Abstract
The International Federation of Societies of Toxicologic Pathologists (IFSTP) proposes a common global framework for training future toxicologic pathologists who will support regulatory-type nonclinical toxicology studies. Trainees optimally should undertake a scientific curriculum of at least 5 years at an accredited institution leading to a clinical degree (veterinary medicine or medicine). Trainees should then obtain 4 or more years of intensive pathology practice during a residency and/or on-the-job “apprenticeship,” at least 2 years of which must be focused on regulatory-type toxicologic pathology topics. Possession of a recognized pathology qualification (i.e., certification) is highly recommended. A non-clinical pathway (e.g., a graduate degree in medical biology or pathology) may be possible if medically trained pathologists are scarce, but this option is not optimal. Regular, lifelong continuing education (peer review of nonclinical studies, professional meetings, reading, short courses) will be necessary to maintain and enhance one’s understanding of current toxicologic pathology knowledge, skills, and tools. This framework should provide a rigorous yet flexible way to reliably train future toxicologic pathologists to generate, interpret, integrate, and communicate data in regulatory-type, nonclinical toxicology studies.
Keywords: toxicologic pathologists, proposed curriculum, training standards, regulatory-type non-clinical safety studies
Abbreviations
ACVP American College of Veterinary Pathologists
ALAPT Associação Latino-americana de Patologia Toxicológica (Latin American Society of Toxicologic Pathology)
BSTP British Society of Toxicological Pathologists
CRP/TP Commissie Registratie Proefdierpathologen / Toxicologisch Pathologen (Committee for the Registration of Laboratory Animal / Toxicological Pathologists in the Netherlands)
DESV-APV Diplôme d’Études Spécialisées Vétérinaires en Anatomie Pathologique Vétérinaire (French Diploma of Special Studies in Veterinary Anatomic Pathology)
Dipl Diplomate
ECVCP European College of Veterinary Clinical Pathology
ECVP European College of Veterinary Pathologists
ESTP European Society of Toxicologic Pathology
ERT European Registered Toxicologist
FATS Fellow of the Academy of Toxicological Sciences
FIATP Fellow, International Academy of Toxicologic Pathology
FRCPath ToxPath
Fellow of the Royal College of Pathologists, sub-specializing n Toxicologic Pathology (United Kingdom)
FRIPH Fellow of the Royal Institute of Public Health
FTA klin Lab
Fachtierarzt für klinische Laboratoriumsmedizin (German Certification for Clinical Laboratory Medicine)
FTA Tox Pathol
Fachtierarzt für Pathologie, Toxikologische Pathologie (German Certification for Veterinary Pathology, sub-specializing in Toxicologic Pathology)
IFSTP International Federation of Societies of Toxicologic Pathologists
JCVP Japanese College of Veterinary Pathology
JSTP Japanese Society of Toxicologic Pathology
KBT Korean Board of Toxicology
KCVP Korean College of Veterinary Pathology
KSTP Korean Society of Toxicologic Pathology
MRCVS Member of the Royal College of Veterinary Surgeons (United Kingdom)
NVT Nederlandse Vereniging voor Toxicologie (Netherlands Society of Toxicology)
-
SFPT Société Française de Pathologie Toxicologique (French Society of Toxicologic Pathology)
SIPTS Società Italiana di Patologia Tossicologica e Sperimentale (Italian Society of Experimental and Toxicologic Pathology)
STP Society of Toxicologic Pathology (of North America)
STP-I Society of Toxicologic Pathology—India
SVTP Schweizerische Vereinigung für Tierpathologie (Swiss Association of Veterinary Pathology)
Introduction
Defining the optimal range of training activities should increase the consistency with which individuals are educated in regulatory-type toxicologic pathology across various geographic regions even in the absence of a uniform global recognition system. Such a framework must acknowledge the diverse educational opportunities, on-the-job training activities, cultural mores, institutional and societal expectations, and roles for toxicologic pathologists around the world. Nevertheless, the framework should represent a high bar for professional practice that will be applicable to toxicologic pathologists in both developed nations (i.e., those with long-established conventions for conducting the pathology analyses of regulatory-type, nonclinical toxicity studies, and for training the individuals who fulfill this role) and emerging nations (i.e., those in which such pathology practices and training programs have yet to be fully formalized).
A reasonable approach to developing a workable global framework of training activities is to propose “best practices” to impart the core theoretical knowledge and applied skills that are essential prerequisites for pathologists who participate in regulatory-type, nonclinical toxicity studies. Topics that must be addressed when formulating such a proposal can be summarized in five basic questions.
What roles do regulatory-type toxicologic pathologists serve?
What are the core knowledge and practical work-related skills required to function as a regulatory-type toxicologic pathologist?
What are suitable educational approaches for imparting core knowledge to regulatory-type toxicologic pathologists?
What experiences are most suitable for acquiring practical work-related skills in regulatory-type toxicologic pathology?
How much training do regulatory-type toxicologic pathologists require?
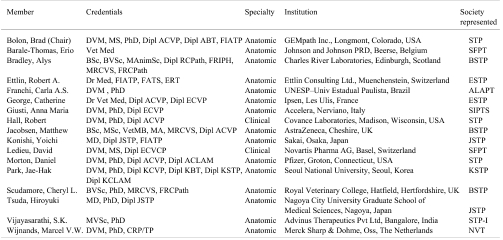
A committee of toxicologic pathologists with substantial experience in performing regulatory-type nonclinical toxicology studies, representing all ten IFSTP member societies, was assembled (Table 1) to answer these questions and produce the present international proposal for training regulatory-type toxicologic pathologists. Given the variability in current training approaches across regions, this document will identify optimal (“best”) practices while acknowledging alternative routes that might be suitable under certain circumstances.
Table 1. Members of the IFSTP Committee on International Best Practices for Training.
1. What roles do regulatory-type toxicologic pathologists serve?
Toxicologic pathologists contributing to regulatory-type, nonclinical toxicity studies are typically engaged in generating, interpreting, and communicating anatomic pathology data (e.g., organ weights, macroscopic and microscopic examination of tissues) OR clinical pathology data (clinical chemistry, hematology, coagulation, urinalysis, flow cytometry, biomarker validation, etc.) OR both anatomic pathology and clinical pathology data. The choice of role depends on individual, institutional, and societal preferences. For example, clinical pathology specialists are routinely engaged full-time in support of regulatory-type nonclinical toxicity studies in North Americaa1 and Europe, while clinical pathologists are not utilized in this capacity in Japan. Regardless of their role, individual toxicologic pathologists accept responsibility for the integrity and reliability of their work and affirm the validity of their contributions by signing the pathology reports that they produce. Single-author pathology reports are acceptable if the toxicologic pathologist has been trained to integrate anatomic pathology and clinical pathology findings; this model comprises the traditional “study pathologist” role found in many regions of the world. Alternatively, an anatomic pathologist may interpret anatomic pathology data and a clinical pathologist may interpret clinical pathology data within separate or combined pathology reports, with each specialist signing a report reflecting his or her contributions. This multi-participant model of integrating pathology data is common in many institutions with the means to build such expert teams.
Wide-ranging command of basic and applied scientific and medical knowledge coupled with substantial practice in a broad array of essential pathology skills is the foundation for proficiency in toxicologic pathology, regardless of any subsequent individual choice to concentrate one’s academic and work-related experiences to a specific toxicologic pathology role. Therefore, the means by which toxicologic pathologists acquire their particular knowledge, skills, and practical experience should be qualitatively similar for regulatory-type toxicologic pathologists throughout the world. National or regional differences in societal expectations, cultural mores, and training resources would then serve to modify the quantitative nature of the toxicologic pathology training experience (e.g., how much time is spent in anatomic pathology vs. clinical pathology functions), as long as the overall quality of the program and its final product—toxicologic pathologists capable of performing in the role that they fill—are always maintained.
2. What are the core knowledge and practical work-related skills required to function as a regulatory-type toxicologic pathologist?
A pathologist is a biomedical scientist with extensive clinical training (e.g., in veterinary medicine or medicine) as well as a thorough understanding of normal biological structures (tissues and fluids) and functions as well as their perturbations resulting from disease. Proficiency as a pathologist requires comprehension at multiple levels of biological organization (e.g., whole animal, cellular, molecular), and the ability to integrate this information with fundamental medical principles to formulate differential diagnoses as well as to identify and characterize disease etiologies and mechanisms (Scudamore and Smith, 2007).
Acceptable performance as a regulatory-type toxicologic pathologist requires that an individual have a solid background in the comparative aspects of normal anatomy (at the gross and light microscopic levels), physiology, and medicine of animals and humans; causes and mechanisms of major background and xenobiotic-induced diseases of common laboratory animal species and humans; principal techniques routinely used for evaluating pathologic changes in tissues (e.g., gross dissection, light microscopy, routine histochemistry and immunocytochemistry) and body fluids (clinical chemistry, cytology, hematology, urinalysis, etc.); and essential principles, practices, and regulations applicable to risk and safety assessment. Such knowledge is necessary for the practice of regulatory-type toxicologic pathology regardless of whether an individual engages primarily in tissue analysis (i.e., anatomic pathology), cell and fluid assessment (i.e., clinical pathology), or some combination of the two.
Other skills and knowledge will obviously be important for toxicologic pathologists in the 21st century. Examples of specific skills include ultrastructural analysis (mainly transmission electron microscopy), innovative microscopy techniques (confocal, fluorescence, etc.), morphometry, and stereology. Toxicologic pathologists should also have at least some familiarity with contemporary research tools, especially such molecular methods as in situ hybridization and laser capture microdissection, and evolving scientific disciplines (genomics, metabolomics, proteomics, toxicogenomics, etc.). Many regulatory-type, nonclinical studies will not utilize these advanced skills and knowledge, and the toxicologic pathologists assigned to such projects thus may not need training in these topics. However, if a regulatory-type, nonclinical toxicity study does employ such sophisticated skills and knowledge, then the toxicologic pathologist chosen for the study clearly must be appropriately trained to interpret the resulting data set.
3. What are suitable educational approaches for imparting core knowledge to regulatory-type toxicologic pathologists?
Substantial challenges were encountered in formulating flexible but rigorous “universal” training practices for toxicologic pathologists. The two main obstacles were the multiplicity of educational approaches that have been employed historically in various regions of the world and the lack of common training standards for presumably equivalent professional degrees among countries (e.g., veterinary medical training in China [4 or 5 years leading to a bachelor’s degree2] vs. the United States [6 to 8 years leading to a doctoral degree;3), or even within a country (e.g., traditional advanced pathology instruction leading to a graduate degree vs. a new certifying examination4). Nevertheless, a workable common framework for training regulatory-type toxicologic pathologists may be readily defined within the educational systems that have been developed in geographic regions with well-established conventions for conducting regulatory-type, nonclinical toxicity studies.
The baseline education for toxicologic pathologists includes the foundational sciences listed in Table 2. Toxicologic pathologists must integrate fundamental knowledge from many basic science disciplines with core clinical concepts and mechanisms, and do so across multiple species. The optimal practice for training toxicologic pathologists in the future will be successful completion of a clinical degree in veterinary medicine (e.g., BVSc, DVM, or the equivalent, emphasizing allopathic [Western] principles of practice) from an accreditedb university or college, followed by additional post-graduate training in pathology. The logic for this approach is that individuals with this background will have acquired their core medical knowledge and toxicologic pathology training in the animal species utilized most commonly for regulatory-type, nonclinical toxicity studies. Naturally, veterinary pathologists should also study human anatomy, physiology, and pathology so that their interpretations of animal findings in nonclinical toxicity studies may be applied in a public health context. The reason for advocating that academic institutions be accredited is that this status provides a minimum level of assurance that appropriate training has been offered by acceptably capable teachers.
Table 2. International Recommendations for Core Subjects to be Included in Training Programs for Regulatory-Type Toxicologic Pathologists.
In some geographic regions, regulatory-type toxicologic pathologists have been trained historically using alternative educational pathways. One approach has been to employ medical pathologists (rather than veterinary pathologists). Similar to their veterinary counterparts, medical pathologists seeking careers in toxicologic pathology will have successfully finished a clinical degree (e.g., MD or the equivalent) from an accredited educational institution followed by advanced post-graduate training in pathology. However, medical pathologists functioning as toxicologic pathologists for regulatory-type, nonclinical toxicity studies also will need to complete several comparative biology courses (including anatomy, physiology, and pathology) during their post-graduate training to compensate for the absence of such animal-oriented material during their human-oriented clinical curriculum. Another alternative approach has been to co-opt medical biologists with doctoral-level training in a relevant biological field (e.g., pathology, pharmacology, or toxicology) as regulatory-type toxicologic pathologists due to the scarcity of veterinary pathologists and medical pathologists. As with any activity, medical biologists may improve their toxicologic pathology talents by practice. However, acceptable performance of medical biologists in this role generally requires an emphasis in their academic curriculum on core subjects in medicine, pathology, and toxicology as well as an integrative (e.g., “whole animal”) focus to biological investigation rather than mainly cellular or molecular approaches used in mechanistic research, as well as extended periods of intensive on-the-job instruction to compensate for the absence of a rigorous education in clinical medicine.
Persons who lack a clinical (veterinary medical or medical) degree and post-graduate academic training in pathology or a doctoral-level degree in a pathology-related biomedical discipline are unlikely to possess sufficient command of the core theoretical knowledge required of regulatory-type toxicologic pathologists.
4. What experiences are most suitable for acquiring practical work-related skills in regulatory-type toxicologic pathology?
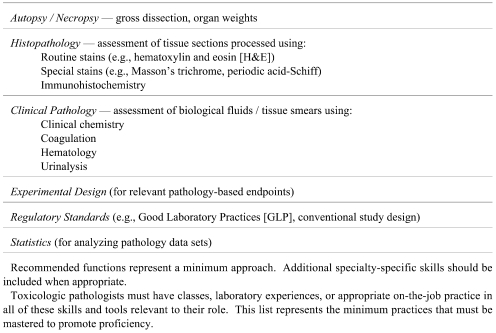
The applied work-related skills and tools required of regulatory-type toxicologic pathologists are listed in Table 3. These elements may be introduced during academic coursework (in laboratory sessions rather than reading assignments). However, the limited experience available in such educational settings necessitates that more extensive practice be gained elsewhere for real aptitude to be developed5. Accordingly, the optimal way to obtain sufficient practical experience as a regulatory-type toxicologic pathologist is via intensive post-graduate training that focuses on generation and interpretation of pathology data. The rationale for this recommendation is that persons with wide-ranging practice using basic pathology skills and tools are much more likely to quickly and easily develop proficiency as toxicologic pathologists.
Table 3. International Recommendations for Applied Skills and Tools to be Used in Training Regulatory-Type Toxicologic Pathologists.
Initial practical training in pathology should include ample diagnostic work in anatomic pathology or clinical pathology (or both), some or all of which should involve problems with natural or experimental cases of toxicity. Training in anatomic pathology and clinical pathology skills and tools is typically initiated in an academic setting during the classroom and clinical rotations of a veterinary medical (or medical) course. In some nations, these basic lessons are then refined during an academic residency in pathology. Such residencies usually emphasize the skills and tools of a particular pathology specialty (e.g., anatomic pathology OR clinical pathology), often as applied to diagnostic cases in a hospital setting. However, optimal residency training will include an absolute requirement that time be spent learning skills relevant to toxicologic pathology (e.g., trainees with educational support from the ACVP/STP Coalition for Veterinary Pathology Fellows) and will generally offer at least some cross-training in both anatomic pathology and clinical pathology. Research training in pathology (as a MS or PhD [or equivalent] student, or as a post-residency clinical fellow in veterinary medicine or medicine) often serves as a useful supplement to such applied programs as some important concepts (e.g., study design, statistical analysis) represent a small portion of the curriculum in many conventional clinically-oriented pathology residencies. However, research training alone is not a suitable substitute for adequate practical experience in pathology.
Applied skills and tools in regulatory-type toxicologic pathology are often gained during an on-the-job apprenticeship (formal or informal) sponsored by one’s first employer. In most countries such experience occurs (1) following a clinical degree in veterinary medicine or medicine or (2) following both a clinical degree and an academic pathology residency. Such apprenticeships tend to stress training in the tasks associated with only one pathology specialty (often anatomic pathology for traditional “study pathologists” in many regions of the world). The main advantage of this arrangement is that “apprentice” toxicologic pathologists are immersed in problems of direct relevance to their employer’s needs. Two possible disadvantages are that the training experience might be curtailed if the employer’s day-to-day expectations and/or financial constraints impinge on training time and opportunities (e.g., access for trainees to external short courses), and that the training may be narrowly focused to the current workload and limited on-site training capabilities of the employer.
Regulatory-type toxicologic pathologists will receive acceptable applied training in either of these two scenarios: academic residency followed by abbreviated (2-year) on-the-job apprenticeship or extended (4-year) on-the-job apprenticeship. However, a likely benefit of the residency-oriented option is that pathology trainees will study many more diagnostic problems and use a larger set of diagnostic tools relative to those needed for typical regulatory-type toxicologic pathology investigations. Such broad exposure is an essential part of a toxicologic pathologist’s knowledge base because many non-toxic etiologies (e.g., infectious and metabolic diseases) as well as spontaneous background findings can impact the genesis, progression, extent, and permanence of toxicant-induced lesions. In the authors’ experience, diagnostic principles relevant to interpreting the significance of complex disease patterns (in which nontoxic and toxicant-induced lesions are intermingled) are better understood in laboratory animal species if the principles were first learned by studying as many animal species as possible (including companion, domestic, and exotic species). The range of disease entities and animal species needed to attain wide-ranging appreciation of diagnostic principles, multiple etiologies, and complex pathophysiologic mechanisms is generally much broader in a hospital or clinical practice setting (i.e., residency) relative to a laboratory focused specifically on toxicologic pathology research.
The possession of a formal pathology certification by regulatory-type toxicologic pathologists is a necessary prerequisite in some institutions and geographic regions but is optional in others. The utility of holding a pathology qualification is clearly acknowledged by the recent IFSTP proposal to develop a global recognition system6. Accordingly, certification in pathology is recommended to tangibly demonstrate that an individual has learned the core knowledge and applied skills needed for professional practice. Widely accepted certification mechanisms used for this purpose include qualifications specifically for toxicologic pathology (e.g., CRP/TP, FRCPath/ToxPath, FTA Pathol/Tox Pathol, FIATP, and JSTP) as well as those for anatomic pathology or clinical pathology (e.g., ACVP, DESV-APV, ECVP, ECVCP, FTA klin Lab, JCVP, and SVTP). The conventions for eligibility and the criteria and mechanisms for awarding these various qualifications vary across regions, but in general they use educational and experiential standards that are similar in the broad sense with those outlined above (Sections 3 and 4).
5. How much training do regulatory-type toxicologic pathologists require?
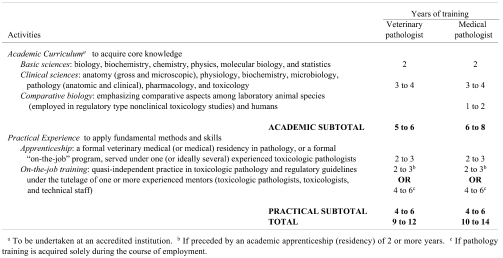
The time and effort required by trainees, teacher(s), training institutions, and employers to prepare an individual for a career in regulatory-type toxicologic pathology will vary somewhat with the chosen approaches to academic coursework and practical experience. The proposed outline of optimal (“best;” Table 4) and realistic (“acceptable;” Table 5) training practices endeavors to balance these individual, institutional, and societal preferences with the absolute requirement that proficiency cannot be attained unless sufficient exposure over time has been obtained for both theoretical knowledge and the applied skills and tools of the toxicologic pathology trade.
Table 4. International Recommendations for Optimal (“Best”) Practices for Training Future Regulatory-Type Toxicologic Pathologists.
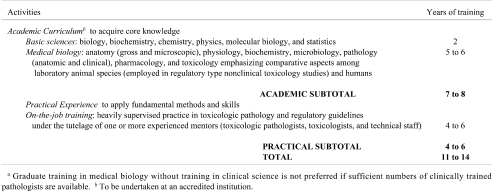
Table 5. Medical Biology without Clinical Education as an Alternative Means for Training Regulatory-Type Toxicologic Pathologistsa.
The education of a regulatory-type toxicologic pathologist typically will require a minimum of 9 years of combined academic coursework and practical training. This time span reflects two factors. First, the number and complexity of subjects that must be studied to gain the theoretical knowledge necessary for this profession (Table 2) cannot be attained in less than 5 years of concentrated (i.e., full-time) study. Second, acquisition and practice of the applied skills and tools used in regulatory-type toxicologic pathology cannot be completed in less than 4 years of applied training. In some nations this concentrated practical experience is undertaken as a 2- to 3-year-long formal veterinary medical residency (or medical residency, or possibly a graduate degree program in medical biology emphasizing hands-on, systems-based research experience in pathology with exposure to diagnostic and interpretative case work) under the tutelage of one or ideally several experienced pathologists, followed by 2 or more years of on-the-job training in regulatory-type toxicologic pathology working with a team of mentors (toxicologic pathologists, toxicologists, and expert technical staff). In other regions, practical experience is attained entirely via an intensive on-the-job training program in regulatory-type toxicologic pathology, lasting at least 4 years, with mentoring by an interdisciplinary team of scientists. Regardless of the training institution and approach, the toxicologic pathology trainee will spend many hours learning and practicing essential skills and techniques of pathology (Table 3) such as sample (tissue and fluid) collection, handling and processing; analytical methods; data interpretation; quality assurance and quality control; and report preparation. Repeated, intense, and broad exposure to these topics is more critical than the number of years spent in training. Nevertheless, true proficiency cannot be obtained in less than 9 years of intensive education in clinical medicine, general pathology, and regulatory-type toxicologic pathology.
Training vs. Proficiency
The academic coursework and on-the-job experiences outlined above have been shown by long historical practice in developed nations to be successful methods for developing the toxicologic pathology expertise of persons who support regulatory-type, nonclinical toxicity studies. That said, neither acquisition of a particular academic degree nor possession of a recognized pathology certification provide a guarantee that true proficiency (i.e., competence) has been achieved, let alone maintained over time. Proficiency is not a static or finite entity. Individuals will not remain proficient indefinitely in all the core knowledge and practical skills of any field. In toxicologic pathology, this gradual regression of proficiency will reflect both the ever-expanding nature of the discipline and the tendency for unused abilities to atrophy. In contrast, individuals will not only retain but also increase their proficiency over time in the core toxicologic pathology knowledge, skills, and tools that they use routinely.
Taken together, these concepts indicate that the initial complex and lengthy training required to educate a regulatory-type toxicologic pathologist represents only the start of a lifelong commitment to professional education. Individuals serving in this role will be expected to expend considerable effort on sharpening known skills and learning new ones throughout their careers. Thus, an essential aspect of training regulatory-type toxicologic pathologists will be to impart a taste for thorough continuing education.
Three mechanisms seem well suited to maintaining and extending proficiency in regulatory-type toxicologic pathologists. The first is habitual attendance at scientific meetings or short courses (ideally at least one per year). The second is constant reading of relevant books and journals in the field. These two options are powerful as they can be undertaken at the individual’s convenience. Therefore, regulatory-type toxicologic pathologists should be encouraged to log such activities as a subjective record of their ongoing training efforts. The third mechanism is regular participation in pathology peer review (either as the study pathologist or the peer review pathologist). Advantages of peer review as a training activity are that documentation of the peer review process will objectively show that an educational exchange occurred, while the subject of the review will automatically address practical aspects of toxicologic pathology practice. The “best practice” for the continuing education of regulatory-type toxicologic pathologists will be to liberally incorporate all three of these options.
Critique of the International Recommendations for Future Training Practices
This IFSTP-proposed global framework for training regulatory-type toxicologic pathologists in the future was prepared in stages. The initial drafts were written by the committee chair, after which the committee members worked together to produce a polished draft, which was then submitted to the IFSTP for distribution to its members societies. The governing bodies of the IFSTP member societies either reviewed the polished draft themselves on behalf of their membership or circulated it to their entire membership. In all, this final proposal incorporates more than 200 comments (many raised by multiple individuals) from 70 toxicologic pathologists (from both anatomic pathology and clinical pathology specialties) representing 8 of the 10 IFSTP member societies.
Where feasible, these remarks have been included specifically in the proposal. However, four other important side issues were raised that had no place within the proposal. These topics are addressed here in “question (Q) and answer (A)” format to further the debate on this proposal. The order in which the items are considered reflects the number of times the issue was raised in the comments on the prior IFSTP-circulated draft. The questions (or comments) have been edited for content and clarity.
Q. This IFSTP proposal on training practices is identical to the IFSTP proposal for a global recognition mechanism6 that was decisively rejected by a vote of the STP membership7. Why circulate the same proposal only 6 months later?
A.The current proposal does not in any way put forward a global mechanism for recognizing regulatory-type toxicologic pathologists. This document is intended only to suggest an internationally accepted framework of rigorous but flexible practices for educating regulatory-type toxicologic pathologists. The proof of this assertion is evident in the text of these two proposals: the international recognition plan6 was designed to provide a point-based scale for measuring a candidate’s past accomplishments, while the present training practice proposition recommends a broad course of study to direct a trainee’s future educational endeavors. True, the more uniform training practices listed here could serve as one facet underpinning any future proposal for global recognition system. However, as noted in the next question, a more consistent approach to educating toxicologic pathologists stands to benefit individuals, employers, and regulatory agencies regardless of whether a recognition mechanism is implemented in the future.
Q. Why is a “best practice” framework for training toxicologic pathologists needed when (1) most IFSTP member societies do not have formal educational standards or certification processes and (2) no mechanism exists for enforcing these international training practice recommendations?
A. This proposal has two purposes. The first is to provide more uniformity of training specifically for those toxicologic pathologists who support regulatory-type, nonclinical toxicity studies. Increased consistency will allow employers and regulatory agencies to more easily compare the educational backgrounds of regulatory-type toxicologic pathologists who were trained in different geographic regions. The second is to offer a defined target— based on long-established, successful training practices that have evolved over time in developed nations—at which toxicologic pathology educators from emerging nations might aim their future training efforts. The rationale is that transfer of the well-recognized training practices from developed nations (which have produced many regulatory-type toxicologic pathologists who are acknowledged as “qualified” by regulatory authorities worldwide) is the best means of assuring that toxicologic pathology education in emerging nations will attain a similar degree of quality, without expending scarce resources in reinventing already effective training techniques. The IFSTP is a reasonable entity for proposing such recommendations because most pathology training programs are not focused on regulatory-type toxicologic pathology, while the individuals belonging to IFSTP members societies are well versed in the training needs of individuals engaged in their profession.
Q. What will prevent this set of training practice recommendations from evolving into a global mechanism for pseudo-certification in toxicologic pathology?
A. No impediment exists to prevent an entity from using these training practice recommendations as one basis for a recognition mechanism (national or global) in the future. However, a widely acknowledged core program of training does not by itself provide a certification method. Employers and regulatory agencies are well acquainted with the difference between training (a course of study) and certification (an assessment that a given body of knowledge, skills, and tools has been assimilated). The current proposal clearly is limited to a recommended course of study, and provides no method for evaluating whether or not a trainee has learned it. Such testing is the responsibility of the training institutions and the relevant certifying bodies in a given nation.
Q. What on-the-job functions will be acceptable training practices for regulatory-type toxicologic pathologists? At what point during an “apprenticeship” will an individual “graduate” from trainee to proficient pathologist?
A. Suitable training tasks would include the full scope of activities undertaken by independent pathologists (e.g., data generation and interpretation appropriate to their specialty, as well as data integration and communication via oral presentations and written pathology reports). Our recommendation (see Section 5 above) is that on-the-job training in regulatory-type toxicologic pathology knowledge, skills, and tools continue for at least 2 years if a trainee has had prior intensive experience (e.g., a formal academic residency) in general pathology, and for at least 4 years if the trainee has had no such prior experience. The rationale for this proposal is that trainees who transition from a formal pathology residency to a toxicologic pathology position have already obtained a substantial amount of applied pathology practice. The main difference between a “pathology trainee” and a “proficient pathologist” is the degree to which daily pathology functions must be supervised by one’s instructor(s). Common practice permits trainees to immediately serve as “study pathologists” (i.e., attest to the validity of their work by signing the final pathology report), but under the close supervision and careful peer review by a pathologist with long experience in the conduct of regulatory-type nonclinical studies. The graduation date will be set by the employer at whatever time the supervisor chooses to affirm that the trainee is capable of performing in a self-sufficient manner.
International Recommendations for Global Training Practices
The current IFSTP proposal suggests a rigorous but flexible framework of training practices for the educational and work-related experiences that should be employed worldwide when educating future toxicologic pathologists who engage in regulatory-type, nonclinical toxicology studies.
Formal Education in Clinical Science and Pathology as the Optimal Training Option
The optimal pathway (“best practice;” Table 4) for educating regulatory-type toxicologic pathologists should produce individuals with the following attributes:
* Theoretical Knowledge: acquired during 5 or more years of scientific and clinical education leading to a degree in veterinary medicine (or medicine).
-
* Practical Experience: acquired by at least 4 years of intensive practice in one of the two following formats:
o Clinically-based residency (2 or more years) followed by an on-the-job toxicologic pathology “apprenticeship” (informal or formal) of 2 years
o On-the-job toxicologic pathology “apprenticeship” of 4 years
The rationale for proposing a clinical education (veterinary medical or medical degree) as a “best practice” is that pathology is a medical discipline that requires clinical training. Because the subjects of regulatory-type, nonclinical toxicity studies are animals, medical pathologists will clearly require additional training in animal-related topics such as species-specific anatomic and physiological variations as well as spontaneous diseases. The logic for the two options for applied post-graduate training are that the knowledge and skills necessary for general pathology understanding and specialized toxicologic pathology performance may be learned in various settings, but since these options do not overlap completely, enough time must be spent to acquire familiarity with both categories of pathology practice.
Medical Biology without Clinical Education as an Alternative Training Pathway
In some geographic regions a number of long-established, regulatory-type toxicologic pathologists have medical biology backgrounds and have been accorded national or international recognition as proficient practitioners by their peers, employers, and regulatory agents on the basis of their many years of professional activity and their successful participation in the pathology peer review process (as both study pathologists and peer review pathologists). The typical features of this option (Table 5) are:
* Theoretical Knowledge: acquired during 7 or more years of science education leading to a doctoral-level degree in a relevant discipline of medical biology (e.g., pathology, pharmacology, toxicology) in the absence of formal training in clinical medicine. Anatomy and physiology as well as pathology, pharmacology, and toxicology must be heavily emphasized in such a nonclinical curriculum, and these subjects must stress comparative and integrated (i.e., “whole animal” rather than limited to cellular / molecular) understanding.
* Practical Experience: acquired through an intensive on-the-job toxicologic pathology “apprenticeship” lasting at least 4 years. Intensive on-the-job training and independent study must fill the gaps in education that will arise when pathology candidates are not classically educated first as veterinarians or physicians.
If veterinary pathologists and medical pathologists are available, this training option should be avoided when designing training programs to educate future generations of toxicologic pathologists. The rationale is that (1) the medical/veterinary medical education and clinical perspective required for a true understanding of pathology is reduced in non-clinical programs, and (2) medical biology training may be focused on cellular and molecular evaluation rather than the system-based (“whole animal”) skills and tools that are the stock-in-trade of regulatory-type toxicologic pathologists with a clinically oriented pathology background. In particular, training institutions in emerging nations should not look to this option as their preferred framework for producing more regulatory-type toxicologic pathologists.
Further Educational Activities
Regardless of the training pathway, individuals employed as regulatory-type toxicologic pathologists are encouraged to acquire a recognized post-graduate pathology qualification (i.e., certification). Such documentation provides tangible evidence that one has understood a core set of pathology-related knowledge, skills, and tools at a discrete point in time.
Pathologists trained by all pathways are also encouraged (and in some locations required) to commit to a lifelong, self-motivated program of regular continuing education activities, such as attending a pertinent scientific meeting or short course, reading professional literature relevant to the role performed on a habitual basis, and participating frequently in pathology peer review for nonclinical toxicology studies. The exact nature of continuing education may be varied according to individual, institutional, and/or societal preferences, but a regular program of ongoing study will remain a necessary training “best practice” for future toxicologic pathologists.
Conclusions
These international recommendations for global training practices have been presented to the IFSTP Executive Committee as well as the governing bodies and/or of all IFSTP member societies. The merits of this proposal have been discussed by these governing bodies, and at their discretion by the members of their societies. At the time of publication, formal endorsements have been conferred by the leadership of the IFSTP as well as 8 of the 10 IFSTP member societies (ALATP, BSTP, ESTP, NVT, SFPT, SIPTS, STP, STP-I). We anticipate that these practices, if implemented consistently, will promote more uniform training among regulatory-type toxicologic pathologists worldwide. We urge all the IFSTP member societies to help training programs for toxicologic pathologists within their nation or region apply these recommendations, keeping in mind that all acceptable training programs for future toxicologic pathologists must seek to assure that high standards of biomedical instruction are always upheld.
For the time being, currently used gauges of training quality (e.g., attaining a relevant national or regional certification in pathology, the employer’s satisfaction with the graduate’s on-the-job performance) will likely remain the metrics of choice for assessing a newly minted toxicologic pathologist’s proficiency. In the future, it is conceivable that a global mechanism to recognize toxicologic pathologists could be developed, using these “best practice” recommendations as the standard for training individuals to prepare for recognition. However, it is equally plausible that consistent worldwide application of the optimal training practices advocated here will ensure the initial proficiency of new toxicologic pathologists so effectively that implementing an international recognition mechanism will be unnecessary. Either way, these “best practices” for training should provide a rigorous yet flexible way to reliably educate toxicologic pathologists from both developed and developing nations to generate, interpret, integrate, and communicate data in regulatory-type, nonclinical toxicology studies.
Footnotes
* The content represents the views of toxicologic pathologists from all ten IFSTP member societies, namely: Associação Latino-americana de Patologia Toxicológica (ALAPT; CASF); British Society of Toxicological Pathologists (BSTP: AB, MJ, CS); European Society of Toxicologic Pathology (ESTP: RAE, CG); Japanese Society of Toxicologic Pathology (JSTP: YK, HT); Korean Society of Toxicologic Pathology (KSTP: JH-P); Nederlandse Vereniging voor Toxicologie (NVT: MVWW); Société Française de Pathologie Toxicologique (SFPT: EB, DL); Società Italiana di Patologia Tossicologica e Sperimentale (SIPTS: AMG); Society of Toxicologic Pathology (STP: BB, RH, DM); Society of Toxicologic Pathology—India (STP-I: SKV). This proposal has been reviewed by the Executive Committee of the International Federation of Societies of Toxicologic Pathologists (IFSTP) as well as the governing bodies and constituencies of the IFSTP member societies mentioned above. As of the publication date, it has been endorsed by the leadership of the ALAPT, BSTP, ESTP, IFSTP, NVT, SFPT, SIPTS, STP, and STP-I. As a service to the international toxicologic pathology community, this paper has been published concurrently in the journals of the BSTP and STP (Toxicologic Pathology), the ESTP (Experimental and Toxicologic Pathology), and the JSTP (Journal of Toxicologic Pathology).
a More than 30 individuals fulfill this function.
b By a national organization.
References
- 1.Schultze AE, Bounous DI, Bolliger AP. Veterinary clinical pathologists in the biopharmaceutical industry. Vet Clin Pathol. 37: 146–158 2008 [DOI] [PubMed] [Google Scholar]
- 2.Yin J-C, Li G-Y, Ren X-F. An overview of veterinary medical education in China: current status, deficiencies, and strategy for improvement. J Vet Med Educ. 33: 238–243 2006 [DOI] [PubMed] [Google Scholar]
- 3.Anonymous AAVMC member institutions. Last retrieved May 21, 2010, from AAVMC (Association of American Veterinary Medical Colleges) website : http://www.aavmc.org/about/about.htm.
- 4.Anonymous The training document of the Indian College of Veterinary Pathologists (ICVP). Last retrieved May 21, 2010, from IAVP (Indian Association of Veterinary Pathologists) website: http://www.iavp.org/ index.php?option=com_content&view=article&id=40&Item id=37.
- 5.Scudamore CL, Smith SH. Future directions in training veterinarians for careers in toxicological pathology in the United Kingdom. J Vet Med Educ. 34: 450–457 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ettlin RA, Bolon B, Pyrah I, Konishi Y, Black HE. Global recognition of qualified toxicologic pathologists: Credential review as a potential route for recognizing the proficiency of pathologists involved in regulatory nonclinical studies. Published in parallel as Exp Toxicol Pathol. 62: 413–422 2010; J Toxicol Pathol. 22: 143,–152 2009. Toxicol Pathol. 37: 553–561 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bolon B, Ochoa R, Mann P. STP debate on the desirability of an international mechanism for recognizing qualified toxicologic pathologists. Toxicol Pathol. 37: 992–996 2009 [DOI] [PubMed] [Google Scholar]