From a distance of the interface, the electric field takes a constant value of 5111 V/m. The arrows indicate direction and velocity of the electroosmotic flow. As it induces a circular bulk flow, it is independent of the phaseguide. The performance limiting influence of EOF is illustrated by the trajectories in Fig. 4. Bacteria enter the channel from the left at different vertical starting points. Close to the top and bottom glass surface the electroosmotic velocity outweighs the oppositely directed electrophoretic migration and bacteria are moved towards the cathode. Due to the circular flow, they turn around at the gel barrier and migrate towards the anode. However, before being captured at the anode by the electric field these bacteria are swept out of the channel (h = 5 μm, 10 μm, 15 μm). As EOF is reversed towards the vertical center (Fig. 3) both the electrophoretic force and EOF are directed towards the anode and bacteria are collected at the gel as desired (h = 40 μm, 60 μm, 95 μm). When EOF is suppressed by addition of HPMC all trajectories end at the gel, independent of the height of the phaseguide. These results verify that that the electric field of 230 V/m is sufficient to capture bacteria at the given flow rate of 15μl/min. Upon variation of the flow rate, the electric field has to be set accordingly. With the drag force of the flow and the perpendicular electrophoretic force, the capture efficiency follows a linear relationship.
Figure 3.
Finite element simulations of the concentration process: Cross section at the anodic side of the channel. Surface plot: Electic field distribution. Arrows: Electroosmotic flow. Lines: Trajectories corresponding to Fig. 4. Simulation parameters: Electric current I = 230 μA, zeta potential ζ = −75 mV, medium conductivity σSB = 250 μS/cm.
Figure 4.
Trajectory simulations of bacteria under the influence of EOF at different heights: Close to the glass surface EOF dominates over the electrophoretic force and bacteria are swept out instead of being captured at the gel. Simulation parameters: Flow rate 15 μl/min, electrophoretic mobility μ = −2 × 10−8 m2/Vs.
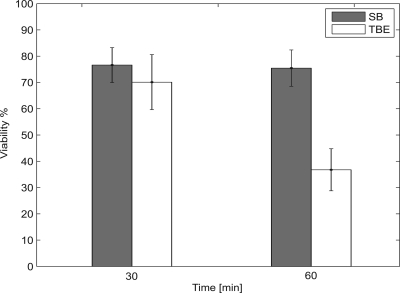
Bacterial viability in the electrophoresis medium was tested by suspending E. coli XL1-blue in SB and TBE (Tris/Borate/EDTA), respectively. The bacteria were plated immediately after suspending as a reference and after 30 min and 60 min at room temperature. Compared with previously used gram positive bacteria, E. coli are far more sensitive to lysis.40 Furthermore, Fig. 5 shows a significant decrease of viable cells in TBE between 30 min and 60 min, whereas the cell number in SB medium almost remains constant within this time frame. Thus, SB turned out to be the better choice for electrophoresis experiments with gram negative bacteria.
Figure 5.
Comparison of E.coli XL1-blue viability in SB and TBE. The faster decrease of viable, culturable bacteria in TBE confirms that SB is the favorable medium for electrophoretic experiments. The bars represent mean values of three independent time series.
As shown by the simulation in Fig. 4, the influence of electroosmosis decreases the capture efficiency of the device. During experiments without HPMC a part of the bacteria visibly followed the simulated trajectories and were swept out of the channel. Thus, the capture efficiency C according to Eq. 2a considerably decreased without the addition of HPMC as a dynamic coating.
The influence of EOF depends on the flow rate and the strength of the electric field. Therefore, in addition to previous results,40 three concentration experiments of E.coli XL1-blue at 15 μl/min without HPMC were performed and showed an capture efficiency of 87.29 ± 6.95%. In contrast, in experiments with 0.1% HPMC and the same species the efficiency was 98.39 ± 1.02% as summarized in Table TABLE I.. By suppressing EOF, the earlier found non-linear variations of the capture efficiency40 cannot be observed anymore.
Table 1.
Results of five independent concentration experiments for E. coli XL-1 blue and E. coli K12, respectively.
| Capture efficiency C (%) | Recovery rate R (%) | Concentration factor fc |
|---|---|---|
| E. coli XL-1 blue | ||
| 98.94 | 77.32 | 47.58 |
| 99.32 | 81.14 | 49.93 |
| 97.46 | 82.43 | 50.73 |
| 99.10 | 87.06 | 53.57 |
| 97.12 | 79.70 | 49.05 |
| 98.39 ± 1.02 | 81.53 ± 3.63 | 50.17 ± 2.23 |
| E. coli K12 | ||
| 99.55 | 72.19 | 44.42 |
| 99.79 | 79.07 | 48.66 |
| 99.94 | 77.23 | 47.53 |
| 99.43 | 78.85 | 48.53 |
| 98.64 | 77.45 | 47.66 |
| 99.47 ± 0.51 | 76.96 ± 2.79 | 47.36 ± 1.72 |
Five independent experiments were conducted with E.coli XL1-blue and K12, respectively. The details of the concentration experiments are presented in Table TABLE I.. Capture efficiencies for both strains were around 99% with small deviations. Variation of parameters, such as the medium conductivity and the flow rate of the μEO pump gave rise to these deviations. The mean value of recovery R was 81.53 ± 3.63% for XL1-blue with a corresponding concentration factor fc of 50.17 ± 2.23. Experiments with the K12 strain showed a mean recovery rate of 76.96 ± 2.79% and a 47.36 ± 1.72-fold concentration. All experiments were conducted at a flow rate of 15 μl/min for a time of 26 min 40 s.
The resuspension process is depicted in Fig. 6 for the fluorescent XL1-blue. Accumulation of bacteria at the gel barrier at the end of the experiment is shown in Fig. 6a. The recovery rates depended on the number of viable and culturable bacteria in the chip and their adherence to the gel. Excellent visibility of the fluorescent bacteria enabled to optimize the resuspension process. A small current of −50 μA to −100 μA applied for 1 min was found to yield optimal resuspension results. Bacterial viability of the initial sample was checked at the end of the experiments. For E.coli K12, the mean viability after experiments was 88.97 ± 5.65%. The viability of Xl1-blue showed more variation (90.78 ± 10.69%) because growth on agar plates was not as reproducible as growth of log-phase K12 in LB medium.
Figure 6.
Resuspension of fluorescent E.coli XL1-blue. (a) Accumulation at the end of the experiment. (b) Application of −50 μA for 1 min to detach bacteria (enhanced online) .
Comparison of the viability and recovery rates reveals a mean loss of 12.01% for K12 and 9.25% for XL1-blue. After inversion of the electric field, the bacteria were resuspended to the medium. As seen from Fig. 6b, the loss can be addressed to irreversible adherence of bacteria to the gel. Thus, it can be concluded that the method itself does not affect bacterial viability, which is important to prevent nucleic acids from enzymatic degradation.
For comparison, Halle et al.38 reported concentration factors of 1.8 for vegetative bacteria and 4.5 for bacterial spores in 9 min 30 s. Balasubramanian et al.39 showed the concentration of bacteria with an efficiency up to 99.9% and a maximum concentration factor of 14.2 in 1 h. In contrast to other groups, we have captured bacteria at the implemented gel barrier. Keeping bacteria away from the electrodes prevents electrode fouling and decreasing capture performance over time. Besides the concentration, subsequent access to the sample is of main importance but often neglected. The controlled release of bacteria from the gel barrier delivers a concentrated sample for further use. Growth based enumeration has proven that the presented method keeps bacteria viable and culturable. After recovering the sample, target molecules can be cleared in the previously developed RNA extraction chip. Neither the used gel nor hydroxypropylmethylcellulose inhibit subsequent PCR amplification.4, 56
CONCLUSION
We have presented a system for on-chip electrophoretic concentration of pathogens to a small volume, required for microfluidic, PCR-based detection systems. E. coli were continuously collected at an embedded polyacrylamide gel, separating the concentration channel from the electrodes. Thus, bacteria were prevented from exposure to high field strengths and electrolysis products at the electrodes. In comparison to previous works,37, 38, 39, 40 several advances were obtained: The small chip volume of 6.5 μl and the flow rate of 15 μl/min enabled high concentration factors in less than 27 min while maintaining capture efficiencies up to 99% by suppression of EOF. Recovering viable and culturable bacteria from the chip yielded factors of 50.17 ± 2.23 for E. coli XL1-blue and 47.36 ± 1.72 for E. coli K12. Phaseguides of half the channel height effectively prevented bubbles from blocking current paths and do away with the elaborate deposition of platinum black. Sodium borate was shown to be the superior medium for the concentration experiments.
The experimental results show the great potential of free flow electrophoresis for the concentration of pathogens. In future work, the performance will be evaluated with inactivated viruses. Furthermore, the presented chip will be combined with the nucleic acid extraction device of Vulto et al.4 to a single device. Avoiding resuspension and transfer of the analyte will further increase the concentration factor, and save time and material. Integration of preconcentration, cell lysis, and nucleic acid isolation and analysis on a single device is a promising step towards rapid detection of pathogens.
ACKNOWLEDGMENTS
This work was supported by the EU Marie Curie Research Training Network (MRTN)’On-Chip Cell Handling and Analysis’ CellCheck, project No. MRTN-CT-2006035854.
References
- Whitesides G. M., Nature (London) 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Lui C., Cady N. C., and Batt C. A., Sensors 9, 3713 (2009). 10.3390/s90503713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineva M. A., Mahilum-Tapay L., and Lee H., Analyst 132, 1193 (2007). 10.1039/b705672a [DOI] [PubMed] [Google Scholar]
- Vulto P., Dame G., Maier U., Makohliso S., Podszun S., Zahn P., and Urban G. A., Lab Chip 10, 610 (2010). 10.1039/b913481f [DOI] [PubMed] [Google Scholar]
- Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems, edited by Zourob M., Elwary S., and Turner A., (Springer, New York, 2008). [Google Scholar]
- Haeberle S. and Zengerle R., Lab Chip 7, 1094 (2007). 10.1039/b706364b [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhang Q., Feng H., Ang S., Chauc F. S., and Liu W.-T., Lab Chip 4, 337 (2004). 10.1039/b401834f [DOI] [PubMed] [Google Scholar]
- Bao N. and Lu C., Appl. Phys. Lett. 92, 3 (2008). [Google Scholar]
- Guan X., Zhang H., Bi Y., Zhang L., and Hao D., Biomed. Microdevices 12, 683 (2010). 10.1007/s10544-010-9421-6 [DOI] [PubMed] [Google Scholar]
- Lien K.-Y., Lee W.-C., Lei H.-Y., and Lee G.-B., Biosens. Bioelectron. 22, 1739 (2007). 10.1016/j.bios.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Beyor N., Seo T. S., Liu P., and Mathies R. A., Biomed. Microdevices 10, 909 (2008). 10.1007/s10544-008-9206-3 [DOI] [PubMed] [Google Scholar]
- Grodzinski P., Yang J., Liu R., and Ward M., Biomed. Microdevices 5, 303 (2003). 10.1023/A:1027357713526 [DOI] [Google Scholar]
- Yi C., Li C.-W., Ji S., and Yang M., Anal. Chim. Acta 560, 1 (2006). 10.1016/j.aca.2005.12.037 [DOI] [Google Scholar]
- Derveaux S., Stubbe B. G., Braeckmans K., Roelant C., Sato K., Demeester J., and Smedt S. C. D., Anal. Bioanal. Chem. 391, 2453 (2008). 10.1007/s00216-008-2062-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten H., Wound Essent. 5, 64 (2010). [Google Scholar]
- Meerhoff T., Houben M., Coenjaerts F., Kimpen J., Hofland R., Schellevis F., and Bont L., Eur. J. Clin. Microbiol. Infect. Dis. 29, 365 (2010). 10.1007/s10096-009-0865-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer K., Maede D., Ellerbroek L., Schulenburg J., Johne R., and Klein G., Food Environ. Virol. 1, 42 (2009). 10.1007/s12560-008-9007-0 [DOI] [Google Scholar]
- Pethig R., Biomicrofluidics 4, 35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen C.-P. and Chang H.-H., Biomicrofluidics 5, 034101 (2011). 10.1063/1.3609263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church C., Zhu J., Huang G., Tzeng T.-R., and Xuan X., Biomicrofluidics 4, 44101 (2010). 10.1063/1.3496358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D., Maheshwari S., and Chang H.-C., Biomicrofluidics 1, 14106 (2007). 10.1063/1.2710191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koklu M., Park S., Pillai S. D., and Beskok A., Biomicrofluidics 4 (2010) 10.1063/1.3479998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapizco-Encinas B. H., Davalos R. V., Simmons B. A., Cummings E. B., and Fintschenko Y., J. Microbiol. Methods 62, 317 (2005). 10.1016/j.mimet.2005.04.027 [DOI] [PubMed] [Google Scholar]
- Schoenfeld F., Griebel A., Konrad R., Rink S., and Karlsen F., JALA 7, 130 (2002). 10.1016/S1535-5535(04)00234-5 [DOI] [Google Scholar]
- Lagally E., Lee S.-H., and Soh H., Lab Chip 5, 10531058 (2005). 10.1039/b505915a [DOI] [PubMed] [Google Scholar]
- Kuczenski R. S., Chang H.-C., and Revzin A., Biomicrofluidics 5, 032005 (2011). 10.1063/1.3608135.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.-K., Kim T.-H., and Lee J.-G., J. Micromech. Microeng. 20, 10 (2010). [Google Scholar]
- Cheng I.-F., Chang H.-C., Hou D., and Chang H.-C., Biomicrofluidics 1, 21503 (2007). 10.1063/1.2723669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. S. W., Mitchell P. D., Oreffo R. O. C., and Morgan H., Biomicrofluidics 4, 022806 (2010) 10.1063/1.3406951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K., Kaprelyants A. S., Salina E. G., and Markx G. H., Biomicrofluidics 4, 022809 (2010) 10.1063/1.3435335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos A., Ramos A., Gonzalez A., Green N., and Morgan H., J. Phys. D: Appl. Phys. 36, 2584 (2003). 10.1088/0022-3727/36/20/023 [DOI] [Google Scholar]
- Oh J., Hart R., Capurroa J., and Noh H., Lab Chip 9, 62 (2009). 10.1039/b801594e [DOI] [PubMed] [Google Scholar]
- Kohlheyer D., Besselink G., Schlautmann S., and Schasfoort R., Lab Chip 6, 374 (2006). 10.1039/b514731j [DOI] [PubMed] [Google Scholar]
- Lu H., Gaudet S., Schmidt M., and Jensen K., Anal. Chem. 76, 5705 (2004). 10.1021/ac049794g [DOI] [PubMed] [Google Scholar]
- Song Y.-A., Chan M., Celio C., Tannenbaum S., Wishnok J., and Han J., Anal. Chem. 82, 2317 (2010). 10.1021/ac9025219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S., Weilbeer C., Howitz S., Becker H., Beushausen V., and Belder D., Lab Chip 11, 309 (2011). 10.1039/c0lc00347f [DOI] [PubMed] [Google Scholar]
- Cabrera C. R. and Yager P., Electrophoresis 22, 355 (2001). [DOI] [PubMed] [Google Scholar]
- Halle K. J., Li J. J., Munson M. S., Monteith J., Guzman E., Feather S., Verba J., Porter Q., Kenning V., Kamholz A. E., Weigl B. H., Bardell P. S. R., and Yager P., in Micro Total Analysis Systems 2003, edited by Northrup M. A., Jensen K. F., and Harrison D. J. (Mesa Monographs, 2003), pp. 559–562. [Google Scholar]
- Balasubramanian A. K., Soni K. A., Beskok A., and Pillai S. D., Lab Chip 7, 1315 (2007). 10.1039/b706559k [DOI] [PubMed] [Google Scholar]
- Podszun S., Vulto P., Heinz H., Hakenberg S., Hermann C., Hankemeier T., and Urban G. A., Enrichment of viable bacteria in a micro-volume by free-flow electrophoresis, Lab Chip, 2012, Advance Article. 10.1039/C1LC20575G [DOI] [PubMed]
- Wilson W. W., Wade M. M., Holman S. C., and Champlin F. R., J. Microbiol. Methods 43, 153 (2001). 10.1016/S0167-7012(00)00224-4 [DOI] [PubMed] [Google Scholar]
- Loosdrecht M. C. M. V., Lyklema J., Norde W., Schraa G., and Zehnder A. J. B., Appl. Environ. Microbiol. 53, 1898 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski M., Szeliga J., Klodziska E., and Buszewski B., Anal. Bioanal. Chem. 391, 2153 (2008). 10.1007/s00216-008-2021-0 [DOI] [PubMed] [Google Scholar]
- Pfetsch A. and Welsch T., Fresenius’ J. Anal. Chem. 359, 198 (1997). 10.1007/s002160050559 [DOI] [Google Scholar]
- Cui H., Horiuchi K., Dutta P., and Ivory C. F., Anal. Chem. 77, 1303 (2005). 10.1021/ac048915+ [DOI] [PubMed] [Google Scholar]
- Frost N. W. and Bowser M. T., Lab Chip 10, 1231 (2010). 10.1039/b922325h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonslow B., Barocas V., and Bowser M., Anal. Chem. 78, 5369 (2006). 10.1021/ac060290n [DOI] [PubMed] [Google Scholar]
- Vulto P., Medoro G., Altomare L., Urban G., Tartagni M., Guerrieri R., and Manaresi N., J. Micromech. Microeng. 16, 1847 (2006). 10.1088/0960-1317/16/9/013 [DOI] [Google Scholar]
- Vulto P., Podszun S., Meyer P., Hermann C., Manz A., and Urban G., Lab Chip, 11, (2011). 10.1039/c0lc00643b [DOI] [PubMed] [Google Scholar]
- Goldschmidtboeing F., Rabold M., and Woias P., J. Micromech. Microeng. 16, 1321 (2006). 10.1088/0960-1317/16/7/029 [DOI] [Google Scholar]
- Lee C.-Y., Linb C.-H., and Fu L.-M., Analyst 129, 931 (2004). 10.1039/b407627n [DOI] [PubMed] [Google Scholar]
- Vulto P., Huesgen T., Albrecht B., and Urban G., J. Micromech. Microeng. 19, 5 (2009). 10.1088/0960-1317/19/7/077001 [DOI] [Google Scholar]
- Brody J. R. and Kern S. E., BioTechniques 36, 214 (2004). [DOI] [PubMed] [Google Scholar]
- Wooley R. and Jones M., Vet. Microbiol. 8, 271 (1983). 10.1016/0378-1135(83)90079-2 [DOI] [PubMed] [Google Scholar]
- Herigstad B., Hamilton M., and Heersink J., J. Microbiol. Methods 44, 121 (2001). 10.1016/S0167-7012(00)00241-4 [DOI] [PubMed] [Google Scholar]
- Vijayakumar R., Kannan V., and Manoharan C., Int. J. Res. Pharm. Sci. 2, 579 (2011). [Google Scholar]