Abstract
Cardiac hypertrophy was observed in a 9-week-old Crl:CD(SD) rat that died unexpectedly. The animal was allocated to the control group of a toxicity study, and no abnormalities in its general conditions, body weight or food intake were observed. Necropsy revealed an increase in heart weight. Gross examination indicated cardiac enlargement with thickening of the right and left ventricular walls. Histopathological examination revealed hypertrophy of the cardiomyocytes in the right and left ventricular walls and the interventricular septum. Electron microscopic examination indicated bizarre nuclei and accumulation of an increased number of various sizes of mitochondria in the perinuclear region of the hypertrophied myocytes. Hypertrophied myocytes connected by intensely folded intercalated disks were also observed. Based on these findings, the animal was diagnosed with cardiac hypertrophy. This is the first case report of cardiac hypertrophy in this strain.
Keywords: SD rat, heart, hypertrophy
Cardiac hypertrophy is cellular adaptation of the heart in response to pressure or volume overload that results from hypertension and cardiovascular malformations such as ventricular septal defect (VSD) and valvular heart disease. Cardiac hypertrophy has been reported in several rat models1–5. For example, cardiac hypertrophy has been experimentally induced in Dahl salt-sensitive rats and rats with constricted aortas. Untreated spontaneously hypertensive rats (SHR) and Wistar Kyoto (WKY) rats also develop cardiac hypertrophy. Increased heart weights and thickening of the ventricular wall have been observed in these rats.
Crl:CD(SD) (SD) rats have been used increasingly in recent toxicity studies, and background data on these rats have been collected. However, few cases of spontaneous cardiac hypertrophy have been reported in this strain. Herein, we describe a case of spontaneous cardiac hypertrophy in a 9-week-old SD rat that died unexpectedly.
This male SD rat was obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) at 5 weeks of age and was handled in accordance with the guidelines for the care and use of laboratory animals established by the Ethical Committee for Animal Experiments of Yakult Central Institute. After an acclimation period of 1 week, the rat was allocated to the control group of a 4-week repeated dose toxicity study, and distilled water was orally administered to the rat daily. However, the rat died unexpectedly on day 22. During the study, no abnormalities were observed in its general conditions, body weight or food intake. Necropsy revealed cardiac enlargement (Fig. 1a) and an increase in heart weight (Table 1)6. Thickening of the right (RV) and left ventricular (LV) walls and slight enlargement of both ventricular cavities were observed in a cross section of the heart (Fig. 1b). The thickening was more prominent in the right ventricle. After fixation, the rat’s lung was pale yellow. No abnormal changes were observed in other organs. The heart and other organs were routinely fixed in 10% phosphate-buffered formalin solution, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE) or using Masson’s trichrome techniques. Immunohistochemical staining for proliferating cell nuclear antigen (PCNA; DAKO, Denmark) or ED1 (Serotec, United Kingdom) was performed using the labeled streptavidin-biotin method. To evaluate cardiomyocyte hypertrophy, the short diameters of the cardiomyocytes were measured in 3 areas (outer layer, inner layer and middle layer) of the RV and LV walls or the interventricular septum (IVS) in HE-stained sections. The short diameters of cardiomyocytes were measured across the nuclei, and 30 cells were counted histologically in each area. For electron microscopic examination, small pieces of the formalin-fixed right ventricle of the heart were first immersed in phosphate-buffered 2.5% glutaraldehyde and then in phosphate-buffered 1% osmium tetroxide for 2 h each. After dehydration through a graded ethanol series, the tissue samples were embedded in Epon 812 resin. Ultrathin sections were prepared and stained with uranyl acetate and lead citrate and then observed under a transmission electron microscope (JEM-1200EX; JEOL, Tokyo, Japan). Histopathological examination indicated cardiomyocyte hypertrophy in the RV and LV walls and IVS. The cardiomyocyte diameter indicated that hypertrophy was most prominent in the RV wall (Figs. 2 and 3). Hypertrophied myocytes had large nuclei, some of which were positive for PCNA. This suggested that cardiomyocytes would be occurred polyploidization7–9. Cardiomyocyte hypertrophy, myocardial degeneration, infiltration of mononuclear cells (histiocytes: positive for ED1) and interstitial/perivascular fibrosis (stained blue by Masson’s trichrome) were also observed in the IVS of the heart (Fig. 4). In addition, disarray of cardiomyocytes was observed at the junction of the IVS and ventricular walls. Furthermore, cellular infiltration of the subepicardium was observed. In the lungs, slight increases in collagen fiber at the alveolar lesion and macrophages containing hemosiderin (heart failure cells) in the alveolar lumen were observed. These findings indicated that pulmonary congestion was a secondary change caused by heart failure due to cardiac hypertrophy. No abnormal histological changes were observed except in the heart and lungs. Electron microscopic examination identified bizarre nuclei in hypertrophied myocytes and accumulation of an increased number of various sizes of mitochondria in the cytoplasm of the hypertrophied myocytes in the perinuclear region. Hypertrophied myocytes connected by intensely folded intercalated disks were also observed (Fig. 5).
Fig. 1.
Gross appearance of cardiac hypertrophy in the 9-week-old SD rat (present case, H). Cardiac enlargement and thickening of the right and left ventricular walls compared with a 10-week old normal SD rat (N) are observed in the (a) whole heart and (b) transverse sections of the heart.
Table 1. Absolute and Relative Organ Weights of the 9-Week-Old SD Rat that Developed Spontaneous Cardiac Hypertrophy (Present Case) and 9-Week-Old Normal SD Rats.
Fig. 2.
Cross sections of the heart (present case, a–d) of the 9-week-old SD rat that developed spontaneous cardiac hypertrophy and (e–h) of a 10-week-old normal SD rat. Hypertrophy of the myocytes can be seen in the (b) right ventricular (RV) wall, (c) interventricular septum (IVS) and (d) left ventricular (LV) wall. Normal myocytes can be seen in the (f) RV wall, (g) IVS and (h) LV wall. HE staining. b, c, d, f, g, and h: ×400.
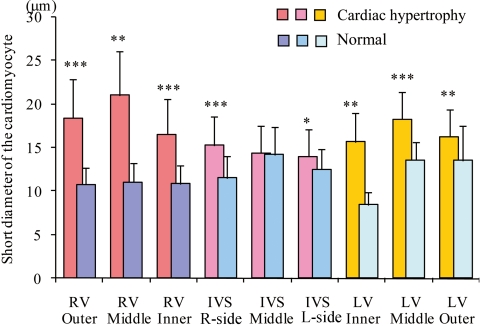
Fig. 3.
Comparison of the short diameters of hypertrophic cardiomyocytes with those in a 10-week-old normal SD rat. The cardiomyocytes of the rat with cardiac hypertrophy are significantly larger in all layers, except for the middle interventricular septum (IVS) layer. *: p < 0.05, **: p < 0.01, ***: p < 0.001. RV, right ventricular wall; LV, left ventricular wall.
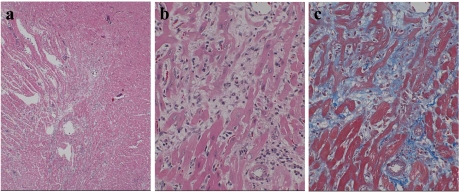
Fig. 4.
(a) Myocardial degeneration, (b) infiltration of mononuclear cells and (c) interstitial/perivascular fibrosis at the interventricular septum (IVS) of the hypertrophic heart. a: HE staining, ×100; b: HE staining, ×400; c: Masson’s trichrome, ×400.
Fig. 5.
Electron microscopic findings of cardiomyocytes obtained from the SD rat that developed spontaneous cardiac hypertrophy. (a) Various sizes of mitochondria and an increase in the number of mitochondria in the cytoplasm, (b) bizarre nuclei and (c) an intensely folded intercalated disk connecting two adjacent myocytes can be seen. (d–f) Cardiomyocytes from a 10-week-old normal SD rat.
SHR and WKY rats are known to spontaneously develop cardiac hypertrophy. SHR rats develop cardiac hypertrophy in response to hypertension, and LV hypertrophy is characteristic of this strain. In addition to these changes in the heart, periarteritis nodosa in mesenteric and pancreatic arteries; fibrinoid deposition in medium and small renal arteries; and arteriolar infarction of the kidney, testis and brain have been reported in SHR rats4. Generalized edema, pleural effusion, ascites, congestion of the lung and liver and nephrosclerosis have also been reported in SHR rats. On the other hand, normotensive WKY rats develop cardiac hypertrophy because of cardiovascular malformations, such as VSD, and LV and biventricular hypertrophy are characteristic in this strain5,10. In the present case, cardiac changes, increase in heart weight, cardiac enlargement, thickening of the ventricular walls and cardiomyocyte hypertrophy were observed that were similar to those observed in SHR and WKY rats. Pulmonary congestion was also observed in the present case. However, there were no obvious changes in other organs and tissues. In the present case, the cause of hypertrophy was unknown; however, (1) the rat had biventricular hypertrophy, (2) no obvious changes in the blood vessels of the kidney or the brain indicating hypertension were observed, (3) the rat died relatively young (at 9 weeks of age) and (4) Crl:CD(SD) rats have been known to develop congenital anomalies of the heart11. Thus, it is possible that the cardiac hypertrophy in this rat could have resulted from cardiovascular malformation or valvular heart disease.
In the present case, the main finding in the heart was cardiomyocyte hypertrophy, although myocardial degeneration, infiltration of histiocytes and fibrosis were also observed at the IVS. Furthermore, the electron microscopic findings reflected the fact that the myocardial hypertrophy of the present case had progressed to a stage of adaptation or compensated hyperfunction. Based on these findings, the SD rat that died unexpectedly at 9-weeks old was diagnosed with cardiac hypertrophy.
References
- 1.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 39: 89–105 1998 [DOI] [PubMed] [Google Scholar]
- 2.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 39: 60–76 1998 [DOI] [PubMed] [Google Scholar]
- 3.Mercadier JJ, Lompre AM, Wisnewsky C, Samuel JL, Bercovici J, Swynghedauw B, Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 49: 525–532 1981 [DOI] [PubMed] [Google Scholar]
- 4.Okamoto K, Aoki K, Nosaka S, Fukushima M. Cardiovascular diseases in the spontaneously hypertensive rat. Jpn Circ J. 28: 943–952 1964 [DOI] [PubMed] [Google Scholar]
- 5.Aiello EA, Villa-Abrille MC, Escudero EM, Portiansky EL, Perez NG, Hurtado MCC, Cingolani HE. Myocardial hypertrophy of normotensive Wistar-Kyoto rats. Am J Physiol Heart Circ Physiol. 286: H1229–H1235 2003 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Kondou K, Ashino T, Tsukamoto S, Hongo C, Kosazuma T. Background data of general toxicological parameters in Crj:CD(SD)IGS rats at 9 and 13 weeks of age. In: Biological Reference Data on CD(SD)IGS Rats – 1999. Matsuzawa T and Inoue H (eds). BEST PRINTING, Tokyo. 46–51. 1999 [Google Scholar]
- 7.Meessen H. Structural bases of myocardial hypertrophy. Br Heart J. 33: 94–99 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koda M, Takemura G, Okada H, Kanoh M, Maruyama R, Esaki M, Li Y, Miyata S, Kanamori H, Li L, Ogino A, Kondo T, Minatoguchi S, Fujiwara T, Fujiwara H. Nuclear hypertrophy reflects increased biosynthetic activities in myocytes of human hypertrophic hearts. Circ J. 70: 710–718 2006 [DOI] [PubMed] [Google Scholar]
- 9.Baroja A, de la Hoz C, Alvarez A, Ispizua A, Bilbao J, de Gandarias JM. Genesis and evolution of high-ploidy tumour cells evaluated by means of the proliferation markeers p34cdc2, cyclin B1, PCNA and 3[H]-thymidine. Cell Prolif. 29: 89–100 1996 [PubMed] [Google Scholar]
- 10.Slama M, Susic D, Varagic J, Frohlich ED. High rate of ventricular septal defects in WKY rats. Hypertension. 40: 175–178 2002 [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi T, Okuda H, Kasahara Y, Ushigome S, Niikura E, Mizutani M, Matsushima T. Background control data of reproductive and developmental toxicity study in Crj:CD(SD)IGS rats – 2000. In: Biological Reference Data on CD(SD)IGS Rats – 2000. Matsuzawa T and Inoue H (eds). BEST PRINTING, Tokyo. 136–140. 2002 [Google Scholar]