Abstract
Epidemiological studies suggest that alcohol consumption increases the risk of developing colorectal cancer. However, the data are confounded by numerous cosegregating variables. To cast further light on the relationships between alcohol intake and colon cancer development, 21-day-old male F344/DuCrj rats were fed 200 ppm 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) in their diet for 8 weeks and doses of 0, 0.1, 0.3, 1, 3, 10 and 20% of ethanol in their drinking water ad libitum for 16 weeks thereafter. The rats were sacrificed after 24 weeks of experiment, and aberrant crypt foci (ACF), surrogate lesions for colon cancer, were examined under a light microscope at low magnification. Ethanol was found not to affect the ACF formation at any dose compared with the initiated-controls. Furthermore, ethanol did not alter colon epithelial cell proliferation. These data, obtained by analysis of a colon cancer surrogate marker lesion, indicate that ethanol lacks promotion activity for MeIQx-initiated rat colon carcinogenesis.
Keywords: ethanol, 2-amino-3, 8-dimethylimidazo[4, 5-f]quinoxaline (MeIQx), rat colon carcinogenesis
Introduction
There is abundant epidemiological evidence showing that excessive and chronic alcohol consumption contribute to cancer development in organs such as the oral cavity, pharynx, larynx, esophagus, liver and breast1. The epidemiologic data concerning the association between alcohol consumption and colorectal cancer are not as clear as those concerning cancers of the upper aerodigestive tract. Most studies, however, have detected a positive correlation2. In 1999, a consensus conference of the World Health Organization on Nutrition and Colorectal Cancer concluded that chronic alcohol ingestion, even with low daily intake (one to three drinks or 10 to 40 g per day), results in a 1.5- to 3.5-fold increase in risk of rectal cancer and a lesser increase in risk of colonic cancer in both sexes3. This conclusion also gained support at a meeting on alcohol and cancer at the International Agency for Research on Cancer4.
Experimental animal studies are critical in clarifying the role of alcohol in organ carcinogenesis. It has been reported that ethanol per se does not have carcinogenic activity even after lifelong exposure in rodents5. The hypothesis is therefore that alcohol may act as a co-carcinogen or tumor-promoter for colon carcinogenesis1. However, experimental data on alcohol and colorectal cancer are controversial and complex, depending on the experimental design6–8. In general, it can be concluded that alcohol stimulates colorectal carcinogenesis primarily during the preinitiation and initiation phases, as interaction may occur between ethanol and procarcinogenic metabolism in these phases2.
Heterocyclic amines (HCAs) are ubiquitously present in cooked foods and are known to form DNA adducts in experimental animals and human9. Recent epidemiological studies have indicated that increased risk of colorectal cancer development is associated with high intake of red meat and HCAs, such as 2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline (MeIQx)10,11. MeIQx is strongly mutagenic in the Ames test12 and is carcinogenic in rodents when fed at high doses, inducing tumors in the livers, Zymbal glands, skin and clitoral glands of rats at a dose of 400 ppm in their diet13 and in the livers, lungs, and hematopoietic systems of mice at a dose of 600 ppm14. It has also been documented that MeIQx induces aberrant crypt foci (ACFs), surrogate marker lesions for colon cancers, in rats15 and mice16.
Exposure of humans to HCAs is all but inescapable, and many people drink alcohol; however, only a limited amount of data are available concerning the effects of interaction between HCAs and alcohol with regard to colon carcinogenesis, especially from in vivo experiments. In the present study, we therefore examined the potential for the ability of ethanol to promote MeIQx-induced colon carcinogenesis in rats. For this purpose, we focused on ACFs in tissue samples from a previous study that demonstrated dose-dependent promotion of MeIQx-induced hepatocarcinogenesis by ethanol in male rats17.
Materials and Methods
Chemicals and antibodies
Ethanol (99.5%) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and MeIQx was purchased from the Nard Institute (Osaka, Japan). Mouse anti-proliferating cell nuclear antigen (PCNA) monoclonal antibodies were obtained from Dako (Carpinteria, CA, USA).
Animals
A total of 125 male, 20-day-old, F344/DuCrj rats were purchased from Charles River Japan (Atsugi, Japan). Their housing conditions and treatment were as described previously17.
Ethics
The experiments were conducted in accordance with the guidelines for Animal Experiments at Osaka City University Medical School, which are in line with other domestic and international guidelines and laws concerning animal rights.
Experimental design
One hundred and twenty-five, 21-day-old, F344/DuCrj rats were divided into eleven experimental groups. The numbers of rats in the groups were 15 (groups 1 to 7) or 5 (groups 8 to 11) per group. In groups 1 to 7 and 11, the rats were fed MF pellet diet mixed with 200 ppm MeIQx (Oriental Yeast Co., Ltd., Tokyo, Japan), and water was provided ad libitum for the first 8 weeks; groups 8 to 10 were fed MF pellet diet without MeIQx. The animals in groups 10 and 11 were euthanized at experimental week 8. Thereafter, the remaining rats received doses of 0 (groups 1 and 8), 0.1 (group 2), 0.3 (group 3), 1 (group 4), 3 (group 5), 10 (group 6) and 20% ethanol (vol/vol; groups 7 and 9) in their drinking water and MF pellet diet ad libitum for 16 weeks. During the period of ethanol administration, the drinking water was changed six times per week. At experimental week 24, all rats were euthanized after overnight withdrawal of food. At sacrifice, the animals were anesthetized with diethyl ether, and their colons were quickly removed. After evaluation of ACF, two samples of the proximal, middle and distal colon were cut into strips and routinely processed for embedding in paraffin. Sections were stained with hematoxylin and eosin (H&E) for histopathological and immunohistochemical examination. Diagnosis and classification of tumors and hyperplasias were performed according to the nomenclature for classification of colon tumors and preneoplastic lesions in the rat proposed by IARC18. The hyperplastic lesions recognized in the present study were all atypical hyperplasias and did not include a focal or reactive hyperplasia.
ACF counts
Colons were quickly excised, flushed with saline, inflated by intraluminal injection of 10% phosphate-buffered formalin solution, slit open along the longitudinal median axis from the cecum to anus and fixed flat between two pieces of filter paper in 10% phosphate-buffered formalin. After fixation for at least 24 h at 4°C, all colons were stained with 0.2% methylene blue (in H2O) for 3–5 min and then examined for ACF by light microscopy at 40× and 100× magnification using the following criteria for identification: (1) increased size compared with normal crypts, (2) enlarged pericryptal zone, (3) slight elevation above the surrounding mucosa and (4) more frequent occurrence of an elongated luminal opening. ACFs were assessed for the number of aberrant crypts in each focus (1 crypt, 2 crypts, 3 crypts and ≥4 crypts).
Immunohistochemistry for PCNA
Immunoenzymatic staining for PCNA was performed after sequential treatment with 3% H2O2, exposure to an anti-PCNA mouse monoclonal antibody at room temperature for 1 hr and then horseradish peroxidase coupled to an inert polymer backbone (Dako EPOS, Dako Cytomation, Carpenteria, CA, USA). The sites of peroxidaase binding were demonstrated by DAB. More than 500 epithelial cells of well-visualized crypts from normal-appearing mucosa were counted per colonic segment under a light microscope. PCNA indices were estimated as the numbers of positive nuclei per 100 epithelial cells (%). In groups 1 to 7, 6 animals were evaluated. Immunohistochemical evaluation of PCNA was performed in a blind manner.
Statistical analysis
Statistical analysis was performed with Statistical Analysis System (SAS) version 9.1.3 (SAS Institute Incorporated, Cary, North Carolina, USA). For the incidences of colon tumors, hyperplasias and ACFs, the Fisher’s exact probability test was employed. For experiments using single dose levels of MeIQx or ethanol, the data for each group were first analyzed with the F-test for homogeneity of variance. If homogeneous, the data were analyzed by the Student’s t test, and if not, they were by the Aspin-Welch’s t test. For the experiment using multiple dose levels of ethanol, the data were initially tested for homogeneity using Bartlett’s analysis. For data found to be homogeneous, Dunnett’s multiple analysis was performed. If the data were not homogeneous, Steel’s multiple analysis was conducted. The significance of differences was determined based on probability levels of 1% and 5%. The data are presented as means or means ± standard deviation (SD).
Results
Final body weights, food consumption and water intake
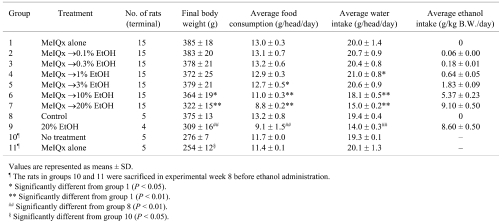
At week 8, all rats were in good general condition, and there were no significant differences among the groups with regard to food consumption and water intake (data not shown). After week 8, food consumption of the rats in Groups 5, 6 and 7, which were administered a dose of 3, 10 or 20% ethanol, was significantly decreased compared with the initiated control Group 1 (Table 1). Furthermore, water intake of the rats in Groups 6 and 7, which were administered a doses of 10 and 20% ethanol, was significantly decreased compared with the initiated control Group 1. In Group 4, water intake was significantly increased compared with the initiated control Group 1, but there was no dose-dependence. Therefore, the increased water intake in Group 4 was not considered to have any toxicological implications. One non-initiated rat administrated 20% ethanol (Group 9) died in experimental week 11, and emaciation was observed at autopsy. The final body weights of rats in Groups 6 and 7 were significantly reduced compared with Group 1. In the groups without MeIQx-treatment (Group 8 and 9), administration of 20% ethanol also reduced final body weight, food consumption and water intake (Table 1).
Table 1. Body Weight, Food Consumption, Water Intake and Ethanol Intake Data for Rats Treated with MeIQx (8 Weeks) and/or Ethanol (16 Weeks).
Effects of ethanol on ACF formation
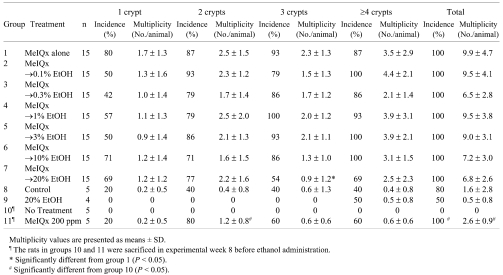
At week 8, ACFs were observed in all MeIQx-treated rats (Group 11); however, no ACFs were observed in the no-treatment rats (Group 10; Table 2). At experimental week 24, the animals treated with MeIQx developed ACFs without relation to ethanol treatment (Fig. 1A–D). The incidences and multiplicities of ACFs in rats administered doses of 0.1 to 10% ethanol (Groups 2 to 6) were not different from those of the control Group 1 (Table 2). In the 20% ethanol treatment group (Group 7), there were no differences in the incidences of any type of ACF compared to the control group (Group 1). There was no difference in ACF multiplicity between Group 7 and the control group with exception of a significant decrease in ACFs with 3 crypts. No statistically significant differences in formation of ACFs were observed between the non-treatment (Group 8) and ethanol only treatment groups (Group 9).
Table 2. Formation of Aberrant Crypt Foci (ACF) in Rats Treated with MeIQx (8 Weeks) and/or Ethanol (16 Weeks).
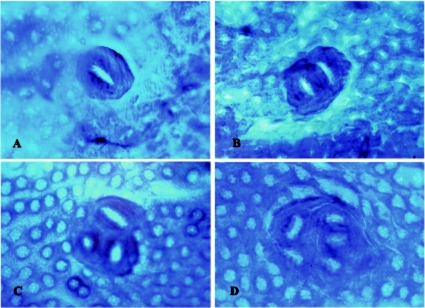
Fig. 1.
Formation of aberrant crypt foci (ACFs) in the colons of F344 rats administered ethanol for 16 weeks after MeIQx initiation for 8 weeks. A-D show typical ACFs consisting of 1 Crypt (A), 2 Crypts (B), 3 Crypts (C) and 4 Crypts or more (D). The samples are stained with 0.2% methylene blue stain. The original magnification is 100×.
Effects of ethanol on formation of proliferative lesions
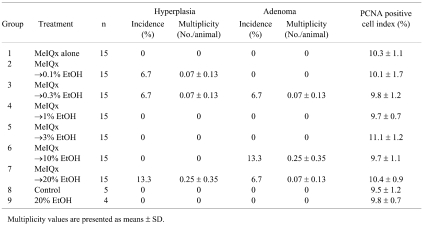
In animals administered doses of 0.1, 0.3, 10 and 20% ethanol after MeIQx treatment, a few hyperplasias and adenomas were observed in the colon at experimental week 24. However, there was no relationship between formation of hyperplastic and neoplastic lesions and ethanol intake (Table 3).
Table 3. Formation of Hyperplasita and Adenoma and Cell Proliferation Indices in Rats Treated with MeIQx (8 Weeks) and/or Ethanol (16 Weeks).
Evaluation of cell proliferation by immunohistochemistry for PCNA
The results of immunohistochemical staining for PCNA, a marker for cell proliferation, are presented in Table 3. The PCNA-positive indices for the colon mucosa did not differ between the ethanol treatment groups at any dose and the initiated controls. Ethanol also had no effect on cell proliferation in the non-MeIQx treatment groups.
Discussion
In past epidemiological studies, it has been proposed that almost 90% of large intestinal cancers might be caused by dietary factors19. Many researchers have pointed to relationships between dietary habits and colon cancer. HCAs and ethanol itself could be direct risk factors for human colon cancer, with other factors (fruits, vegetables, fiber and fecal weight) implicated as modulating their influences2,20,21. However, in the present study, we could not find any evidence that ethanol promotes MeIQx-induced ACF formation, although it has previousely been found to promote the formation of hepatocellular adenomas and/or carcinomas at doses of 3% or more via induction of cytochrome P450 2E1 (CYP 2E1)17. Previous studies of colorectal cancer using carcinogen-treated (azoxymethane or 1,2-dimethylhydrazine) rodent models have also suggested that ethanol does not promote colon carcinogenesis7,8.
On the other hand, ethanol may be considered a co-carcinogen, and the mechanisms of colorectal carcinogenesis stimulation of ethanol could well be related to metabolism of procarcinogens2. To clarify the effects of interaction between HCAs and ethanol in colon carcinogenesis, further studies after treatment with potent colon carcinogenic HCAs, PhiP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) or MeIQ (2-amino-3-methylimidazo[4,5-f]quinoline), are needed.
In the present study, ACF formation seemed to be affected in the 20% ethanol treatment group (Group 7) compared with the MeIQx-alone group (Group 1). The multiplicity of 3 crypt ACFs in Group 7 was significantly decreased, although other findings, such as the incidences of ACF, tumor formation and cell proliferation, were not affected. A similar tendency without statistical significance was observed for formation of ACFs in the ethanol only treatment group (Group 9) compared with the non-treatment group (Group 8). Several studies have revealed that food restriction inhibits development of ACFs and colon tumors22,23. In this context, it is interesting that food consumption and final body weight were significantly decreased in the 20% ethanol treatment groups (Group 7 and 9). These data suggest that the cause of decreased ACF formation was body weight loss due to decreased food intake, rather than the ethanol itself. Indeed, it remains a possibility that body weight loss nullified any promotion effects of ethanol on ACF formation. To clarify whether calorie restriction affects the effects of ethanol on ACF formation, further studies with an isocaloric diet are needed.
In conclusion, the present analysis of a surrogate maker for colon cancer indicates that ethanol has no promotional effect on MeIQx-initiated rat colon carcinogenesis, although it does promote rat hepatocarcinogenesis17.
Acknowledgments
This investigation was supported by a grant from the Ministry of Health, Labour and Welfare of Japan. We acknowledge the expert technical support of Kaori Touma and other technicians in the histological examination.
References
- 1.Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 39: 155–165 2004 [DOI] [PubMed] [Google Scholar]
- 2.Seitz HK, Poschl G, Stickel F. Alcohol and colorectal cancer. In: Exogenous Factors in Colonic Carcinogenesis. W Scheppach, M Scheuerle. (eds). Kluwer, Dordrecht, Boston, London. 128–141. 2003 [Google Scholar]
- 3.Scheppach W, Bingham S, Boutron-Ruault MC, Gerhardsson de Verdier M, Moreno V, Nagengast FM, Reifen R, Riboli E, Seitz HK, Wahrendorf J. WHO consensus statement on the role of nutrition in colorectal cancer. Eur J Cancer Prev. 8: 57–62 1999 [DOI] [PubMed] [Google Scholar]
- 4.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 8: 292–293 2007 [DOI] [PubMed] [Google Scholar]
- 5.Holmberg B, Ekstrom T. The effects of long-term oral administration of ethanol on Sprague-Dawley rats–a condensed report. Toxicology. 96: 133–145 1995 [DOI] [PubMed] [Google Scholar]
- 6.Seitz HK, Czygan P, Waldherr R, Veith S, Raedsch R, Kassmodel H, Kommerell B. Enhancement of 1,2-dimethylhydrazine-induced rectal carcinogenesis following chronic ethanol consumption in the rat. Gastroenterology. 86: 886–891 1984 [PubMed] [Google Scholar]
- 7.Hamilton SR, Sohn OS, Fiala ES. Effects of timing and quantity of chronic dietary ethanol consumption on azoxymethane-induced colonic carcinogenesis and azoxymethane metabolism in Fischer 344 rats. Cancer Res. 47: 4305–4311 1987 [PubMed] [Google Scholar]
- 8.Hamilton SR, Hyland J, McAvinchey D, Chaudhry Y, Hartka L, Kim HT, Cichon P, Floyd J, Turjman N, Kessie G, Nair PP, Dick J. Effects of chronic dietary beer and ethanol consumption on experimental colonic carcinogenesis by azoxymethane in rats. Cancer Res. 47: 1551–1559 1987 [PubMed] [Google Scholar]
- 9.Schut HA, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 20: 353–368 1999 [DOI] [PubMed] [Google Scholar]
- 10.Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A, Custer LJ, Lum-Jones A, Chang W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat Res. 506–507: 205–214 2002 [DOI] [PubMed] [Google Scholar]
- 11.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 506–507: 197–204 2002 [DOI] [PubMed] [Google Scholar]
- 12.Sugimura T. Studies on environmental chemical carcinogenesis in Japan. Science. 233: 312–318 1986 [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Ohgaki H, Hasegawa H, Sato S, Takayama S, Sugimura T. Carcinogenicity in rats of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Carcinogenesis. 9: 71–73 1988 [DOI] [PubMed] [Google Scholar]
- 14.Ohgaki H, Hasegawa H, Suenaga M, Sato S, Takayama S, Sugimura T. Carcinogenicity in mice of a mutagenic compound, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) from cooked foods. Carcinogenesis. 8: 665–668 1987 [DOI] [PubMed] [Google Scholar]
- 15.Tanakamaru Z, Mori I, Nishikawa A, Furukawa F, Takahashi M, Mori H. Essential similarities between spontaneous and MeIQx-promoted aberrant crypt foci in the F344 rat colon. Cancer Lett. 172: 143–149 2001 [DOI] [PubMed] [Google Scholar]
- 16.Okonogi H, Ushijima T, Shimizu H, Sugimura T, Nagao M. Induction of aberrant crypt foci in C57BL/6N mice by 2-amino-9H-pyrido[2,3-b]indole (A alphaC) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Cancer Lett. 111: 105–109 1997 [DOI] [PubMed] [Google Scholar]
- 17.Kushida M, Wanibuchi H, Morimura K, Kinoshita A, Kang JS, Puatanachokchai R, Wei M, Funae Y, Fukushima S. Dose-dependence of promotion of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline-induced rat hepatocarcinogenesis by ethanol: evidence for a threshold. Cancer Sci. 96: 747–757 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohr U. Digestive System. In: International classification of rodent tumours, part 1. In The Rat. U Mohr (ed). Lyon, 56– 64. 1997 [Google Scholar]
- 19.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 66: 1191–1308 1981 [PubMed] [Google Scholar]
- 20.Nowell S, Coles B, Sinha R, MacLeod S, Luke Ratnasinghe D, Stotts C, Kadlubar FF, Ambrosone CB, Lang NP. Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: contribution of metabolic variation to risk. Mutat Res. 506–507: 175–185 2002 [DOI] [PubMed] [Google Scholar]
- 21.Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL. Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst. 85: 884–891 1993 [DOI] [PubMed] [Google Scholar]
- 22.Lasko CM, Bird RP. Modulation of aberrant crypt foci by dietary fat and caloric restriction: the effects of delayed intervention. Cancer Epidemiol Biomarkers Prev. 4: 49–55 1995 [PubMed] [Google Scholar]
- 23.Kakuni M, Morimura K, Wanibuchi H, Ogawa M, Min W, Hayashi S, Fukushima S. Food restriction inhibits the growth of intestinal polyps in multiple intestinal neoplasia mouse. Jpn J Cancer Res. 93: 236–241 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]