Abstract
Toxicity assessment of nanoparticles, now widespread in our environment, is an important issue. We have focused attention on the carcinogenic potential of copper oxide (CuO) and titanium dioxide (TiO2). In experiment 1, a sequential pilot study, the effectiveness of a carcinogenic bioassay featuring intraperitoneal injection (i.p.) of 20 mg 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) or 0.1% N-bis(2-hydroxypropyl)nitrosamine (DHPN) in drinking water for 2 weeks was examined. Based on the results, DHPN, as the lung carcinogen, and evaluation at week 30 were selected as the most appropriate for our purposes in Experiment 1. In experiment 2, the carcinogenic bioassay was used to assess the carcinogenic potentials of instilled nanoparticles of CuO and TiO2. There were no significant intergroup differences in the lung neoplastic lesions induced by DHPN, although the neoplastic lesions induced by the nanoparticles in the CuO or TiO2 intratracheal instillation (i.t.) groups, demonstrated a tendency to increase compared with the microparticles administration. At the very least, the carcinogenic bioassay with DHPN proved useful for assessment of the modifying effects of instilled particles, and further assessment of the carcinogenic potential of nanoparticles appears warranted.
Keywords: nanoparticles, lung carcinogenicity, intratracheal instillation, NNK, DHPN
Introduction
The health risk posed by fine particles, especially nanoparticles in the air, has attracted much greater attention with the development of nanotechnology. The nanoparticle is a source of air pollution1, but there are very few established evaluation methods for detection of lung carcinogenicity.
We have previously described a rat in vivo bioassay for detection of hazards due to fine particles by intratracheal instillation (i.t.)2, which can be used for risk assessment, at an early stage, posed by materials inhaled into deep lung tissue. Sequential analysis of the effects of quartz (DQ-12, 4 mg/rat), a typical lung toxic agent3, revealed days 1 and 28 after instillation to be most appropriate for detection of acute and subacute inflammatory changes, respectively. For further validation, the toxicity of fine particles from various materials (quartz, hydrotalcite, potassium octatitanate, palladium oxide and carbon black) was examined in our in vivo bioassay4; 16 particle types, including nanoparticles were also examined5. With the same volume of particles, 2 mg/rat i.t., the nanoparticles of both CuO and NiO caused much more severe acute toxicity, such as acute death and severe inflammatory changes in neutrophil infiltration and edema, than micrometer-diameter particles of the same materials. These experiments confirmed the suitability of this bioassay for fine particle toxicity assessment. However, it cannot predict carcinogenicity.
We therefore aimed to establish a bioassay using the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) as an initiating carcinogen. In the lungs of A/J female mice, the initial event in this model is reported to be formation of O6-methylguanine-DNA, a major promutagenic adduct that leads to GC>AT transitional mispairing and subsequent activation of the K-ras proto-oncogene6,7. The bioassay model of NNK inducing lung tumor using A/J mice is used extensively, and we have previously reported the inhibitory effects of 8-methoxypsoralen, a potent human CYP2A6 inhibitor8,9. For carcinogenic assessment of fine particles by i.t., however, rats are more suitable because their tracheas are thick enough to allow instillation. There have been a number of reports of NNK-induced bioassay models using rats10,11, and in the present study, experiment 1, a pilot study, was conducted to investigate the effectiveness of NNK for a carcinogenic bioassay model using F344 rats. For comparison, N-bis(2-hydroxypropyl)nitrosamine (DHPN), a carcinogen targeting the lung, nasal cavity, paranasal cavity, liver, thyroid gland, kidney, esophagus, colon and urinary bladder12–14, was applied. Quartz was employed as a typical lung toxic agent to assess the influence of its inflammatory effects on lung tumor development.
In experiment 2, the bioassay model with DHPN assessed in experiment 1 was used to assess the carcinogenic potentials of nanoparticles of copper oxide (CuO) and titanium dioxide (TiO2) in comparison with the same materials of micrometer order size.
Materials and Methods
Chemicals
NNK (Toronto Research Chemicals, Toronto, ON, Canada) and DHPN (Nacalai Tesque Inc., Kyoto, Japan) were employed for initiation. Quartz dust (DQ-12) was obtained from Deutsche Montan Technologie (GmbH, Germany) with a particle diameter of less than 7 μm. Titanium dioxide with a particle diameter of less than 5 μm (Rutile form, Lot. TCG4139) (TiO2 micro) and titanium dioxide with a particle diameter of 80 nm (Lot. DPN0960) (TiO2 nano) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Copper oxide with a particle diameter of less than 5 μm (CAS1317-38-0; CuO micro) and copper oxide with a particle diameter of 33 nm (CAS1317-38-0; CuO nano) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The particles were all suspended in saline (Otsuka Isotonic sodium chloride solution, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan).
Animal treatment
Male F344/DuCrlCrj rats (4 weeks of age) purchased from Japan Charles River (Kanagawa, Japan) were maintained in the Kagawa University Animal Facility according to the Institutional Regulations for Animal Experiments. The protocols of the experiments were approved by the Animal Care and Use Committee. The animals were housed in polycarbonate cages with white wood chips for bedding and given free access to drinking water and a basal diet, CE-2 (CLEA Japan Inc., Tokyo, Japan), under controlled conditions of humidity (60 ± 10%), lighting (12-h light/dark cycle) and temperature (24 ± 2°C). The experiments were started after a 2-week acclimation period.
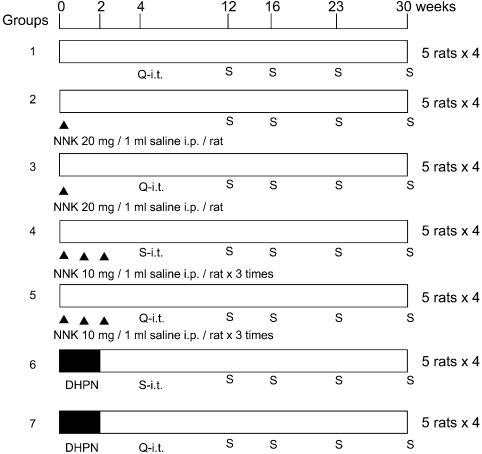
Experiment 1: The experimental design is shown in Fig. 1. A total of 140, 6-week-old, male F344 rats were randomly separated into 7 groups of 20 rats each. At the start of the experiment, groups 2 and 3 were administered a single intraperitoneal injection (IP) of 20 mg NNK suspended in 1 ml saline, groups 4 and 5 received 3 IPs of 10 mg NNK suspended in 1 ml saline in weeks 0, 1 and 2 and groups 6 and 7 were administered 0.1% DHPN in drinking water for 2 weeks. In week 4, groups 1, 3, 5 and 7 were exposed by i.t. to 0.5 mg quartz suspended in saline (0.2 ml) using an aerosolizer (PennCentury Philadelphia, PA, USA), and groups 4 and 6 were exposed by i.t. to 0.2 ml saline as vehicle control groups. The test solutions were sonicated to maintain as much suspension as possible and 0.2 ml solutions were instilled into each rate with 1 ml of air so that the solution would be spread throughout in the lung. Subgroups of 5 rats each were sacrificed in weeks 12, 16, 23 and 30 thereafter.
Fig. 1.
Design of experiment 1. A total of 140, 6-week-old, male F344 rats were employed. Groups 2 and 3: a single IP of 20 mg NNK/1 ml saline. Groups 3 and 4: 3 × IP of 10 mg NNK/ 1 ml saline at weeks 0, 1 and 2. Groups 6 and 7: 0.1% DHPN in drinking water for 2 weeks. At week 4, groups 1, 3, 5 and 7 received i.t. of 0.5 mg quartz (DQ-12)/0.2 ml saline (Q-i.t.), and groups 4 and 6 received 0.2 ml of saline (S-i.t.). Subgroups of 5 rats each were sacrificed (S) at weeks 12, 16, 23 and 30 thereafter.
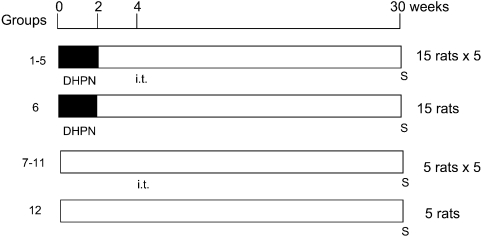
Experiment 2: The experimental design is shown in Fig. 2. A total of 120 rats (6-week-old) were randomly separated into 6 groups of 15 animals and an additional 6 groups of 5 animals each. Groups 1-6 were administered 0.1% DHPN in drinking water for 2 weeks. Groups 1–5 and 7–11 were exposed by i.t. to 0.5 mg/rat quartz (groups 1 and 7), CuO micro (groups 2 and 8), CuO nano (groups 3 and 9), TiO2 micro (groups 4 and 10) and TiO2 nano (groups 5 and 11) in saline (0.2 ml) using an aerosolizer in week 4. Group 6 was a DHPN control group, and Group 12 was an untreated control group. All rats were sacrificed in week 30.
Fig. 2.
Design of experiment 2. A total of 120 rats (6-week-old) were employed. Groups 1–6: 0.1% DHPN in drinking water for 2 weeks. Groups 1–5 and 7–11: i.t. of 0.5 mg/rat quartz (groups 1 and 7), CuO micro (groups 2 and 8), CuO nano (groups 3 and 9), TiO2 micro (groups 4 and 10) and TiO2 nano (groups 5 and 11) in week 4. Group 6 was a DHPN control group, and Group 12 was an untreated control group. All rats were sacrificed (S) at week 30.
At autopsy, the lungs, liver and kidneys were removed. The lungs, including the trachea and heart, were weighed, infused with 10% phosphate buffered formalin and then rinsed in 10% phosphate buffered formalin. The weights of the lungs were calculated by subtraction of the weight of the remaining trachea and heart. The organs were immersed in 10% phosphate buffered formalin for a week, and slices were routinely processed for embedding in paraffin for histopathological examination of H and E stained sections. The histopathological specimens were routinely 2 slices of the left lobe and 1 slice each of the other lobes.
Histopathological analysis
Lung lesions were categorized as hyperplasia, adenoma and adenocarcinoma in accordance with the established criteria15. Incidences were assessed along with numbers of hyperplasias, adenomas and adenocarcinomas in experiment 1. In experiment 2, in addition to the numbers, the areas of adenomas and adenocarcinomas were assessed using an Image Processor for Analytical Pathology (IPAP-WIN, Sumika Technoservice Corporation, Osaka, Japan)16. The numbers and areas in experiment 2 were indicated as: No. per 1 cm2, which was calculated as (number of lesions / total area cm2 of lung per a slide), and % Area, which was calculated as (total area of lesions / total area of lung per a slide) ×100.
Statistics
Body and organ weights and numbers and areas of the induced lung lesions were analyzed by the Tukey-Kramer post-hoc test. P values less than 0.05 were considered significant.
Results
Experiment 1
After intratracheal instillation, no symptoms of dyspnea were observed. The general conditions of the rats in all the groups did not change remarkably during the experimental period.
The average body weight of group 7 (DHPN and quartz i.t. group), 337.4 ± 19.6 (g), was greater than that of group 5 (10 mg NNK and quartz i.t. group), 297.9 ± 14.6, in week 23. There were no significant intergroup differences in body weight in the other groups. The absolute and relative weights of the lungs treated with quartz (groups 3, 5 and 7) were increased significantly compared with the control groups (groups 2, 4 and 6, respectively; data not shown).
In regard to macroscopic findings, white spots were seen on the surfaces of the lungs in the quartz i.t. groups. White nodules were observed in groups 6 and 7 (DHPN groups) in week 12, and these nodules grew sequentially thereafter. In the NNK and DHPN groups (groups 2–7), there were no differences in nodule appearance with or without quartz i.t.
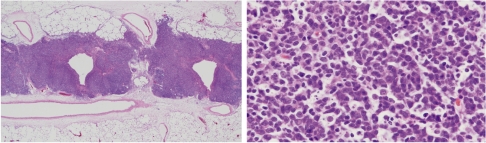
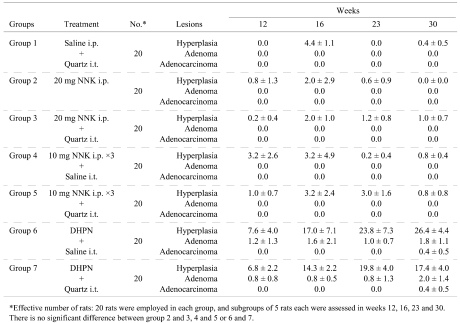
In regard to histopathological changes in the lungs, hyperplastic lesions with mild inflammatory changes were observed in groups 2–5 (NNK i.p. groups) at week 12, but the hyperplastic lesions then decreased with time. After week 23, large granular lymphocytic lymphomas (LGL lymphomas) were found around the trachea were apparent in all rats treated with NNK i.p. (groups 2–5; Fig. 3). The data for the numbers of lung lesions in the various groups are summarized in Table 1. The NNK IP groups (groups 2–5) did not demonstrate obvious development of epithelial tumors in the lungs despite the inflammatory changes caused by quartz. In the DHPN groups (groups 6 and 7), lung tumors and many hyperplasias were observed that increased in number and size with time. In the quartz i.t. groups (groups 1, 3, 5 and 7), severe inflammatory changes with neutrophil infiltration, histiocyte infiltration, pulmonary edema and granuloma formation were observed at weeks 12, 16 and 23. At week 30, they were somewhat reduced. The treatment with quartz i.t. did not influence the lung tumor development in this experiment. The livers and kidneys did not demonstrate any significant histopathological alteration in any group.
Fig. 3.
Histopathological appearance of a large granular lymphocytic lymphoma (LGL lymphoma) in group 2 (20 mg NNK i.p. group) in experiment 1. A, magnification 12.5×; B, magnification 400×.
Table 1. Number of Lesions in Lung Tissues Sections at Different Time Points (Experiment 1).
Experiment 2
No symptoms of dyspnea were observed after i.t. treatment, and the general conditions of the rats in all the groups did not change remarkably during the experimental period.
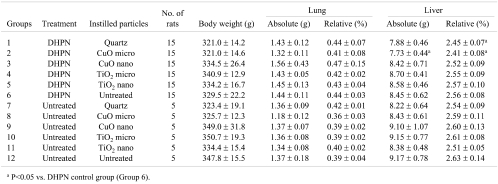
The final data for the body and organ weights in week 30 are shown in Table 2. The absolute and relative liver weights in group 2 (DHPN and CuO micro i.t.) were decreased significantly compared with group 6 (DHPN control group), while the absolute liver weights in group 1 (DHPN and quartz i.t.) were increased. The body, lung and kidney weights indicated no significant intergroup differences.
Table 2. Body and Organ Weights of the Rats (Experiment 2).
In regard to macroscopical findings, nodules in the lungs were observed in the DHPN groups 1–6, but there was no intergroup variation in number or area. In the DHPN untreated groups (groups 7–12), quartz i.t. (group 7) caused some white spots on the surface of the lungs, while CuO micro or nano i.t. (groups 8 and 9) induced mild nodulated changes. However, no lung lesions were found with TiO2 micro or nano i.t. (groups 10 and 11).
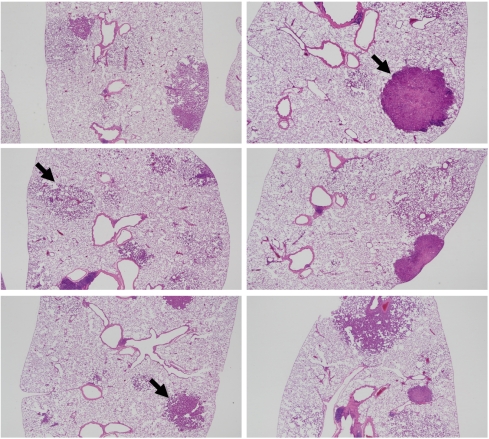
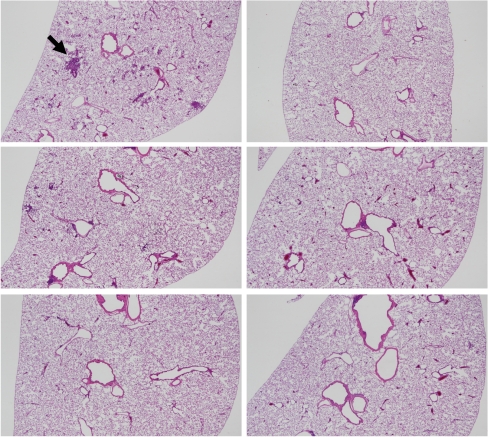
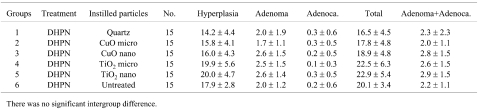
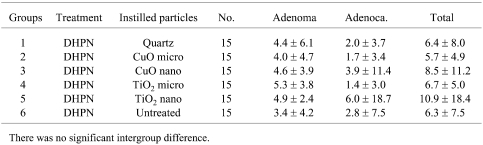
In regard to histopathological assessment, all the DHPN groups (groups 1–6) contained hyperplasias, adenomas and adenocarcinomas in the lungs (Fig. 4). In the DHPN untreated groups (Fig. 5), quartz i.t. (group 7) and, to a lesser extent, CuO micro i.t. (group 8) caused granulomas. CuO nano i.t. (group 9) induced a milder inflammatory change. Both TiO2 micro i.t. (group 10) and TiO2 nano i.t. (group 11) were not associated with any appreciable inflammatory change. The data for histopathological lesions in the DHPN groups (groups 1–6) are summarized in Tables 3 (the number of lesions per cm2 in a histopathological sections) and 4 (% areas). There were no significant intergroup differences in the numbers and areas of hyperplasias, adenomas and adenocarcinomas.
Fig. 4.
Histopathological findings for the DHPN groups (groups 1–6) in experiment 2. A, group 1 (quartz); B, group 6 (DHPN control), adenocarcinoma indicated by the arrow; C, group 2 (CuO micro), hyperplasia indicated by the arrow; D, group 3 (CuO nano); E, group 4 (TiO2 micro), adenoma indicated by the arrow; F, group 5 (TiO2 nano). The magnification in A–F is 12.5×.
Fig. 5.
Histopathological findings for the DHPN untreated groups (groups 7–12) in experiment 2. A, group 7 (quartz), granuloma indicated by the arrow; B, group 12 (untreated); C, group 8 (CuO micro); D, group 9 (CuO nano); E, group 10 (TiO2 micro); F, group 11 (TiO2 nano). The magnification is A–F is 12.5×.
Table 3. Number of Histopathological Lesions per cm2 of Lung (Experiment 2).
Table 4. Areas (%) of the Histopathological Lesions in the Lung Tissue Sections (Experiment 2).
Discussion and Conclusions
In experiment 1 of the present study, DHPN was found to be more effective for induction of lung lesions than NNK, the latter causing a 100% incidence of LGL lymphomas. The LGL lymphoma may be observed as a spontaneous neoplastic lesion in Fischer rats15; however Chung, et al. reported that 1.5 mg/kg body weight NNK administered by subcutaneous injection 3 times weekly for 21 weeks induces a 36% incidence in F344 rats11. In the DHPN groups (groups 6 and 7), lung hyperplasia, adenoma and adenocarcinoma increased sequentially, but quartz had no effect on their development. At an early stage of the experiment, severe inflammatory changes, especially neutrophil infiltration, were observed in the acute phase that became milder with time. Based on the results of experiment 1, DHPN, employed as a lung carcinogen, and evaluation at week 30 were concluded to be most appropriate for our purposes.
The results of experiment 2 showed that the numbers and areas of neoplastic lesions induced by DHPN exhibited no significant intergroup differences in groups 1–6. However, in groups 3 and 5, the CuO or TiO2 nanoparticle i.t. groups, the numbers and areas of neoplastic lesions tended to increase compared with the data for microparticles (groups 2 and 4). Copper undergoes redox-cycling reactions and produces reactive oxygen species17, though it has an important role in the biological systems of various metalloenzymes18,19. Abe et al. reported about the carcinogenic risk of copper gluconate, a copper-containing compound, using a rat medium-term multi-organ carcinogenicity bioassay model20. Their report showed that copper gluconate admixed in a diet at a concentration of 300–6000 ppm might pose carcinogenic risks for the liver and forestomach at high dose levels. TiO2 has been classified as belonging to Group 2B (possibly carcinogenic to humans) in an International Agency for Research on Cancer (IARC) publication21. It has also been reported that TiO2 has carcinogenic potential22 and causes formation of DNA adducts23. On the other hand, it has also been reported that TiO2 should not exert carcinogenic effects on the human lung based on an overview epidemiology and toxicology studies in mice, rats and hamsters24.
In general, nanoparticles are likely to exert a greater impact because of the areas and characters of their surfaces25. The causes of the lack of clear effects in experiment 2 might be related to the low doses or frequencies of instillation used. Intratracheal administration of nanoparticles induces severe inflammatory changes in the acute phase in the rat lung at high doses5, but given chronically, the nanoparticles may be eliminated easily and may induce only mild inflammatory changes. It is well known that nanoparticles spread from the primary target-organ to other organs. For example, in an oral administration study in mice, nanoparticles of titanium dioxide were found to be retained in many organs, such as the liver, spleen, kidneys and lung tissues, after uptake through the gastrointestinal tract26. This spreading ability to other organs of nanoparticles may indicate that the nanoparticle is cleared from the primary organ, like the lungs, earlier than microparticles. However there are no reports supporting this hypothesis, and further experimentation is needed to validate the evidence.
In conclusion, there were no significant intergroup differences in the neoplastic lesions induced by DHPN with nanoparticles of CuO or TiO2 by i.t. despite a tendency for increase compared with the microparticle i.t. groups. However, this carcinogenic bioassay with DHPN appears useful for detection of the modified effects of instilled particles. Assessment of the harmful effects of nanoparticles is an important priority and should be pursued in more investigations, such as with intraperitoneal application of multiwall carbon nanotubes, which have been observed induce mesotheliomas in the p53+/- mouse27.
Acknowledgments
This work was supported by a Grants-in-Aid for Scientific Research (KAKENHI; No. 18790272) and for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and a grant from the Japan Society for the Promotion of Science (JSPS). We thank Dr. Malcolm A. Moore, a native English-speaking scientist, for critical reading of the manuscript.
References
- 1.Donaldson K, Seaton A. The Janus faces of nanoparticles. J Nanosci Nanotechnol. 7: 4607–4611 2007 [PubMed] [Google Scholar]
- 2.Yokohira M, Takeuchi H, Yamakawa K, Saoo K, Ikeda M, Matsuda Y, Zeng Y, Hosokawa K, Maeta H, Imaida K. Establishment of a bioassay system for detection of lung toxicity due to fine particle instillation: Sequential histopathological changes with acute and subacute lung damage due to intratracheal instillation of quartz in F344 male rats. J Toxicol Pathol. 18: 13–18 2005 [Google Scholar]
- 3.Bruch J, Rehn B, Song W, Gono E, Malkusch W. Toxicological investigations on silicon carbide. 2. In vitro cell tests and long term injection tests. Br J Ind Med. 50: 807–813 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokohira M, Takeuchi H, Yamakawa K, Saoo K, Matsuda Y, Zeng Y, Hosokawa K, Imaida K. Bioassay by intratracheal instillation for detection of lung toxicity due to fine particles in F344 male rats. Exp Toxicol Pathol. 58: 211–221 2007 [DOI] [PubMed] [Google Scholar]
- 5.Yokohira M, Kuno T, Yamakawa K, Hosokawa K, Matsuda Y, Hashimoto N, Suzuki S, Saoo K, Imaida K. Lung toxicity of 16 fine particles on intratracheal instillation in a bioassay model using F344 male rats. Toxicol Pathol. 36: 620–631 2008 [DOI] [PubMed] [Google Scholar]
- 6.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 51: 5557–5564 1991 [PubMed] [Google Scholar]
- 7.Ronai ZA, Gradia S, Peterson LA, Hecht SS. G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis. 14: 2419–2422 1993 [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H, Saoo K, Yokohira M, Ikeda M, Maeta H, Miyazaki M, Yamazaki H, Kamataki T, Imaida K. Pretreatment with 8-methoxypsoralen, a potent human CYP2A6 inhibitor, strongly inhibits lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Cancer Res. 63: 7581–7583 2003 [PubMed] [Google Scholar]
- 9.Takeuchi H, Saoo K, Matsuda Y, Yokohira M, Yamakawa K, Zeng Y, Miyazaki M, Fujieda M, Kamataki T, Imaida K. Dose dependent inhibitory effects of dietary 8-methoxypsoralen on NNK-induced lung tumorigenesis in female A/J mice. Cancer Lett. 234: 232–238 2006 [DOI] [PubMed] [Google Scholar]
- 10.Huynh HT, Teel RW. Effects of intragastrically administered Pycnogenol on NNK metabolism in F344 rats. Anticancer Res. 19: 2095–2099 1999 [PubMed] [Google Scholar]
- 11.Chung FL, Kelloff G, Steele V, Pittman B, Zang E, Jiao D, Rigotty J, Choi CI, Rivenson A. Chemopreventive efficacy of arylalkyl isothiocyanates and N-acetylcysteine for lung tumorigenesis in Fischer rats. Cancer Res. 56: 772–778 1996 [PubMed] [Google Scholar]
- 12.Kitamura Y, Umemura T, Kanki K, Ishii Y, Kuroiwa Y, Masegi T, Nishikawa A, Hirose M. Lung as a new target in rats of 2-amino-3-methylimidazo[4,5-f]quinoline carcinogenesis: results of a two-stage model initiated with N-bis(2-hydroxypropyl)nitrosamine. Cancer Sci. 97: 368–373 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seike N, Wanibuchi H, Morimura K, Nishikawa T, Kishida H, Nakae D, Hirata K, Fukushima S. Lack of promoting effect due to oral administration of dimethylarsinic acid on rat lung carcinogenesis initiated with N-bis(2-hydroxypropyl)nitrosamine. Cancer Lett. 175: 113–119 2002 [DOI] [PubMed] [Google Scholar]
- 14.Konishi Y, Kondo H, Ikeda T, Kawabata A, Shoji Y, Denda A. Effect of dose on the carcinogenic activity of orally administered N-bis(2-hydroxypropyl)nitrosamine in rats. Gann. 69: 573–577 1978 [PubMed] [Google Scholar]
- 15.Gary AB, Scot LE, Michel RE, Charles AM, William FM. Pathology of the Fischer Rat, Reference and Atlas. Academic Press, 350–357. 1990 [Google Scholar]
- 16.Watanabe T, Katsura Y, Yoshitake A, Masataki H, Mori T. IPAP : Image processor for analytical pathology. J Toxicol Pathol. 7: 353–361 1994 [Google Scholar]
- 17.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 12: 1161–1208 2005 [DOI] [PubMed] [Google Scholar]
- 18.Prohaska JR, Geissler J, Brokate B, Broderius M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp Biol Med (Maywood). 228: 959–966 2003 [DOI] [PubMed] [Google Scholar]
- 19.Wasser IM, de Vries S, Moenne-Loccoz P, Schroder I, Karlin KD. Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NO(x) redox chemistry. Chem Rev. 102: 1201–1234 2002 [DOI] [PubMed] [Google Scholar]
- 20.Abe M, Suzuki N, Yoshida M, Usuda K, Furukawa S, Juneja LR, Okubo T, Nakae D. Possible carcinogenic risks of copper gluconate and their prevention by co-administered green tea catechins evaluated by a rat medium-term multi-organ carcinogenicity bioassay protocol. Food Chem Toxicol. 46: 1760–1770 2008 [DOI] [PubMed] [Google Scholar]
- 21.Overall Evaluations of Carcinogenicity to HumansGroup 2B: Possibly carcinogenic to humans. IARC Monograph. 93: 1–5 2006 [Google Scholar]
- 22.Lee KP, Trochimowicz HJ, Reinhardt CF. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 79: 179–192 1985 [DOI] [PubMed] [Google Scholar]
- 23.Gallagher J, Heinrich U, George M, Hendee L, Phillips DH, Lewtas J. Formation of DNA adducts in rat lung following chronic inhalation of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis. 15: 1291–1299 1994 [DOI] [PubMed] [Google Scholar]
- 24.Hext PM, Tomenson JA, Thompson P. Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup Hyg. 49: 461–472 2005 [DOI] [PubMed] [Google Scholar]
- 25.Powers KW, Brown SC, Krishna VB, Wasdo SC, Moudgil BM, Roberts SM. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol Sci. 90: 296–303 2006 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 168: 176–185 2007 [DOI] [PubMed] [Google Scholar]
- 27.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53+/– mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 33: 105–116 2008 [DOI] [PubMed] [Google Scholar]