Abstract
The FVB/N (FVB) mouse has been a popular background strain for constructing transgenic mice. However, behavioral phenotyping of the resultant mice is complicated due to severe visual impairment in the FVB background strain. Previous studies reported cognitive impairments with the FVB strain, suggesting the background as unsuitable for behavioral analysis. In this study, we compared FVB mice to the well characterized C57BL/6 (B6) strain in a battery of hippocampus dependent tasks that had several non-visual cues. The tasks included: trace eyeblink conditioning, spontaneous alternation in the Y-maze, social recognition, trace and contextual fear conditioning, and odor habituation-dishabituation. FVB mice were able to learn all the tasks, often to similar levels as B6 mice. In contrast to previous reports, our data suggest FVB mice are not cognitively deficient with temporal memory tasks when the tasks do not rely heavily upon vision. Thus, the FVB strain may be used as the genetic background for behavioral phenotyping when non-visual hippocampal dependent tasks are utilized.
Keywords: eyeblink conditioning, fear conditioning, Y-maze, social recognition, habituation-dishabituation
The FVB/N (FVB) mouse has been a popular background strain for genetic manipulation due to large and dominant pronuclei (Auerbach et al., 2003; Taketo et al., 1991). However, behavioral phenotyping of the resultant mice is complicated due to severe visual impairment of this strain. The visual impairment is conferred by two mutations: first, a loss of function at the tyrosinase locus resulting in albinism (Errijgers et al., 2007), and second, the Pdebrd1 gene mutation resulting in retinal degeneration at approximately 35 days post-natal (Gimenez & Montoliu, 2001). Those two mutations render the mouse virtually blind as early as two months of age (Errijgers et al., 2007).
These visual defects suggest that behavioral tests which are visually demanding may give the impression of poor learning. FVB mice show impaired performance on hippocampal dependent tasks such as the radial arm maze (Mineur & Crusio, 2002) and Morris water maze (Owen, Logue, Rasmussen, & Wehner, 1997; Pugh, Ahmed, Smith, Upton, & Hunter, 2004; Royle, Collins, Rupniak, Barnes, & Anderson, 1999; Voikar, Koks, Vasar, & Rauvala, 2001). However, performance in the Morris water maze is positively correlated with visual abilities (Brown & Wong, 2007). FVB mice are unimpaired at conditioned odor preference (Brown & Wong, 2007) and fear conditioning (Owen et al, 1997); tasks which rely less heavily on visual input. In addition, FVB mice that had been crossed with a variant of the 129 strain, and backcrossed to eliminate albinism and rd genes were able to learn the Morris water maze (Errijgers et al 2007).
In this study, we sought to determine whether FVB mice are learning impaired on hippocampal dependent tasks when the need for visual cues is minimized. The FVB mice used in this study were at least 3 months of age at the time of testing, which is past the age of previously reported retinal degeneration (Errijgers et al., 2007; Gimenez & Montoliu, 2001). We utilized five learning tasks: trace eyeblink conditioning (EBC), spontaneous alternation in the Y-maze, social recognition, trace and contextual fear conditioning, and habituation-dishabituation to odor stimuli. We compared the FVB strain side-by-side with that of the well characterized behavior of the C57BL/6 (B6) strain.
METHODS
Animals
Behavioral assays were performed at the Northwestern University Behavioral Phenotyping Core. Male FVB/NJ and C57BL/6J mice were acquired from The Jackson Laboratories, (Bar Harbor, ME) and group housed by strain. The holding room was maintained at a constant temperature and humidity (23°C, 45%). Mice had continuous access to food and water and were housed in clear polypropylene cages (30 × 19 × 13 cm L × W × H) with a 14/10 hour light/dark cycle. All behavior was tested during the light period. Naive cohorts were used in all tasks, except habituation-dishabituation, to avoid confounding effects from task interactions. Sixty-two unique mice were used across all tasks in this study. Mice were 3–4 months of age at the time of data collection. Mice were handled and housed in accordance with the standards approved by the Institutional Animal Care and Use Committee of Northwestern University and the National Institutes of Health.
Trace Eyeblink Conditioning
Prior to training, mice were affixed with a headbolt as previously described (Galvez, Cua, & Disterhoft, 2009; Tseng, Guan, Disterhoft, & Weiss, 2004). Briefly, mice received an anesthesic cocktail, i.p., of ketamine (87 mg/kg) and xylazine (13 mg/kg). A custom fabricated headbolt was connected to the skull with instant adhesive at the base (Loctite® 454), then dental cement (Grip® Cement, Caulk Dentsply, Milford, DE) to protect the adhesive and headbolt. The headbolt contained five wires soldered to male pins within aligned holes. The ground wire was wrapped around a screw that was implanted into the skull. Two wires were passed through the upper eyelid to record electromyographic (EMG) activity from the obicularis oculi muscle, and two wires emerged just caudal to the eye to transmit a periorbital shock.
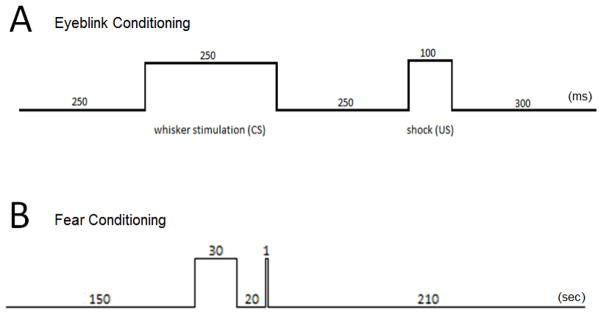
Animals were allowed a 5–6 day recovery period in their home cages. For training, a tether was connected to the headbolt so mice could move freely about the training chamber. Mice were trained one session per day for eleven days. Each session had 30 trials with a mean inter-trial interval (ITI) of 45-s ±15-s. Mice received two paired stimuli: a conditioned stimulus (CS), a deflection of the whiskers on one side of the muzzle driven by a piezo actuator attached to the tether (60 Hz, 250 μm deflection) and an unconditioned stimulus (US), a periorbital shock sufficient to cause reliable eyeblinks (0.25–2 mA train of biphasic square wave shocks, 60 Hz, 0.5 ms pulses) (Galvez, Weiss, Cua, & Disterhoft, 2009). Training consisted of one day of habituation to the eyeblink chamber (no CS/US), followed by five days of training (paired CS/US) and five days of extinction (CS alone). Pairings of CS/US were separated by a 250 ms stimulus-free trace interval (Fig. 1A), which is sufficient to require the hippocampus (Tseng et al., 2004). Shock thresholds were adjusted at the start of each session to provide the minimal current required to reliably elicit a blink and mild head twitch. A computer using custom software coded in LabView controlled stimulus presentation procedures and data collection, storage, and analysis routines. Any trials with spontaneous EMG activity in the baseline period greater than 1 standard deviation from the mean were excluded. Adaptive conditioned responses were defined as EMG activity during the 200 ms prior to US onset that was at least 4 SD larger than the mean baseline activity for a minimum of 15 ms.
Figure 1.
(A) Time intervals used in trace eyeblink conditioning. Units are in milliseconds. (B) Trace fear conditioning intervals. Units are in seconds. After 150-s baseline period, a 5 Hz white noise pulse (CS) sounded for 30-s followed by a 20-s stimulus free trace period and a 1-s 0.7 mA foot shock.
Spontaneous Alternation in the Y Maze
Y-maze performance was assessed in a similar manner to Ohno and colleagues (2004) (L. Holcomb et al., 1998; M. Ohno et al., 2004). The Y-maze was within a fluorescently lit (30 lux), sound attenuated chamber. Each arm of the symmetrical Y-maze was 46 cm long, 17 cm high, 3 cm wide at the bottom and 16.5 cm wide at the top. Video tracking was collected with LimeLight, (Actimetrics, Wilmette, IL) which uses a pixel-based algorithm to track the animal as it explores the maze. Each arm of the Y-maze was arbitrarily assigned as zone A, B or C. The maze was cleaned with 70% ethanol prior to training each mouse. All mice were placed in the stem of the Y and allowed to explore for 8 minutes. The software automatically tabulated each arm entry. Video was analyzed by an observer to be sure arm entry was not counted unless all four limbs were with the arm (L. A. Holcomb et al., 1999). Percent alternation was calculated as the number of non-repeating triads (i.e. ABC, CBA, BAC…) divided by the maximum possible alternations (total number of arms entered minus 2) × 100. A score of 50% indicated random performance.
Social Recognition
We modified the social recognition protocol used by Kogan and colleagues (Kogan, Frankland, & Silva, 2000; Ohno et al., 2004). This paradigm had four stages: 1) a 15-minute habituation period to the open field [55 × 55 × 30 cm], 2) a 5-minute habituation to an empty cylinder cage positioned within the open field, 3) a 5-minute stimulus trial where a test mouse was exposed to a novel juvenile within the cylinder cage, and 4) a repeat of step three after a 3-hour ITI. The open field was cleaned with 75% ethyl alcohol after each part of the experiment to remove odor cues. The LimeLight system tracked the animal’s path throughout the arena. Prior to the cage habituation, the open field video was reviewed to assure the cage was not placed in a quadrant to which the animal showed bias. For the stimulus trial, each test mouse was paired with a unique and novel juvenile mouse that was placed within a cylinder cage with an inner diameter of 9 cm and a height of 11 cm (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH). Juvenile mice (21–25 days postnatal) are a relatively neutral stimulus and minimize aggressive and mating behaviors (Terranova, Laviola, de Acetis, & Alleva, 1998). Investigation of the juvenile mouse by the test mouse was quantified by the cumulative sniffing duration over five minutes. Cumulative sniffing duration for all trials was tallied by a trained observer. Sniffing was defined as the snout directed toward the cylinder cage at a distance ≤ 1 cm. Behavior was then scored using a recognition index: the duration of investigation time during the second exposure divided by the initial investigation time × 100. Mice scoring an index less than 1 are considered capable of social recognition.
Trace Fear Conditioning
Fear conditioning cages (30 × 25 × 30 cm) were placed within dimly lit (≈10 lux) sound attenuated chambers. The floor of the cage was equipped with stainless steel rods for shock delivery. We used a trace fear conditioning protocol similar to what has been previously described by our laboratory (Kaczorowski & Disterhoft, 2009). After a baseline period (150-sec), mice were presented with three pairings of the CS, (30-sec of pulsed white noise at 5Hz, 5 ms rise/fall, 75dB), and the US (a 1-sec, 0.7 mA scrambled shock to the cage floor). The CS and US were separated by a 20-sec stimulus-free trace interval, which makes this a hippocampus dependent task (Chowdhury, Quinn, & Fanselow, 2005). The ITI was 210-sec (Fig. 1B). After the last shock delivery, mice remained in the chamber for 30-sec. A white noise CS was chosen to minimize any potential hearing impairment for the B6 cohort (McFadden, Ding, & Salvi, 2001). Olfactory cues were also employed as part of the fear context; Sparkle® (window cleaner) was wiped on the walls of the conditioning chamber. Behavior was video recorded with FreezeFrame3 (Actimetrics) which uses a motion detection algorithm. Mice were tested for contextual memory 24 hours after training. They were reintroduced to the training context and behavior was monitored for 5 minutes with no CS or US presentation. Three hours later, trace cue memory was tested. The animal was placed in a novel context, which included a smooth polypropylene cage floor, different lighting ( ≈30 lux), wall color (white, from tan), cage size (40 × 50 × 20 cm,), and a novel scent (Clidox®) wiped on the walls. In this novel context, we monitored freezing bouts in response to the white noise CS, comparing 60-sec pre-CS onset to 60-sec post CS onset. Freezing behavior was defined as cessation of movement except for respirations for a minimum of one second. Behavior scoring was automatically tabulated by FreezeFrame and reviewed by a trained observer. The two odors selected for this paradigm are readily available. Both are ammonia and alcohol free, and appear to lack noxious qualities in naive mice. Sparkle® is a window cleaner mainly comprised of glycol derivatives and has a flowery smell. Clidox® is routinely used as a disinfectant for animal behavior equipment. It contains 500 ppm of chlorine dioxide as its active ingredient and emits a citrus odor. Further, Sparkle® and Clidox® are not pungent odors at the concentrations we used. The banana extract was diluted to 1% concentration such that it was judged by a human observer to have the same intensity as Sparkle® and Clidox®.
Habituation-dishabituation
30-days after fear conditioning the same cohort was tested in habituation-dishabituation to an odor stimulus (Wrenn, Harris, Saavedra, & Crawley, 2003; Yang & Crawley, 2001). This paradigm was conducted to verify that mice detected the olfactory cues from the previous fear context (Sparkle®) and novel context (Clidox®). In this task, the animal is presented with the same odor three times (to measure habituation) and then a novel odor (to measure dishabituation). Mice were acclimated for 30-minutes to a clean cage identical in size to their home cages. The cage was empty except for one cotton tip applicator (Puritan® 6″) protruding inward from the cage lid to 4 cm above the bedding. Immediately after the acclimation period, the mouse was exposed for two minutes to a cotton applicator dipped in water. After the two-minute trial, a new cotton applicator replaced the existing one and sniffing behavior was recorded. A third trial was conducted under the same conditions. The ITI was approximately 30 seconds. The use of water as the first scent acclimated the animal to the testing paradigm. We tested three additional odors; each odor being presented three times before moving on to the next: 1% banana extract, Sparkle®, and Clidox®. All trials were video recorded with the Limelight system and scored by a trained observer. Sniffing was defined as the animal’s nose within 2 cm of the cotton tip (Yang & Crawley, 2001).
Statistical Analysis
All data were sorted using Microsoft Excel and all statistical measures were analyzed with Stat View. Differences were evaluated using t-tests, one-way analysis of variance (ANOVA), repeated measures ANOVA, and Fisher’s protected least significant difference post hoc tests where appropriate. Alpha for all statistical tests was 0.05. All data are reported as means ±SEM.
RESULTS
Trace Eyeblink Conditioning
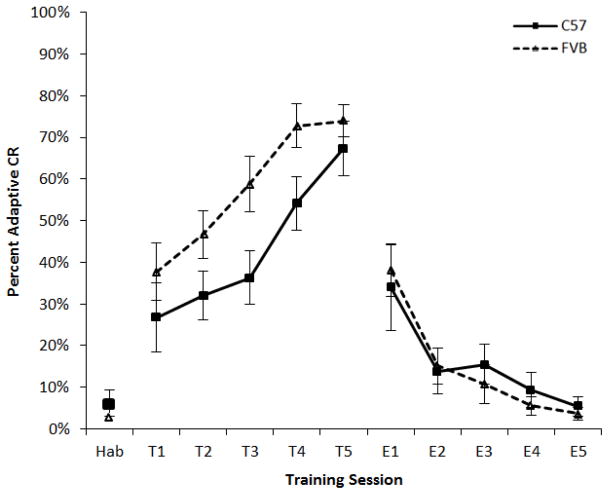
Three month-old FVB and B6 mice (each n = 10) were trace eyeblink conditioned. FVB mice acquired significantly more conditioned blink responses than the B6 mice (F1,18 = 6.89, p = 0.017). Throughout all training sessions a higher percentage of adaptive CRs was seen in FVB mice (Fig. 2). Interestingly, the mean shock threshold to elicit a blink was less for FVB mice than that of B6 mice (1.1 ±0.13 mA vs 2.2 ±0.16 mA), and a repeated measures ANOVA revealed a significant difference of shock threshold levels throughout training (F1,18 = 6.06, p = 0.024). This suggests an enhanced sensitivity of FVB mice to the periorbital shock. Overall, a significant increase in the percentage of adaptive CRs was seen in combined group analysis over the five training sessions (F4,72 = 17.29, p < 0.0001), i.e. both B6 and FVB mice acquired the task. The largest difference between the strains was during sessions three and four, but the two groups were at similar levels of performance during the last conditioning session. This result makes the following extinction data easier to interpret since both groups started at the same level of performance before the first extinction session.
Figure 2.
Percent of trials with adaptive conditioned responses for three phases of training. Habituation (Hab) session shows baseline activity that met the criteria for being scored as a response even though no stimuli were presented. Both strains increased their percentage of adaptive CRs throughout training (T1–T5). No strain-session interaction was identified. FVB mice achieved significantly more adaptive CRs (see Results). Extinction (E1–E5) sessions showed learning of the dissociation of CS and US. No interactions of strain and session were observed in training or extinction.
During CS alone extinction sessions, there was a significant decrease in percent adaptive CRs (strains combined, F4,72 = 16.88, p < 0.0001), indicating that there was learning of the CS/US dissociation. However, contrary to acquisition, there was no strain difference during extinction sessions (F1,18 = 0.41). Nor was there a significant interaction of strain and extinction session.
Spontaneous Alternation in the Y Maze
Percent spontaneous alternation was analyzed over an 8 minute trial of Y maze exploration, known to be sensitive to working memory (Weiss, Shroff, & Disterhoft, 1998). Both strains (each n = 10) completed the task with a similar degree of percent alternation (Fig. 3) and both performed above chance (approximately 72%). FVB mice demonstrated significantly increased locomotor activity within the maze as compared to B6 mice (41.3±3.05 entries vs 26±3.62 entries, p = 0.0046), as well as more non-repeating triads (p = 0.0025). These data indicate that these strains have equivalent working memory, although the FVB mice are more active in this task.
Fig 3.
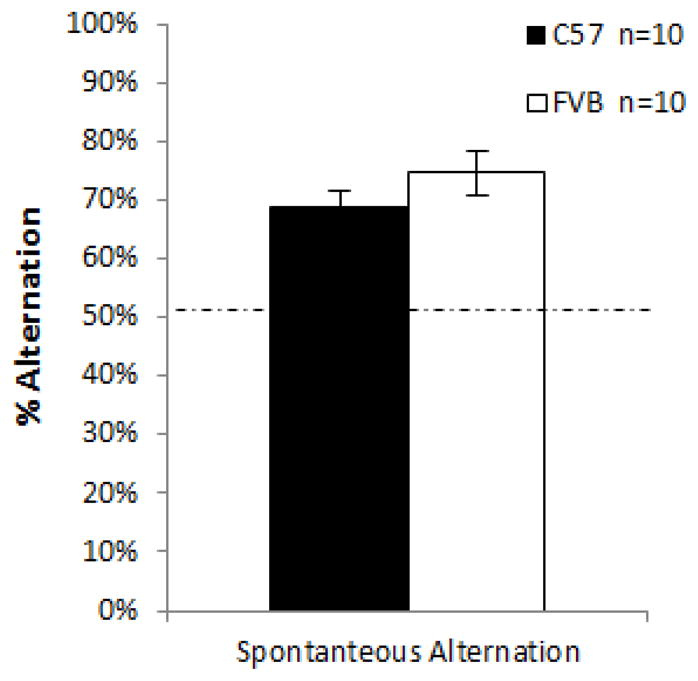
Mean percent spontaneous alternation over an 8-minute period. Both groups exhibited spontaneous alternation, i.e. >50%. FVB = 75% ±3.8%, B6 = 69% ±2.6%. No significant difference was found between the strains (p = 0.26).
Social Recognition
Eleven mice of each strain were tested in social recognition. Social recognition indices were calculated based on differential investigations times between two trials. Both strains demonstrated they recognized the juvenile mouse, scoring indices less than 1 (Fig. 4A). B6 mice scored a lower mean recognition index (better recognition) of 0.45 ±0.073, and FVB mice scored 0.62 ±0.057. The difference between the two groups was not statistically significant. Baseline level of locomotor activity was recorded during the habituation period. The mean locomotor activity (Fig. 4B) of the two strains also did not reveal any significant difference. FVB mice exhibited longer sniffing durations than B6 mice during the stimulus and recognition trials (Fig. 4C) while the number of sniffing bouts between the strains (data not shown) were not significantly different. This longer sniffing may be in part due to the particularly social behaviors in the FVB background as previously noted (Bolivar, Walters, & Phoenix, 2007; Moy et al., 2004).
Figure 4.
(A) No significant differences in social recognition index (t-test, p=0.09). Both strains scored an index below a value of 1. (B) Total distance (cm) in locomotion for each strain during habituation (15 minutes) to the open field. FVB = 8071 ±531 cm, B6 = 6976 ±432 cm, (t-test, p=0.13). (C) Cumulative sniffing over the three trials of social recognition. The FVB mice exhibited significantly longer investigation times than B6 mice during the stimulus and recognition trials *p<0.0005 for each of the two trials.
Trace Fear conditioning
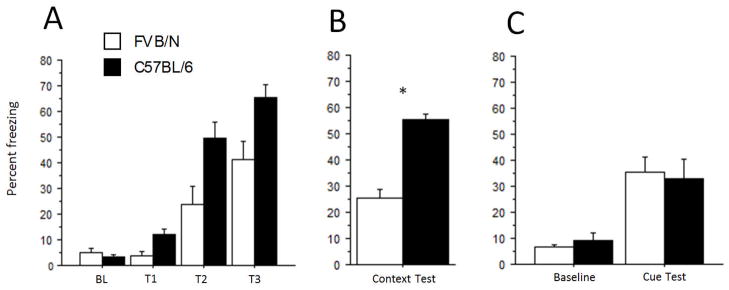
Ten mice of each strain were tested in trace fear conditioning. Training day baseline freezing levels were similar between strains, as was their freezing behavior during the first CS exposure. Further, no percent freezing interaction was detected between the baseline and the first CS exposure for the two strains (F1,18 = 1.29), indicating the CS was initially non-aversive to either strain. Increases in percent freezing were seen in both strains during the three training trials (Fig. 5A). FVB mice consistently froze less than B6 mice (strain effect, F1,18 = 19.0, p = 0.0004) and there was no interaction of strain and training trials (F2,36 = 1.63).
Figure 5.
Contextual and trace fear conditioning. (A) Training day percent freezing for three consecutive trials. BL = 150-s baseline, T=trial. Percent freezing measured 50-s following CS onset (30-s CS + 20-s trace). A strain effect was revealed in freezing over the three training trials (see Results). There was no significant interaction of strain and trials. (B) Contextual fear test. Significant strain differences in freezing to the context were observed with a t-test (*p<0.0001), B6 = 55%±2.1%, FVB = 25%±2.2%. Within strain comparisons (paired t-test) from training day baseline to context test showed significant increases (p<0.0001) in freezing for both strains. (C) Cue test. Both strains exhibited freezing to the cue within a novel context, but there was no significant difference between the strains. B6 = 33%±7.3%, FVB = 35%±5.6%, p> 0.05.
Twenty-four hours after training mice were returned to the same context. Both strains froze significantly more during the context test (Fig. 5B) compared to their respective baseline periods from the training day (p < 0.0001). However, B6 mice froze more than FVB mice in the training context (strain effect, F1,18 = 29.56, p < 0.0001). Thus, while FVB mice are capable of contextual fear conditioning, they do not freeze as much as B6 mice. For the auditory trace cue test we compared the 60-sec pre-CS baseline period in a novel context to the 60-sec following CS onset. Contrary to the contextual fear test, there was no difference (Fig. 5C) between strains (F1,18 = 0.0002). Combining the strains revealed significant differences between the two periods (F1,1 = 29.57, p < 0.0001). Thus, FVB mice learn trace cued fear conditioning to levels equal to that of B6 mice.
Habituation-dishabituation
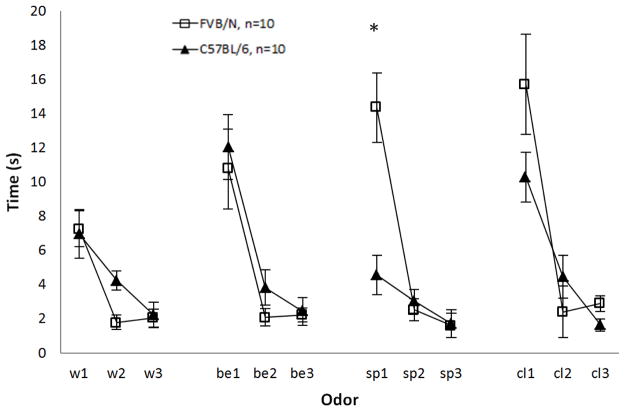
The purpose of the habituation-dishabituation task was to test the animal’s olfactory abilities and to determine if the two strains could detect the stimuli used in fear conditioning. Odor recognition memory engages the hippocampus (Eichenbaum, Kuperstein, Fagan, & Nagode, 1987), and it has been demonstrated to be dependent on the integrity of the hippocampus in both rodents and humans (Bunsey & Eichenbaum, 1995; (Dudchenko, Wood, & Eichenbaum, 2000; Levy, Hopkins, & Squire, 2004). The order of the odor presentation was the following: water, 1% banana extract (a neutral stimulus), Sparkle® (which was paired with the shock in fear conditioning), and Clidox® (which was paired with the novel context in fear conditioning). There were clear significant differences (p < 0.001) in cumulative sniffing duration between the first two presentations of each odor for both strains, with the exception of Sparkle® for B6 mice (p = 0.65), demonstrating unimpaired habituation for both strains (Fig. 6). Also, cumulative sniffing significantly increased (p < 0.0001) for all odors for both strains when a novel odor was presented, indicating successful dishabituation. The only difference between the two strains for this task was during the exposure to Sparkle®. Repeated measures analysis for the three exposures to this scent identified a strain effect (F1,18 = 4.386, p = 0.05) and a strain-sniffing interaction (F2,36 = 21.7, p < 0.0001). Post-hoc tests (Fischer’s PLSD) revealed the difference to be between the first and second exposures to Sparkle®, p < 0.0001 (Fig. 6). This is likely due to fear conditioning with Sparkle® (see Discussion). Overall, the data demonstrate both FVB and B6 mice have functional recognition memory for repeated exposures to olfactory stimuli.
Figure 6.
Odor habituation-dishabituation. FVB n=10, B6 n=10. w=water, be=1% banana extract, sp=Sparkle®, cl=Clidox®. Each trial is cumulative sniffing for a 2-minute period. FVB mice habituated to all stimuli. B6 mice habituated to all stimuli except Sparkle, where transitioning from sp1-sp2 resulted in no significant changes in sniffing, p=0.65 (see Discussion). Unpaired t-test of first exposure to Sparkle (sp1) revealed a significant difference between the strains, *p=0.0009. Increases in cumulative sniffing to novel stimuli were seen in both strains.
DISCUSSION
The use of genetically modified mice has transformed the field of neuroscience (Havekes & Abel, 2009). There is a growing awareness that the behavioral phenotyping of these mice needs to be as dynamic as their genetic constructs. Backcrossing transgenes developed in one strain to another is time consuming and requires a large number of animals. In some cases, it may be best to change the behavioral task instead of the animal. This choice needs to be balanced against the mass of behavioral data already reported for B6 mice. Here, we demonstrate that the FVB strain is an appropriate background for behavioral phenotyping if the confound of vision impairment is minimized by utilizing tasks based on non-visual cues. Our results indicate that the olfactory, auditory, and tactile abilities of these mice are sufficient to allow hippocampus dependent learning.
The relative hyperactivity of FVB mice has been suggested as one reason for poor learning in this strain. However, previous studies have yielded conflicting results as to whether FVB mice are hyperactive as measured by spontaneous locomotion. Logue et al (1997) reported no differences between B6 mice and FVB mice in the open field, while Voikar et al (2001) and Mineur et al (2002) reported that FVB mice show higher activity. We did not see a significant difference in the amount of activity during baseline periods for either social recognition or fear conditioning. However, we did observe FVB mice to be much more active in the Y maze, a task which tends to motivate ambulation due to the narrow alleys. Variation in duration of the assay, size of arenas, and luminance of the testing environment can affect the animals’ spontaneous locomotion (J. Crawley et al., 1997; J. N. Crawley, 2007; Mrosovsky, 1994), likely leading to the conflicting reports in the literature. Here, baseline locomotion was controlled for in all tasks in which animal movement was a factor.
Trace eyeblink conditioning is an associative learning task. The nature of the task, learning that a vibration of the whiskers predicts a periorbital shock, makes it ideal for FVB mice since it requires no visual input. To our knowledge, there are no previous reports of eyeblink conditioning in FVB mice. FVB mice performed significantly better than B6 mice throughout training. The lack of strain and training session interaction during eyeblink conditioning (near parallel learning curves) suggests that the FVB mice are more sensitive to the whisker CS than B6 mice. Sensory compensation rooted from the loss of photoreceptors in FVB mice may be an explanation of this potential enhanced vibrissa sensitivity.
Spontaneous alternation in the Y maze has been extensively used as a measure of working memory. Despite the difference in visual abilities in the two strains, both exhibited spontaneous alternation rather than random exploration. It is likely that FVB mice used other sensory modalities than vision to perform the task, such as whisking their vibrissa against the maze walls for navigation, or formed a place memory based on the direction they travelled (Packard & McGaugh, 1996). Our social recognition data clearly illustrates that both strains’ investigation of the social environment is sensitive to the presence of novel or repeated social stimuli (Fig. 4C). FVB mice showed a higher level of investigation over all trials (including when a social stimulus was absent) which, similar to the increased exploratory behavior in the Y maze, could be a compensatory effect of their intact sensory modalities.
FVB and B6 mice learned both trace cue and contextual fear conditioning. The performance of FVB mice was no different than B6 mice in response to the cue, however, they froze significantly less to the training context. One likely reason for this difference is the nature of the sensory stimuli. The salient stimulus was the auditory cue, and was readily learned by FVB mice. Contextual fear conditioning, on the other hand, consisted of visual, olfactory, and tactile stimuli. While we tried to maximize the olfactory and tactile stimuli, the impaired performance of the FVB mice could suggest that mice rely more heavily upon visual stimuli for contextual fear conditioning.
Habituation-dishabituation was the only task in which non-naive mice were used: these mice had previously been fear conditioned. FVB and B6 mice demonstrated both habituation to repeated presentations of the same odor, and dishabituation to the novel odor. However, there was an interesting difference between the strains in response to the Sparkle®, which was included as part of the context during fear conditioning. B6 mice spent less time investigating the novel presentation of Sparkle® than did FVB mice. A possible reason for the decrease in sniffing is because these mice were avoiding the scent that previously cued an accompanying foot shock. If this is indeed the reason, then it is interesting that FVB mice did not also avoid the Sparkle. This could suggest that FVB mice have impaired recollection of fear conditioning thirty days later. A possible explanation for this could be decreased adult neurogenesis in the dentate gyrus in the FVB strain (Schauwecker, 2006), which can hinder storage of a memory trace into the cortex (Kitamura et al., 2009).
The habituation-dishabituation data presented here may be behavioral evidence for memory consolidation impairments in FVB mice. More rigorous tests would be necessary to confirm this. Still, we only detected the remote memory impairment in FVB mice with a remote, 30-day memory test. Electrophysiological experiments by Bampton et al, have shown that potentiation of excitatory postsynaptic potentials leading to long-term potentiation (LTP) can be induced and maintained in FVB mice for at least 60 minutes (Bampton, Gray, & Large, 1999). This ability for LTP is positively correlated with learning and memory (Bliss & Collingridge, 1993) and is reflected in the learning paradigms presented here.
In conclusion, hippocampus dependent tasks are clearly within the cognitive capacity of FVB mice. It is important that these tasks do not rely heavily on visual cues. Our data from trace eyeblink conditioning, trace cued fear conditioning, social recognition, Y-maze spontaneous alternation and habituation-dishabituation of odor recognition reflect that FVB mice have the ability to form temporal associations as well as B6 mice. Given the number of existing strains of transgenic mice on the FVB background, the time and expense of backcrossing them to B6 mice for behavioral phenotyping is not necessary if appropriate tasks are utilized.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Auerbach AB, Norinsky R, Ho W, Losos K, Guo Q, Chatterjee S, Joyner AL. Strain-dependent differences in the efficiency of transgenic mouse production. Transgenic Res. 2003;12(1):59–69. doi: 10.1023/a:1022166921766. [DOI] [PubMed] [Google Scholar]
- Bampton ETW, Gray RA, Large CH. Electrophysiological characterisation of the dentate gyrus in five inbred strains of mouse. Brain Research. 1999;841(1–2):123–134. doi: 10.1016/s0006-8993(99)01811-9. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. [10.1038/361031a0] Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behavioural Brain Research. 2007;176(1):21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Wong AA. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14(3):134–144. doi: 10.1101/lm.473907. 14/3/134 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5(6):546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning withlong, but not short, trace intervals in mice. Behav Neurosci. 2005;119(5):1396–1402. doi: 10.1037/0735-7044.119.5.1396. 2005-13804-026 [pii] [DOI] [PubMed] [Google Scholar]
- Crawley J, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Paylor R. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? : Behavioral phenotyping of transgenic and knockout mice. 2. Hoboken, N.J: Wiley-Interscience; 2007. [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7(3):716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D’Hooge R, Kooy RF. Fvb.129p2-pde6b(+) tyr(c-ch)/ant, a sighted variant of the fvb/n mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6(6):552–557. doi: 10.1111/j.1601-183X.2006.00282.x. GBB282 [pii] [DOI] [PubMed] [Google Scholar]
- Galvez R, Cua S, Disterhoft JF. Age-related deficits in a forebrain-dependent task, trace-eyeblink conditioning. Neurobiology of Aging, In Press, Corrected Proof. 2009 doi: 10.1016/j.neurobiolaging.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Cua S, Disterhoft J. A novel method for precisely timed stimulation of mouse whiskers in a freely moving preparation: Application for delivery of the conditioned stimulus in trace eyeblink conditioning. Journal of Neuroscience Methods. 2009;177(2):434–439. doi: 10.1016/j.jneumeth.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (pdebrd1) in fvb/n-derived transgenic mice. Lab Anim. 2001;35(2):153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- Havekes R, Abel T. Chapter 1 genetic dissection of neural circuits and behavior in mus musculus. In: Stephen FG, editor. Advances in genetics. Vol. 65. Academic Press; 2009. pp. 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Duff K. Accelerated alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. [10.1038/nm0198-097] Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: Lack of association with amyloid deposits. Behav Genet. 1999;29(3):177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learning & Memory. 2009;16(6):362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell1. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10(1):47–56. doi: 10.1002/(sici)1098-1063(2000)10:1<47::aid-hipo5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Levy DA, Hopkins RO, Squire LR. Impaired odor recognition memory in patients with hippocampal lesions. Learning & Memory. 2004;11(6):794–796. doi: 10.1101/lm.82504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and f1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80(4):1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40(6):313–321. [PubMed] [Google Scholar]
- Mineur YS, Crusio WE. Behavioral and neuroanatomical characterization of fvb/n inbred mice. Brain Res Bull. 2002;57(1):41–47. doi: 10.1016/s0361-9230(01)00635-9. S0361923001006359 [pii] [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Crawley JN. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes, Brain and Behavior. 2004;3(5):287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. In praise of masking: Behavioural responses of retinally degenerate mice to dim light. Chronobiol Int. 1994;11(6):343–348. doi: 10.3109/07420529409057251. [DOI] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Disterhoft JF. Bace1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of alzheimer’s disease. Neuron. 2004;41(1):27–33. doi: 10.1016/s0896-6273(03)00810-9. S0896627303008109 [pii] [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the morris water task and fear conditioning in inbred mouse strains and f1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80(4):1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pugh PL, Ahmed SF, Smith MI, Upton N, Hunter AJ. A behavioural characterisation of the fvb/n mouse strain. Behavioural Brain Research. 2004;155(2):283–289. doi: 10.1016/j.bbr.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Collins FC, Rupniak HT, Barnes JC, Anderson R. Behavioural analysis and susceptibility to cns injury of four inbred strains of mice. Brain Research. 1999;816(2):337–349. doi: 10.1016/s0006-8993(98)01122-6. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Genetic influence on neurogenesis in the dentate gyrus of two strains of adult mice. Brain Research. 2006;1120(1):83–92. doi: 10.1016/j.brainres.2006.08.086. [DOI] [PubMed] [Google Scholar]
- Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Hansen CT. Fvb/n: An inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. Journal of Comparative Psychology. 1998;112(1):3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14(1):58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72(1–2):271–281. doi: 10.1016/s0031-9384(00)00405-4. S0031-9384(00)00405-4 [pii] [DOI] [PubMed] [Google Scholar]
- Weiss C, Shroff A, Disterhoft JF. Spatial learning and memory in aging c57bl/6 mice. Neuroscience Research Communications. 1998;23(2):77–92. doi: 10.1002/(sici)1520-6769(199809/10)23:2<77::aid-nrc2>3.0.co;2-y. [DOI] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: Methodology and application to galanin-overexpressing transgenic mice. Behavioral Neuroscience. 2003;117(1):21–31. doi: 10.1037/0735-7044.117.1.21. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. John Wiley & Sons, Inc; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]