Abstract
While ethanol intake at high levels (3-4 or more drinks), either in acute (occasional binge drinking) or chronic (daily) settings, increases the risk for myocardial infarction and ischemic stroke, an inverse relationship between regular consumption of alcoholic beverages at light to moderate levels (1-2 drinks per day) and cardiovascular risk has been consistently noted in a large number of epidemiologic studies. Although initially attributed to polyphenolic antioxidants in red wine, subsequent work has established that the ethanol component contributes to the beneficial effects associated with moderate intake of alcoholic beverages regardless of type (red versus white wine, beer, spirits). Concerns have been raised with regard to interpretation of epidemiologic evidence for this association including heterogeneity of the reference groups examined in many studies, different lifestyles of moderate drinkers versus abstainers, and favorable risk profiles in moderate drinkers. However, better controlled epidemiologic studies and especially work conducted in animal models and cell culture systems have substantiated this association and clearly established a cause and effect relationship between alcohol consumption and reductions in tissue injury induced by ischemia/reperfusion (I/R), respectively. The aims of this review are to summarize the epidemiologic evidence supporting the effectiveness of ethanol ingestion in reducing the likelihood of adverse cardiovascular events such as myocardial infarction and ischemic stroke, even in patients with co-existing risk factors, to discuss the ideal quantities, drinking patterns, and types of alcoholic beverages that confer protective effects in the cardiovascular system, and to review the findings of recent experimental studies directed at uncovering the mechanisms that underlie the cardiovascular protective effects of antecedent ethanol ingestion. Mechanistic interrogation of the signaling pathways invoked by antecedent ethanol ingestion may point the way towards development of new therapeutic approaches that mimic the powerful protective effects of socially relevant alcohol intake to limit I/R injury, but minimize the negative psychosocial impact and pathologic outcomes that also accompany consumption of ethanol.
Keywords: ischemia/reperfusion; ethanol preconditioning; alcohol; myocardial infarction; stroke, coronary disease; inflammation; signaling; epidemiology
1. Introduction
A large number of epidemiologic studies suggest that regular ingestion of alcoholic beverages at low to moderate levels (1-2 drinks per day) may induce tolerance to ischemia, even in the presence of co-existing risk factors. Subsequent studies conducted in a variety of animal models support this contention and have provided important mechanistic insight that may allow for development of new therapeutic strategies to limit I/R injury that remain effective despite the presence of co-morbid risk factors in humans. The development of such alternate pharmacologic approaches is an important focus of scientific inquiry because compliance with other cardioprotective lifestyle interventions such as exercise is often difficult to achieve, while use of alcoholic beverages is contraindicated for individuals susceptible to the development of alcoholism, especially the large majority who cannot be reliably identified by objective criteria (such as familial alcoholism) as being at risk prior to beginning ethanol consumption. In addition, ethanol ingestion increases the morbidity and mortality associated with hemorrhagic stroke, induces liver injury, aggravates high-fat diet-induced steatohepatitis, is toxic to neurons, increases the risk for development of breast, oral and gastrointestinal cancers, and has psychosocial consequences (e.g., impaired driving ability and performance of employment responsibilities), even when consumed at low to moderate levels [1-11].
The aims of this review are first to summarize the epidemiologic evidence supporting the association between ethanol ingestion and reductions in the likelihood of adverse cardiovascular events, even in patients with co-existing risk factors. As part of this objective, we will discuss the ideal quantities, drinking patterns, and types of alcoholic beverages that confer protective effects in the cardiovascular system. Second, we will review the findings of recent studies conducted in animal models and cell culture systems that are directed at uncovering the mechanisms that underlie the protective effects of antecedent ethanol ingestion, with an eye towards development of new therapeutic approaches that mimic the powerful protective effects of socially-relevant alcohol intake but minimize the negative psychosocial impact and pathologic effects of ethanol consumption.
Throughout this review, ethanol and alcohol are used interchangeably to denote ethyl alcohol. In addition, the term standard drink refers to one 5 ounce glass of wine, 12 ounces of beer, or 1.5 ounces of 80 proof spirits, each of which contain approximately 15-20 g of ethanol. Use of the phrase light to moderate drinking denotes ethanol consumption in the range of 1-2 standard drinks per day, while heavy alcohol consumption refers to intake of 3 or more standard drinks per day.
2. Epidemiologic evidence for the association between regular moderate ethanol intake and cardiovascular protection
Given the well-established pathologic effects of heavy drinking, wine lovers world-wide no doubt rejoiced when large-scale epidemiological studies first emerged showing that regular consumption of light to moderate amounts of alcoholic beverages, in particular red wine, was associated with a cardioprotective effect. One of the first studies showing a significant negative association between alcohol consumption and the risk of a subsequent first myocardial infarction that was well-controlled for cigarette smoking and other risk factors was published in 1974 [12]. Over the following 4 decades, numerous epidemiological studies including several meta-analyses (for example [13-51]) have consistently reported that an average alcohol consumption in the range of 0.5 to 2 standard drinks per day reduces coronary heart disease-related risks and ischemic stroke compared to non-drinkers.
Interestingly, initial work suggested that the protective effects associated with light to moderate intake of alcoholic beverages may be due to the polyphenols in grapes, wine, and dark beer. In particular, the stilbene resveratrol has been a major focus of research interest because this and other red wine constituent polyphenols exert powerful antioxidant actions, upregulate eNOS gene expression, enhance NO production and have been found to reduce morbidity and mortality due to cardiovascular disease [52-58]. In the human diet, red wine is one of the richest sources of polyphenols and moderate red wine drinkers consume these compounds at levels well above the population average [58]. Despite the demonstrated protective actions of resveratrol and flavonoids, discrimination between the effects of these red wine constituent polyphenols versus ethanol per se to induce protection associated with consumption of alcoholic beverages is controversial, in part owing to low levels of the former achieved in the blood following red wine ingestion [49]. Indeed, flavonoids and other wine polyphenols are extensively metabolized during absorption, resulting in formation of glucuronidated, sulfated, and methylated derivatives of the parent polyphenol, which have not been extensively evaluated for their protective effects [49,53,54]. As a consequence, the highest plasma concentrations achieved after ingestion of resveratrol-rich beverages by humans range between 1 and 10 μM. These concentrations are lower than those typically evaluated in cell culture models and in animal studies. To further complicate the distinction between ethanol- and resveratrol-specific effects, the downstream molecular mechanisms so far identified for the two compounds show substantial overlap (reviewed in [59]). Nevertheless, important mechanistic distinctions exist, particularly with regard to the effects of resveratrol to induce the expression of several longevity genes, which have also been implicated in the cardioprotective effects of caloric restriction [52,53,58]. Some epidemiological studies have shown that consumption of red wine is more protective than other types of alcohol [29], whereas other studies failed to identify an additional advantage associated with red wine [38].
The health effects of ethanol are dependent on the amount of alcohol consumed and the pattern of intake. Nearly all epidemiologic studies report a J-shaped curve, whereby light to moderate ethanol consumption (1-2 standard drinks per day) exhibit less risk for adverse cardiovascular events and overall mortality than abstainers, while heavy drinkers (3-4 or more drinks per day) demonstrate increased risk [13-51]. As with liver injury induced by ethanol, this varies by sex, with women benefiting from 1 standard drink per day, whereas daily consumption of 1-2 drinks by males was associated with reduced total mortality [18,20]. On the other hand, increased mortality occurs in females with daily intake of 2 or more alcohol beverages and in men consuming more than 3 drinks per day.
The effects of light to moderate ethanol consumption appear to be most clearly related to cardiovascular benefits, with most studies reductions in risk for heart disease by 30-35% [31,39]. Regular alcohol consumption at low to moderate levels is associated with significant reductions in the incidence of myocardial infarction in both males and females, regardless of age in adults [61]. Importantly, this effect was noted in higher risk populations, including individuals with diabetes, hypertension, hypercholesterolemia, known heart disease, or who are overweight, as well as in cigarette smokers [13,16,19,23,24,28,34,43,47,49,51,59,61-73].
Cardiovascular disease risk is also lowered by moderate ethanol consumption in individuals adhering to healthy lifestyle behaviors. Indeed, it has been reported that men who exercised regularly for at least 30 min/day, abstained from smoking, adhered to a healthy diet, and maintained their body mass index at less than 25 kg/m2 derived a health benefit from regular alcohol intake at moderate levels, demonstrating a 40-50% reduction in risk for myocardial infarction [37]. Moderate wine drinking also appears to strengthen the cardioprotective effects of fish consumption, an observation that links two important components of the Mediterranean diet, namely omega-3 fatty acids and wine [74].
In addition to reducing the incidence and severity of myocardial infarction, low to moderate alcohol consumption is also associated with lower risk for ischemic stroke, dementia, congestive heart failure, peripheral artery disease, intestinal and hepatic I/R injury, and frequency of Raynaud’s phenomenon [5,6,32,41,67,75-84]. Heart rate variability, a marker of autonomic imbalance, is also improved by consumption of alcoholic beverages, with wine intake demonstrating a stronger association with this effect than beer or spirits [84]. Reductions in C-reactive protein, fibrinogen, interleukin-6, and tumor necrosis factor alpha also occur with regular moderate alcohol consumption [82,85-88]. The aforementioned observations clearly indicate that the health benefits of alcohol consumption extend beyond the heart.
Although the concept that moderate intake of ethanol exerts protective effects in the cardiovascular system is now well accepted, important issues have been raised which challenge this premise. For example, it has been argued that uncontrolled confounding influences by lifestyle factors may play a role in the association between moderate alcohol intake and cardiovascular risk. Some work has led to the suggestion that individuals who regularly consume alcohol beverages exhibit healthier habits with regard to diet and/or exercise and enjoy superior sociodemographic factors, which could explain the reduced risk for ischemic myocardial disease [66,89-95]. However, systematic review and meta-analysis of interventional studies directed at the association between alcohol consumption and disease markers associated with risk for cardiovascular disease in adults without known cardiovascular disease argues against this supposition, as do the results of studies designed to better control for confounding sociodemographic factors and differences in dietary patterns and exercise adherence [18,20].
It has now become apparent that the pattern of drinking (which refers to the frequency and quantity of alcohol intake) exerts important effects on the association alcohol consumption and risk for cardiovascular disease. Binge drinking, which is defined as consumption of 3 or more alcoholic beverages within 1-2 hrs, is now recognized to increase the possibility for adverse cardiovascular risks [42,48,73]. Individuals pursuing this pattern of drinking often only consume alcohol one or two days during the week, so that their average intake over a given week may fall in the range of 1-2 drinks/day, an amount that exerts protection when ingested regularly each day. The fact that binge drinking exerts deleterious effects points to the importance of querying daily consumption versus number of drinks per week in evaluating data for the assessment of the health effects of ethanol consumption.
While the association between alcohol consumption and decreased cardiovascular risk is clear, it remains uncertain as to whether these correlative findings imply causation. However, a number of points regarding the aforementioned epidemiologic findings suggest that the association may indeed represent a cause-and-effect relationship [13-51]. First, there is a temporal relation between alcohol use and prevention of cardiovascular disease. Second, greater protection is observed with increasing ethanol dose over the protective range of alcohol intake. Third, the protective association between ethanol consumption and adverse cardiovascular events has been consistently observed in diverse patient populations and in both men and women. Fourth, the reduction in risk remains significant even after the influences of potential confounders such as cigarette smoking, diet, and exercise are factored into the analysis. Fifth, the association is specific for lowering rates of cardiovascular disease but does correlate with protection in other conditions such as cancer. Finally, the coupling of the aforementioned epidemiologic associations with the interventional mechanistic studies discussed below, provides compelling support for the notion that ethanol intake may indeed confer protection against the deleterious effects of I/R.
3. Cardioprotective mechanisms induced by ethanol ingestion: direct influences on cardiomyocytes vs indirect extra-cardiac effects
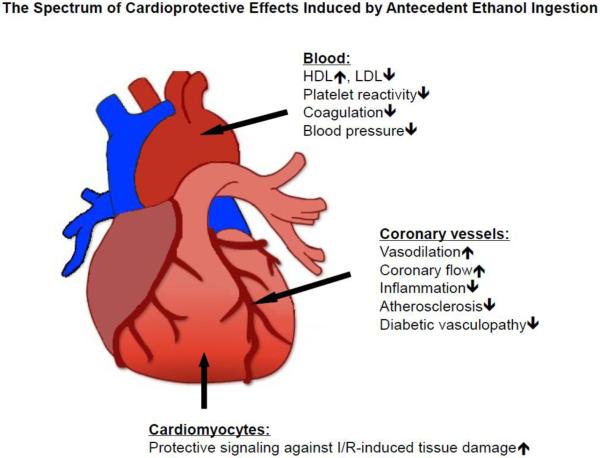
Epidemiologic studies have also established associations which provide important clues regarding the mechanisms whereby ethanol intake may exert cardioprotective effects. Ethanol ingestion influences blood lipoprotein profiles, coagulation and fibrinolytic cascades, platelet aggregation, oxidative stress, insulin sensitivity, and endothelial function to reduce the risk of adverse cardiovascular events [13,22,25,26,35,40,42,45,50,70,72,85,96-110]. Use of experimental animal models and more simplified (reductionist) cell culture systems to examine the molecular mechanisms whereby ethanol confers cardioprotection has led to concept that ethanol may also directly induce cell survival programs in parenchymal cells that render them resistant to the deleterious effects of I/R. From this work, it has become apparent that alcohol consumption has beneficial effects on numerous targets that may be acting synergistically. As summarized in Figure 1, regular light-to-moderate alcohol consumption has direct effects on cardiomyocytes as well as vascular and extra-cardiac effects that positively influence strong risk factors of coronary heart disease.
Figure 1.
Antecedent ethanol ingestion protects the myocardium by direct effects to activate cell survival signaling programs in cardiac myocytes and indirectly via affects to reduce risk factors such as hypertension and atherosclerosis, decrease platelet reactivity and coagulation, and modify coronary vessel function.
With regard to indirect protective effects, chronic moderate alcohol consumption leads to beneficial alterations in blood lipid profile, platelet function, and fibrinolytic activity, all of which are directly linked to the risk of acute coronary events. For example, a 1999 meta-analysis of 42 experimental studies assessing the effects of moderate alcohol consumption on concentrations of high density lipoprotein cholesterol, apolipoprotein A I, fibrinogen, triglycerides, and other biological markers concluded that alcohol consumption over only 1-9 weeks leads to significant changes in blood lipids and clotting factors [46]. Based on published associations between these biomarkers and risk of coronary heart disease, the authors estimated that 30 g of alcohol a day would cause an estimated reduction of 24.7% in risk of coronary heart disease. Moreover, regular ethanol intake at light to moderate levels reduces oxidative stress, improves insulin sensitivity, and restores endothelium-dependent vasodilator responses in animal models and patients at risk for myocardial infarction, effects that persist for at least 24 hrs after ingestion [13,22,25,26,35,40,42,45,50,70,72,85,96-113].
Furthermore, regular light-to-moderate alcohol consumption also has beneficial effects with regard to diabetes and hypertension, two major risk factors of coronary artery disease. In epidemiological studies, alcohol consumption was associated with improved insulin sensitivity in diabetic patients after myocardial infarction [112] as well as in healthy non-diabetic women [11-113]. Moreover, moderate drinking also reduces the risk of newly developing type II diabetes [114]. In addition, regular light-to- moderate alcohol consumption also appears lower blood pressure. In young men, the lowest systolic blood pressure was found in subjects taking 1-3 drinks per day [115]. However, whether this is also true in other age groups or can be reproduced in patients who are already hypertensive patients is not yet known. Our understanding of the mechanisms underlying the effects of ethanol ingestion to improve insulin resistance and reduce blood pressure is also unclear.
Apart from improving the cardiovascular risk profile, ethanol also has direct effects on the coronary vasculature to increase myocardial blood flow. Ethanol-induced vasodilation occurs as a result of NO generation secondary to increased nitric oxide synthase (NOS) expression and activity and via activation of transient receptor potential vanilloid 1 (TRPV1) channels on perivascular sensory nerve terminals, which subsequently release the potent vasodilator calcitonin gene-related peptide (CGRP), thereby increasing coronary blood flow [116-119]. Interestingly, polyphenols in red wine also increase NOS activity [120,121] and ingestion of red wine increases brachial artery blood flow in humans [122]. However, the increase in coronary blood flow following consumption was nearly identical to that produced by intake of an equivalent volume of ethanol per se (ingestion of which produced similar increments in blood alcohol), when evaluated in the same group of individuals [122]. These data suggest that the absorption of red wine polyphenols does not augment the vasodilator response induced by ethanol alone. Irrespective of the responsible mediator of coronary vasodilation induced by intake of alcoholic beverages, it is unlikely that this hyperemic effect is directly responsible for cardioprotection during I/R that occurs hours later, because this action of ethanol does not persist once the alcohol is metabolized. Indeed, the appearance of a protected phenotype induced by ethanol ingestion does not become manifest until the alcohol is removed from the experimental system [123-125]. Moreover, the concomitant presence of ethanol prevents the appearance of preconditioning induced by other interventions [123-126]. On the other hand, antecedent ethanol exposure enhances the beneficial actions of ischemic preconditioning in studies where sufficient time elapsed to allow the ingested alcohol to be metabolized [127].
In addition to the indirect beneficial effects associated with consumption of alcoholic beverages, ethanol ingestion increases the tolerance of cardiomyocytes to I/R by direct activation of cell survival programs. Indeed, acute and chronic alcohol administration in animal models has been found to decrease postischemic cell death and infarct size and to induce expression of known cardioprotective proteins such as NOS, heat shock proteins, and superoxide dismutase. Interestingly, antecedent ethanol ingestion appears to trigger activation of signaling cascades that are similar to those involved in another well-studied cardioprotective intervention, ischemic preconditioning (IPC). In IPC, a tissue is exposed to cycles of brief I/R prior to induction of prolonged period of coronary artery occlusion (referred to as index ischemia) and reperfusion [128]. Like antecedent ethanol ingestion, IPC markedly reduces myocardial I/R injury and does so in two temporal phases. The early phase (or classical IPC) arises within minutes after the bouts of preconditioning ischemia, relies on preexisting mediators, and disappears after 2-4 hrs. A second window of protection (SWOP, late phase or delayed IPC) reemerges 24 hrs later that is mediated by the expression of protective proteins and persists for up to 48-72 hrs [129]. In the next section, we will discuss in detail why and to what extent alcohol-induced cardioprotection resembles IPC.
4. Molecular signaling mechanisms in ethanol-induced preconditioning of the heart
Complementing and extending the results reported in epidemiological studies, work conducted in animal models has provided deeper insight into the molecular signaling mechanisms that underlie the infarct-sparing effects of antecedent ethanol exposure. Cardioprotective effects triggered by alcohol ingestion/exposure have been observed in various rodent models (mice, rats, guinea pigs, rabbits) as well as in dogs. Principally, two different approaches for ethanol exposure have been used: either chronic low-to-moderate alcohol consumption over at least a few weeks to simulate human drinking patterns or acute administration of a one-time dose of alcohol shortly before an ischemic insult.
The fact that both acute and chronic alcohol administration elicits protection against subsequent ischemic damage suggests that its underlying mechanisms may be akin to that elicited by ischemic preconditioning, which is also characterized by a first and a second window of protection. Not all signaling mediators and in particular the end-effector(s) of ischemic preconditioning have been identified to date. However, strong evidence has been presented for the critical roles of adenosine, bradykinin, opioids, protein kinase C, reactive oxygen species, the mitochondrial KATP channel, among others, in the first window (= early phase or classic) preconditioning (reviewed in [80,130]). In second window (=late or delayed phase) preconditioning, signaling molecules such as ROS or NO, protein kinases such protein kinase C, Akt, and MAPK, and transcription factors such as NFkB or STAT1/3 are recruited by the preconditioning stimulus leading to the transcription and synthesis of de novo proteins such as COX-2, heat shock protein, iNOS, aldehyde dehyrogenase, among others, that then protect the heart against ischemic damage via potential effects on the mitochondrial permeability transition pore (reviewed in [80,131]).
This wealth of information concerning the signaling cascades in early and late phase ischemic preconditioning provided an excellent starting point to unravel how alcohol intake can reduce myocardial infarct size. In animal models of acute alcohol-induced protection, a single low or moderate dose (in most studies equivalent to 0.5 - 2 drinks) given within 2 hours before the onset of ischemia/reperfusion led to a significant reduction in infarct size. This short-term protective effect could be abolished by pharmacological inhibition of protein kinase C (either with broad spectrum PKC inhibitors or agents that specifically target the PKCε isoform), protein tyrosine kinases, and mitochondrial KATP channels [132,133]. However, blockade of adenosine receptors or treatment with a free radical scavenger was ineffective in counteracting alcohol-induced protection [123]. Taken together, these data show that PKCε activation plays a central role both in early ischemic preconditioning and in alcohol-induced protection. Furthermore, the mitochondrial KATP channel appears to be important in either form of cardioprotection. However, upstream mechanisms at the receptor level appear to differ substantially, but this has not yet been characterized in great detail except for the contribution (or not) of adenosine receptors.
Mechanistic studies in animals that were fed alcohol at low-to-moderate doses over a long period of time before the ischemic insult (3-12 weeks) involve cardioprotective signaling elements that have been identified with known mediators of late phase IPC. In rat hearts, low-dose alcohol consumption over 3 weeks increased the expression levels of cardioprotective proteins such as hsp70, heme oxygenase-1, and manganese superoxide dismutase (MnSOD) [134]. Chronic alcohol feeding also increased the levels of activated PKCε and Akt [135,136], and also upregulated eNOS expression and activity and NO generation [118,120]. Interestingly, the mitochondrial KATP channel is also involved in the protective effects of chronic alcohol feeding [137-139]. Furthermore, α1-adrenoreceptors, adenosine A1-receptors, and phospholipase C have been implicated in the infarct-sparing effects associated with chronic ethanol intake in some species [140-142]. However, the temporal sequence of the signaling cascade invoked by chronic ethanol exposure has not been interrogated. Thus, it is unclear how these signaling elements are linked in a cascade that ultimately enables the appearance of a protected phenotype or whether species differences dictate how alcohol triggers cardioprotection.
Recently, another powerful cardioprotective mechanism has been described wherein tissues exposed to intermittent myocardial ischemia and reperfusion in the first few minutes after re-establishing reperfusion after prolonged ischemia significantly reduces the extent of myocardial tissue damage. Termed ischemic postconditioning or staccato reperfusion, this form of cardioprotection provides unequivocal evidence that reperfusion injury is distinct from that induced by ischemia per se. However, no studies have been conducted to date to suggest that alcohol may also exert a postconditioning-like protective effect if given at the onset of reperfusion. However, some of the signaling mediators identified in ischemic postconditioning such as adenosine receptors, PKC, and members of the RISK pathway (reviewed in [80,143]) are very likely to be activated by ethanol in the early reperfusion phase as well, suggesting that this approach should be evaluated.
5. Toxic ethanol metabolites and cardioprotection
After drinking an alcoholic beverage, the absorbed ethanol is metabolized predominantly by alcohol dehydrogenase to acetaldehyde. Acetaldehyde is far more reactive than ethanol itself, and is highly cytotoxic. Acetaldehyde is then further metabolized by mitochondrial aldehyde dehydrogenase to form acetate and NADH. Cardiac myocytes do not express alcohol dehydrogenase, but do express aldehyde dehydrogenase. Interestingly, ectopic expression of alcohol dehydrogenase in the hearts of transgenic mice exacerbates the toxic effects (e.g., depression of cardiac contractility) induced by acute alcohol administration [144,145]. In contrast, when the ability of the myocardium to detoxify aldehydes is enhanced by transgenic overexpression of aldehyde dehydrogenase-2 (ALDH2), the detrimental effects of chronic alcohol intake are ameliorated [146].
Recently, Chen and co-workers demonstrated that mitochondrial ALDH2 plays an important cardioprotective role [147]. These investigators discovered that in rat hearts that pretreatment either with ethanol or with a PKCε activator before undergoing I/R resulted in the phosphorylation and thus activation of ALDH2, an effect that was inversely correlated to the extent of ischemic tissue damage. Using a high-throughput screening approach, this group identified small-molecule activators of aldehyde dehydrogenase-2 (designated Alda-1 and Alda-44), which when administered to rats before an ischemic event, greatly reduced infarct size [147,148]. These findings were independently confirmed by another group through use of a transgenic approach to overexpress ALDH2, which was associated with an infarct-sparing effect and improved contractile function during reperfusion [149].
The underlying mechanism of ALDH2-induced cardioprotection against ischemia is thought to be due to the resulting reduction in the formation of cytotoxic aldehydes such as 4-hydroxynonenal (4-HNE). Indeed, hearts subjected to I/R after ethanol pretreatment or IPC demonstrate significantly lower levels of 4-HNE adducts, indicating that this mechanism is shared by both cardioprotective interventions [150]. Furthermore, Chen and co-workers used an isoform-specific inhibitor to demonstrate that PKCε is responsible for ischemia- as well as ethanol-induced activation of ALDH2 in rat hearts [147]. These findings were confirmed in a subsequent study which showed that ethanol failed to provide protection in PKCε knockout mice whereas administration of an ALDH2 activator (Alda-44) effectively reduced infarct size in these animals [148]. These studies suggest that pretreatment of patients with ALDH2-activators before cardiac ischemia (such as coronary bypass surgery) may represent a promising new therapeutic approach to limit I/R injury. In particular, patients expressing inactive mutants of ALDH2, as often found in East Asian populations, may greatly benefit from such new treatments.
While the vast majority of publications demonstrate beneficial effects of moderate ethanol consumption, a few early studies reported that either acute or chronic ethanol administration failed to elicit cardioprotection [151-154]. However, the interpretation of these findings is clouded by the fact that ethanol was present during I/R in most of these studies, which was subsequently shown to prevent the appearance preconditioned phenotype in animal models [123-125]. In two of these studies, ethanol was administered acutely 10 min prior to I/R [151,153], which does not allow sufficient time for metabolic degradation to occur. In one of the chronic alcohol feeding studies that failed to show protection, significant blood alcohol levels were detected at the onset of the ischemia [31]. Subsequent work demonstrated that ethanol is cardioprotective, but only if ethanol is washed out of the tissues or sufficiently metabolized prior to the onset of I/R [123,124]. These findings have been extended to humans by Niccoli and co-workers [125] who found that ischemic preconditioning was abolished and the extent of ischemia worsened in patients given 40 g of alcohol shortly before coronary angioplasty. Although this is just a single study conducted in a small number of patients, these data strongly suggest caution against providing ethanol to patients with acute chest pain or who are in danger of suffering an acute myocardial infarction within the next few hours.
While the mechanisms that contribute to the abrogating effects of the continued presence of ethanol on preconditioning are not clear, it has been suggested that alcohol dehydrogenase-catalyzed formation of acetaldehyde may interfere with removal of other harmful aldehydes such as 4-hydroxy-2-nonenol and malondialdehyde, both of which are generated during I/R by peroxidation of membrane lipids. Thus, insufficient ethanol washout or metabolism prior to I/R may lead to accumulation of cytotoxic aldehydes that worsen postischemic tissue injury by inactivating enzymes and disrupting structural proteins through the formation of protein adducts [123,124,155]. It has also been suggested that ethanol may activate protein kinase C isoforms that play opposing roles in I/R, with effects dependent on ethanol dose and timing for response evaluations [155].
However, such timing issues do not account for the discrepant findings reported in a third acute study wherein ethanol was provided long enough before the onset of ischemia to allow for its metabolism and removal of toxic by-products [153]. Furthermore, lack of washout was also not an issue in another study testing the effects of chronic alcohol feeding, since the hearts were isolated and perfused with ethanol-free buffer for the I/R protocol [27]. Therefore, these findings suggest that there might be other, as yet unidentified factors that influence the ability of ethanol to induce cardioprotection that may be uniquely explored in the I/R models used in these studies.
6. Protective effects of moderate ethanol ingestion in stroke
A considerable body of epidemiologic literature also associates moderate alcohol consumption with significantly reduced risks for cerebrovascular (ischemic) stroke, although at a less robust level than the reported for ischemic coronary disease [59]. In addition, ethanol ingestion has been shown to be effective in reducing dementia (induced by cerebral exposure to HIV-1 protein gp-120 or amyloid-β, respectively), which may have an ischemic component [156-159], and stroke-induced injury in experimental animal models [160-165]. However, our understanding of the mechanisms whereby antecedent ethanol exposure limits cerebral I/R is less well-developed as compared to the cardiac literature.
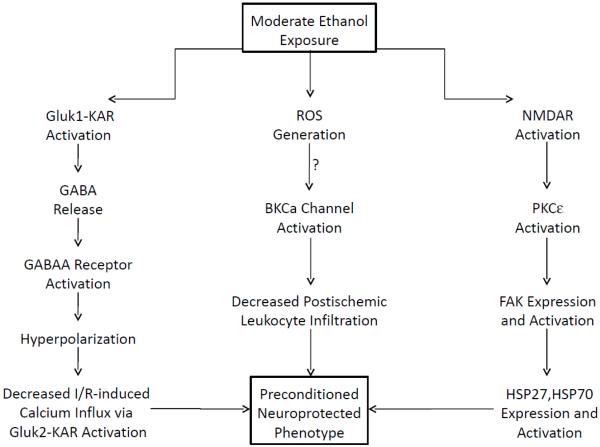
One of the first studies in this area was reported in 2007 and indicated that ethanol ingestion 24 hrs prior to induction of cerebral I/R ameliorated protected the brain against postischemic delayed neuronal death, neuronal and dendritic degeneration, oxidative DNA damage, and glial cell activation, changes that were associated with improved behavioral deficits [164]. These beneficial effects were attenuated by coincident treatment with a NADPH oxidase inhibitor, indicating that antecedent ethanol ingestion at socially relevant levels induces neuroprotective effects in I/R by a mechanism that is triggered by ROS produced through NADPH oxidase [164]. In subsequent work from this group, it was shown that I/R-induced brain injury and inflammation (leukocyte rolling and adhesion) was attenuated not only by antecedent ethanol or but also by pretreatment with an activator of large conductance, Ca2+-activated potassium (BKCa) channels [165]. Since the protective effects of both forms of preconditioning were prevented by pretreatment with a BKCa channel inhibitor [165] or prior neutrophil depletion (unpublished observations), it appears that the neuroprotective effects of antecedent ethanol ingestion may also involve activation of these potassium channels and prevention of neutrophil-dependent neuronal injury. Finally, recent work has demonstrated that ethanol-induced neuroprotection involves activation of ionotropic glutamate receptors [166,167] in I/R, as depicted in Figure 2. Moderate ethanol ingestion also prevents the neurodegeneration induced by β-amyloid or other proinflammatory peptides (eg, HIV-1 glycoprotein 120) that have been linked to dementia by a mechanism involving alcohol-induced activation of synaptic N-methyl-d-aspartate receptors [168-170] (Figure 2).
Figure 2.
Mechanisms for induction of a preconditioned neuroprotected phenotype by ethanol in stroke. Ethanol exposure activates ionotropic glutamate receptors of the kainite Gluk1 receptor (Gluk1-KAR) subtype to release gamma-aminobutyric acid (GABA) from intraneuronal synaptic terminals, which subsequently activates postsynaptic GABAA receptors (left arm of figure). The ensuing influx of chloride ions through activated GABAA receptors hyperpolarizes the membrane, thereby decreasing I/R-induced calcium influx through kainite Gluk2 receptors (Gluk2-KAR) via an effect to suppress assembly of Gluk2, postsynaptic density protein-95, and mixed-lineage kinase 3 signaling module induced by ischemia, which in turn inhibits the c-Jun N-terminal kinase 3 (JNK3) apoptotic pathway. Gluk1-KAR activation also decreases tyrosine phosphorylation of synaptic N-methyl-d-apartate receptors (NMDAR), which may also contribute to neuroprotection. The central arm illustrates the effect of ingestion of moderate levels of ethanol to produce an anti-inflammatory phenotype such that postischemic leukocyte/endothelial interactions are suppressed. The attenuation of neutrophil-dependent neuronal injury and cell death appears to be initiated by the effect of ethanol at moderate levels to invoke the formation of reactive oxygen species (ROS) and activation of large conductance, calcium-activated potassium (BKCa) channels, cytoprotective events that may be mechanistically linked since oxidants can activate these potassium channels. Interestingly, NMDAR are thought to be initial pro-survival sensors for ethanol, engendering protection against dementia-inducing neuroinflammatory proteins (right arm of figure) via activation of protein kinase C epsilon (PKCε) and focal adhesion kinase (FAK), which in turn induce elevations in protective heat shock proteins (HSP). Since age-dependent cognitive decline and/or dementia are thought to have an ischemic component, the disparate neuroprotective roles of NMDA receptor activation versus inhibition in dementia vs ischemic stroke implies dramatically different receptor regulation of downstream signaling elements in these conditions.
7. Adaptive cytoprotection in splanchnic organs after ethanol exposure
The ability of antecedent ethanol exposure to act as a mild irritant and induce the adaptive metamorphosis to a protected phenotype in the gastric mucosa was first described in 1979 by Robert and coworkers, a phenomenon referred to as adaptive cytoprotection in this literature [171-174]. Subsequent studies directed at examining the mechanisms whereby low concentrations of ethanol confer protection against noxious perturbations in the stomach have revealed an important role for prostaglandins, especially PGE2 acting via EP1 receptors [171-180]. Other work has implicated a co-regulatory relationship between NO and prostaglandins, increased glutathione, activation of gastric dopamine or β2-adrenergic receptors, improved mucosal blood flow, enhanced mucus secretion, stimulation of the internal enteric reflex, increased salivary secretions, prevention of intracellular calcium accumulation, and the formation of a protective layer of surface debris in the development of the defensive phenotype induced by ethanol exposure [176-179,181]. In addition to these mechanisms, antecedent ethanol exposure has been shown to limit the production of proinflammatory mediators such as leukotriene C4, an effect which may limit disturbances in microcirculatory function that otherwise would produce severe mucosal injury during subsequent exposure to necrotizing stimuli [176]. The latter observation led Dinda and coworkers [182] to propose that ethanol-induced adaptive cytoprotection is due to inhibition of neutrophil infiltration.
While most studies investigating the mechanisms of adaptive cytoprotection have involved assessment of mucosal damage in the stomach, several investigators have observed similar protective effects of antecedent ethanol exposure in the small intestine and liver. The mechanisms underlying ethanol-induced adaptive cytoprotection in the small bowel also appear to involve the production of prostaglandins [176,179,182]. However, it is important to emphasize that the intraluminal ethanol levels (20-100% ethanol) to which the small intestine was exposed in these studies, while relevant to the stomach, far exceeded those present in downstream segments of the gastrointestinal tract following oral intake. Indeed, the jejunum, ileum, and colon are exposed to ingested ethanol primarily via the bloodstream secondary to gastric absorption. Thus, the physiologic relevance of the mechanistic information obtained from studies employing luminal exposure, especially at a dose of 20-100% ethanol, is questionable. A more relevant ethanol exposure protocol was employed in a study examining hepatocellular protection and survival rates in rats exposed to liver I/R. In this report, rats received 40% ethanol at a dose of 5 g/kg by gavage, which produced increases in plasma ethanol that peaked at 180 mg/dl within two hours of ingestion, 24 hrs prior to induction of hepatic I/R. Postischemic liver injury, complement activation and deposition, and hepatic oxidant production were reduced and 14-day survival rates were remarkably improved by antecedent ethanol ingestion [78].
8. Ethanol exerts anti-inflammatory effects in I/R: cellular signaling mechanisms
Recognition of the fact that reperfusion can initiate a cascade of deleterious processes that exacerbate the tissue injury induced by ischemia has resulted in an intensive research effort directed at defining the cellular and molecular events that underlie I/R injury. Indeed, work conducted over the past 15 years has led to the development of the concept that oxidant-induced leukocyte/endothelial cell interactions are largely responsible for the microvascular dysfunction induced by reperfusion (reviewed in 80,183-186]. Reactive oxygen species generated by xanthine oxidase and other enzymes (eg, NAD(P)H oxidase) promote the formation of proinflammatory stimuli, modify the expression of adhesion molecules on the surface of leukocytes and endothelial cells, and reduce levels of the potent anti-adhesive agent nitric oxide. This latter effect is exacerbated by a postischemic decline in endothelial nitric oxide synthase activity and oxidation of soluble guanylyl cylase (sGC), which serves to amplify the intense inflammatory responses elicited by I/R by reducing the bioavailability of NO and ability of downstream signaling elements to respond to this antiadhesive signaling molecule [166]. Coincident with these changes, perivascular cells (eg, macrophages, mast cells) become activated and release other inflammatory mediators (eg, TNFα and other cytokines, PAF, LTB4). As a consequence of these events, leukocytes begin to form adhesive interactions with postcapillary venular endothelium. Platelets also play an important role in the adhesion of leukocytes to the postischemic microvasculature. The activated leukocytes emigrate into the tissues, inducing microvascular barrier dysfunction via release of oxidants and hydrolytic enzymes. In addition to these changes, leukocytes also contribute to postischemic nutritive perfusion failure (fewer perfused capillaries, ie, capillary no-reflow), endothelium-dependent vasoregulatory dysfunction in arterioles, and parenchymal cell dysfunction. Thus, leukocyte-endothelial cell adhesive interactions, which precipitate the development of arteriolar, capillary, and postcapillary venular dysfunction in the microcirculation, are among the earliest signs of tissue dysfunction and injury elicited by I/R [80,183-186].
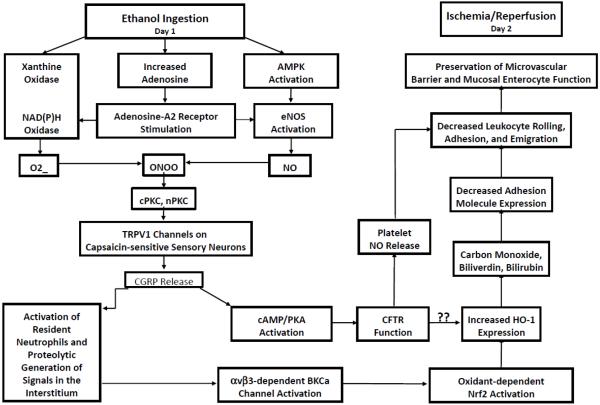
A growing body of evidence indicates that antecedent ethanol ingestion abrogates P-selectin expression, leukocyte-platelet/endothelial cell interactions, oxidant generation, capillary no-reflow, mitochondrial dysfunction, release of proinflammatory mediators, impaired arteriolar endothelium-dependent vasoregulation, and microvascular barrier disruption induced by I/R 24 hrs later [187-204]. As depicted in Figure 3, these beneficial actions are instigated by ethanol-induced activation of xanthine oxidase and NADPH oxidase, which produce superoxide, coincident with adenosine-A2 receptor-dependent activation of the α2 isoform of AMPK, which phosphorylates and activates eNOS [164,189,191,199-201]. Concomitant production of superoxide and NO leads to the formation of reactive nitrogen oxide species (such as peroxynitrite), which may stimulate cysteine γ-lyase and/or cystathionine β synthase to produce hydrogen sulfide (H2S). RNOS and H2S activate KATP channels on capsaicin-sensitive sensory neurons, sensitizing them to ethanol and rendering them resistant to down-regulation, thereby provoking release of CGRP [190,195,206-209]. CGRP activates cystic fibrosis transmembrane regulator (CFTR) function by a cAMP/PKA-dependent mechanism, thereby enhancing platelet NO (effects that were absent in CFTRΔ506 mice and inhibited by CFTRinh1-72, unpublished observations), and also large conductance, calcium-activated potassium channels (BKCa), which may induce the expression of heme oxygenase-1 (HO-1) during I/R 24 hrs later [163,204,205] (Figure 3). The reaction products of HO-1 (CO, biliverdin and secondarily derived bilirubin) act to abrogate adhesion molecule expression and thus prevent postischemic leukocyte/endothelial cell adhesive interactions. This preserves endothelial barrier function, which is now protected from neutrophil-dependent injury mechanisms during I/R. Although inhibitor studies ruled out a role for IKCa and SKCa in the effect of H2S to induce tolerance to ischemia, other pharmacologic activators of these channels can produce preconditioning [203], providing another potential avenue for exploration in the development of anti-ischemia agents. Ethanol-induced signaling also upregulates HO-1 by an ARE/Nrf2-dependent mechanism [210], which may serve to protect soluble guanylyl cyclase from redox inactivation in reperfused tissues [211] (Figure 3).
Figure 3.
Ethanol ingestion 24 hrs at moderate levels prior to the onset of ischemia/reperfusion induces the appearance of an anti-inflammatory phenotype such that postcapillary venules fail to express endothelial cell adhesion molecules, thereby attenuating leukocyte recruitment to ischemic tissues. Development of this protected phenotype is instigated by the effect of ethanol to trigger the generation of superoxide (O2-) secondary to activation of xanthine oxidase and NADPH oxidase and endothelial nitric oxide synthase (eNOS)-dependent formation of nitric oxide (NO) in the vascular wall. Superoxide interacts with NO to form reactive nitrogen oxide species such as peroxynitrate (ONOO), which together with O2- and NO, activate transient receptor potential vanilloid 1 (TRPV1) channels on capsaicin-sensitive sensory neurons. This initiates release of calcitonin gene-related peptide release (CGRP) produces downstream signals by at least two mechanisms. First, CGRP activates cAMP/protein kinase A-dependent signaling to stimulate cystic fibrosis transmembrane regulator (CFTR) function in endothelial cells and/or platelets. While it is not clear how this chloride channel couples to downstream signaling elements to effect the anti-inflammatory phenotype, it likely links with expression of pro-survival protein expression (such as heme oxygenase-1 or HO-1). Another possibility relates to CFTR-stimulated production the antiadhesive gasotransmitter NO by platelets. CGRP also activates resident neutrophils, thereby causing release of proteases that sever extracellular matrix molecules to expose cryptic sites within or release bioactive cleavage products from these interstitial structures. This results in αvβ3 integrin-dependent activation of large conductance, calcium-activated potassium (BKCa) channels, which results in oxidant-dependent Nrf2 activation. These events culminate in increased expression and activity of HO-1. This enzyme catalyzes the rate limiting step in heme metabolism, forming the anti-adhesive molecule carbon monoxide, as well as the powerful anti-oxidants biliverdin and bilirubin. These reaction products decrease endothelial cell adhesion molecule expression, thereby reducing leukocyte rolling, adhesion and emigration and preventing leukocyte-dependent reperfusion injury.
In addition to its influence on vascular endothelial cells, moderate ethanol consumption also exerts anti-inflammatory effects in human adipose tissue. While promoting the expression of adiponectin, a fat-derived adipokine that exerts powerful anti-inflammatory effects, ethanol or wine ingestion also reduced the release of inflammatory cytokines and pro-inflammatory mediators from human adipose tissue under basal conditions and following exposure to lipopolysaccharides [13,212,213].
Importantly, the aforementioned anti-inflammatory effects are manifest in animals ingesting ethanol at volumes that produce peak plasma concentrations (40-50 mg/dl blood) that occur in humans consuming 1-2 alcoholic beverages, but are absent in mice consuming ethanol at levels that raise blood alcohol to 300 mg/dl. Moreover, the postischemic anti-inflammatory effects induced by moderate ethanol consumption persist in mice with co-existing risk factors, as discussed above. The latter observation is exceedingly important because numerous studies suggest that the effectiveness of IPC as an anti-ischemic intervention is impaired or eliminated when tested in hypertensive, hypercholesterolemic or diabetic animal models (reviewed in 80). This may explain why IPC does not always appear to produce beneficial effects in humans, who typically present with co-morbid risk factors. When taken together, these latter two findings will no doubt fuel a concerted effort to define why the presence of risk factors impairs IPC, with a focus on developing new therapeutic strategies that circumvent the cellular processes that limit the effectiveness of this intervention in the presence of co-morbidities. Examination of the cellular mechanisms by which antecedent ethanol ingestion confers protection may provide important clues in this regard.
9. Summary
Abundant epidemiologic evidence and the results interventional mechanistic studies conducted in animal models and cell culture systems strongly support the notion that antecedent ethanol exposure at moderate levels confers protective cardiovascular effects. Mechanistic interrogation of the signaling pathways invoked by antecedent ethanol ingestion may point the way towards development of new therapeutic approaches that mimic the powerful protective effects of socially relevant alcohol intake to limit I/R injury, but minimize the negative psychosocial impact and pathologic outcomes that also accompany consumption of alcoholic beverages. Indeed, preconditioning can be induced by pharmacologic administration of agents identified as triggers (eg, adenosine and CGRP receptor agonists, NO donors), downstream mediators (BKCa channel agonists), and effectors (eg, agents such as hemin, which upregulates HO-1 expression) in lieu of ethanol. Finally, exciting new findings indicate that intake of alcoholic beverages at moderate levels enhances neovascularization and capillary densities in ischemic tissues of diabetic mice by enhanced mobilization of circulating endothelial progenitor cells to these regions [214]. These and other provocative findings should provide the impetus for continued exploration of novel pathways to invoke cell survival programs that limit ischemic injury.
Highlights.
Epidemiologic evidence that ethanol ingestion is cardioprotective is summarized.
Molecular signaling mechanisms invoked by antecedent ethanol consumption.
Protective actions of ethanol ingestion in stroke and peripheral vascular disease.
Anti-inflammatory signaling mechanisms invoked by ethanol ingestion.
Acknowledgements
The authors’ work is supported by grants from the National Institutes of Health (AA-014595 and HL-094585) and a Scientist Development Grant from the American Heart Association. The authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int J Environ Res Public Health. 2010;7:1540–57. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G, Luo J. Anthocyanins: are they beneficial in treating ethanol neurotoxicity? Neurotox Res. 2010;17:91–101. doi: 10.1007/s12640-009-9083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis. 2011;12:3–9. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miranda-Mendez A, Lugo-Baruqui A, Armendariz-Borunda J. Molecular basis and current treatment for alcoholic liver disease. Int J Environ Res Public Health. 2010;7:1872–88. doi: 10.3390/ijerph7051872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukamal K. Alcohol intake and noncoronary cardiovascular diseases. Ann Epidemiol. 2007;17:S8–S12. doi: 10.1016/j.annepidem.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types--a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reidy J, McHugh E, Stassen LF. A review of the relationship between alcohol and oral cancer. Surgeon. 2011;9:278–83. doi: 10.1016/j.surge.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Seitz HK, Cho CH. Contribution of alcohol and tobacco use in gastrointestinal cancer development. Methods Mol Biol. 2009;472:217–41. doi: 10.1007/978-1-60327-492-0_9. [DOI] [PubMed] [Google Scholar]
- 9.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–41. 4–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Bower KA, Chen G, Shi X, Dong Z, Ke Z, et al. Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin. Alcohol Clin Exp Res. 2010;34:751–60. doi: 10.1111/j.1530-0277.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Seitz HK, Wang XD. Moderate alcohol consumption aggravates high-fat diet induced steatohepatitis in rats. Alcohol Clin Exp Res. 2010;34:567–73. doi: 10.1111/j.1530-0277.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatsky AL, Friedman GD, Siegelaub AB. Alcohol consumption before myocardial infarction. Results from the Kaiser-Permanente epidemiologic study of myocardial infarction. Ann Intern Med. 1974;81(3):294–301. doi: 10.7326/0003-4819-81-3-294. [DOI] [PubMed] [Google Scholar]
- 13.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camargo CA, Jr., Hennekens CH, Gaziano JM, Glynn RJ, Manson JE, Stampfer MJ. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157(1):79–85. [PubMed] [Google Scholar]
- 15.Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95(10):1505–23. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation. 2010;121:1951–9. doi: 10.1161/CIRCULATIONAHA.109.865840. [DOI] [PubMed] [Google Scholar]
- 17.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–47. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 19.Di Castelnuovo A, Costanzo S, di Giuseppe R, de Gaetano G, Iacoviello L. Alcohol consumption and cardiovascular risk: mechanisms of action and epidemiologic perspectives. Future Cardiol. 2009;5:467–77. doi: 10.2217/fca.09.36. [DOI] [PubMed] [Google Scholar]
- 20.Di Castelnuovo A, Costanzo S, Donati MB, Iacoviello L, de Gaetano G. Prevention of cardiovascular risk by moderate alcohol consumption: epidemiologic evidence and plausible mechanisms. Intern Emerg Med. 2010;5:291–7. doi: 10.1007/s11739-010-0346-0. [DOI] [PubMed] [Google Scholar]
- 21.Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–8. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dufour MC, Caces MF, Whitmore CC, Hanna EZ. Alcohol consumption and death from acute myocardial infarction in a national longitudinal cohort. Alcohol Clin Exp Res. 1996;20:97A. [Google Scholar]
- 23.Femia R, Natali A, L’Abbate A, Ferrannini E. Coronary atherosclerosis and alcohol consumption: angiographic and mortality data. Arterioscler Thromb Vasc Biol. 2006;26:1607. doi: 10.1161/01.ATV.0000222929.99098.1f. [DOI] [PubMed] [Google Scholar]
- 24.Freiberg MS, Samet JH. Alcohol and coronary heart disease: the answer awaits a randomized controlled trial. Circulation. 2005;112:1379–81. doi: 10.1161/CIRCULATIONAHA.105.568030. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–50. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 26.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–34. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 27.Gibson BT, Ong JH, Starnes JW, Farrar RP. Effects of chronic moderate and heavy ethanol consumption on myocardial recovery from ischemia. Alcohol Clin Exp Res. 1998;22(9):2086–92. [PubMed] [Google Scholar]
- 28.Ginter E, Simko V. Ethanol and cardiovascular diseases: epidemiological, biochemical and clinical aspects. Bratisl Lek Listy. 2008;109:590–4. [PubMed] [Google Scholar]
- 29.Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133(6):411–9. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 30.Jackson R, Broad J, Connor J, Wells S. Alcohol and ischaemic heart disease: probably no free lunch. Lancet. 2005;366:1911–2. doi: 10.1016/S0140-6736(05)67770-7. [DOI] [PubMed] [Google Scholar]
- 31.Kabagambe EK, Baylin A, Ruiz-Narvaez E, Rimm EB, Campos H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am J Clin Nutr. 2005;82:1336–45. doi: 10.1093/ajcn/82.6.1336. [DOI] [PubMed] [Google Scholar]
- 32.Klatsky AL. Alcohol and cardiovascular diseases. Expert Rev Cardiovasc Ther. 2009;7:499–506. doi: 10.1586/erc.09.22. [DOI] [PubMed] [Google Scholar]
- 33.Klatsky AL. Alcohol and cardiovascular health. Integr Comp Biol. 2004;44:324–8. doi: 10.1093/icb/44.4.324. [DOI] [PubMed] [Google Scholar]
- 34.Klatsky AL. Alcohol and cardiovascular mortality: common sense and scientific truth. J Am Coll Cardiol. 2010;55:1336–8. doi: 10.1016/j.jacc.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 35.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66:1237–42. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 36.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55:1328–35. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukamal KJ, Chiuve SE, Rimm EB. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Arch Intern Med. 2006;166:2145–50. doi: 10.1001/archinte.166.19.2145. [DOI] [PubMed] [Google Scholar]
- 38.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr., Stampfer MJ, Willett WC, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 39.Mukamal KJ, Jensen MK, Gronbaek M, Stampfer MJ, Manson JE, Pischon T, et al. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation. 2005;112:1406–13. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Kawi C, Kinoshito M, Moss AJ. Moderate alcohol intake and outcome after an acute coronary event. Circulation. 1995;92:I–708. [Google Scholar]
- 41.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b. [DOI] [PubMed] [Google Scholar]
- 42.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–14. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 43.Rajpathak SN, Freiberg MS, Wang C, Wylie-Rosett J, Wildman RP, Rohan TE, et al. Alcohol consumption and the risk of coronary heart disease in postmenopausal women with diabetes: Women’s Health Initiative Observational Study. Eur J Nutr. 2010;49:211–8. doi: 10.1007/s00394-009-0065-3. [DOI] [PubMed] [Google Scholar]
- 44.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 45.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 46.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–8. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruidavets JB, Ducimetiere P, Evans A, Montaye M, Haas B, Bingham A, et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) BMJ. 2010;341:c6077. doi: 10.1136/bmj.c6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saremi A, Arora R. The cardiovascular implications of alcohol and red wine. Am J Ther. 2008;15:265–77. doi: 10.1097/MJT.0b013e3180a5e61a. [DOI] [PubMed] [Google Scholar]
- 50.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–73. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 51.Tanasescu M, Hu FB, Willett WC, Stampfer MJ, Rimm EB. Alcohol consumption and risk of coronary heart disease among men with type 2 diabetes mellitus. J Am Coll Cardiol. 2001;38:1836–42. doi: 10.1016/s0735-1097(01)01655-2. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee S, Lekli I, Narasimman G, Bertelli AAA, Das DK. Expression of longevity proteins in both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Rad Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Wu JM, Hsieh T-c. Resveratrol: a cardioprotective substance. NY Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigo R, Miranda A, Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 2011;12:410–24. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 55.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, et al. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34:810–7. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 56.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–83. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q, Sun AY, Simonyi A, Miller DK, Smith RE, Luchtefeld RG, et al. Oral administration of grape polyphenol extract ameliorates cerebral ischemia/reperfusion-induced neuronal damage and behavioral deficits in gerbils: comparison of pre- and post-ischemic administration. J Nutr Biochem. 2009;20:369–77. doi: 10.1016/j.jnutbio.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shinmura K. Cardiovascular protection afforded by caloric restriction: essential role of nitric oxide synthase. Geriatr Gerontol Int. 2011;11:143–156. doi: 10.1111/j.1447-0594.2010.00675.x. [DOI] [PubMed] [Google Scholar]
- 59.Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, et al. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–19. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eagon PK. Alcoholic liver injury: influence of gender and hormones. World J Gastroenterol. 2010;16:1377–84. doi: 10.3748/wjg.v16.i11.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 62.Beulens JW, Rimm EB, Ascherio A, Spiegelman D, Hendriks HF, Mukamal KJ. Alcohol consumption and risk for coronary heart disease among men with hypertension. Ann Intern Med. 2007;146:10–9. doi: 10.7326/0003-4819-146-1-200701020-00004. [DOI] [PubMed] [Google Scholar]
- 63.Gigleux I, Gagnon J, St-Pierre A, Cantin B, Dagenais GR, Meyer F, et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136:3027–32. doi: 10.1093/jn/136.12.3027. [DOI] [PubMed] [Google Scholar]
- 64.Klatsky AL. Alcohol and stroke: an epidemiological labyrinth. Stroke. 2005;36:1835–6. [PubMed] [Google Scholar]
- 65.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–25. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 66.Mukamal KJ, Ding EL, Djousse L. Alcohol consumption, physical activity, and chronic disease risk factors: a population-based cross-sectional survey. BMC Public Health. 2006;6:118. doi: 10.1186/1471-2458-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr., Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–13. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 68.O’Keefe JH, Carter MD, Lavie CJ. Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc. 2009;84:741–57. doi: 10.4065/84.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schminke U, Luedemann J, Berger K, Alte D, Mitusch R, Wood WG, et al. Association between alcohol consumption and subclinical carotid atherosclerosis: the Study of Health in Pomerania. Stroke. 2005;36:1746–52. doi: 10.1161/01.STR.0000173159.65228.68. [DOI] [PubMed] [Google Scholar]
- 70.Greenfield JR, Samaras K, Hayward CS, Chisholm DJ, Campbell LV. Beneficial postprandial effect of a small amount of alcohol on diabetes and cardiovascular risk factors: modification by insulin resistance. J Clin Endocrinol Metab. 2005;90:661–72. doi: 10.1210/jc.2004-1511. [DOI] [PubMed] [Google Scholar]
- 71.Gorelick PB, Rodin MB, Langenberg P, Hier DB, Costigan J. Weekly alcohol consumption, cigarette smoking, and the risk of ischemic stroke: results of a case-control study at three urban medical centers in Chicago, Illinois. Neurology. 1989;39:339–43. doi: 10.1212/wnl.39.3.339. [DOI] [PubMed] [Google Scholar]
- 72.Turner BC, Jenkins E, Kerr D, Sherwin RS, Cavan DA. The effect of evening alcohol consumption on next-morning glucose control in type 1 diabetes. Diabetes Care. 2001;24:1888–93. doi: 10.2337/diacare.24.11.1888. [DOI] [PubMed] [Google Scholar]
- 73.van de Wiel A, de Lange DW. Cardiovascular risk is more related to drinking pattern than to the type of alcoholic drinks. Neth J Med. 2008;66:467–73. [PubMed] [Google Scholar]
- 74.de Leiris J, Besse S, Boucher F. Diet and heart health: moderate wine drinking strengthens the cardioprotective effects of fish consumption. Curr Pharm Biotechnol. 2010;11:911–21. doi: 10.2174/138920110793262024. [DOI] [PubMed] [Google Scholar]
- 75.Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–20. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Djousse L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation. 2007;115:34–9. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 78.Ma ZW, Feng XB, Zheng SG, Bie P, Wang SG, Li K, et al. Ethanol preconditioning reduces hepatic I/R injury by inhibiting the complement system activation. J Surg Res. 2011;166:314–23. doi: 10.1016/j.jss.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 79.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–42. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 80.Kalogeris T, Baines C, Krenz M, Korthuis Invited Review: Cell biology of ischemia/reperfusion injury: Prevention of Ischemic Injury by Preconditioning and Stem Cell Therapy. Int Rev Cell Mol Biol. 2010 Under review. [Google Scholar]
- 81.Liu Y, Kalogeris T, Wang M, Zuidema M, Wang Q, Dai H, Davis MH, Hill MA, Korthuis RJ. Hydrogen sulfide preconditioning or neutrophil depletion attenuates ischemia/reperfusion-induced mitochondrial dysfunction in rat small intestine. Am J Physiol. 2011 doi: 10.1152/ajpgi.00413.2010. provisionally accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–24. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 83.Suter LG, Murabito JM, Felson DT, Fraenkel L. Smoking, alcohol consumption, and Raynaud’s phenomenon in middle age. Am J Med. 2007;120:264–71. doi: 10.1016/j.amjmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Janszky I, Ericson M, Blom M, Georgiades A, Magnusson JO, Alinagizadeh H, et al. Wine drinking is associated with increased heart rate variability in women with coronary heart disease. Heart. 2005;91:314–8. doi: 10.1136/hrt.2004.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–7. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 86.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, et al. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care. 2004;27:184–9. doi: 10.2337/diacare.27.1.184. [DOI] [PubMed] [Google Scholar]
- 87.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56:1130–6. doi: 10.1038/sj.ejcn.1601459. [DOI] [PubMed] [Google Scholar]
- 88.Zairis MN, Ambrose JA, Lyras AG, Thoma MA, Psarogianni PK, Psaltiras PG, et al. C Reactive protein, moderate alcohol consumption, and long term prognosis after successful coronary stenting: four year results from the GENERATION study. Heart. 2004;90:419–24. doi: 10.1136/hrt.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson G. Gender differences in cardiovascular disease prevention. Menopause Int. 2008;14:13–7. doi: 10.1258/mi.2007.007031. [DOI] [PubMed] [Google Scholar]
- 90.Naimi TS, Brown DW, Brewer RD, Giles WH, Mensah G, Serdula MK, et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking U.S. adults. Am J Prev Med. 2005;28:369–73. doi: 10.1016/j.amepre.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ellison RC. Continuing reluctance to accept emerging scientific data on alcohol and health. AIM Digest. 2002;11:6–7. [Google Scholar]
- 93.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116(11):1306–17. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 94.Lee SJ, Sudore RL, Williams BA, Lindquist K, Chen HL, Covinsky KE. Functional limitations, socioeconomic status, and all-cause mortality in moderate alcohol drinkers. J Am Geriatr Soc. 2009;57:955–62. doi: 10.1111/j.1532-5415.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walzem RL. Wine and health: state of proofs and research needs. Inflammopharmacology. 2008;16:265–71. doi: 10.1007/s10787-008-8027-6. [DOI] [PubMed] [Google Scholar]
- 96.Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipiol. 2010;21:346–51. doi: 10.1097/MOL.0b013e32833c1f41. [DOI] [PubMed] [Google Scholar]
- 97.Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91(4):1182–8. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 98.Fumeron F, Betoulle D, Luc G, Behague I, Ricard S, Poirier O, et al. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J Clin Invest. 1995;96:1664–71. doi: 10.1172/JCI118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keller JW, Folts JD. Relative effects of cigarette smoke and ethanol on acute platelet thrombus formation in stenosed canine coronary arteries. Cardiovasc Res. 1988;22(1):73–8. doi: 10.1093/cvr/22.1.73. [DOI] [PubMed] [Google Scholar]
- 100.McKenzie CR, Abendschein DR, Eisenberg PR. Sustained inhibition of whole-blood clot procoagulant activity by inhibition of thrombus-associated factor Xa. Arterioscler Thromb Vasc Biol. 1996;16:1285–91. doi: 10.1161/01.atv.16.10.1285. [DOI] [PubMed] [Google Scholar]
- 101.Miceli M, Alberti L, Bennardini F, Di Simplicio P, Seghieri G, Rao GH, et al. Effect of low doses of ethanol on platelet function in long-life abstainers and moderate-wine drinkers. Life Sci. 2003;73:1557–66. doi: 10.1016/s0024-3205(03)00473-9. [DOI] [PubMed] [Google Scholar]
- 102.Mukamal KJ, Massaro JM, Ault KA, Mittleman MA, Sutherland PA, Lipinska I, et al. Alcohol consumption and platelet activation and aggregation among women and men: the Framingham Offspring Study. Alcohol Clin Exp Res. 2005;29:1906–12. doi: 10.1097/01.alc.0000183011.86768.61. [DOI] [PubMed] [Google Scholar]
- 103.Pikaar NA, Wedel M, van der Beek EJ, van Dokkum W, Kempen HJ, Kluft C, et al. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism. 1987;36:538–43. doi: 10.1016/0026-0495(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 104.Renaud SC, Beswick AD, Fehily AM, Sharp DS, Elwood PC. Alcohol and platelet aggregation: the Caerphilly Prospective Heart Disease Study. Am J Clin Nutr. 1992;55:1012–7. doi: 10.1093/ajcn/55.5.1012. [DOI] [PubMed] [Google Scholar]
- 105.Ridker PM, Vaughan DE, Stampfer MJ, Glynn RJ, Hennekens CH. Association of moderate alcohol consumption and plasma concentration of endogenous tissue-type plasminogen activator. JAMA. 1994;272:929–33. doi: 10.1001/jama.1994.03520120039028. [DOI] [PubMed] [Google Scholar]
- 106.Tozzi Ciancarelli MG, Di Massimo C, De Amicis D, Ciancarelli I, Carolei A. Moderate consumption of red wine and human platelet responsiveness. Thromb Res. 2011;128:124–9. doi: 10.1016/j.thromres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 107.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337. doi: 10.1136/bmj.b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veenstra J, Ockhuizen T, van de Pol H, Wedel M, Schaafsma G. Effects of a moderate dose of alcohol on blood lipids and lipoproteins postprandially and in the fasting state. Alcohol Alcohol. 1990;25:371–7. [PubMed] [Google Scholar]
- 109.Vliegenthart R, Oei HH, van den Elzen AP, van Rooij FJ, Hofman A, Oudkerk M, et al. Alcohol consumption and coronary calcification in a general population. Arch Intern Med. 2004;164:2355–60. doi: 10.1001/archinte.164.21.2355. [DOI] [PubMed] [Google Scholar]
- 110.Volcik KA, Ballantyne CM, Fuchs FD, Sharrett AR, Boerwinkle E. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: differences between Whites and African Americans of the ARIC study. Ann Epidemiol. 2008;18:101–7. doi: 10.1016/j.annepidem.2007.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287(19):2559–62. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 112.Marfella R, Cacciapuoti F, Siniscalchi M, Sasso FC, Marchese F, Cinone F, et al. Effect of moderate red wine intake on cardiac prognosis after recent acute myocardial infarction of subjects with Type 2 diabetes mellitus. Diabet Med. 2006;23(9):974–81. doi: 10.1111/j.1464-5491.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 113.Joosten MM, Beulens JW, Kersten S, Hendriks HF. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia. 2008;51:1375–81. doi: 10.1007/s00125-008-1031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conigrave KM, Rimm EB. Alcohol for the prevention of type 2 diabetes mellitus? Treat Endocrinol. 2003;2(3):145–52. doi: 10.2165/00024677-200302030-00001. [DOI] [PubMed] [Google Scholar]
- 115.Gillman MW, Cook NR, Evans DA, Rosner B, Hennekens CH. Relationship of alcohol intake with blood pressure in young adults. Hypertension. 1995;25(5):1106–10. doi: 10.1161/01.hyp.25.5.1106. [DOI] [PubMed] [Google Scholar]
- 116.Kiviniemi TO, Saraste A, Toikka JO, Saraste M, Raitakari OT, Parkka JP, et al. A moderate dose of red wine, but not de-alcoholized red wine increases coronary flow reserve. Atherosclerosis. 2007;195(2):e176–81. doi: 10.1016/j.atherosclerosis.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Kleinhenz DJ, Sutliff RL, Polikandriotis JA, Walp ER, Dikalov SI, Guidot DM, et al. Chronic ethanol ingestion increases aortic endothelial nitric oxide synthase expression and nitric oxide production in the rat. Alcohol Clin Exp Res. 2008;32(1):148–54. doi: 10.1111/j.1530-0277.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abou-Agag LH, Khoo NK, Binsack R, White CR, Darley-Usmar V, Grenett HE, et al. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39(4):540–8. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Gazzieri D, Trevisani M, Tarantini F, Bechi P, Masotti G, Gensini GF, et al. Ethanol dilates coronary arteries and increases coronary flow via transient receptor potential vanilloid 1 and calcitonin gene-related peptide. Cardiovasc Res. 2006;70:589–99. doi: 10.1016/j.cardiores.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 120.Booyse FM, Pan W, Grenett HE, Parks DA, Darley-Usmar VM, Bradley KM, et al. Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Ann Epidemiol. 2007;17:S24–31. doi: 10.1016/j.annepidem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 121.Zenebe W, Pechanova O, Andriantsitohaina R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol Res. 2003;52(4):425–32. [PubMed] [Google Scholar]
- 122.Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, et al. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol. 2008;294:H605–12. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- 123.Krenz M, Baines CP, Heusch G, Downey JM, Cohen MV. Acute alcohol-induced protection against infarction in rabbit hearts: differences from and similarities to ischemic preconditioning. J Mol Cell Cardiol. 2001;33(11):2015–22. doi: 10.1006/jmcc.2001.1465. [DOI] [PubMed] [Google Scholar]
- 124.Krenz M, Baines CP, Yang XM, Heusch G, Cohen MV, Downey JM. Acute ethanol exposure fails to elicit preconditioning-like protection in in situ rabbit hearts because of its continued presence during ischemia. J Am Coll Cardiol. 2001;37(2):601–7. doi: 10.1016/s0735-1097(00)01125-6. [DOI] [PubMed] [Google Scholar]
- 125.Niccoli G, Altamura L, Fabretti A, Lanza GA, Biasucci LM, Rebuzzi AG, et al. Ethanol abolishes ischemic preconditioning in humans. J Am Coll Cardiol. 2008;51(3):271–5. doi: 10.1016/j.jacc.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 126.Krenz M, Yang XM, Qin Q, Downey JM, Cohen MV. Dose-response relationships of the protective and antiprotective effects of acute ethanol exposure in isolated rabbit hearts. Heart Dis. 2002;4(5):276–81. doi: 10.1097/00132580-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 127.McDonough KH. Chronic alcohol consumption causes accelerated myocardial preconditioning to ischemia-reperfusion injury. Alcohol Clin Exp Res. 1997;21:869–73. [PubMed] [Google Scholar]
- 128.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 129.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83(4):1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 130.Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24(3):225–34. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther. 2010;24(3):235–54. doi: 10.1007/s10557-010-6237-9. [DOI] [PubMed] [Google Scholar]
- 132.Chen CH, Gray MO, Mochly-Rosen D. Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: role of epsilon protein kinase C. Proc Natl Acad Sci U S A. 1999;96(22):12784–9. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inagaki K, Mochly-Rosen D. DeltaPKC-mediated activation of epsilonPKC in ethanol-induced cardiac protection from ischemia. J Mol Cell Cardiol. 2005;39(2):203–11. doi: 10.1016/j.yjmcc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 134.Sato M, Fraga C, Das DK. Induction of the expression of cardioprotective proteins after mild-to-moderate consumption of alcohol. Pathophysiology. 2004;10(2):139–45. doi: 10.1016/j.pathophys.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 135.Miyamae M, Rodriguez MM, Camacho SA, Diamond I, Mochly-Rosen D, Figueredo VM. Activation of epsilon protein kinase C correlates with a cardioprotective effect of regular ethanol consumption. Proc Natl Acad Sci U S A. 1998;95(14):8262–7. doi: 10.1073/pnas.95.14.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol. 2002;283(1):H165–74. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- 137.Pagel PS, Krolikowski JG, Kehl F, Mraovic B, Kersten JR, Warltier DC. The role of mitochondrial and sarcolemmal K(ATP) channels in canine ethanol-induced preconditioning in vivo. Anesth Analg. 2002;94(4):841–8. doi: 10.1097/00000539-200204000-00012. table of contents. [DOI] [PubMed] [Google Scholar]
- 138.Pagel PS, Toller WG, Gross ER, Gare M, Kersten JR, Warltier DC. K(ATP) channels mediate the beneficial effects of chronic ethanol ingestion. Am J Physiol Heart Circ Physiol. 2000;279(5):H2574–9. doi: 10.1152/ajpheart.2000.279.5.H2574. [DOI] [PubMed] [Google Scholar]
- 139.Zhu P, Zhou HZ, Gray MO. Chronic ethanol-induced myocardial protection requires activation of mitochondrial K(ATP) channels. J Mol Cell Cardiol. 2000;32(11):2091–5. doi: 10.1006/jmcc.2000.1233. [DOI] [PubMed] [Google Scholar]
- 140.Miyamae M, Camacho SA, Zhou HZ, Diamond I, Figueredo VM. Alcohol consumption reduces ischemia-reperfusion injury by species-specific signaling in guinea pigs and rats. Am J Physiol. 1998;275(1 Pt 2):H50–6. doi: 10.1152/ajpheart.1998.275.1.H50. [DOI] [PubMed] [Google Scholar]
- 141.Miyamae M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 1997;94(7):3235–9. doi: 10.1073/pnas.94.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyamae M, Domae N, Zhou HZ, Sugioka S, Diamond I, Figueredo VM. Phospholipase C activation is required for cardioprotection by ethanol consumption. Exp Clin Cardiol. 2003;8(4):184–8. [PMC free article] [PubMed] [Google Scholar]
- 143.Hausenloy DJ. Signalling pathways in ischaemic postconditioning. Thromb Haemost. 2009;101(4):626–34. [PubMed] [Google Scholar]
- 144.Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, et al. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282(4):H1216–22. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- 145.Liang Q, Carlson EC, Borgerding AJ, Epstein PN. A transgenic model of acetaldehyde overproduction accelerates alcohol cardiomyopathy. J Pharmacol Exp Ther. 1999;291(2):766–72. [PubMed] [Google Scholar]
- 146.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119(14):1941–9. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321(5895):1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Budas GR, Disatnik MH, Chen CH, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCvarepsilon) knockout mice. J Mol Cell Cardiol. 2010;48:757–64. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32(8):1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46(2):278–84. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bellows SD, Hale SL, Kloner RA. Acute Ethanol Does Not Protect Against Ischemic/Reperfusion Injury in Rabbit Myocardium. J Thromb Thrombolysis. 1996;3(3):181–4. doi: 10.1007/BF00181659. [DOI] [PubMed] [Google Scholar]
- 152.Dow JS, Hale SL, Kloner RA. Can moderate alcohol intake limit the size of myocardial infarction? J Cardiovasc Pharmacol. 2001;37(6):662–7. doi: 10.1097/00005344-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 153.Hale SL, Kloner RA. Ethanol does not exert myocardial preconditioning in an intact rabbit model of ischemia/reperfusion. Heart Dis. 2001;3(5):293–6. doi: 10.1097/00132580-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 154.Itoya M, Morrison JD, Downey HF. Effect of ethanol on myocardial infarct size in a canine model of coronary artery occlusion-reperfusion. Mol Cell Biochem. 1998;186(1-2):35–41. [PubMed] [Google Scholar]
- 155.Churchill EN, Disatnik MH, Budas GR, Mochly-Rosen D. Ethanol for cardiac ischemia: the role of protein kinase c. Ther Adv Cardiovasc Dis. 2008;2(6):469–83. doi: 10.1177/1753944708094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de la Torre JC. Impaired cerebromicrovascular perfusion. Summary of evidence in support of its causality in Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:136–52. doi: 10.1111/j.1749-6632.2000.tb05572.x. [DOI] [PubMed] [Google Scholar]
- 157.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68(Suppl 2):S74–87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rhodin JA, Thomas T. A vascular connection to Alzheimer’s disease. Microcirculation. 2001;8:207–20. doi: 10.1038/sj/mn/7800086. [DOI] [PubMed] [Google Scholar]
- 159.Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:40–50. doi: 10.1196/annals.1379.004. [DOI] [PubMed] [Google Scholar]
- 160.Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-beta-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. Neuroreport. 2004;15:2093–6. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- 161.Belmadani A, Neafsey EJ, Collins MA. Human immunodeficiency virus type 1 gp120 and ethanol coexposure in rat organotypic brain slice cultures: Curtailment of gp120-induced neurotoxicity and neurotoxic mediators by moderate but not high ethanol concentrations. J Neurovirol. 2003;9:45–54. doi: 10.1080/13550280390173409. [DOI] [PubMed] [Google Scholar]
- 162.Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104:769–81. doi: 10.1016/s0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q, Kalogeris TJ, Wang M, Jones AW, Korthuis RJ. Antecedent ethanol attenuates cerebral ischemia/reperfusion-induced leukocyte-endothelial adhesive interactions and delayed neuronal death: role of large conductance, Ca2+-activated K+ channels. Microcirculation. 2010;17:427–38. doi: 10.1111/j.1549-8719.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, et al. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007;43:1048–60. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 166.Qi SH, Liu Y, Hao LY, Guan QH, Gu YH, Zhang J, et al. Neuroprotection of ethanol against ischemia/reperfusion-induced brain injury through decreasing c-Jun N-terminal kinase 3 (JNK3) activation by enhancing GABA release. Neuroscience. 2010;167:1125–37. doi: 10.1016/j.neuroscience.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 167.Qi SH, Liu Y, Wang WW, Wang M, Zhang GY. Neuroprotection of ethanol against cerebral ischemia/reperfusion induced brain injury through GABA receptor activation. Brain Res. 2009;1276:151–8. doi: 10.1016/j.brainres.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 168.Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. 2010;41:420–5. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mitchell RM, Neafsey EJ, Collins MA. Essential involvement of the NMDA receptor in ethanol preconditioning-dependent neuroprotection from amyloid-betain vitro. J Neurochem. 2009;111:580–8. doi: 10.1111/j.1471-4159.2009.06351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sivaswamy S, Neafsey EJ, Collins MA. Neuroprotective preconditioning of rat brain cultures with ethanol: potential transduction by PKC isoforms and focal adhesion kinase upstream of increases in effector heat shock proteins. Eur J Neurosci. 2010;32:1800–12. doi: 10.1111/j.1460-9568.2010.07451.x. [DOI] [PubMed] [Google Scholar]
- 171.Chaudhury TK, Robert A. Prevention by mild irritants of gastric necrosis produced in rats by sodium taurocholate. Dig Dis Sci. 1980;25:830–6. doi: 10.1007/BF01338524. [DOI] [PubMed] [Google Scholar]
- 172.Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761–7. [PubMed] [Google Scholar]
- 173.Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am J Physiol. 1983;245:G113–21. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 174.Robert A, Nezamis JE, Lancaster C, Hanchar AJ. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–43. [PubMed] [Google Scholar]
- 175.Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983;245:G601–23. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- 176.Ko JK, Cho CH. Adaptive cytoprotection and the brain-gut axis. Digestion. 2011;83(Suppl 1):19–24. doi: 10.1159/000323400. [DOI] [PubMed] [Google Scholar]
- 177.Ko JK, Cho CH. Co-regulation of mucosal nitric oxide and prostaglandin in gastric adaptive cytoprotection. Inflamm Res. 1999;48:471–8. doi: 10.1007/s000110050489. [DOI] [PubMed] [Google Scholar]
- 178.Ko JK, Cho CH. Histological study of mechanisms of adaptive cytoprotection on ethanol-induced mucosal damage in rat stomachs. Dig Dis Sci. 1998;43:1248–57. doi: 10.1023/a:1018807808088. [DOI] [PubMed] [Google Scholar]
- 179.Kokoska ER, Smith GS, Deshpande Y, Rieckenberg CL, Miller TA. Adaptive cytoprotection induced by ethanol in human intestinal cells: role of prostaglandins and calcium homeostasis. Ann Surg. 1998;228:123–30. doi: 10.1097/00000658-199807000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takeuchi K, Araki H, Umeda M, Komoike Y, Suzuki K. Adaptive gastric cytoprotection is mediated by prostaglandin EP1 receptors: a study using rats and knockout mice. J Pharmacol Exp Ther. 2001;297:1160–5. [PubMed] [Google Scholar]
- 181.Yamamoto H, Tanaka A, Kunikata T, Hirata T, Kato S, Takeuchi K. Inducible types of cyclooxygenase and nitric oxide synthase in adaptive cytoprotection in rat stomachs. J Physiol Paris. 1999;93:405–12. doi: 10.1016/s0928-4257(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 182.Dinda PK, Wasan S, Beck IT, Kossev P. Adaptive cytoprotection against ethanol-induced small intestinal mucosal injury. Can J Physiol Pharmacol. 1996;74:598–602. [PubMed] [Google Scholar]
- 183.Gute DC, Korthuis RJ. Inflammation in ischemia/reperfusion-induced skeletal muscle injury: Role of neutrophil adhesion and ischemic preconditioning. Cell Biochem. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- 184.Gute DC, Korthuis RJ. Role of leukocyte adherence in reperfusion-induced mircovascular dysfunction and tissue injury. In: Granger DN, Schmid-Schobein GW, editors. Physiology and Pathophysiology of Leukocyte Adhesion. Vol. 19. Oxford Univ Press; 1995. pp. 359–380. [Google Scholar]
- 185.Korthuis RJ, Granger DN. Mechanisms of Ischemia/reperfusion. Ann Rev Physiol. 1995 doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- 186.Panes J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–90. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 187.Dayton C, Yamaguchi T, Kamada K, Carter P, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic leukocyte adhesion and P-selectin expression by a protein kinase C-dependent mechanism. Dig Dis Sci. 2005;50:684–90. doi: 10.1007/s10620-005-2557-1. [DOI] [PubMed] [Google Scholar]
- 188.Dayton C, Yamaguchi T, Kamada K, Carter P, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic P-selectin expression in murine small intestine. Microcirculation. 2004;11:709–18. doi: 10.1080/10739680490521014. [DOI] [PubMed] [Google Scholar]
- 189.Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2007;292:H326–32. doi: 10.1152/ajpheart.00744.2006. [DOI] [PubMed] [Google Scholar]
- 190.Gaskin FS, Kamada K, Yusof M, Durante W, Gross G, Korthuis RJ. AICAR preconditioning prevents postischemic leukocyte rolling and adhesion: role of K(ATP) channels and heme oxygenase. Microcirculation. 2009;16:167–76. doi: 10.1080/10739680802355897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gaskin FS, Kamada K, Zuidema MY, Jones AW, Rubin LJ, Korthuis RJ. Isoform-selective 5′-AMP-activated protein kinase-dependent preconditioning mechanisms to prevent postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2011;300:H1352–60. doi: 10.1152/ajpheart.00944.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goral J, Karavitis J, Kovacs EJ. Exposure-dependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–47. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Imhof A, Blagieva R, Marx N, Koenig W. Drinking modulates monocyte migration in healthy subjects: a randomised intervention study of water, ethanol, red wine and beer with or without alcohol. Diab Vasc Dis Res. 2008;5:48–53. doi: 10.3132/dvdr.2008.009. [DOI] [PubMed] [Google Scholar]
- 194.Kamada K, Dayton CB, Yamaguchi T, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic microvascular dysfunction. Pathophysiology. 2004;10:131–7. doi: 10.1016/j.pathophys.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 195.Kamada K, Gaskin FS, Yamaguchi T, Carter P, Yoshikawa T, Yusof M, et al. Role of calcitonin gene-related peptide in the postischemic anti-inflammatory effects of antecedent ethanol ingestion. Am J Physiol Heart Circ Physiol. 2006;290:H531–7. doi: 10.1152/ajpheart.00839.2005. [DOI] [PubMed] [Google Scholar]
- 196.Sacanella E, Estruch R. The effect of alcohol consumption on endothelial adhesion molecule expression. Addict Biol. 2003;8:371–8. doi: 10.1080/13556210310001656376. [DOI] [PubMed] [Google Scholar]
- 197.Sacanella E, Vazquez-Agell M, Mena MP, Antunez E, Fernandez-Sola J, Nicolas JM, et al. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: a randomized trial. Am J Clin Nutr. 2007;86:1463–9. doi: 10.1093/ajcn/86.5.1463. [DOI] [PubMed] [Google Scholar]
- 198.Vrsalovic M, Vrsalovic MM, Presecki AV, Lukac J. Modulating role of alcohol and acetaldehyde on neutrophil and monocyte functions in vitro. J Cardiovasc Pharmacol. 2007;50:462–5. doi: 10.1097/FJC.0b013e31812378fb. [DOI] [PubMed] [Google Scholar]
- 199.Yamaguchi T, Dayton C, Shigematsu T, Carter P, Yoshikawa T, Gute DC, et al. Preconditioning with ethanol prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol. 2002;283:H1019–30. doi: 10.1152/ajpheart.00173.2002. [DOI] [PubMed] [Google Scholar]
- 200.Yamaguchi T, Dayton CB, Ross CR, Yoshikawa T, Gute DC, Korthuis RJ. Late preconditioning by ethanol is initiated via an oxidant-dependent signaling pathway. Free Radic Biol Med. 2003;34:365–76. doi: 10.1016/s0891-5849(02)01292-3. [DOI] [PubMed] [Google Scholar]
- 201.Yamaguchi T, Kamada K, Dayton C, Gaskin FS, Yusof M, Yoshikawa T, et al. Role of eNOS-derived NO in the postischemic anti-inflammatory effects of antecedent ethanol ingestion in murine small intestine. Am J Physiol Heart Circ Physiol. 2007;292:H1435–42. doi: 10.1152/ajpheart.00282.2006. [DOI] [PubMed] [Google Scholar]
- 202.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO− and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–76. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zuidema MY, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, et al. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 2010;299:H1554–67. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent Hydrogen Sulfide Elicits an Anti-Inflammatory Phenotype in Postischemic Murine Small Intestine: Role of Heme Oxygenase-1. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00432.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A. 2009;106:20097–102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent Hydrogen Sulfide Elicits an Anti-Inflammatory Phenotype in Postischemic Murine Small Intestine: Role of Heme Oxygenase-1. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00432.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol. 2004;141(8):1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181–95. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 209.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–71. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84–93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wandler A, Bruun JM, Nielsen MP, Richelsen B. Ethanol exerts anti-inflammatory effects in human adipose tissue in vitro. Mol Cell Endocrinol. 2008;296:26–31. doi: 10.1016/j.mce.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 212.Jones AW, Durante W, Korthuis RJ. Heme oxygenase-1 deficiency leads to alteration of soluble guanylate cyclase redox regulation. J Pharmacol Exp Ther. 2010;335:85–91. doi: 10.1124/jpet.110.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vazquez-Prieto MA, Renna NF, Diez ER, Cacciamani V, Lembo C, Miatello RM. Effect of red wine on adipocytokine expression and vascular alterations in fructose-fed rats. Am J Hypertens. 2011;24:234–40. doi: 10.1038/ajh.2010.214. [DOI] [PubMed] [Google Scholar]
- 214.Huang PH, Tsai HY, Wang CH, Chen YH, Chen JS, Lin FY, et al. Moderate intake of red wine improves ischemia-induced neovascularization in diabetic mice--roles of endothelial progenitor cells and nitric oxide. Atherosclerosis. 2010;212:426–35. doi: 10.1016/j.atherosclerosis.2010.06.034. [DOI] [PubMed] [Google Scholar]