Abstract
Although omega-3 (n-3) fatty acids negatively regulate triglyceride biosynthesis, the mechanisms mediating this effect are poorly understood, and emerging evidence suggests that stearoyl-CoA desaturase (Scd1) is required for de novo triglyceride biosynthesis. To investigate this mechanism, we determined the effects of perinatal n-3 deficiency and postnatal repletion on rat liver Scd1 mRNA expression and activity indices (liver 16:1/16:0 & 18:1/18:0 ratios), and determined relationships with postprandial (non-fasting) plasma triglyceride levels. Rats were fed conventional diets with or without the n-3 fatty acid precursor α-linolenic acid (ALA, 18:3n-3) during perinatal development (E0-P100), and a subset of rats fed the ALA− diet were switched to the ALA+ diet post-weaning (P21-P100, repletion). Compared with controls, rats fed the ALA− diet exhibited significantly lower liver long-chain n-3 fatty acid compositions and elevations in monounsaturated fatty acid composition, both of which were normalized in repleted rats. Liver Scd1 mRNA expression and activity indices (16:1/16:0 & 18:1/18:0 ratios) were significantly greater in n-3 deficient rats compared with controls and repleted rats. Among all rats, liver Scd1 mRNA expression was positively correlated with liver 18:1/18:0 and 16:1/16:0 ratios. Plasma triglyceride levels, but not glucose or insulin levels, were significantly greater in n-3 deficient rats compared with controls and repleted rats. Liver Scd1 mRNA expression and activity indices were positively correlated with plasma triglyceride levels. These preclinical findings demonstrate that n-3 fatty acid status is an important determinant of liver Scd1 mRNA expression and activity, and suggest that down-regulation of Scd1 is a mechanism by which n-3 fatty acids repress constitutive triglyceride biosynthesis.
Keywords: Omega-3 fatty acid, stearoyl-CoA desaturase-1 (Scd1), triglyceride, glucose, liver, rat
1. Introduction
Elevated fasting plasma triglyceride (TG) levels are an independent risk factor for cardiovascular disease [1], and postprandial (non-fasting) TG levels are a strong predictor of future cardiovascular risk [2–4]. Supplementation with long-chain omega-3 (n-3) fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), dose-dependently decrease elevated TG levels in patients with hypertriglyceridemia [5,6], and lower levels of long-chain n-3 fatty acids are associated with increased cardiovascular disease risk [7,8]. Recurrent mood disorders, including major depressive disorder and bipolar disorder, are associated with long-chain n-3 fatty acid deficits [9], elevated TG levels [10,11], and excess mortality attributable in part to premature cardiovascular-related disease [12]. While these clinical observations suggest that n-3 fatty acid status is an important determinant of lipid homeostasis, the mechanisms mediating this relationship remain poorly understood.
An emerging body of evidence suggests that stearoyl-CoA desaturase (Scd1, delta9-desaturase) plays a central role in regulating hepatic TG biosynthesis. Scd1 is the rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids (MUFA), palimitoleic acid (16:1n-7) and oleic acid (18:1n-9), from saturated fatty acids (SFA), palmitic acid (16:0) and stearic acid (18:0), respectively. Oleic acid is a required substrate for the synthesis of TG [13], and Scd1 mutant mice exhibit deficits in TG biosynthesis [14–16]. Pharmacological inhibition of the Scd1 enzyme reduces elevated TG levels in rodent disease models [17]. In rodents [16] and human subjects [18,19], liver Scd1 mRNA expression is correlated with activity indices (16:1/16:0 and 18:1/18:0 ratios) in liver and plasma triglyceride fractions, and elevations in the plasma 18:1/18:0 ratio (‘desaturation index’) are positively correlated with plasma TG levels [18]. This body of evidence suggests that Scd1 plays a central role in the regulation of hepatic TG biosynthesis.
Several in vitro and ex vivo studies have found that short and long-chain n-3 and n-6 polyunsaturated fatty acids (PUFA), but not oleic acid (18:1n-9) or stearic acid (18:0), decrease Scd1 expression at the level of transcription and mRNA stability [20–22]. Additionally, dietary supplementation with long-chain n-3 fatty acids (fish oil) decrease Scd1 activity in liver microsomes ex vivo [23] and reduce liver TG synthesis and/or secretion in different rodent models [24–29]. Moreover, a prior study found that dietary supplementation with EPA (20:5n-3) decreased mouse liver Scd1 mRNA expression and hepatic TG content [30]. While these findings implicate n-3 fatty acids as a negative regulator of liver Scd1 mRNA expression, another study found that chronic dietary n-3 fatty acid deficiency resulting in a depletion of rat liver n-3 fatty acids did not significantly alter liver Scd1 mRNA expression [31]. To further evaluate this mechanism, we investigated the effect of perinatal n-3 fatty acid deficiency, which produces robust reductions in peripheral n-3 fatty acid levels in adulthood, and repletion on liver Scd1 mRNA expression and activity indices (liver 18:1/18:0 & 16:1/16:0 ratios), and investigated relationships with non-fasting plasma TG concentrations. In view of evidence also implicating Scd1 expression/activity in insulin sensitivity [32,33], we additionally investigated relationships with plasma glucose and insulin concentrations. Our primary hypothesis was that n-3 fatty acid deficiency would increase liver Scd1 expression/activity in association with plasma TG levels, and that this response would be corrected by normalization of n-3 fatty acid status.
2. Materials and methods
2.1. Diets
Diets were either α-linolenic acid (ALA, 18:3n-3)-fortified (ALA+, TD.04285) or ALA− free (ALA−, TD.04286)(Harlan-TEKLAD, Madison, WI). The compositions of ALA+ and ALA− diets are presented in Table 1. Diets were vacuum packaged and stored at 4°C. Both diets provided 3.8 Kcal/g, and were matched for percent kcal from protein (19.2%), carbohydrate (64.4%), and fat (16.5%). Analysis of diet fatty acid composition by gas chromatography confirmed that both diets were closely matched in saturated fatty acids, monounsaturated fatty acids, and the n-6 fatty acid precursor linoleic acid (18:2n-6), and that neither diet contained preformed n-3 or n-6 fatty acids including DHA and AA, respectively (Table 1).
Table 1.
Diet Compositions
| Ingredient1 | ALA+ | ALA− |
|---|---|---|
| Casein, vitamine free | 20 | 20 |
| Carbohydrate | ||
| Cornstarch | 20 | 20 |
| Sucrose | 27 | 27 |
| Dextrose | 9.9 | 9.9 |
| Maltose-dextrin | 6 | 6 |
| Cellulose | 5 | 5 |
| Mineral mix (AIMN-93G-MX) | 3.5 | 3.5 |
| Vitamin mix (AIN-93-VX) | 1 | 1 |
| L-Cystine | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 |
| Fat | ||
| Hydrogenated coconut oil | 4.5 | 5.1 |
| Safflower | 1.9 | 1.9 |
| Flaxseed | 0.6 | 0 |
| Fatty acid composition2 | ||
| C8:0 | 4.3 | 5.0 |
| C10:0 | 3.8 | 4.2 |
| C12:0 | 29 | 32.8 |
| C14:0 | 11 | 12.5 |
| C16:0 | 8.3 | 8.7 |
| C18:0 | 9.4 | 10.2 |
| 18:1n-9 | 6.7 | 4.7 |
| 18:2n-6 | 22.7 | 21.9 |
| 20:4n-6 | nd | nd |
| 18:3n-3 | 4.6 | nd |
| 22:6n-3 | nd | nd |
g/100 g diet
wt % of total fatty acids
nd = not detected
2.2. Animals
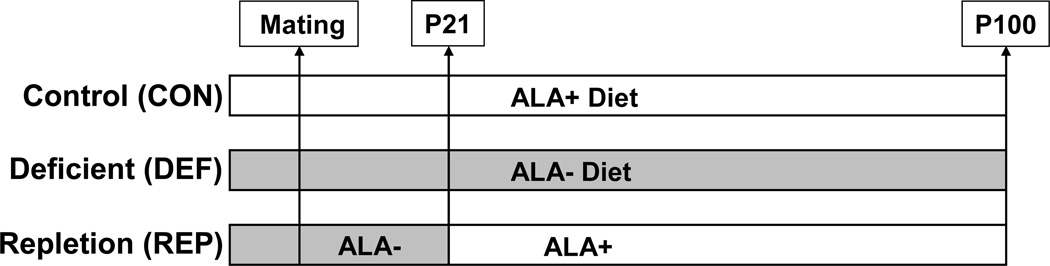
The experimental design used for generating the different treatment groups is illustrated in Figure 1. Male offspring bred in-house to nulliparous female Long-Evans hooded rats were used. For perinatal n-3 deficiency, dams were fed the n-3-deficient diet for 1 month prior to mating through weaning, and male offspring were maintained on the ALA− diet post-weaning (P21) to adulthood (P100)(n=10). Controls were born to dams maintained on the ALA+ diet, and received the ALA+ diet post-weaning (P21) to adulthood (P100)(n=10). Repleted rats were offspring of dams maintained on the ALA− diet, and switched to ALA+ diet post-weaning (P21) to adulthood (P100)(n=10). Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard vivarium conditions on a 12:12 h light:dark cycle. Food (g/kg/d) and water (ml/kg/d) intake and body weight (kg) were recorded. Rats were sacrificed by decapitation on P100–101 between 9:00–12:00 am in a counter-balanced manner relative to the common removal of food hoppers at 9:00 am. Trunk blood was collected into EDTA-coated tubes, and plasma isolated by centrifugation at 4°C. Liver was harvested and flash frozen in liquid nitrogen. All samples were stored at −80°C. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
Figure 1.
Diagram illustrating the experimental design used to generate control (CON), n-3-deficient (DEF), and n-3-repleted (REP) rats. Controls were born to nulliparous dams maintained on diet fortified with α-linolenic acid (ALA+ diet), and received the ALA+ diet post-weaning (P21) to adulthood (P100). For perinatal n-3 deficiency, dams were fed the ALA− diet for 1 month prior to mating through weaning, and offspring were maintained on the ALA− diet post-weaning (P21) to adulthood (P100). Repleted rats were offspring of dams maintained on the ALA− diet for 1 month prior to mating, and switched to ALA+ diet post-weaning (P21) to adulthood (P100).
2.3. Fatty acid composition
The gas chromatography procedure used to determine liver fatty acid composition has been described in detail previously [34]. Briefly, total fatty acid composition was determined with a Shimadzu GC-2010 (Shimadzu Scientific Instruments Inc., Columbia MD). The column is a DB-23 (123-2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 µM (J&W Scientific, Folsom CA). The carrier gas is helium with a column flow rate of 2.5 ml/min. Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 7.4 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). The limit of detection was 7 µg of an individual fatty acid in a 100 mg sample of liver tissue. Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All analyses were performed by a technician blinded to treatment. We focused our primary analysis on the principle substrates (16:0, 18:0) and products (16:1n-7, 18:1n-9) of Scd1, and liver 16:1/16:0 and 18:1/18:0 ratios were calculated as indices of liver Scd1 activity.
2.4. Plasma TG, glucose, and insulin levels
Plasma TG (GPO-PAP, RANDOX Laboratories Ltd., Antrim UK), glucose (GOD-POD, Genzyme Diagnostics P.E.I. Inc., Charlottetown, PE, Canada), and insulin (ELISA, Linco Research, St. Charles MI, USA) concentrations were determined using commercially available kits according to the manufacturer’s instructions. All analyses were performed by a technician blinded to treatment.
2.5. Liver Scd1 mRNA expression
Frozen liver was homogenized (BioLogics Model 300 V/T ultrasonic homogenizer, Manassas, VA) in Tri Reagent (MRC Inc., Cincinnati, OH), and total RNA isolated and purified using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Total RNA was treated to remove potential DNA contamination using RNase-free DNase (Qiagen, Valencia, CA), and RNA quantified using a Nanodrop instrument (Nanodrop Instruments, Wilmington, DE). RNA quality was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). cDNA was prepared from 2 µg total RNA using a high-capacity RT cDNA Archive Kit (Applied Biosystems, Foster City, CA) along with no RT controls to confirm lack of genomic DNA contamination. Liver mRNA levels of stearoyl-CoA desaturase (Scd1, Rn00594894_g1) were measured by real-time quantitative PCR using an ABI 7900HT500 Real Time PCR System (Applied Biosystems, Foster City, CA). The nucleotide sequences of primer/probe sets can be obtained from www.appliedbiosystems.com. Sample were run in Microamp Fast 96 well reaction plates using 20 µl reaction volumes consisting of 10 µl of 2X TaqMan Fast Universal PCR Matermix (Applied Biosystems, Foster City, CA), 1 µl of TaqMan gene expression assay, and 9 µl of cDNA. No template controls (NTC) substituting DEPC-treated water for cDNA were run with each plate to verify lack of cross contamination. Thermal cycling conditions were: 95°C for 10 min followed by 95°C for 1 sec denaturing step and 60°C for 20 sec, annealing step for 40 cycles. Data were analyzed by comparing the difference between target gene and endogenous control (GAPDH, Rn99999916_s1) cycle thresholds for each sample using the comparative Ct method [35].
2.6. Statistical analysis
Group (control, deficient, repleted) differences in fatty acid composition, plasma TG, glucose, and insulin levels, and liver gene expression were evaluated with a one-way ANOVA, and individual group differences compared with unpaired t-tests (2-tail, α=0.05). Parametric (Pearson) correlation analyses were performed to determine relationships between liver fatty acid data, plasma analytes, and gene expression data (2-tail, α=0.05). Analyses were performed with GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Food/water intake and body weight
There were no significant group differences in food intake, F(2,29)=0.6, p=0.58 (CON: 60.8±2.6; DEF: 58±2.5; REP: 60.0±2.1 g/kg/d) or water intake, F(2,29)=2.4, p=0.1 (CON: 45.9±5.2; DEF: 45.7±6.2; REP: 42.1±2.9 ml/kg/d). There were no significant group differences in baseline (P60) body weight, F(2,29)=1.0, p=0.38 (CON: 349.9±8.6; DEF: 345±10; REP: 351±9.2 kg) or endpoint body weight, F(2,29)=1.1, p=0.34 (CON: 461±12; DEF: 431±14; REP: 441±14 kg).
3.2. Liver PUFA composition
Group differences in liver fatty acid composition are presented in Table 2. There was a significant main effect of treatment (diet) for liver DHA (22:6n-3) composition, F(2,29)=221, p≤0.0001, arachidonic acid (AA, 20:4n-6) composition, F(2,29)=4.7, p=0.01, and linoleic acid (18:2n-6) composition, F(2,29)=110, p≤0.0001. In all cases, these fatty acids were significantly lower in n-3 deficient rats compared with both controls and repleted rats, and did not differ between control and repleted rats. The short-chain ALA (18:3n-3) and long-chain n-3 fatty acids EPA (20:5n-3) and docosapentaenoic acid (22:5n-3) were depleted (not detectable) in n-3 deficient rat liver.
Table 2.
Liver fatty acid composition
| Fatty Acid1 | CON (n=10) | DEF (n=10) | REP (n=10) | P-value2 |
|---|---|---|---|---|
| C14:0 | 0.46 ± 0.03 | 1.63 ± 0.07 *** | 0.38 ± 0.01 ### | P<0.0001 |
| C16:0 | 18.33 ± 0.24 | 22.67 ± 0.84 *** | 18.20 ± 0.21 ### | P<0.0001 |
| C18:0 | 18.09 ± 0.37 | 15.50 ± 0.52 *** | 18.32 ± 0.29 ### | P<0.0001 |
| Total SFA | 36.88 ± 0.20 | 39.80 ± 0.62 *** | 36.91 ± 0.18 ### | P<0.0001 |
| 16:1n-7 | 1.01 ± 0.10 | 4.71 ± 0.41 *** | 1.00 ± 0.07 ### | P<0.0001 |
| 18:1n-9 | 7.14 ± 0.25 | 15.01 ± 1.18 *** | 7.06 ± 0.29 ### | P<0.0001 |
| 18:1n-7 | 3.06 ± 0.14 | 4.66 ± 0.24 *** | 2.67 ± 0.05 ### | P<0.0001 |
| Total MUFA | 11.21 ± 0.42 | 24.38 ± 1.44 *** | 10.72 ± 0.36 ### | P<0.0001 |
| 18:2n-6 | 18.07 ± 0.78 | 7.30 ± 0.60 *** | 18.60 ± 0.37 ### | P<0.0001 |
| 18:3n-6 | 0.21 ± 0.01 | 0.17 ± 0.02 | 0.20 ± 0.01 | P=0.17 |
| 20:3n-6 | 0.71 ± 0.04 | 0.61 ± 0.05 | 0.68 ± 0.02 | P=0.21 |
| 20:4n-6 | 23.11 ± 0.51 | 20.19 ± 1.14 * | 22.96 ± 0.39 # | P=0.017 |
| 22:4n-6 | 0.41 ± 0.03 | 0.48 ± 0.04 | 0.46 ± 0.02 | P=0.25 |
| 22:5n-6 | 0.19 ± 0.02 | 4.70 ± 0.23 *** | 0.22 ± 0.02 ### | P<0.0001 |
| Total n-6 | 43.08 ± 0.52 | 33.76 ± 1.84 *** | 43.49 ± 0.46 ### | P<0.0001 |
| 18:3n-3 | 0.54 ± 0.05 | nd *** | 0.61 ± 0.02 ### | P<0.0001 |
| 20:5n-3 | 0.29 ± 0.02 | nd *** | 0.29 ± 0.01 ### | P<0.0001 |
| 22:5n-3 | 0.93 ± 0.03 | nd *** | 0.95 ± 0.02 ### | P<0.0001 |
| 22:6n-3 | 6.63 ± 0.36 | 0.47 ± 0.03 *** | 6.80 ± 0.21 ### | P<0.0001 |
| Total n-3 | 8.39 ± 0.28 | 0.47 ± 0.03 *** | 8.65 ± 0.21 ### | P<0.0001 |
Data are mean fatty acid wt % of total fatty acids ± SEM (nd = not detected)
One-way ANOVA (2-tailed),
P ≤ 0.05,
P ≤ 0.001 versus CON,
P ≤ 0.05,
P ≤ 0.001 versus DEF.
3.3. Liver Scd1 mRNA expression and product/precursor ratios
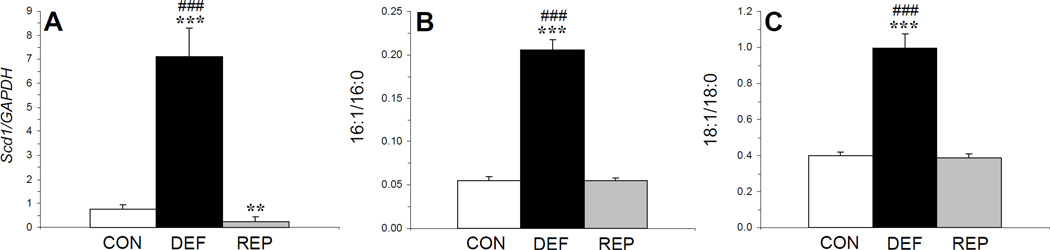
The main effect of treatment was not significant for GAPDH mRNA expression, F(2,26)=2.1, p=0.14. There was a significant main effect of treatment for liver Scd1/GAPDH mRNA expression, F(2,26)=41.5, p≤0.0001 (Fig. 2A). Scd1 mRNA expression was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and was lower in repleted rats compared with controls (p=0.008). The main effect of treatment was significant for the liver 16:1/16:0 ratio F(2,29)=119, p≤0.0001 (Fig. 2B). The 16:1/16:0 ratio was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.96). The main effect of treatment was significant for the liver 18:1/18:0 ratio F(2,29)=29, p≤0.0001 (Fig. 2C). The 18:1/18:0 ratio was significantly greater in n-3-deficient rats compared with both controls (p≤0.0001) and repleted rats (p≤0.0001), and did not differ between control and repleted rats (p=0.74).
Figure 2.
Liver Scd1/GAPDH mRNA expression (A), and liver 16:1/16:0 (B) and 18:1/18:0 (C) ratios in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. **p≤0.01, ***p≤0.0001 vs. controls, ###p≤0.0001 vs. DEF rats.
3.4. Plasma TG, glucose, and insulin levels
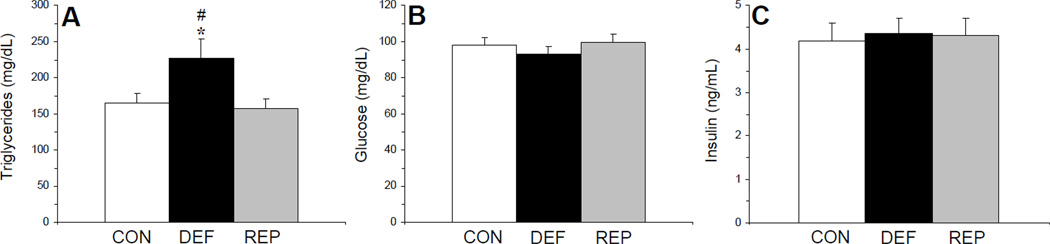
There was a significant main effect of treatment for plasma TG concentrations, F(2,29)=4.1, p=0.03 (Fig. 3A). TG concentrations were significantly higher in n-3-deficient rats compared with both controls (p=0.04) and repleted rats (p=0.03), and did not differ between control and repleted rats (p=0.67). The main effect of treatment was not significant for plasma glucose concentrations, F(2,29)=0.6, p=0.56 (Fig. 3B) or plasma insulin concentrations, F(2,29)=0.05, p=0.95 (Fig. 3C).
Figure 3.
Plasma triglyceride (A), glucose (B), and insulin (C) concentrations in control (CON, n=10), n-3-deficient (DEF, n=10), and n-3-repleted (REP, n=10) rats. Values are group mean ± S.E.M. *p≤0.05 vs. controls, #p≤0.05 vs. DEF rats.
3.5. Linear regression analyses
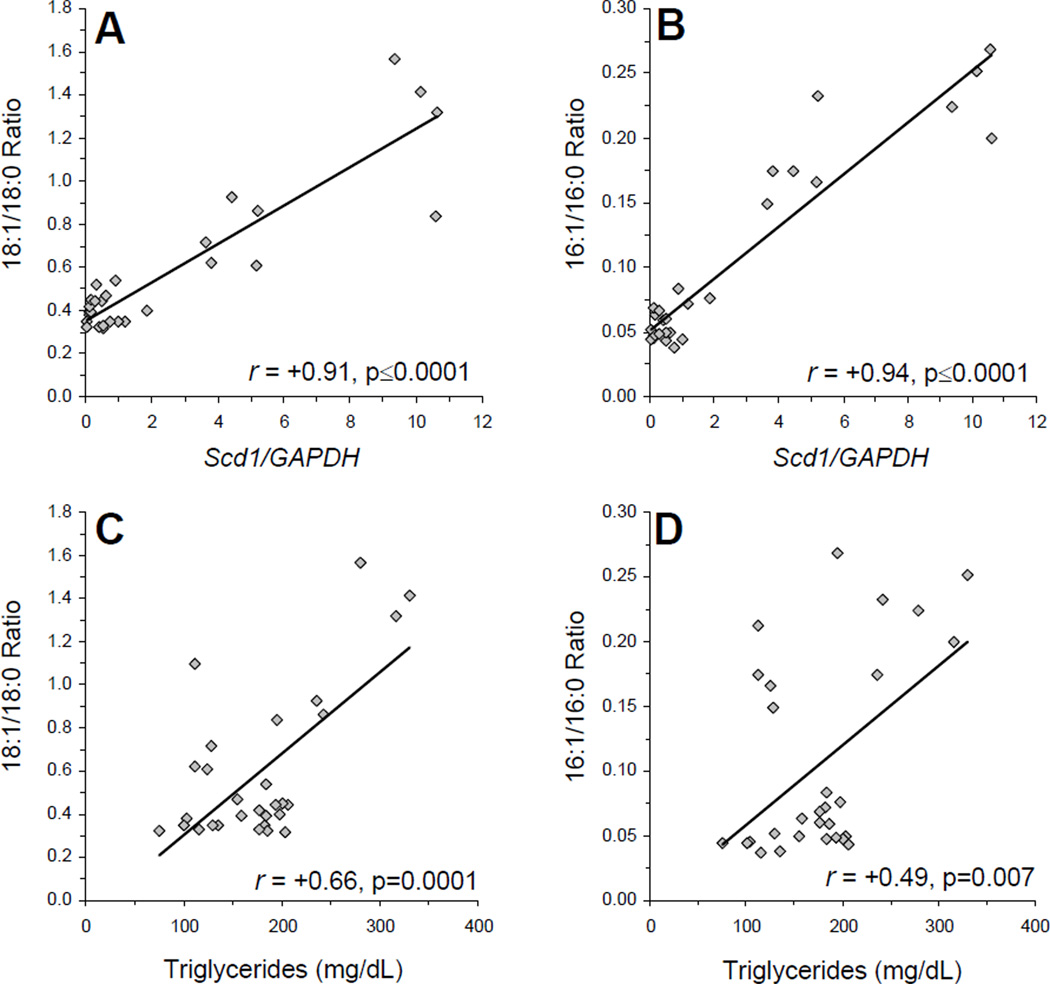
Among all rats (n=30), liver Scd1 mRNA expression was positively correlated with the liver 18:1/18:0 (r = +0.91, p≤0.0001)(Fig. 4A) and 16:1/16:0 (r = +0.94, p≤0.0001)(Fig. 4B) ratios, and inversely correlated with liver DHA (r = −0.84, p≤0.0001), AA (r = −0.69, p≤0.0001), and LA (r = −0.89, p≤0.0001) compositions. The 18:1/18:0 ratio was inversely correlated with liver DHA (r = −0.86, p≤0.0001), AA (r = −0.85, p≤0.0001), and LA (r = −0.86, p≤0.0001) compositions. Similarly, the 16:1/16:0 ratio was inversely correlated with liver DHA (r = −0.91, p≤0.0001), AA (r = −0.63, p=0.0002), and LA (r = −0.93, p≤0.0001) compositions. Plasma TG concentrations were positively correlated with Scd1 mRNA expression (r = +0.50, p=0.004), as well as 18:1/18:0 (r = +0.66, p=0.0001)(Fig. 4C) and 16:1/16:0 (r = +0.49, p=0.007)(Fig. 4D) ratios. Plasma TG concentrations were inversely correlated with liver DHA (r = −0.33, p=0.04), AA (r = −0.63, p=0.0002), and LA (r = −0.36, p=0.04) compositions. No significant correlations were observed for plasma glucose or insulin concentrations.
Figure 4.
Relationships between liver Scd1 mRNA expression the liver 16:1/16:0 (A) and 18:1/18:0 (B) ratios, and relationship between plasma TG concentrations and liver 16:1/16:0 (C) and 18:1/18:0 (D) ratios among all rats (n=30). Pearson correlation coefficients and associated p-values (two-tailed) are presented.
4. Discussion
This study demonstrates that chronic dietary n-3 fatty acid deficiency robustly up-regulates Scd1 mRNA expression in rat liver, and that this response is prevented by normalization of liver n-3 fatty acid composition. Greater liver Scd1 mRNA expression was associated with greater liver Scd1 activity indices (16:1/16:0 & 18:1/18:0), and liver Scd1 mRNA expression was positively correlated with both 16:1/16:0 and 18:1/18:0 ratios. We additionally demonstrate that n-3 fatty acid deficiency significantly increases non-fasting plasma TG concentrations, and that this response is positively correlated with liver Scd1 mRNA expression and activity indices. These effects could not be attributed to group differences in dietary fat intake, and were not associated with greater body weight gain or plasma glucose of insulin concentrations. Together, these findings demonstrate that n-3 fatty acids negatively regulate liver Scd1 expression/activity in vivo, and support prior evidence that this mechanism is associated with elevated TG biosynthesis.
The finding of greater liver Scd1 mRNA expression in n-3 deficient rats is not consistent with a prior study finding that chronic dietary n-3 fatty acid deficiency did not significantly alter rat liver Scd1 mRNA expression [31]. Potentially relevant differences between the present and this prior study include when n-3 fatty acid deficiency was initiated (perinatal vs. post-weaning), rat strain (Long-Evans hooded vs. Fisher-344), and diet composition. Despite similar 97–99% depletions of long-chain n-3 fatty acids in the livers of n-3 deficient rats in both studies, the Igarshi et al. [31] study found liver AA composition was increased in n-3 deficient rats, whereas it was reduced in the present study. Moreover, reductions in liver LA (18:2n-6) composition were more robust in the present study. Importantly, AA and LA are both potent inhibitors of Scd1 transcription in vitro and in vivo [21,22,36], and liver AA and LA compositions were both inversely correlated with Scd1 mRNA expression in the present study. Taken together, these data suggest that up-regulation of liver Scd1 mRNA expression in response to n-3 fatty acid deficiency may require concomitant reductions liver AA and LA composition.
The observation that liver Scd1 mRNA expression was positively correlated with liver 16:1/16:0 and 18:1/18:0 ratios is consistent with elevated liver Scd1 enzyme activity in n-3 deficient rats [16,18,19]. This finding is also consistent with the prior finding that n-3 deficient rats exhibit elevated indices of Scd1 activity in adipocytes [37], and the finding that feeding long-chain n-3 fatty acids significantly decrease Scd1 enzyme activity in liver microsomes ex vivo [23] and in vivo mouse liver [30]. Furthermore, the present finding that liver 16:1/16:0 and 18:1/18:0 ratios were both positively correlated with plasma TG levels is consistent with prior in vivo studies demonstrating that liver Scd1 expression and activity indices are required for hepatic TG biosynthesis [14,15]. Together, these data suggest that up-regulation of liver Scd1 expression/activity is a mechanism contributing to elevated TG concentrations in response to n-3 fatty acid deficiency.
Prior preclinical and clinical studies have found that Scd1 activity indices are positively associated with excess adiposity and obesity [38]. For example, Scd1 mutant mice exhibit reduced adiposity independent of body weight gain, and are resistance to diet-induced obesity [39]. In the present study, elevations in liver Scd1 expression and activity in n-3 deficient rats were not associated with significantly greater body weight gain. Indeed, n-3 deficient rats exhibited a trend towards lower endpoint body weight compared with controls. Interestingly, a prior study also found that n-3 deficient mice exhibited a significant decrease in body weight despite greater liver TG content [40]. Although we did not investigate visceral adiposity in the present study, these findings suggest that elevated liver Scd1 expression/activity and TG synthesis resulting from n-3 fatty acid deficiency do not lead to excessive weight gain under the current dietary conditions. Additional studies are warranted to further evaluate the relationship liver Scd1 expression/activity and visceral adiposity in the n-3 fatty acid deficient rat model.
A role for n-3 fatty acids in the regulation of glucose metabolic homeostasis is supported by a number of findings [41], and several findings suggest that elevations in liver Scd1 activity and TG biosynthesis are associated with insulin resistance. For example, Scd1 mutant mice exhibit both impaired TG biosynthesis [14,15] and increased insulin sensitivity [33]. Pharmacological inhibition of the Scd1 enzyme reduces elevated TG and glucose levels in rodent disease models [17,32]. In human subjects, elevations in the plasma 18:1/18:0 ratio, an index of SCD1 enzyme activity (‘desaturation index’), are associated with greater plasma TG levels and insulin resistance [18,42]. In the present study, elevations in liver Scd1 expression/activity and plasma TG levels were not accompanied by significant alterations in plasma glucose or insulin levels. Indeed, n-3 deficient rats exhibited a trend towards lower glucose levels. Although additional studies will be required to further characterize this relationship, this finding suggest that elevated Scd1 expression/activity and plasma TG levels are not sufficient to induce a dysregulation in glucose homeostasis under the current dietary conditions. In agreement with a prior study [43], these findings also suggest that elevated liver Scd1 expression/activity in response to n-3 fatty acid deficiency is not mediated through an insulin-dependent mechanism.
In summary, the present data demonstrate that n-3 fatty acid deficiency up-regulates liver Scd1 mRNA expression and activity indices and increase plasma TG concentrations in vivo. The present data also demonstrate that normalization of liver n-3 fatty acid status normalizes liver Scd1 mRNA expression/activity and plasma TG levels. Taken in conjunction with prior findings, these data suggest that up-regulation of liver Scd1 expression/activity may represent a mechanism contributing to elevated plasma TG concentrations in response to n-3 fatty acid deficiency. These and previous data encourage future clinical studies to investigate the relationship between Scd1 mRNA expression/activity and plasma TG levels in disorders associated with n-3 fatty acid deficiency to evaluate pathophysiological relevance.
Acknowledgments
This work was supported in part by NIH grants MH083924 (to R.K.M.), DK59630 (to P.T.), and DK056863 (to S.C.B.). The authors thank S. Hofmann and E. Donelan for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 2.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 3.Eberly LE, Stamler J, Neaton JD Multiple Risk Factor Intervention Trial Research Group. Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163:1077–1083. doi: 10.1001/archinte.163.9.1077. [DOI] [PubMed] [Google Scholar]
- 4.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 5.McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715–728. doi: 10.1592/phco.27.5.715. [DOI] [PubMed] [Google Scholar]
- 6.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–252. doi: 10.3945/ajcn.110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008;10:503–509. doi: 10.1007/s11883-008-0078-z. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 9.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baghai TC, Varallo-Bedarida G, Born C, Häfner S, Schüle C, Eser D, Rupprecht R, Bondy B, von Schacky C. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 index. J Clin Psychiatry. 2011 doi: 10.4088/JCP.09m05895blu. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller DD, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–137. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 13.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024. [PubMed] [Google Scholar]
- 16.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 17.Uto Y, Ogata T, Kiyotsuka Y, Ueno Y, Miyazawa Y, Kurata H, Deguchi T, Watanabe N, Konishi M, Okuyama R, Kurikawa N, Takagi T, Wakimoto S, Ohsumi J. Novel benzoylpiperidine-based stearoyl-CoA desaturase-1 inhibitors: Identification of 6-[4-(2-methylbenzoyl)piperidin-1-yl]pyridazine-3-carboxylic acid (2-hydroxy-2-pyridin-3-ylethyl) amide and its plasma triglyceride-lowering effects in Zucker fatty rats. Bioorg Med Chem Lett. 2010;20:341–345. doi: 10.1016/j.bmcl.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 18.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Peter A, Cegan A, Wagner S, Lehmann R, Stefan N, Königsrainer A, Königsrainer I, Häring HU, Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin Chem. 2009;55:2113–2120. doi: 10.1373/clinchem.2009.127274. [DOI] [PubMed] [Google Scholar]
- 20.Landschulz KT, Jump DB, MacDougald OA, Lane MD. Transcriptional control of the stearoyl-CoA desaturase-1 gene by polyunsaturated fatty acids. Biochem Biophys Res Commun. 1994;200:763–768. doi: 10.1006/bbrc.1994.1516. [DOI] [PubMed] [Google Scholar]
- 21.Ntambi JM, Sessler AM, Takova T. A model cell line to study regulation of stearoyl-CoA desaturase gene 1 expression by insulin and polyunsaturated fatty acids. Biochem Biophys Res Commun. 1996;220:990–995. doi: 10.1006/bbrc.1996.0520. [DOI] [PubMed] [Google Scholar]
- 22.Sessler AM, Kaur N, Palta JP, Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. J Biol Chem. 1996;271:29854–29858. doi: 10.1074/jbc.271.47.29854. [DOI] [PubMed] [Google Scholar]
- 23.Garg ML, Wierzbicki AA, Thomson AB, Clandinin MT. Dietary cholesterol and/or n-3 fatty acid modulate delta 9-desaturase activity in rat liver microsomes. Biochim Biophys Acta. 1988;962:330–336. doi: 10.1016/0005-2760(88)90262-7. [DOI] [PubMed] [Google Scholar]
- 24.Hassanali Z, Ametaj BN, Field CJ, Proctor SD, Vine DF. Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes Metab. 2010;12:139–147. doi: 10.1111/j.1463-1326.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Borthwick F, Hassanali Z, Wang Y, Mangat R, Ruth M, Shi D, Jaeschke A, Russell JC, Field CJ, Proctor SD, Vine DF. Chronic dietary n-3 PUFA intervention improves dyslipidaemia and subsequent cardiovascular complications in the JCR:LA-cp rat model of the metabolic syndrome. Br J Nutr. 2011;31:1–11. doi: 10.1017/S0007114510005453. [DOI] [PubMed] [Google Scholar]
- 26.Mustad VA, Demichele S, Huang YS, Mika A, Lubbers N, Berthiaume N, Polakowski J, Zinker B. Differential effects of n-3 polyunsaturated fatty acids on metabolic control and vascular reactivity in the type 2 diabetic ob/ob mouse. Metabolism. 2006;55:1365–1374. doi: 10.1016/j.metabol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Oarada M, Tsuzuki T, Gonoi T, Igarashi M, Kamei K, Nikawa T, Hirasaka K, Ogawa T, Miyazawa T, Nakagawa K, Kurita N. Effects of dietary fish oil on lipid peroxidation and serum triacylglycerol levels in psychologically stressed mice. Nutrition. 2008;24:67–75. doi: 10.1016/j.nut.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Qi K, Fan C, Jiang J, Zhu H, Jiao H, Meng Q, Deckelbaum RJ. Omega-3 fatty acid containing diets decrease plasma triglyceride concentrations in mice by reducing endogenous triglyceride synthesis and enhancing the blood clearance of triglyceride-rich particles. Clin Nutr. 2008;27:424–430. doi: 10.1016/j.clnu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Ryan AS, Bailey-Hall E, Nelson EB, Salem N., Jr The hypolipidemic effect of an ethyl ester of algal-docosahexaenoic acid in rats fed a high-fructose diet. Lipids. 2009;44:817–826. doi: 10.1007/s11745-009-3330-6. [DOI] [PubMed] [Google Scholar]
- 30.Kajikawa S, Harada T, Kawashima A, Imada K, Mizuguchi K. Highly purified eicosapentaenoic acid prevents the progression of hepatic steatosis by repressing monounsaturated fatty acid synthesis in high-fat/high-sucrose diet-fed mice. Prostaglandins Leukot Essent Fatty Acids. 2009;80:229–238. doi: 10.1016/j.plefa.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Issandou M, Bouillot A, Brusq JM, Forest MC, Grillot D, Guillard R, Martin S, Michiels C, Sulpice T, Daugan A. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur J Pharmacol. 2009;618:28–36. doi: 10.1016/j.ejphar.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci U S A. 2003;100:11110–11115. doi: 10.1073/pnas.1934571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Mutch DM, Grigorov M, Berger A, Fay LB, Roberts MA, Watkins SM, Williamson G, German JB. An integrative metabolism approach identifies stearoyl-CoA desaturase as a target for an arachidonate-enriched diet. FASEB J. 2005;19:599–601. doi: 10.1096/fj.04-2674fje. [DOI] [PubMed] [Google Scholar]
- 37.Oguzhan B, Sancho V, Acitores A, Villanueva-Peñacarrillo ML, Portois L, Chardigny JM, Sener A, Carpentier YA, Malaisse WJ. Alteration of adipocyte metabolism in omega3 fatty acid-depleted rats. Horm Metab Res. 2006;38:789–798. doi: 10.1055/s-2006-956180. [DOI] [PubMed] [Google Scholar]
- 38.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pachikian BD, Neyrinck AM, Cani PD, Portois L, Deldicque L, De Backer FC, Bindels LB, Sohet FM, Malaisse WJ, Francaux M, Carpentier YA, Delzenne NM. Hepatic steatosis in n-3 fatty acid depleted mice: focus on metabolic alterations related to tissue fatty acid composition. BMC Physiol. 2008;8:21. doi: 10.1186/1472-6793-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–440. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 42.Sampath H, Ntambi JM. Role of stearoyl-CoA desaturase in human metabolic disease. Future Lipidology. 2008;3:163–173. [Google Scholar]
- 43.Waters KM, Ntambi JM. Polyunsaturated fatty acids inhibit hepatic stearoyl-CoA desaturase-1 gene in diabetic mice. Lipids. 1996;31 Suppl:S33–S36. doi: 10.1007/BF02637047. [DOI] [PubMed] [Google Scholar]