Abstract
Although the medial prefrontal cortex (mPFC) has been shown to be integrally involved in extinction of a number of associative behaviors, its role in extinction of alcohol (ethanol)-induced associative learning has received little attention. Previous reports have provided evidence supporting a role for the mPFC in acquisition and extinction of amphetamine-induced conditioned place preference (CPP) in rats, however, it remains unknown if this region is necessary for extinction of ethanol (EtOH)-induced associative learning in mice. Using immunohistochemical analysis of phosphorylated and unphosphorylated cAMP response element-binding protein (CREB), the current set of experiments first showed that the prelimbic (PL) and infralimbic (IL) subregions of the mPFC exhibited dynamic responses in phosphorylation of CREB to a Pavlovian-conditioned, EtOH-paired cue. Interestingly, CREB phosphorylation within these regions was sensitive to manipulations of the EtOH-cue contingency—that is, the cue-induced increase of pCREB in both the PL and IL was absent following extinction. In order to confirm a functional role of the mPFC in regulating the extinction process, we then showed that electrolytic lesions of the mPFC following acquisition blocked subsequent extinction of EtOH-CPP. Together, these experiments indicate a role for the PL and IL subregions of the mPFC in processing changes of the EtOH-cue contingency, as well as in regulating extinction of EtOH-induced associative learning in mice.
Keywords: Extinction, Medial Prefrontal Cortex, Ethanol, Associative Learning, Conditioned Place Preference, Mice
1. INTRODUCTION
Rehabilitation from drug- and alcohol-associated addictive behaviors represents a uniquely difficult task in the clinic. Because patients who pursue rehabilitation treatments have most often spent many years with an addiction, breaking the control that these drug-seeking behaviors exert over a patient’s everyday life is a daunting task—one that most often ends in relapse. Development of, and relapse to, addictive disorders is thought to be strongly influenced by a broad spectrum of drug-associated environmental cues and contexts that, following years of drug-related learning, are able to initiate and maintain drug-seeking behaviors (for review see Duka et al., 2010). It is for this reason that rehabilitation treatments of drug- and alcohol-associated addictive disorders have included cue-exposure therapy (CET)—a type of extinction training during which previously drug-paired cues are presented in a nonreinforced manner in hopes of reducing their valence and subsequent behavioral control (e.g., Drummond & Glautier, 1994). However, despite the effectiveness of extinction to reduce drug-seeking behavior in animals (for review see Millan et al., 2011), cue-exposure therapy has been largely ineffective at increasing rates of abstinence (Conklin & Tiffany, 2002). Therefore, it is important to continue to further our understanding of the neurobiological substrates underlying extinction of drug- and alcohol-seeking behavior in animals in order to improve rehabilitation treatments, including CET, in humans.
In the conditioned place preference (CPP) model of drug-induced associative learning, a neutral stimulus attains significant incentive value after being repeatedly paired with a drug of abuse such as ethanol (EtOH) (Cunningham et al., 2011). Following this straightforward Pavlovian-conditioning procedure, approach behavior to the EtOH-paired conditioned stimulus (CS) is assessed during a preference test. In subsequent extinction of CPP, animals are exposed to the same set of cues, but in a nonreinforced manner (i.e., in the absence of EtOH). Similar to acquisition, extinction of CPP represents associative learning that depends on reward-prediction error, and both phases of learning can be represented by negative exponential functions of associative strength, as reflected in the model described by Rescorla & Wagner (1972). The extinction phase of associative behaviors such as CPP is thought to weaken the conditioned response elicited by the CS through an inhibitory mechanism (Pavlov, 1927; Konorski, 1967). Furthermore, the behavioral inhibition exhibited during extinction is thought to involve de novo memory formation that leaves the original association intact (Bouton, 2004).
Recent evidence suggests that the medial prefrontal cortex (mPFC) is involved in both the expression and extinction of conditioned fear and operant drug-seeking behavior (for review see Peters et al., 2009). Specifically, the prelimbic (PL) subregion has been suggested to be primarily involved in expression of conditioned behaviors while the infralimbic (IL) subregion is involved in consolidation (and therefore also retrieval) of extinction (Vidal-Gonzalez et al., 2006; Quirk & Mueller, 2008). Following extinction, exposure to cues capable of reinstating drug-seeking behavior has been shown to cause significant activation of both the PL and IL subregions of the mPFC (Zavala et al., 2007). With regards to expression of non-operant drug-induced associative learning, a brief cocaine-CPP expression test resulted in significant activation of the PL (Miller & Marshall, 2005) while inactivation of the mPFC (both PL & IL) impaired extinction of amphetamine-CPP (Hsu & Packard, 2008). Additionally, inactivation of the mPFC reinstated heroin-CPP, an effect the authors attributed to a disinhibition of place preference expression (Ovari & Leri, 2008). Contrary to these findings however, Zavala et al. (2003) reported that PL lesions had no effect on acquisition, expression, or extinction of cocaine-induced CPP. Thus, despite its broad support for a role in extinction of conditioned fear and drug-induced operant behavior, there remains conflicting evidence for the role of the mPFC in extinction of CPP.
The involvement of the mPFC in extinction of ethanol (EtOH)-induced associative learning has received even less attention and it remains unknown if the PL and IL subregions within the mPFC are necessary for extinction of Pavlovian-conditioned EtOH-seeking behaviors such as EtOH-CPP in mice. Previous reports that involved operant EtOH procedures have shown inconsistent evidence for the involvement of mPFC in extinction of EtOH-seeking behavior. Specifically, Dayas et al. (2007) reported cue-induced activation of the mPFC following exposure to cues capable of reinstating self-administration in rats after extinction. In contrast, however, Hamlin et al. (2007) reported no significant effect of extinction on mPFC activation after contextual renewal of EtOH-responding. Interestingly, Topple et al. (1998) showed significant PL activation following exposure to the EtOH-paired operandum during an extinction session. Thus, although the data remain somewhat inconsistent, it appears that the mPFC may be activated in situations when EtOH is expected but not received (i.e., a large EtOH-reward prediction error exists), such as extinction and/or reinstatement. However, the aforementioned experiments were performed using operant procedures in rats and thus it remains unknown how extinction of the EtOH-cue contingency would affect mPFC activation elicited by a Pavlovian-conditioned cue in the absence of an instrumental response.
Therefore, although the literature supports the assertion that the PL and IL subregions exhibit differential roles in expression and extinction of cue-induced behaviors (e.g., Peters et al., 2009), these studies have primarily been performed in operant procedures in rats with drugs other than EtOH, and it is therefore unclear if the distinction between these subregions generalizes across species and to extinction of EtOH-induced associative learning in mice.
The current experiments were performed in order to first understand how extinction of an EtOH-cue association affects cue-induced activation of the mPFC and second, to examine the effects of direct manipulations of the mPFC on extinction of EtOH-induced associative learning in mice. Experiment 1 utilized immunohistochemical (IHC) analysis of the activated transcription factor, phospho-cAMP response element-binding protein (pCREB), to examine the effects of extinction on mPFC (PL and IL) activation following a brief exposure to an EtOH-paired cue capable of eliciting approach behavior in mice. Experiment 2 then used electrolytic lesions of the mPFC to confirm a functional role of this region in extinction of EtOH-CPP in mice. It was hypothesized that 1) the mPFC would show cue-induced expression of pCREB that was sensitive to changes in the EtOH-cue contingency following extinction, and 2) mPFC lesions would impair extinction of EtOH-CPP. These experiments were intended to further clarify the roles of the PL and IL subregions of the mPFC in processing changes of EtOH-cue contingencies that underlie extinction of EtOH-induced associative learning in mice.
2. METHODS & MATERIALS
2.1 Subjects
Adult, male DBA/2J mice (n=111) were obtained from Jackson Laboratory (Sacramento, CA) at 6–7 weeks of age and allowed to acclimate to the animal colony for 2 weeks before experiments commenced. Mice were housed, four to a cage, in cob bedding in a Thoren rack with water and food available ad libitum throughout each experiment. All experiments were conducted during the light phase (7:00–19:00). The Oregon Health & Science University IACUC approved all experimental procedures.
2.2 Drugs
Ethanol (20% v/v in isotonic saline) was administered intraperitoneally (IP) at a dose of 2 g/kg (12.5 ml/kg)
2.3 Conditioning Apparatus
All behavioral procedures were performed in custom made, acrylic and aluminum conditioning boxes (30 × 15 x 15 cm), each of which was enclosed in a sound-attenuating chamber (Model E10–20, Colbourn Instruments, Allentown, PA). Six infrared emitters and detectors, mounted 5 cm apart and 2.2 cm above the floor of the box, were used to obtain spatial location and locomotor activity data throughout conditioning, extinction and testing. The CSs consisted of two distinct tactile cues-grid and hole floors. Grid floors (2.3 mm stainless steel rods, 6.4 mm apart) and hole floors (16-gauge stainless steel perforated with 6.4-mm round holes) were interchangeable allowing for either full- or split-cue configurations during conditioning/extinction and testing, respectively. These cues are unbiased in that drug-and experimentally-naïve DBA/2J mice show equal preference for the two floors (Cunningham et al., 2003).
2.4 Experiment 1
The purpose of Experiment 1 was to determine how changes in the EtOH-cue contingency effect cue-induced CREB activation within the PL and IL subregions of the mPFC in mice.
2.4.1 EtOH-Cue Conditioning Procedure
The experiment involved a 2 × 2 design with the following four groups: Paired-No Extinction, Paired-Extinction, Unpaired-No Extinction, Unpaired-Extinction. Thus, each of the two Paired groups had a control group matched for cumulative drug and cue exposure.
Habituation
On Day 1, mice were given a saline injection (12.5 ml/kg) and habituated to the conditioning apparatus, equipped with white paper flooring, for 5 mins.
Conditioning
On Days 2–5, Paired animals received daily conditioning trials during which EtOH (2 g/kg) was paired with the Grid floor cue while saline was paired with the Hole floor cue on alternating days, similar to the conditioning procedure described by Hill et al. (2007). The order in which the animals received the pairings over the 4 days (S-E-S-E or E-S-E-S) was fully counterbalanced. Two to three hrs after the end of the conditioning trial, animals received a saline injection (12.5 ml/kg) in their home cage. In contrast to the Paired groups, Unpaired animals received saline injections before both of the cues (S-S-S-S) followed by an EtOH injection in the home cage 2–3 hrs after the Grid trials. Thus, the experimental (Paired) and control (Unpaired) groups received equal amounts of EtOH during conditioning (Cunningham, 1993).
Test 1
On Day 6, all animals received a drug-free, 15-min test during which both tactile cues were presented and place preference for the EtOH-paired floor (Grid floor) was assessed.
Extinction
Three days after Test 1, animals in the Paired-Extinction and Unpaired-Extinction groups received 4 days of extinction (Days 7–10). Extinction consisted of four, non-reinforced exposures to each of the CS+ and CS- cues separately (each preceded by a saline injection) for 30 mins. Nonreinforced cue exposures occurred in the morning (AM) and afternoon (PM) of each of the 4 days and trials were separated by 3–4 hrs. The No Extinction groups were weighed daily during this phase and were otherwise left undisturbed.
Test 2
Twenty-four hrs after the last extinction trial (Day 11) all animals received a second, drug-free 15-min preference test.
Cue Exposure
Two days following Test 2, all animals received a brief, 5-min exposure to the CS+ cue and were subsequently sacrificed by asphyxiation with CO2 12–15 mins later and brains were processed for IHC.
2.4.2 Immunohistochemistry Procedure
The general IHC procedure has previously been described in detail (see Bachtell et al., 2002). Briefly, brains were postfixed with 2% paraformaldehyde in phosphate-buffered saline (PBS), immersed in 20% & 30% sucrose, 0.1%NaN3, 1mM NaF in PBS, then frozen and sectioned (30µm) with a cryostat (Leica CM1900). The phosphatase inhibitor, NaF, was added to all buffers (1mM) and incubation solutions (0.1mM) in order preserve the protein phosphorylation state. Blocking was performed with 4% normal goat serum. CREB (1:500) and pCREB (1:250) (both obtained from Cell Signaling Technology, Beverly, MA) immunoreactivity (IR) was detected with Vectastain ABC kit (Vector Laboratories, Burlingam, CA), and enzymatic development was performed with Metal Enhanced DAB kit (Pierce Chemical, Rockford, IL). For each subject, two separate adjacent coronal slices were used for pCREB and CREB staining and subsequent cell quantification within the PL and IL subregions of the mPFC. Additional analysis of pCREB and CREB-IR in the core and shell subregions of the nucleus accumbens (NAc) and basolateral amygdala (BLA) was also included. The slices analyzed for each region were from the following anterior/posterior (AP) coordinates as determined from Paxinos & Franklin (2001): PL and IL subregions of the mPFC (AP: +1.70mm), BLA (AP: −1.46mm), core and shell subregions of the NAc (AP: +1.10mm). These coordinates and slice acquisition parameters were determined from previous reports including IHC analysis of these regions (e.g., Hill et al., 2007).
Slice images were obtained using an Olympus BX51 microscope and Macintosh computer equipped with Q-Capture software. Slices were viewed, and cells were counted “blinded” (i.e., with no knowledge of group assignment) using Image J software. CREB- and pCREB-containing cells were counted bilaterally in a rectangular fixed-size region (173 µm x 104 µm) randomly applied to an area well within each brain region’s boundaries determined from Paxinos & Franklin, 2001. Three independent counts were obtained in each brain area for every subject, averaged, and analyzed as raw pCREB and CREB counts as well as a ratio of pCREB/CREB-IR for each group. Similar to previous reports of IHC analysis following cue exposure (e.g., Hill et al., 2007), a single slice from each subject that most closely represented the desired Bregma level for each brain region was included in each analysis.
2.5 Experiment 2
The purpose of Experiment 2 was to determine the effects of electrolytic lesions of the PL and IL (mPFC) on extinction of EtOH-CPP in mice.
2.5.1 Conditioned Place Preference Procedure
Habituation
On Day 1, mice were given a saline injection (12.5 ml/kg) and habituated to the conditioning apparatus, equipped with white paper flooring, for 5 mins.
Conditioning
One day later animals received daily CPP conditioning trials during which EtOH (2 g/kg) was paired with one of the tactile cues (e.g., Grid) while saline was paired with the other cue (e.g., Hole) on alternating days using the standard, one-compartment procedure (Cunningham et al., 2006). The floor with which EtOH was paired (Conditioning Subgroup) and trial-type order (S-E-S-E or E-S-E-S) were fully counterbalanced (Cunningham et al., 2003).
Test 1
One day after the last conditioning trial, all animals received a drug-free, 30-min preference test during which both tactile cues were presented and place preference was assessed. Floor orientations were counterbalanced within each group.
Surgery
Animals received a single, midline electrolytic lesion of the mPFC (see 2.5.2 Surgical Procedure for details) or sham surgery and received 6–7 days to recover. Animals in the No Sx group remained in the home cage undisturbed.
Extinction
After fully recovering from surgery, animals received 4 days of extinction. Extinction consisted of AM and PM sessions during which animals received four, 30-min non-reinforced exposures to each of the CS+ and CS- cues separately as previously described (see Groblewski et al., 2011). Specifically, for the AM session, animals were weighed, injected with saline, and immediately placed on the CS- cue for 30 mins. Approximately 3 hrs later during the PM session, animals were again weighed and injected with saline and immediately placed on the CS+ floor for 30 mins.
Test 2
One day after the last extinction trial all animals received a second, drug-free 30-min preference test identical to Test 1.
2.5.2 Surgical Procedure
Electrolytic Lesion Surgery
A single, midline electrolytic lesion of the mPFC was performed based on the lesion parameters described previously (see Gremel and Cunningham, 2008). Specifically, after receiving meloxicam (0.2 mg/kg, subcutaneously) to reduce post-surgery pain and inflammation, animals were induced and maintained under isoflurane anesthesia. Animals were placed in a stereotax (Model 1900, KOPF Instruments, Tujunga, CA) with the skull horizontal. A midline burr hole was drilled 1.75 mm rostral to bregma and an electrode (Rhodes Medical Instruments, Woodland Hills, CA) was lowered into brain with the tip of the electrode targeted at the border between the PL and IL subgregions of the mPFC (AP: 1.8 mm, ML: 0.0 mm, DV: −2.5 mm) determined from Paxinos & Franklin (2001). A 0.5 mA current was then passed through the tip of the electrode for 5 secs. Sham animals received an identical surgery but no current was passed through the lowered electrode. Mice were given 5–6 days to recover before the behavioral procedure resumed. Animals in the No Sx group remained in the home cage, undisturbed, during the surgery and recovery period.
Histology
Upon completion of each experiment, animals received an overdose of pentobarbital (150 mg/kg) and brains were removed and postfixed with 2% paraformaldehyde in PBS. Brains were cryoprotected with 20% then 30% sucrose in PBS and 0.1% NaN3. Frozen slices (40µm) were obtained on a cryostat (Leica CM1900) and thionin-stained for quantification of lesion location and size.
2.6 Statistical Analysis
Group differences in EtOH-induced associative learning, as modeled using variations of the standard EtOH-CPP procedure, were assessed using analysis of variance (ANOVA) of the percentage of each test spent on the EtOH-paired (CS+) floor (% Time on EtOH-paired floor). Extinction was defined a priori as a significant reduction in place preference that was determined by Bonferroni-adjusted within-subject comparisons of preferences on Test 1 and 2 (paired t-tests) for each group following an initial two-way ANOVA (Group x Test) (Groblewski et al., 2011). Additional between-group analyses of the time spent on the Grid floor were performed including the Conditioning Subgroup (G+ and G−) as a third factor (Cunningham et al., 2003) and these data are reported in Table 1 for both experiments. However, because in Experiment 1 all Paired animals had EtOH paired with the Grid floor (i.e., all animals were G+), the comparison between Conditioning Subgroups was not possible. For IHC analysis, between-group differences in pCREB- and CREB-positive cell counts were initially determined separately using one-way ANOVAs followed by Bonferroni-adjusted between-groups comparisons (independent t-tests). However, because there were no significant group differences in the number of CREB-positive cells in any of the brain regions analyzed (see Supplementary Table 1), IHC data were subsequently analyzed and presented as the ratio pCREB/CREB-IR for each region. Overall α was set at .05 for all statistical tests.
Table 1.
Preference test data including Conditioning Subgroup (expressed as Time on Grid Floor). Data are expressed as mean (± SEM).
| Exp. | Group | Conditioning Subgroup |
n | Test 1: Time on Grid Floor (sec/min) |
Post hoc tests |
Test 2: Time on Grid Floor (sec/min) |
Post hoc tests |
|---|---|---|---|---|---|---|---|
| 1 | Paired-No Extinction | G+ | 10 | 49.0 ± 2.1 | na | 43.8 ± 3.1 | |
| Paired-Extinction | G+ | 12 | 49.7 ± 1.9 | na | 27.5 ± 5.3 | # | |
| Unpaired-No Extinction | G+ | 12 | 29.9 ± 6.2 | na | 27.4 ± 5.7 | ||
| Unpaired-Extinction | G+ | 12 | 23.6 ± 5.4 | na | 28.8 ± 5.5 | ||
| 2 | No Sx | G+ | 7 | 41.1 ± 2.1 | ] * | 27.0 ± 5.1 | |
| G− | 7 | 16.4 ± 1.9 | 25.9 +3.2 | ||||
| Sham | G+ | 12 | 42.8 ± 1.3 | ] * | 29.7 ± 4.5 | ||
| G− | 11 | 17.2± 1.5 | 22.4 + 4.8 | ||||
| Lesion | G+ | 7 | 44.6 ± 2.2 | ] * | 38.7 ± 5.4 | ] * | |
| G− | 5 | 19.7 ± 2.2 | 16.1 ± 3.3 |
Experiment 1. Significant reduction in place preference on (#) as determined by Bonferroni-corrected within-subject comparisons of Test 1 & 2 (p < .05).
Experiment 2. Significant place preference (*) as determined by Bonferroni-corrected post-hoc comparisons of G+ and G− subgroups (p < .05).
Because the goal of these experiments was to specifically manipulate extinction of an acquired EtOH-conditioned approach behavior (CPP), we decided, a priori, to remove animals that failed to express a place preference of greater than 50% on Test 1. These experiments revealed that less than 25% of all subjects failed to express significant preference following the 2-trial conditioning procedure—a finding that is consistent with previous reports that have also included removal of non-learners from extinction analyses (e.g., Groblewski et al., 2011)
3. RESULTS
3.1 Experiment 1
A total of 2 animals (approximately 9%) were removed from analysis for exhibiting less than 50% preference on Test 1.
3.1.1 EtOH-Cue Conditioning
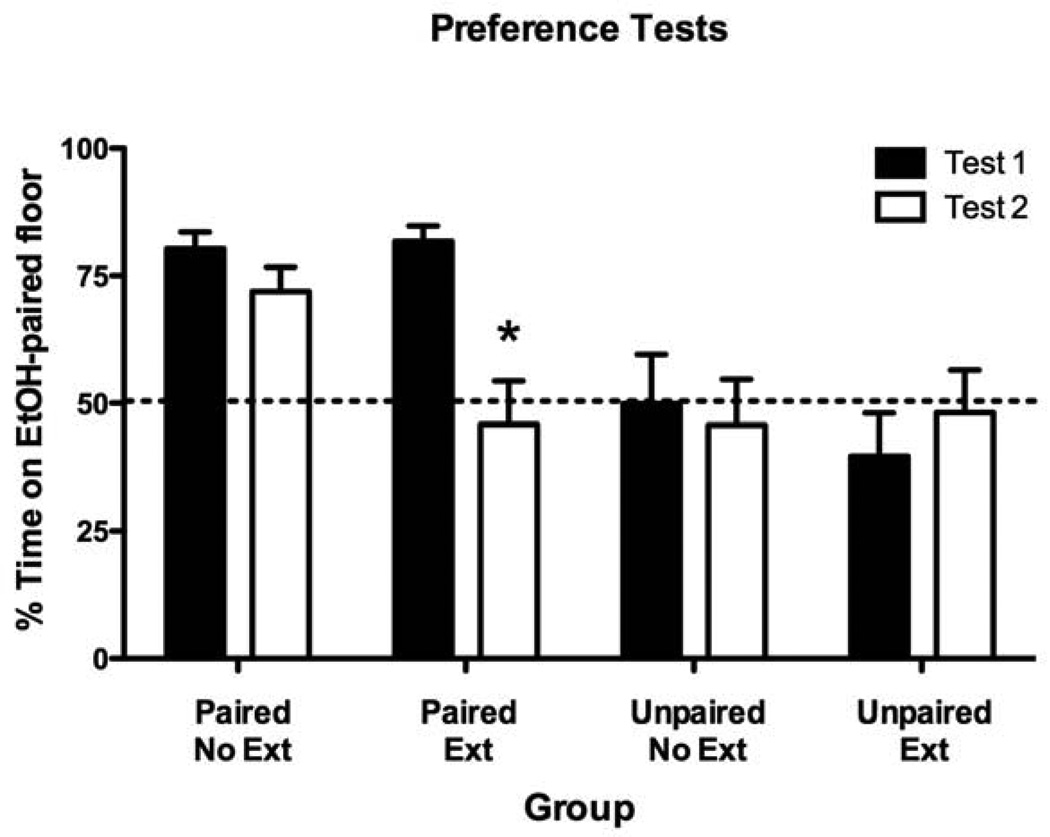
On Test 1, animals in both Paired groups showed significant preference for the EtOH-paired cue (Figure 1). As expected, neither of the Unpaired groups showed significant preference for either cue. Following the extinction phase (Test 2), the Paired-Extinction group exhibited a significant reduction in place preference (i.e., extinction) whereas the Paired-No Extinction group continued to express significant CPP equal in strength to that expressed on Test 1 (Figure 1). These findings were supported by a significant Group x Test interaction [F(3,42) = 6.5, p < .01] as well as significant main effects of Group [F(3,42) = 4.9, p < .01] and Test [F(1,42) = 7.1, p < .05]. Separate paired t-tests revealed that only the Paired-Extinction group showed a significant change in preference following the extinction phase [t(11) = 3.8 p < .005]. Both Unpaired groups continued to show an indifference for the two cues regardless of whether they underwent extinction or not. These results were further supported by the within-subject analyses of Grid Time data presented in Table 1. The behavioral results of Experiment 1 demonstrated acquisition of EtOH-induced associative learning in mice as characterized by Pavlovian-conditioned approach behavior. Moreover, this approach behavior was eliminated following extinction (Paired-Extinction) but conserved following the passage of an equivalent amount of time (Paired-No Extinction).
Figure 1. Pavlovian ethanol-cue conditioning induced significant approach behavior that was reduced following extinction.
Preference Test data (expressed as Percent Time on EtOH-Paired Floor) before (Test 1) and after (Test 2) extinction. Significant reduction in place preference (*) as determined by Bonferroni-corrected post-hoc comparisons of Tests 1 and 2 for each group (p < .05). Data are expressed as mean (± SEM).
3.1.2 Immunohistochemistry
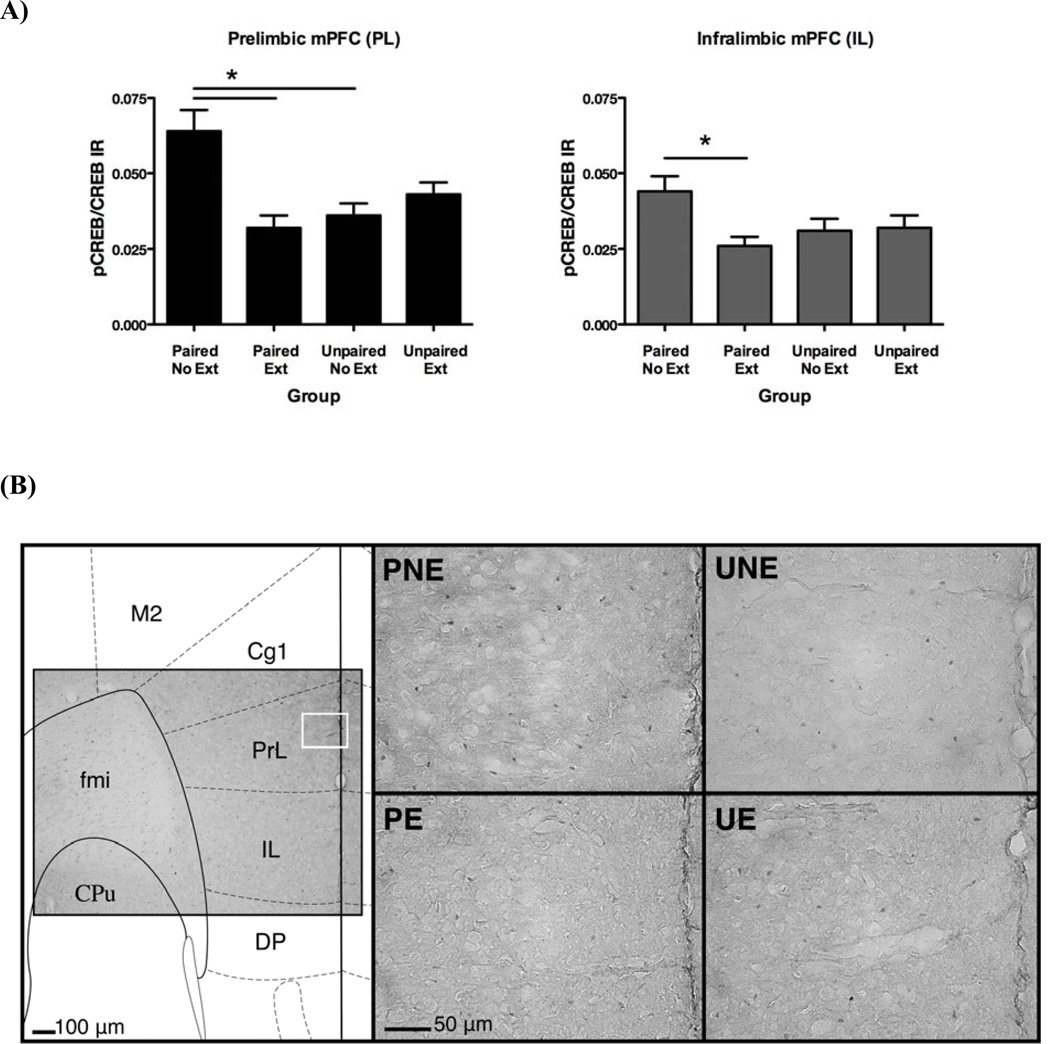
Analyses of the PL and IL subregions of the mPFC revealed a significant increase in pCREB/CREB-IR in the Paired-No Extinction group (Figure 2a). This cue-induced increase did not exist following extinction, as evidenced in the Paired-Extinction group. In the PL, an initial one-way ANOVA revealed a significant main effect of group [F(3,38) = 7.8, p < .001] and subsequent post-hoc comparisons showed that the Paired-No Extinction group had significantly greater pCREB/CREB-IR in the PL than both the Paired-Extinction (p < .001) and Unpaired-No Extinction groups (p < .005). In the IL, there was also a main effect of group [F(3,38) = 2.9, p < .05] and post-hoc comparisons revealed that the Paired-No Extinction group was significantly different than the Paired-Extinction group (p < 05). The effects on pCREB/CREB-IR were attributable only to differences in pCREB as there were no group differences in CREB-positive cell counts (p’s > 0.05; raw data counts for pCREB supplementary ed in -Supplementary Table 1). Therefore, these findings suggested that exposure to an EtOH-paired cue resulted in significant increases in pCREB/CREB-IR in the mPFC and this effect was absent when the EtOH-cue contingency was extinguished.
Figure 2. Cue-induced increases in phosphorylation of CREB in the PL and IL subregions of the mPFC were absent following extinction.
(A) Immunohistochemistry data from the prelimbic (PL) and infralimbic (IL) subregions of the mPFC. Data expressed as a ratio of pCREB-positive cells to CREB-positive cells (mean ± SEM). Significant differences (*) as determined by Bonferroni-corrected post-hoc comparisons (p < .05). (B) Representative photomicrographs of regions of analysis within the PL of coronal sections approximately 1.7mm anterior (rostral) to Bregma (Paxinos & Franklin, 2001).
Additional analysis of the NAc (core and shell) and BLA revealed no significant group differences in cue-induced changes in pCREB or CREB cell counts (-Supplementary Table 1) or pCREB/CREB-IR (Table 2). These observations were further supported by statistical analyses that revealed no main effect of group in any of the initial ANOVAs (p’s > .05).
Table 2.
Immunohistochemistry data from the nucleus accumbens (NAc) and basolateral amygdala (BLA). Data expressed as a ratio of pCREB-positive cells to CREB-positive cells (mean ± SEM).
| Group | NAc | BLA | |
|---|---|---|---|
| Core | Shell | ||
| Paired-No Extinction | 0.05 ± 0.006 | 0.059 ± 0.008 | 0.265 ± 0.042 |
| Paired-Extinction | 0.045 ± 0.007 | 0.055 ± 0.008 | 0.22 ± 0.027 |
| Unpaired-No Extinction | 0.044 ± 0.004 | 0.052 ± 0.004 | 0.242 ± 0.027 |
| Unpaired-Extinction | 0.061 ± 0.01 | 0.048 ± 0.007 | 0.241 ± 0.029 |
3.2 Experiment 2
A total of 14 animals (approximately 22%) were removed from analysis for exhibiting less than 50% preference on Test 1.
3.2.1 Histology
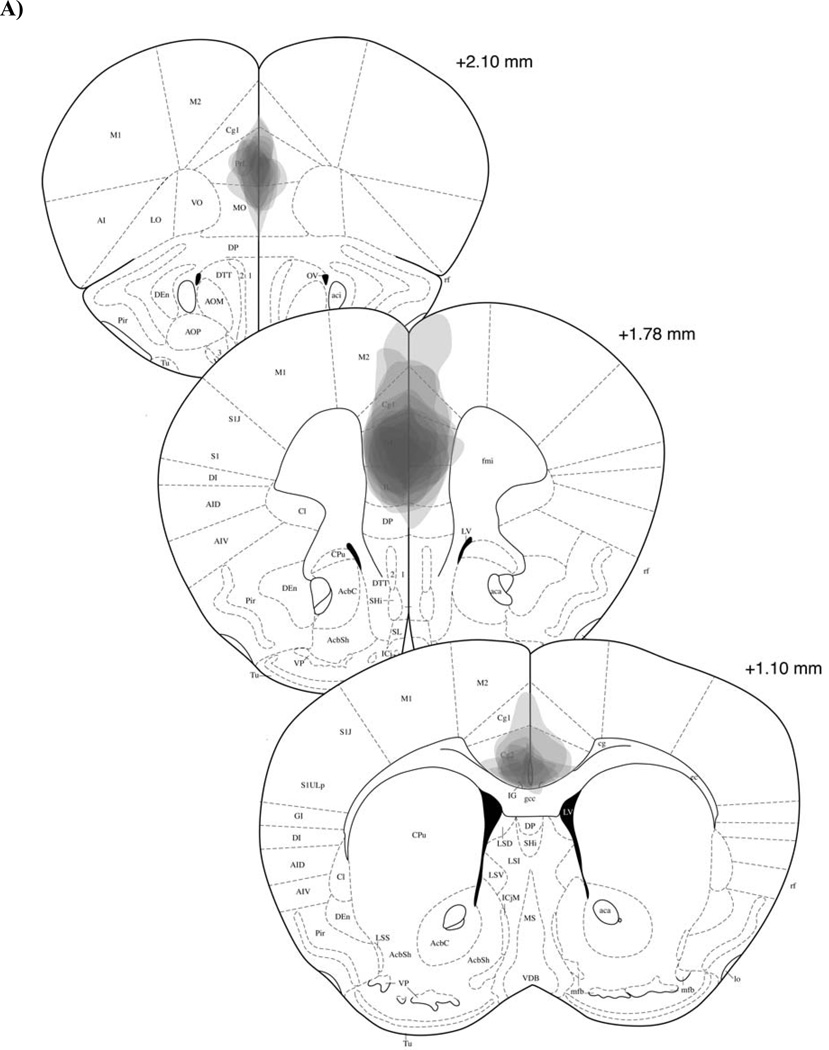
Histological analysis of the animals that received surgery indicated that damage caused by the electrolytic lesion affected both the PL and IL subregions of the mPFC (Figures 3a & b). Although there was some observable cell proliferation that extended dorsally into the anterior cingulate (ACC) subregion of the mPFC in some of the subjects, the area of overlap for all subjects was limited to the PL and IL. Follow-up behavioral analyses revealed that the 5 subjects with damage that extended into the ACC did not exhibit different levels of extinction when compared to the other 7 animals with damage restricted to only the PL/IL at Bregma +1.78 (i.e., there were no differences in Subgroup extinction and no Subgroup x Test interaction). These analyses suggested that any extinction effect seen in the Lesion group resulted from damage to the PL and IL mPFC and was not caused by secondary damage to portions of the ACC. In contrast to the Lesion group, animals in the Sham group showed no detectable tissue damage that resulted from insertion of the electrode (data not shown). As such, no animals from either group were excluded on the basis of histology.
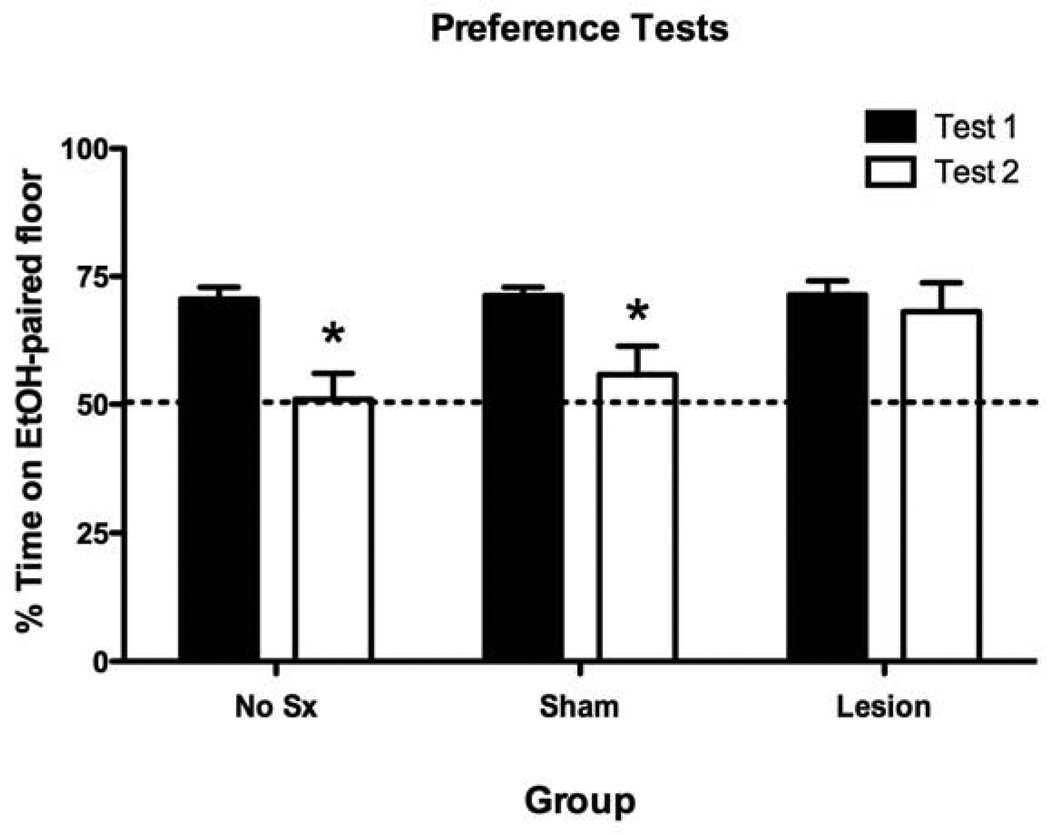
Figure 3. Histological analysis of lesion-induced tissue damage in the mPFC.
(A) Lesion damage across three stereotaxic levels (adapted from Paxinos & Franklin, 2001) for each subject, represented at 15% opacity in order to show overlap. (B) Representative photomicrograph of an electrolytic lesion of the mPFC (AP: 1.8mm, ML: 0.0mm, DV: −2.5mm).
3.2.2 Conditioned Place Preference
The results of the CPP expression tests revealed that electrolytic lesions of the mPFC prevented the normal extinction of EtOH-CPP exhibited by the Sham and No Sx groups(Figure 4). Initial analysis of the place preference results (Percent Time on EtOH-paired floor) from Tests 1 and 2 revealed a significant main effect of Test [F(1,46) = 13.3, p < .005] but no Group x Test interaction (F(2,46) = 1.7, p > .1). The a priori Bonferroni-corrected paired t-tests comparing preferences on Tests 1 and 2 for each group were performed and revealed a significant reduction in preference (i.e., extinction) in the No Sx (t(13) =4.8 p < .001) and Sham (t(22) = 2.8 p < .05) groups, but not in the Lesion group (p > .05). These findings were further supported by the additional between-group analyses of Time on Grid Floor data for each of the Conditioning Subgroups (see Table 1). Specifically, all three groups exhibited significant place preference on Test 1, as indicated by a significant difference between the Conditioning Subgroups within each group. However, on Test 2following extinction, a significant difference between Conditioning Subgroups existed only in the Lesion group (p < .05). Additionally, there was a significant Test x Conditioning Subgroup interaction for the No Sx (F(1,12) = 22.7, p < .001) and Sham (F(1,21) = 7.4, p < .05) groups, but not the Lesion group (p > .05). Together, these analyses showed that electrolytic lesions of the mPFC prevented extinction of EtOH-CPP.
Figure 4. Electrolytic lesions of the mPFC blocked extinction of ethanol-induced associative learning in mice.
Preference Test data (expressed as Percent Time on EtOH-Paired Floor) before (Test 1) and after (Test 2) extinction. Significant reduction in place preference (*) as determined by Bonferroni-corrected post-hoc comparisons of Tests 1 and 2 for each group (p < .05). Data are expressed as mean (± SEM).
4. DISCUSSION
4.1 Cue-induced activation of mPFC-CREB is sensitive to extinction of EtOH-induced associative learning in mice
The results of Experiment 1 revealed that the PL and IL subregions of the mPFC were activated following a brief exposure to an EtOH-paired cue that was capable of eliciting Pavlovian approach behavior. Interestingly, the cue-induced CREB activation observed in both of these regions was absent when the EtOH-cue contingency was extinguished. In contrast to the mPFC, analysis of the NAc (core and shell) and BLA revealed no evidence of activation induced by exposure to the EtOH-paired cue. These data suggest that the PL and IL subregions of the mPFC may play a critical role in the expression and/or extinction of EtOH-induced cue-evoked approach behavior in mice.
The use of pCREB-IR as an indicator of neuronal activation has been described in previous studies (Herdegen & Leah, 1998). However, although it has been shown that phosphorylation of CREB results in transcription of a number of immediate early genes (IEGs) including c-fos and Zif268 (Adams et al., 2000) and blocking phosphorylation of CREB can prevent subsequent Fos expression (Vanhoutte et al., 1999), it has also been reported that pCREB can be uncoupled from CREB-mediated gene expression (Hardingham et al., 1999). One advantage of analyzing pCREB instead of Fos is its shorter detection window as changes in pCREB can be detected within 5–15 mins whereas detection of Fos requires 90–120 mins (Kovacs & Sawchenko, 1996; Shiromani et al., 1995; Stanciu et al., 2001). Thus, although pCREB is not sufficient for CREB-mediated gene expression, it is rapidly detectable and reflects activation of hundreds of extracellular signals and upstream transduction cascades that are initiated by cellular depolarization (Johannessen et al., 2004).
Activation of the PL and IL regions following exposure to previously-conditioned drug-paired cues is not entirely surprising given the wealth of evidence implicating the PL and IL in expression and extinction of drug-seeking behavior (Peters et al., 2009). Interestingly, however, animals that received extinction of the EtOH-cue association (and concomitant cue-induced approach behavior) did not exhibit mPFC activation. These results are in agreement with previous reports showing significant activation of the PL and IL by a cocaine-paired cue capable of eliciting drug-seeking behavior in rats using an operant procedure (Kufahl et al., 2009; Zavala et al. 2007). Importantly, Zavala et al. (2007) also showed that extinction (accompanied by a reduction in seeking behavior) eliminated the cue-induced activation in both of these mPFC subregions. However, because in these studies the animals were tested for cue-induced lever pressing, it is unclear if the increases in mPFC activation were cue-induced or behavior-induced, or both. By performing IHC analysis immediately following the 5-min passive CS+ exposure, the current IHC experiment eliminated the possible influence of the drug-seeking behavior on CREB activation. Therefore, the current results showed that the changes in PL- and IL-CREB activation following acquisition and extinction of an EtOH-cue association are not due simply to changes in conditioned-approach behavior.
It is interesting that in neither the current study nor the study by Zavala et al. (2007) did expression of extinction cause an increase in IL-activation. Extrapolating from the model proposed by Peters et al. (2009), one would expect IL activation (and no PL activation) following exposure to an extinguished drug-paired cue. However, the current experiment as well as a number of previously published extinction studies showed concurrent cue-induced activation of both of these regions (e.g., Kim et al., 2010; Zavala et al., 2007; Kufahl et al., 2009; Dayas et al., 2007). Together these data suggest that it may be possible for these two subregions of the mPFC to be simultaneously activated by cues before and/or during expression or extinction behavior. Although speculative in nature, possible mechanisms have been discussed previously (e.g., Groblewski & Stafford, 2010). It is possible that these subregions could be simultaneously functioning through activation of inhibitory interneurons within each region, connections between the two regions, and/or projections that differentially regulate multiple downstream nuclei to result in a common behavioral output (Vidal-Gonzalez et al., 2006; Quirk & Mueller, 2008; Peters et al., 2009).
The findings that neither the BLA nor the NAc were significantly activated by exposure to a drug-paired cue are somewhat surprising given the extensive data that have implicated these two areas in drug-related behaviors. A study by Radwanska et al. (2008) showed that exposure to an EtOH-paired cue capable of reinstating self-administration resulted in significant activation of the BLA (indicated by an increase in BLA-pERK levels). However, a previous report involving a Pavlovian EtOH-conditioning procedure similar to the current study showed that passive exposure to an EtOH-paired cue did not cause significant activation of the BLA or NAc (Hill et al., 2007). In another similar study, Bernardi et al. (2009) examined BLA activation following exposures to both the CS+ and CS- cues alone or the CS+/CS- cues together (as in the CPP expression test) following Pavlovian cue-conditioning with cocaine. The results of the IHC analyses showed that Fos expression was significantly increased in the BLA following the choice test (CS+/CS-) condition, but not after exposure to either of the cues alone. Bernardi et al. concluded that the BLA may play a more important role in the actual expression of approach behavior (such as that exhibited during a CPP test) but may not be significantly activated following simple presentation of a drug-paired cue. The current results are in agreement with this assertion in that a brief exposure to the EtOH-paired cue did not induce changes in pCREB/CREB-IR within the BLA.
The IHC results of Experiment 1 suggest that areas of the mPFC, but not NAc or BLA, exhibit increases in activity upon exposure to an EtOH-paired cue as indicated by an increase in pCREB/CREB-IR. Moreover, the conditioned increase of pCREB/CREB-IR in the PL and IL was absent when the EtOH-cue association was inhibited following extinction (measured behaviorally as a reduction in cue-induced approach behavior). Thus the mPFC is not only responsive to a brief exposure to an EtOH-paired cue, but it also possesses dynamic properties that are sensitive to changes in the EtOH-cue contingency following acquisition and extinction. Similar to the findings of Topple et al. (1998), the mPFC showed the greatest activation when EtOH was expected but not received during the nonreinforced cue exposure. These results suggest that the mPFC may play an important role in processing the reward prediction error component of extinction learning and would therefore be integrally involved in extinction of EtOH-induced associative learning in mice. Because of the nature of the current IHC experiments, however, it is not possible to confirm a causal role of CREB transcription in mediating extinction with this procedure. Therefore, we cannot rule out the possibility that the absence of a cue-induced change in pCREB/CREB-IR in the Paired-Extinction group could reflect an extinction-specific activation of a mechanism(s) that repress pCREB. Interestingly, it has been reported that activation of CREB via phosphorylation at Ser133 can be blocked by CaMKII-induced phosphorylation of CREB at Ser142 (Sun et al., 1994). Thus, future experiments involving direct manipulations of CREB activation within the mPFC will certainly help to identify a causal role of this transcriptional pathway within the mPFC in controlling extinction of ethanol-induced associative learning in mice.
4.2 Extinction of EtOH-induced associative learning in mice requires intact mPFC function
The results of Experiment 2 showed that electrolytic lesions of the mPFC that encompassed both the PL and IL subregions significantly impaired extinction of EtOH-CPP in mice. These results are in agreement with the correlative findings of Experiment 1, and together, these experiments are the first to show that the mPFC plays an essential role in extinction of EtOH-induced associative learning in mice.
The preference data of Experiment 2 revealed that a group with lesions of the mPFC showed significantly impaired extinction when compared to sham-lesioned animals (Sham group) or animals that did not receive surgery (No Surgery group). These data suggest that the mPFC is involved in extinction, but not necessarily expression, of approach behavior to an EtOH-paired cue in mice. Specifically, animals in the Lesion group continued to express significant preference for the EtOH-paired cue after surgery and extinction. In contrast, the Sham group showed significant preference only before extinction. If the mPFC were required for initiation and/or maintenance of approach behavior, the lesion group would have showed impaired place preference following surgery, regardless of whether extinction training was administered. Thus it appears that the mPFC is involved in extinction and not necessarily required for expression of EtOH-CPP.
The current experiment is in agreement with the findings of Hsu & Packard (2008) that showed that reversible lesions of the mPFC, achieved by temporary inactivation with bupivacaine, blocked extinction of amphetamine-CPP in rats. The current findings are also in agreement with the suggestion that the mPFC plays a key role in actively inhibiting expression of CPP during extinction (Ovari & Leri, 2008). In contrast to these findings, Zavala et al. (2003) showed that pre-conditioning quinolinic acid lesions of the PL region of the mPFC did not prevent acquisition or subsequent extinction of cocaine-CPP in rats. In fact, the extinction data reported by Zavala et al. (2003) suggested that these PL lesions may have even facilitated extinction of cocaine-CPP. Although the reasons for this discrepancy in results is not completely clear, it is important to note that the lesions administered by Zavala et al. were primarily limited to the PL subregion of the mPFC and were administered prior to conditioning. On the other hand, the areas affected by the manipulations reported here, as well as by Hsu & Packard, encompassed both the PL and IL subregions of the mPFC and were administered after conditioning but before extinction. As previously mentioned, it is thought that these subregions may be differentially involved in expression and extinction of conditioned behaviors in rats (Peters et al., 2009). Although it is possible that these two subregions are also differentially involved in extinction of CPP, our data suggest that lesions that encompass both subregions of the mPFC had an overall effect of impairing extinction of EtOH-CPP in mice.
Although effects of distinct PL and IL lesions in mice have been previously reported (Lehmann & Herkenham, 2011) we believe that it is difficult, if not impossible, to accurately and reproducibly lesion only one of these subregions of the mouse mPFC. Therefore, it is currently not possible to discern the differential effects of PL and IL electrolytic lesions on extinction of CPP in mice with this procedure. As seen in Figure 3A, a subset of the subjects that received electrolytic lesions aimed at the PL/IL showed damage that extended into portions of the ACC—an area that has been shown to be involved in expression of CPP (Gremel et al., 2011) but not necessarily extinction in rodents (Vidal-Gonzalez et al., 2006). However, our analysis of this subset of animals showed that they did not exhibit CPP behavior different from animals with damage limited to the PL/IL. Therefore, it appears that the extinction-impairing effects of the mPFC lesion were caused by damage to the PL/IL and not the ACC. Future studies utilizing other intracranial techniques may allow for greater distinction to be made between the subregions of the mouse mPFC and would help to further characterize the effect of mPFC lesions on extinction of EtOH-induced associative learning in mice reported here. These studies will also help to determine if the PL and IL of the mouse mPFC do in fact exert bidirectional control over extinction as previously described in rats (Quirk & Mueller, 2008).
4.3 The mPFC and response-inhibition during extinction of EtOH-induced conditioned approach behavior
Together with the IHC data of Experiment 1, these results suggest that the mPFC may be selectively activated at the onset of extinction when the reward prediction error is greatest. Because the brief cue exposure was preceded by only a saline injection, the 5-min trial was effectively an extinction trial. During both extinction and the brief cue exposure, nonreinforced presentation of the CS+ created a large prediction error, in that the Paired No-Extinction animals may have still expected EtOH’s effects in the presence of the cue. This discrepancy between reward expectation and actual outcome may have resulted in the observed mPFC recruitment as the mPFC has been implicated in assessment of reward salience and prediction error encoding (for review see Rushworth & Behrens, 2008). Additionally, lesions of the PL have been shown to impair sensitivity to changes in reward-specific contingencies that underlie goal-directed behavior in rats (Balleine & Dickinson, 1998). In contrast to the Paired-No Extinction group, the prediction error experienced by the Paired-Extinction group during the cue exposure was minimal because of the repeated nonreinforced CS+ exposures previously experienced during extinction. Therefore, the increased pCREB/CREB-IR within the mPFC of the Paired-No Extinction group could have represented a prediction error-induced initiation of extinction learning, and not simply a cue-induced initiation of approach behavior.
One caveat to this interpretation, however, is that the IHC data in Experiment 1 did not actually identify the type of pCREB-containing cells within the mPFC. For example, if the mPFC cells that were activated by cue-exposure were inhibitory interneurons, it is possible that an increase in pCREB in these cells actually resulted in a net decrease of mPFC output. In fact, Miller & Marshall (2004) reported that exposure to a cocaine-paired cue resulted in an increase in activity of GABA-ergic PL neurons, thereby reducing overall PL inhibition of the NAc and BLA. The mPFC lesions described in Experiment 2, which were large and nonspecific enough to effectively destroy any cells within this region, would have effectively decreased overall mPFC output. If, as Miller & Marshall (2004) hypothesized, decreased PL output is necessary for expression of CPP, then it is possible that the mPFC lesions actually caused an expression of CPP that was resistant to extinction. This lesion-induced persistence of CPP may have been caused by an inability of the mPFC to process the inhibitory association and/or inhibit the actual approach behavior, during extinction. This suggestion is supported by reports showing the involvement of the mPFC in the formation of inhibitory associations (Rhodes & Killcross, 2007) and expression of inhibitory extinction learning (Ovari & Leri, 2008).
4.4 Conclusions
The current findings strongly support a role for the PL and IL subregions of the mPFC in extinction of EtOH-induced associative learning in mice. Although these subregions have been implicated in extinction of other behaviors in rats such as conditioned fear and drug self-administration, the current study is the first to identify a functional role for the mPFC in extinction of Pavlovian-conditioned EtOH-induced approach behavior in mice. Using IHC, lesion, and behavioral techniques, these experiments showed mPFC-CREB activation following a brief, nonreinforced exposure to an EtOH-paired cue—a response that was sensitive to changes in the EtOH-cue contingency. Additionally, the results of these experiments confirmed a functional role of the mPFC in regulating extinction-induced changes in EtOH-paired associations, as lesions of the mPFC blocked extinction of EtOH-CPP. Together, these data showed that the mPFC plays an integral role in processing the changes in EtOH-cue contingencies that are required for extinction of EtOH-induced associative learning in mice and further support a role for the mPFC in extinction of the drug-associated memories that drive drug-seeking behavior.
Highlights.
Exposure to an ethanol-paired cue increased pCREB-IR in the mPFC of mice
Extinction eliminated cue-induced increases in mPFC pCREB
mPFC lesions blocked extinction of ethanol-CPP in mice
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by grants from the National Institutes of Health (AA018052, AA007702, AA007468) and awards from the American Psychological Association and the N.L. Tartar Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JP, Roberson ED, English JD, Selcher JC, Sweatt JD. MAPK regulation of gene expression in the central nervous system. Acta Neurobiol Exp (Wars) 2000;60:377–394. doi: 10.55782/ane-2000-1357. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. The Journal of pharmacology and experimental therapeutics. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learning & memory. 2009;16:777–789. doi: 10.1101/lm.1648509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: Haaren F, editor. Methods in behavioral pharmacology. Amsterdam: Elsevier; 1993. [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature protocols. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Groblewski PA, Voorhees CM. Place Conditioning. Totawa, NJ: Humana Press; 2011. [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. Journal of consulting and clinical psychology. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Duka T, Crombag HS, Stephens DN. Experimental medicine in drug addiction: towards behavioral, cognitive and neurobiological biomarkers. Journal of psychopharmacology. 2010 doi: 10.1177/0269881110388324. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Young EA, Cunningham CL. Blockade of opioid receptors in anterior cingulate cortex disrupts ethanol-seeking behavior in mice. Behavioural brain research. 2011;219:358–362. doi: 10.1016/j.bbr.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Franken FH, Cunningham CL. Inhibition of extracellular signal-regulated kinase (ERK) activity with SL327 does not prevent acquisition, expression, and extinction of ethanol-seeking behavior in mice. Behavioural brain research. 2011;217:399–407. doi: 10.1016/j.bbr.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Stafford JM. When the medial prefrontal cortex fails: implications for extinction and posttraumatic stress disorder treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7124–7126. doi: 10.1523/JNEUROSCI.1413-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain research. Brain research reviews. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hill KG, Ryabinin AE, Cunningham CL. FOS expression induced by an ethanol-paired conditioned stimulus. Pharmacology, biochemistry, and behavior. 2007;87:208–221. doi: 10.1016/j.pbb.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiology of learning and memory. 2008;89:504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cellular signalling. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:832–837. doi: 10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. An interdisciplinary approach. Chicago, London: University of Chicago Press; 1967. Integrative activity of the brain. [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental Enrichment Confers Stress Resiliency to Social Defeat through an Infralimbic Cortex-Dependent Neuroanatomical Pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking Behavioural brain research. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. The European journal of neuroscience. 2005;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neuroscience letters. 2008;444:52–55. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. New York: Dover Publications, Inc; 1927. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, Kaczmarek L. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33:1835–1846. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. The European journal of neuroscience. 2007;26:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature neuroscience. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Magner M, Winston S, Charness ME. Time course of phosphorylated CREB and Fos-like immunoreactivity in the hypothalamic supraoptic nucleus after salt loading. Brain research. Molecular brain research. 1995;29:163–171. doi: 10.1016/0169-328x(94)00242-7. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain research. Molecular brain research. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes & development. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neuroscience letters. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Molecular and cellular biology. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & memory. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain research. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.