Summary
Activation of pattern recognition receptors on dendritic cells (DCs) and macrophages leads to secretion of cytokines that control differentiation of CD4+ T cells. The current understanding is that interleukin (IL)-6 in combination with transforming growth factor-β (TGF-β) leads to generation of T helper-17 (Th17) lineage cells. Here, we have discovered that the cytokine requirements for Th17 cell polarization depend on the site of priming. While IL-6 played a critical role in Th17 cell lineage priming in the skin and mucosal tissues, it was not required for Th17 cell priming in the spleen. In contrast, IL-1 played an irreplaceable role for priming of Th17 cell lineage cells in all tissues. Importantly, we have demonstrated that IL-6 independent and dependent pathways of Th17 cell differentiation are guided by DCs residing in various tissues. These results reveal fundamental differences by which the systemic, mucosal and cutaneous immune systems guide Th17 cell lineage commitment.
Introduction
Activation of the innate immune system is critical for inducing priming of antigen specific naïve CD4+ T cells (Janeway, 1989; Medzhitov, 2001). Dendritic cells (DCs) are equipped with a broad array of pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) (Iwasaki and Medzhitov, 2004), Retinoic acid inducible gene I (RIG-I)-like receptors (Meylan et al., 2006), Nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors (NLRs) (Williams et al., 2010) and C-type lectin receptors (Geijtenbeek and Gringhuis, 2009), all of which sense pathogen associated molecular patterns (PAMPs) and trigger DC maturation. Maturation of DCs is characterized by high expression of major histocompatibility complex (MHC) and costimulatory molecules, as well as the production of inflammatory cytokines and chemokines, which play critical roles in activation of naïve T cells (Palm and Medzhitov, 2009).
In addition to naïve T cell priming, cytokines secreted by DCs following PRR engagement govern the fate of activated CD4+ T cells, and regulate their survival and lineage commitment (Zhu et al., 2010). Cytokines such as IL-12 and IL-18 initiate or promote T helper-1 (Th1) cell commitment of primed T cells, which protect the host against various bacterial and viral pathogens (Hsieh et al., 1993; Takeda et al., 1998). A newly defined lineage of T cells, called T helper-17 (Th17) cells, has been shown to be critical for protection against certain bacterial and fungal infections, and also to be responsible for several autoimmune diseases (Korn et al., 2009). The orphan nuclear receptor RORγt has been shown to be both necessary, and sufficient, for Th17 cell differentiation (Ivanov et al., 2006; Yang et al., 2008b). A combination of interleukin (IL)-6 and transforming growth factor-β (TGF-β), in vitro, leads to induction of the transcription factor RORγt and differentiation of murine naïve T cells into Th17 lineage cells (Bettelli et al., 2006; Manel et al., 2008; Mangan et al., 2006; Veldhoen et al., 2006). Other studies have demonstrated that IL-1 enhances Th17 cell differentiation induced by a combination of IL-6, TGF-β, IL-23 or IL-21 (Acosta-Rodriguez et al., 2007; Korn et al., 2007; Nurieva et al., 2007; Volpe et al., 2008; Yang et al., 2008a; Zhou et al., 2007). Furthermore, interleukin-1 receptor-deficient (Il1r1−/− ) mice have been shown to be resistant to experimental autoimmune encephalomyelitis (EAE) (Sutton et al., 2006) and, most recently, IL-6 has been shown to control Th17 cell differentiation through regulation of IL-1R on CD4+ T cells (Chung et al., 2009). However, the relative contributions of IL-1 and IL-6 in Th17 cell differentiation are not completely understood.
Multicellular organisms constantly encounter microbial stimuli, both from commensals as well as invading pathogens. Most microbes invade their hosts through the mucosal surfaces such as the intestine, the respiratory tract, uro-genital tract, as well as the skin. Adaptive immune responses to pathogens are generated in the draining lymph nodes of the site of infection. However pathogens also penetrate into the blood stream and cause systemic infection and adaptive immune responses to such pathogens are generated in the spleen. The mucosal immune system, the cutaneous immune system, and the systemic immune system face unique challenges in dealing with infectious agents. The former two are in constant contact with commensal micro-organsims while the spleen is largely a sterile environment. In addition, unique DC populations reside in different tissues. Broadly, DCs can be categorized into tissue-resident and lymphoid organ-resident DCs (Villadangos and Schnorrer, 2007). The former reside in peripheral tissues such as mucosa, skin, and non-lymphoid organs, and migrate from peripheral tissues into the corresponding lymph nodes, through the afferent lymphatics (Randolph et al., 2005), both at steady state and during infection. Lymphoid organ-resident or blood DCs enter the spleen and lymph nodes as bone-marrow derived precursors and develop within those organs without trafficking through peripheral tissues (Liu et al., 2007). The spleen contains exclusively blood-derived DCs; whereas, the lymph nodes in addition to blood-derived DC subsets contain migratory DCs coming from peripheral tissues (Henri et al., 2001). While we understand that DCs play a critical role in activation of naïve T cells the importance of anatomical location of DCs for T cell differentiation remains largely unexplored.
In the current study we discovered that Th17 cell lineage commitment has differential cytokine requirements depending on the site of priming. We have shown that IL-6, which is thought to be a necessary differentiation factor for Th17 cell lineage development, was critical in the mucosal tissues of the gut and lungs as well as in the skin draining lymph nodes but not in the spleen. However, IL-1R signaling was critical for induction of IL-17 secreting T cells in all tissues. We have further demonstrated that DC populations resident in the mucosal tissues, skin and spleen were responsible for the differential cytokine requirements for Th17 cell priming. This study provides important insights into how priming microenvironments guide the cytokine requirements for development of Th17 lineage cells and demonstrates that DC populations from sterile microenvironments and commensal inhabited microenvironments impose different rules for Th17 cell priming.
Results
MyD88 adaptor signaling in T cells is critical for inducing antigen specific Th17 cells
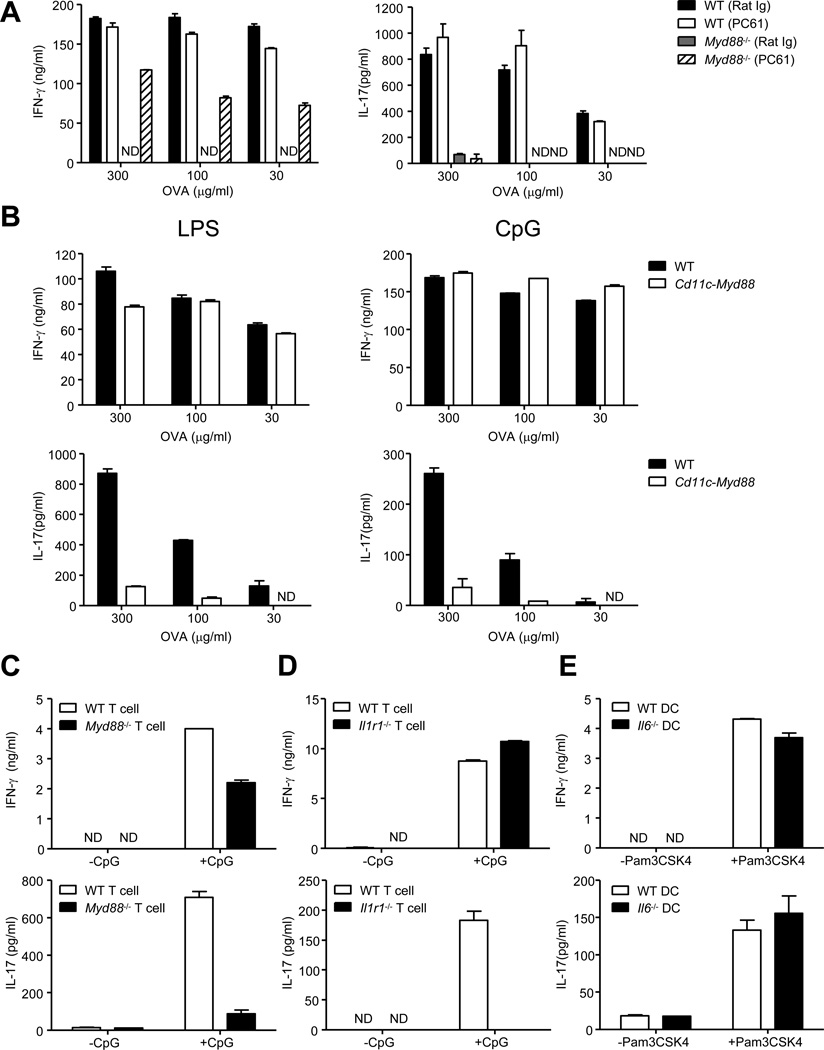
Depletion of CD4+CD25+ regulatory T (Treg) cells in Myd88−/− mice restores otherwise defective antigen specific CD4+ T cell priming as well as Th1 cell commitment (Pasare and Medzhitov, 2004). We investigated whether such priming would also restore Th17 cell lineage commitment in Myd88−/− mice. As demonstrated before, immunization of Myd88−/− mice using antigen mixed with TLR4 ligand lipopolysaccharide (LPS), after in vivo depletion of Treg cells (Figures S1A and S1B), led to priming of interferon-γ (IFN-γ) producing T cells (Figure 1A). Intriguingly, CD4+ T cells from Treg cell depleted Myd88−/− mice failed to secrete detectable IL-17A (IL-17) (Figure 1A). To explore the possibility that a lack of IL-6 and IL-23 secretion by DCs could be the cause of defective Th17 cell priming in Myd88−/− mice, we used a transgenic mouse expressing MyD88 only in DCs and macrophages under the control of the Cd11c promoter (Pasare and Medzhitov, 2005) (Cd11c-Myd88 Tg). DCs from the Cd11c-Myd88 Tg mice had normal TLR induced IL-6 and IL-23 secretion (Figure S1C). Strikingly, these mice had normal Th1 cell priming but defective Th17 cell priming (Figure 1B), demonstrating that restoring TLR signaling in DCs was insufficient to induce antigen specific Th17 cell priming
Figure 1. MyD88-dependent IL-1R signaling in T cells is required for Th17 cell priming and IL-6 is dispensable for Th17 cell priming in vitro.
(A) WT and Myd88−/− mice received rat anti-mouse CD25 antibody (PC61) or control antibody (Rat Ig) by intravenous route (25µg/mouse). Three days later, Treg cell depletion was confirmed (Figure S1A) and mice were immunized in the footpads (fp) with Ovalbumin (OVA) (50µg/fp) and LPS (5µg/fp) emulsified in incomplete Freund’s adjuvant (IFA). CD4+ T cells were purified from the draining lymph nodes at day 7 post-immunization and cultured with Tlr2−/−Tlr4−/− B cells as antigen presenting cells (APC)s in the presence of titrating doses of OVA for 72 hours. IFN-γ (left) and IL-17 (right) concentrations in the culture supernatants were determined by ELISA. (B) WT and Cd11c-Myd88 Tg mice were immunized in the fp with OVA (50µg/fp) and LPS (5µg/fp) (left panels) or OVA (50µg/fp) and CpG (5µg/fp) (right panels) emulsified in IFA. CD4+ T cells were activated to measure IFN-γ (upper panels) and IL-17 (lower panels) as described above. (C, D and E) Purified naïve CD4+ T cells (3×105) from the indicated strains were cultured in the presence of purified WT splenic DCs or Il6−/− splenic DCs (6×104) and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR ligands. Culture supernatants were collected and assayed for IFN-γ top panels) and IL-17 (bottom panels) by ELISA. Data are representative of three to four independent experiments. Bar graphs represent mean ± SEM.
In our in vitro priming assays, WT T cells differentiated into both Th1 and Th17 cell lineages (Figure 1C). However, Myd88−/− T cells differentiated into IFN-γ producing Th1 cells but not IL-17 producing Th17 cells (Figure 1C). Since MyD88 is an adaptor molecule shared by TLRs and the IL-1 receptor (Wesche et al., 1997), we tested the ability of Il1r1−/− T cells to differentiate into Th17 cells and found that, Il1r1−/− CD4+ T cells behaved like Myd88−/− CD4+ T cells in terms of IL-17 production (Figure 1D). This is consistent with previously suggested role for IL-1R signaling in CD4+ T cells for Th17 cell differentiation (Chung et al., 2009; Sutton et al., 2006). A very surprising result however was that Il6−/− DCs were able to induce Th17 cell differentiation comparable to their WT counterparts (Figure 1E). These data suggest that IL-1 is critical and IL-6 is dispensable for Th17 cell lineage commitment. Since the current understanding is that IL-6 is critical for Th17 cell priming (Korn et al., 2009), these results led us to revisit the paradigm and further investigate in vivo requirements for Th17 cell lineage commitment.
Th17 cells are present in the peripheral secondary lymphoid organs but not in the intestinal lamina propria of Il6−/− mice
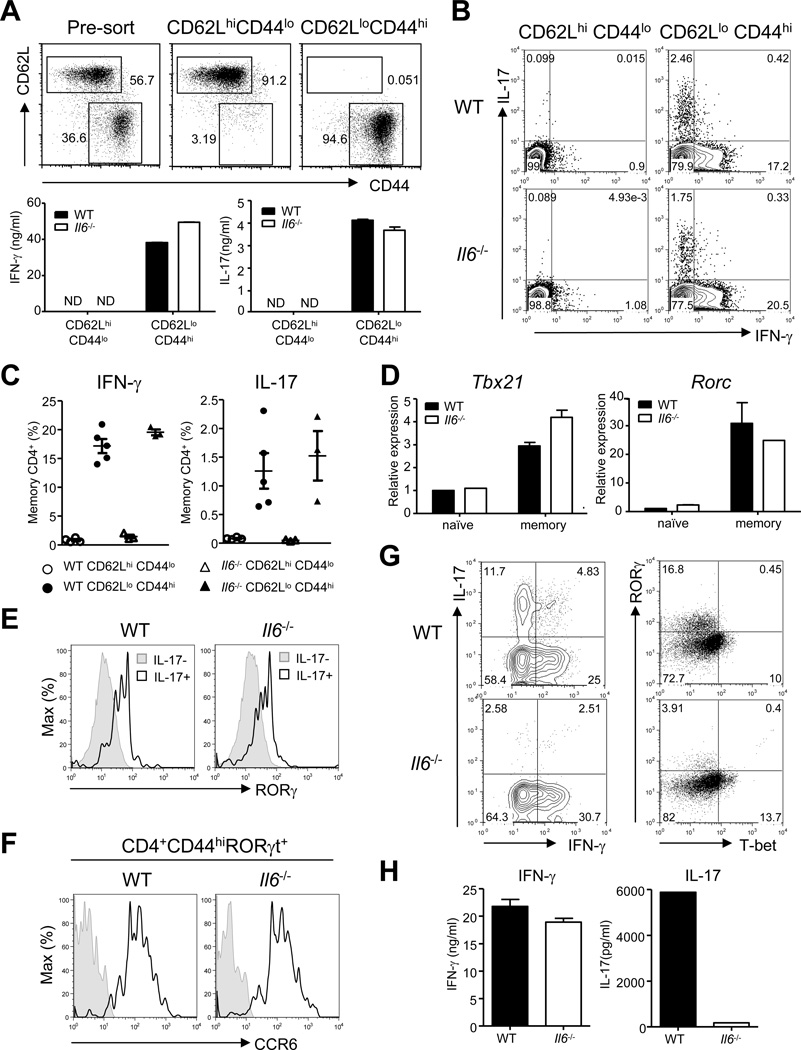
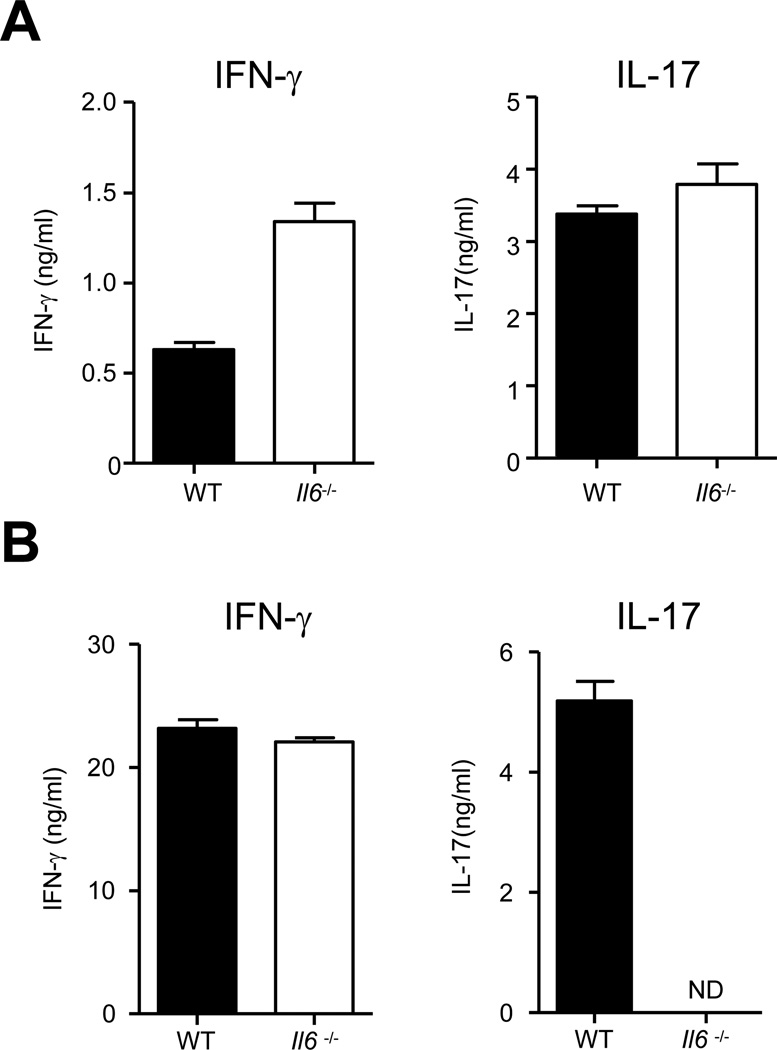
Since our in vitro data suggested that IL-6 is dispensable for Th17 cell lineage commitment, we investigated the status of Th17 cells in Il6−/− mice. In the spleens of Il6−/− mice, we found that CD62LloCD44hi memory CD4+ T cells secreted IFN-γ when activated ex vivo, similar to their WT counterparts (Figure 2A). Surprisingly, memory cells derived from the spleens of Il6−/− mice also secreted IL-17 comparable to the quantities secreted by WT CD4+ T cells. The percentage of memory CD4+ T cells making IL-17 was also comparable between WT and Il6−/− mice (Figures 2B and 2C). In agreement with our in vitro data, memory CD4+ T cells from both Myd88−/− and Il1r1−/− mice were defective in the secretion of IL-17 (Figure S2A). We next measured the expression of the Th17 cell -lineage master regulator RORγt, and found that memory T cells obtained from both WT and Il6−/− mice expressed similar quantities of this transcription factor, both at the mRNA (Figure 2D) and the protein (Figure 2E) level. The RORγt-expressing memory CD4+ T cells from Il6−/− mice also express CCR6, a chemokine receptor known to be preferentially expressed by Th17 cells (Hirota et al., 2007)(Figure 2F). Overall, Il6−/− mice seem to have Th17 cells that are phenotypically indistinguishable from those in the WT mice in the spleen.
Figure 2. Th17 lineage cells are present in the peripheral secondary lymphoid organs but not lamina propria of the intestines of IL-6-deficient mice.
(A–E) CD4+ T cells from the spleens of slightly aged (16–20 weeks) WT and Il6−/− mice were sorted to obtain CD62LhiCD44lo naïve and CD62LloCD44hi memory populations. (A) The sorted cells were cultured on plates coated with anti-CD3 and anti-CD28 for 48 hours and culture supernatants were assayed for IFN-γ and IL-17 by ELISA. (B–C) The sorted cells were stimulated with PMA and ionomycin and stained for intracellular IFN-γ, and IL-17 following permeabilization. Representative plots (B) and combined data (C) of IFN-γ and IL-17 secreting CD4+TCRβ+ cells from several mice are shown. (D) cDNA was made from freshly isolated naïve and memory CD4+ T cells and the transcript amounts of Tbx21 (left) and Rorc (right) were measured by quantitative RT-PCR. (E) CD62LloCD44hi memory populations were stimulated as described in (B) and stained for IL-17 and RORγ. Histograms show the expression of RORγ by CD4+TCRβ+IL-17+ and CD4+TCRβ+IL-17− cells. (F) Splenocytes from WT and Il6−/− mice were stained for surface CD4, CD44, CCR6 and intracellular RORγ. Open histograms show the expression of CCR6 on CD4+CD44hiRORγ+ cells. Shaded histograms, unstained control. (G) Lamina propria lymphocytes (LPLs) were isolated from indicated mice after at least two weeks of cohousing and stained for IFN-γ and IL-17 following stimulation, or RORγ and T-bet directly. (H) Enriched CD4+ T cells from LP of indicates strains of mice were cultured (30,000 CD4+ T cells/well) in the presence of plate-bound anti-CD3 and anti-CD28 for 48 hours and culture supernatants were assayed for IFN-γ IL-17. Data are representative of three to five independent experiments. Bar graphs represent mean ± SEM.
These data differ from earlier findings demonstrating that IL-6 plays a non-redundant role in Th17 cell lineage commitment (Bettelli et al., 2006; Ivanov et al., 2006). However, many earlier reports have used recombinant cytokine cocktails for in vitro differentiation assays. Likewise, in vivo studies have dealt mainly with analyzing cytokine commitment in the CD4+ T cells derived from the lamina propria (LP) of the intestines. We therefore decided to investigate Th17 cell commitment of CD4+ T cells derived from the LP. Of note, because certain components of the intestinal microflora have been shown to favor the induction of Th17 cells (Ivanov et al., 2009), mice were co-housed for at least two weeks to ensure homogenous microflora populations between different genotypes. In contrast to our findings in the spleen, we found that Th17 cell lineage commitment was defective in LP CD4+ T cells in Il6−/− mice (Figure 2G, H). In Myd88−/− and Il1r1−/− mice, CD4+ T cells from the LP behaved like CD4+ T cells in the spleen and were defective in secretion of IL-17 (Figure S2A).
It has been previously reported that TGF-β drives expression of the transcription factor Foxp3 and leads to generation of peripherally-induced Treg cells (Bettelli et al., 2006; Chen et al., 2003; Marie et al., 2005). However, IL-6 antagonizes Foxp3-mediated inhibition of RORγt, thus preventing the generation of Treg cells and promoting the induction of Th17 lineage cells (Zhou et al., 2008). Since we found that Il6−/− mice had normal Th17 lineage cells that express RORγt in the spleen, but both were defective in the LP of the gut, we wanted to determine if the proportion of Foxp3+ Treg cells was influenced in these tissues in Il6−/− mice. We saw a similar proportion of CD4+ T cells from the spleens of WT and Il6−/− mice expressing Foxp3 (Figures S2B and S2C). However, when we examined LP lymphocytes, we found that Il6−/− mice had approximately double the proportion of Foxp3+ CD4+ T cells when compared to WT mice (Figures S2B and S2C). These data suggest that IL-6 affects the lineage choice between Foxp3+ Treg cells and Th17 lineage cells only in the LP of the intestines but not in the spleen.
Differential requirement of IL-6 for Th17 cell differentiation in secondary lymphoid organs and intestinal lamina propria
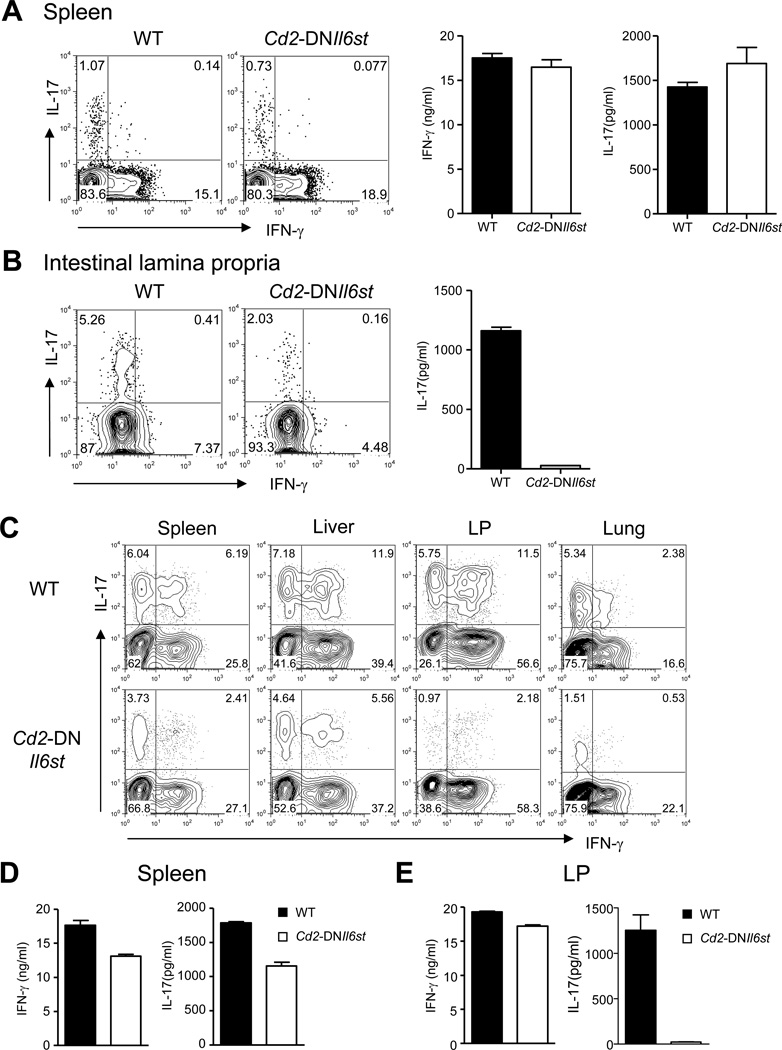
The above observations raised the interesting possibility that IL-6 could be required for Th17 cell lineage commitment in a tissue-specific manner. To test this hypothesis, we generated mice that express a dominant negative form of gp130, the signaling subunit of the IL-6 receptor complex (Hibi et al., 1990; Taga et al., 1989), in T cells under the control of a Cd2 promoter (Cd2-DNIl6st Tg) (Zhumabekov et al., 1995). CD4+ T cells from these mice had diminished phosphorylation of Stat3 in response to IL-6 (Figure S3A) and failed to show an enhanced response to a combination of IL-1β and IL-6 in a proliferation assay with anti-CD3 stimulation (Figure S3B). Analysis of T cells from the spleen and LP of Cd2-DNIl6st Tg mice revealed a phenotype similar to Il6−/− mice, where IL-17 secreting T cells were present in the spleen and lymph nodes, but were reduced in the LP (Figure 3A and 3B).
Figure 3. Differential requirement of IL-6 for Th17 cell lineage commitment in different priming micro-environments under lymphopenic conditions.
(A) Splenic naïve (CD62LhiCD44lo) and memory (CD62LloCD44hi) CD4+ T cells from WT and Cd2-DNIl6st Tg mice were sorted and stimulated with PMA and ionomycin stained for intracellular IFN-γ and IL-17 (left two panels). Naïve and memory cells were also stimulated with plate bound anti-CD3 and anti-CD28 for 48 hours and culture supernatants were assayed for IFN-γ and IL-17 (Right two panels). Data for memory cells are shown. Naïve cells did not secrete detectable IFN-γ or IL-17. (B) LPLs were isolated from WT and Cd2-DNIl6st Tg mice and were either activated with PMA and ionomycin followed by staining for intracellular IL-17 and IFN-γ (left panels) or cultured in the presence of plate-coated anti-CD3 and anti-CD28 for 48 hours after enrichment for CD4+ T cells and assayed for IL-17 secretion in the culture supernatants (right panel). (C–E) Cells from the spleen and lymph nodes of WT or Cd2-DNIl6st Tg mice were transferred intravenously into Rag1−/− recipients. After 7 days, mononuclear cells (MCs) were isolated from the spleen, liver, LP and lung. (C) MCs from the indicated organs were stimulated with PMA and ionomycin and stained for intracellular IFN-γ and IL-17. CD4+TCRβ+ positive cells expressing intracellular IFN-γ and IL-17 are shown. (D-E) CD4+ T cells from indicated organs were stimulated with plate-bound anti-CD3 and anti-CD28 for 48 hours and analyzed for IFN-γ and IL-17 secretion in the culture supernatants. Data are representative of two or three independent experiments. Bar graphs represent mean ± SEM.
In order to look at the differentiation of Th1 and Th17 cells in various tissues, we transferred naïve cells pooled from the spleen and lymph nodes of Cd2-DNIl6st Tg mice into Rag1−/− recipients. Commensal microflora cross the intestinal barrier in Rag1−/− mice and this approach would allow us to assess Th1 and Th17 cell differentiation in various organs. We analyzed T cells from the spleen, liver, LP and lung of the recipients seven days post transfer for the presence of IFN-γ- and IL-17-producing CD4+ T cells. Intracellular staining suggested that both WT and Cd2-DNIl6st Tg CD4+ T cells committed to Th17 lineage cells in the spleen and the liver (Figure 3C). In contrast, Cd2-DNIl6st Tg CD4+ T cells were defective in Th17 cell commitment in the LP and the lungs (Figure 3C). Cd2-DNIl6st Tg CD4+ T cells from the spleens of Rag1−/− recipients secreted appreciable quantities of IL-17, whereas Cd2-DNIl6st Tg CD4+ T cells from the LP of Rag1−/− recipients failed to secrete detectable amounts of IL-17 (Figure 3D and 3E). In contrast, Il1r1−/− CD4+ T cells recovered from the Rag1−/− hosts had defective IL-17 production in all the organs that were examined (Figure S3C).
Lack of Th17 cells selectively in the gut in Il6−/− and Cd2-DNIl6st Tg mice may suggest a requirement of IL-6 for the survival of Th17 cells in the intestine. We tested this possibility by staining intestinal RORγt+ CD4+ T cells with Annexin V, which marks early apoptotic cells. There was indeed a small proportion of RORγt+ Th17 cells in the gut of Il6−/− and Cd2-DNIl6st Tg mice (Figure 2G) and the proportions of these cells that stained positive with Annexin V was comparable to those of WT mice (Figure S3D). These results argue that IL-6 signaling is not specifically required for the survival of Th17 cells in the gut, and favors the hypothesis that IL-6 is a tissue-specific priming factor for Th17 cells.
IL-6 independent priming of Th17 cells in the spleen following systemic infection
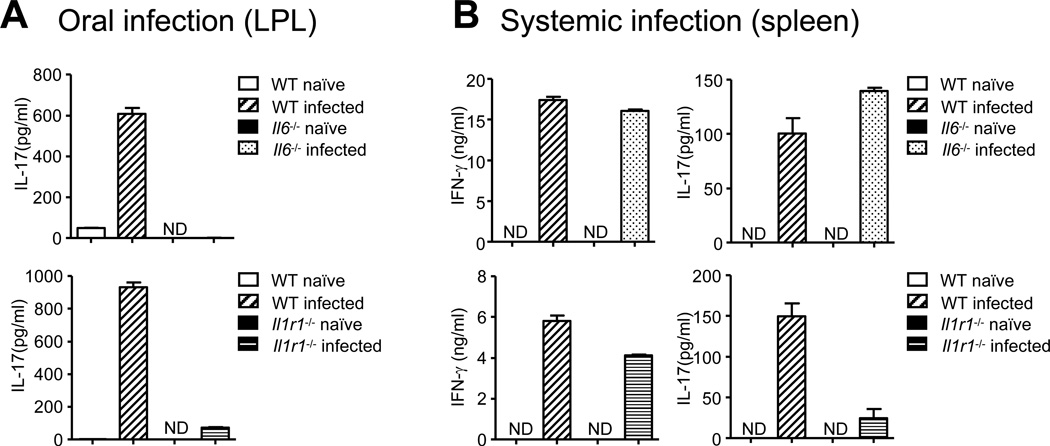
Since our data suggest a selective requirement of IL-6 for Th17 cell commitment in mucosal tissues (the intestines and lungs), but not in spleen both at steady state and during differentiation under lymphopenic conditions (Rag1−/− transfer model), we investigated the generation of Th17 lineage cells during infection. Oral infection using Citrobactor rodentium led to priming of Citrobacter-specific Th17 cells in the LP of WT mice, but Th17 cell priming was defective in both Il6−/− mice as well as Il1r1−/− mice (Figures 4A). We were also not able to detect any Citrobacter-specific Th17 cell response in the mesenteric lymph nodes of Il6−/− mice (Figure S4), indicating that the lack of antigen-specific Th17 cells in the LP is due to a priming defect rather than defective migration of activated cells from the site of priming to the peripheral tissue. Importantly, when mice were infected with S. typhimurium by the intraperitoneal route causing a systemic infection, comparable Salmonella-specific Th17 cell priming was observed in WT and Il6−/− mice (Figure 4B) in the spleen. However, there was defective priming of Salmonella-specific Th17 cells in Il1r1−/− mice (Figure 4B). These data demonstrate that IL-1 is required for generating IL-17-secreting CD4+ T cells, irrespective of the route of infection, whereas IL-6 is important for inducing Th17 cells in the LP of the intestines during oral infection, but is not required for Th17 cell priming in the spleen during a systemic infection.
Figure 4. Differential requirement of IL-6 for Th17 cell lineage commitment during systemic and oral infection.
(A) Mice of indicated genotypes were infected orally with ~ 1–2 × 109 CFU of Citrobacter rodentium. CD4+ T cells were purified from the LP on day 8 post -infection and stimulated with 3µg/ml of Citrobacter rodentium sonicated lysates for 72 hours in the presence of naïve WT B cells as APCs. IL-17 concentrations in the culture supernatants were then measured by ELISA. Citrobacter-specific IFN-γ was not detectable in any of the cultures. (B) Mice of indicated genotypes were infected intraperitoneally with 103 CFU of Salmonella typhimurium. CD4+ T cells from the spleen were isolated on day 7 post infection and stimulated with 3µg/ml of Salmonella typhimurium sonicated lysates for 72 hours in the presence of naïve B cells as APCs. IFN-γ and IL-17 concentrations in the culture supernatants were then measured by ELISA. Cells cultured without Citrobacter or Salmonella sonicated lysates did not secrete any detectable IFN-γ or IL-17. Data are representative of three independent experiments. Bar graphs represent mean ± SEM.
Subcutaneous immunization fails to generate Th17 cells in the absence of IL-6
Because of the similarities between the mucosal and cutaneous immune systems, we sought to determine whether our findings in the intestine are applicable to the cutaneous immune system. Although IL-17 secreting CD4+ T cells are present in the skin-draining lymph nodes in both WT and Il6−/− mice (Figures 5A, S5A and S5B), it is possible that these cells are primed in the spleen and subsequently traffic to the peripheral lymph nodes. Therefore, we tested whether IL-6 is required to generate fresh antigen-specific Th17 cell responses during immune responses initiated from the skin. We transferred OT-II T cells into WT and Il6−/− mice and recipients were immunized in the footpads with ovalbumin and LPS emulsified in incomplete Freund’s Adjuvant (IFA). OT-II T cells from the immunized WT recipients secreted both IFN-γ and IL-17 (Figure 5B). However, OT-II T cells recovered from Il6−/− recipients secreted only IFN-γ but no IL-17 suggesting that IL-6 is required for Th17 cell priming during subcutaneous immunization (Figure 5B). Consistently, Cd2-DNIl6st Tg mice immunized in the footpads generated a much reduced antigen-specific Th17 cell response (Figure S5C), further supporting the notion that IL-6 is required for generating Th17 cells in the skin draining lymph nodes.
Figure 5. The cutaneous immune system primes Th17 cells in an IL-6-dependent manner.
(A) CD4+ T cells from the inguinal, popliteal, brachial, and axillary lymph nodes of WT and Il6−/− mice were stimulated with plate-bound anti-CD3 and anti-CD28 for 48 hours and cytokine production was assayed in the culture supernatants. (B) 2.5−106 purified CD45.1 congenic OT-II T cells were transferred intravenously into WT or Il6−/− mice (CD45.2 background). The recipients were then immunized in the footpads with 50µg/fp of OVA and 5µg/fp of LPS emulsified in IFA. CD4+ T cells were enriched by negative selection from the draining lymph nodes on day 5 post-immunization, and CD4+CD45.1+ OT-II T cells were recovered by cell sorting followed by stimulation with plate-bound anti-CD3 and anti-CD28 for 48 hours. Cytokine secretion was analyzed by ELISA in the culture supernatants. Data are representative of two independent experiments. Bar graphs represent mean ± SEM.
Importantly, CD4+ T cells from the gut of did not appear to have an intrinsic defect in becoming Th17 cells, as naïve CD4+ T cells from the mesenteric lymph nodes (where gut homing T cells are primed) could be polarized towards the Th17 cell lineage, with IL-6 and TGF-β as well as IL-1, IL-23 and TGF-β, as efficiently as splenic CD4+ T cells (Figures S5D–S5F). In addition, lack of IL-6 during development did not affect the ability of CD4+ T cells to become Th17 cells, because Th17 cell polarizing conditions induced similar amounts of IL-17 producing cells from splenic and mesenteric lymph node CD4+ T cells from Il6−/− mice comparing to their WT counterparts (Figures S5D-S5F). In the context of in vitro priming with WT splenic DCs, when splenic and mesenteric lymph node CD4+ T cells from WT and Il6−/− mice were compared, they produced very similar amounts of IFN-γ as well as IL-17 (Figures S5G-S5I). Consistent with our earlier observation, IL-6 neutralization did not affect IL-17 production by all four groups of T cells (Figure S5G); however IL-1 receptor blockade with IL-1 receptor antagonist completely abolished IL-17 secretion in the cultures (Figure S5H). Using Il6−/− splenic DCs in the in vitro culture system showed similar results as IL-6 neutralization, further confirming that IL-6 is not required for priming of Th17 cells when splenic DCs are used (Figure S5I).
Resident DCs in priming micro-environments dictate cytokine requirements for Th17 cell differentiation
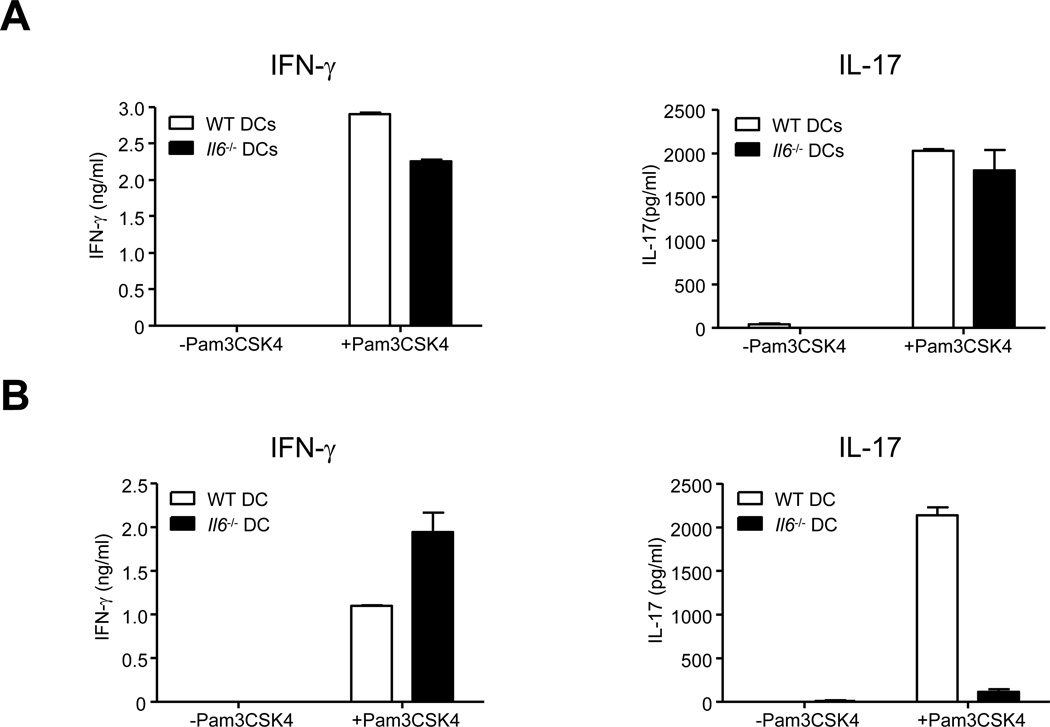
To explore the possibility that DC populations resident in the spleen and LP could primarily be responsible for the differentially controlled lineage choices made by activated T cells in their milieu, we examined the necessity of IL-6 for Th17 cell priming induced by DCs from the spleen and LP. We purified DCs from the spleens and LP of WT and Il6−/− mice and tested their ability to prime naïve WT CD4+ T cells into Th1 and Th17 cell lineages in vitro. Splenic DCs from both WT and Il6−/− mice induced differentiation of T cells that secreted IFN-γ as well as IL-17 (Figure 6A), thus validating that Th17 cell lineage development induced by splenic DCs does not require IL-6. However, Il6−/− LP DCs failed to induce differentiation of IL-17 secreting T cells, but induced differentiation of IFN-γ secreting T cells (Figure 6B). WT LP DCs induced both IFN-γ and IL-17 from activated CD4+ T cells (Figure 6B). These data demonstrate that while both splenic DCs and LP DCs induce differentiation of naïve T cells into Th17 cells, splenic DCs do so independent of IL-6, whereas LP DCs depend on IL-6 to induce Th17 lineage cells.
Figure 6. Dendritic cells from the spleen and lamina propria prime Th17 cells in an IL-6-independent and dependent manner respectively.
(A) Purified naïve WT CD4+ T cells were cultured in the presence of purified splenic DCs from indicated strains and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR2 ligand Pam3CSK4 (100ng/ml). Five days later, culture supernatants were collected and analyzed for IFN-γ (left) and IL-17 (right) by ELISA. (B) Purified naïve WT CD4+ T cells were cultured in the presence of purified lamina propria DCs from indicated strains and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR2 ligand Pam3CSK4 (100ng/ml). Five days later, culture supernatants were collected and analyzed for IFN-γ (left) and IL-17 (right) by ELISA. Data are representative of three independent experiments. Bar graphs represent mean ± SEM.
CD103+ DCs determine the dependency on IL-6 for Th17 cell differentiation
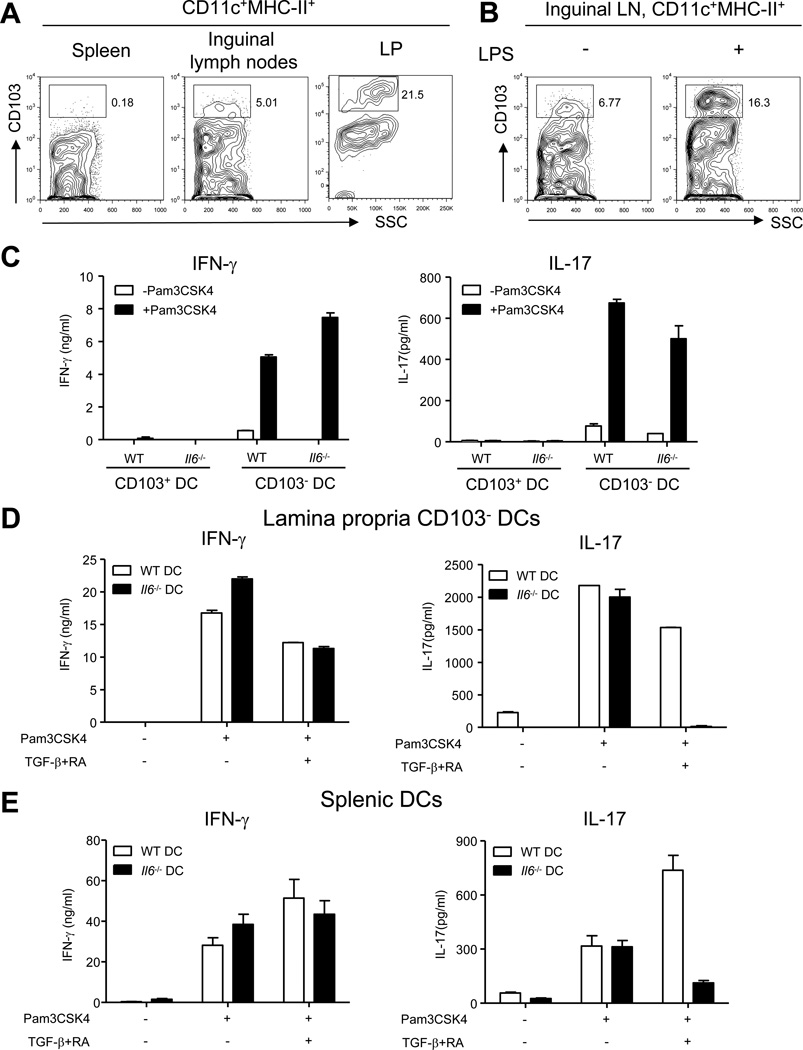
In order to understand the differences in the abilities of spleen and LP DCs to induce Th17 cell priming we began to investigate the differences in DC populations that reside in these tissues. Most strikingly, we noticed that CD103hi DCs are absent in the spleen but comprise ~20% of intestinal DCs (Figure 7A). These CD103hi DCs (hereafter referred to as CD103+ DCs) were also present at a low percentage in the skin-draining lymph nodes but accumulated upon subcutaneous LPS injection (Figures 7A, 7B and S6A). We sorted out CD103+ DCs from the intestine and tested their ability to induce CD4+ T cell differentiation. Both WT and Il6−/− LP CD103+ DCs failed to induce detectable IFN-γ or IL-17 production from in vitro activated CD4+ T cells (Figure 7C), consistent with a previous report showing that these DCs preferentially induce Treg cells (Coombes et al., 2007). We further explored the possibility that CD103+ DCs might impose the requirement of IL-6 for Th17 cell priming. We depleted CD103+ DCs from LP DCs and used the CD103− DC population from Il6−/− mice for in vitro priming assays. Consistent with our prediction, the CD103− DC population from the LP resembled the splenic DCs. WT and Il6−/− CD103− LP DCs induced similar quantities of IFN-γ as well as IL-17, demonstrating that CD103+ DCs in the gut are dominant over CD103− DCs and prevent IL-6 independent priming of Th17 lineage cells (Figure 7C). Also, we found that lymph node resident DCs from naïve mice, which have a very small CD103+ population, were able to prime Th17 cells in vitro in the absence of IL-6 (Figure S6B). However, when CD103+ DCs were recruited to the skin draining lymph nodes, following subcutaneous immunization (Figure 7B), IL-6 was required for Th17 cell priming (Figure 5B).
Figure 7. CD103+ DCs dictate the requirement of IL-6 for Th17 cell priming.
(A) Proportion of CD103+ DCs in the spleen, the inguinal lymph nodes and the LP. Cells from the CD11c+MHC ClassII+ gate are shown. (B) WT mice were injected with 10µg of LPS in the left footpad and PBS in the right footpad. The inguinal lymph nodes were collected 21 hours later and stained for CD11c, MHC Class II and CD103. Cells from the CD11c+MHC Class II+ gate are shown. (C) Purified naïve WT CD4+ T cells were cultured in the presence of purified CD103+ and CD103− LP DCs from indicated strains and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR2 ligand Pam3CSK4 (100ng/ml). Five days later, culture supernatants were collected and analyzed for IFN-γ (left) and IL-17 (right) by ELISA. (D–E) Purified naïve WT CD4+ T cells were cultured with (D) LP CD103− DCs or (E) splenic DCs from indicated strains and anti-CD3 (10ng/ml) for 5 days in the presence or absence of the TLR2 ligand Pam3CSK4 (100ng/ml). TGF-β (0.3ng/ml) and RA (0.1 nM) was added into the culture as indicated. Five days later, culture supernatants were collected and analyzed for IFN-γ (left) and IL-17 (right) by ELISA. Data are representative of three independent experiments. Bar graphs represent mean ± SEM.
It has been previously reported that CD103+ DCs in the gut make high quantities of TGF-β and retinoic acid (RA), both of which have been shown to impact Th17 cell differentiation. Low concentration of TGF-β promotes Th17 cell differentiation while high concentration of TGF-β inhibits it (Manel et al., 2008). RA suppresses Th17 cell differentiation yet enhances inducible regulatory T cell (iTreg) generation(Mucida et al., 2007; Nolting et al., 2009). We first tested the possibility that IL-6 could be required to overcome the suppressive effects of TGF-β. If this were true, neutralization of TGF-β would lead to IL-6 independent priming by LP DCs. However neutralization of TGF-β and inhibition of TGF-β signaling led to total abrogation of Th17 cell priming (Figure S6C), even when WT DCs were used for priming. These experiments suggest that TGF-β is an absolute requirement for Th17 cell priming. We also tested the possibility that RA made by CD103+ DCs was responsible for dictating the requirement of IL-6 for Th17 cell priming in the gut. Addition of RA abrogated Th17 cell priming induced by both WT and Il6−/− DCs without significantly affecting Th1 cell lineage development (Figure S6D). However, when a combination of TGF-β and RA was added to in vitro priming cultures, there was total abrogation of Th17 cell priming induced by Il6−/− CD103− DCs but not by WT DCs (Figure 7D). Similarly, TGF-β and RA also inhibited priming of Th17 cell lineage cells by splenic DCs from Il6−/− mice (Figure 7E). RA has been demonstrated to enhance Smad3 phosphorylation and TGF-β signaling (Mucida et al., 2007; Nolting et al., 2009). Consistent with previous studies, IL-6 diminished the ability of TGF-β to induce Smad3 phosphorylation in CD4+ T cells (Figure S6E). Our data therefore suggest that in the presence of CD103+ DCs that make both TGF-β and RA, IL-6 is required to overcome their effects to allow Th17 cell priming in the gut. In the absence of CD103+ DCs, as is the case in the spleen, Th17 cell priming is independent of IL-6.
Discussion
This study reveals several insights into how different priming microenvironments determine the cytokine requirements for Th17 cell lineage differentiation. Our previous work has shown that Myd88−/− mice fail to mount Th1 cell responses when LPS is used as an adjuvant (Pasare and Medzhitov, 2004). However CD25+ Treg cell depletion restores Th1 cell priming in Myd88−/− mice (Pasare and Medzhitov, 2004). Since several studies show reciprocal regulation of Treg and Th17 cell lineages (Bettelli et al., 2006; Korn et al., 2007), we were interested in exploring whether depletion of Treg cells restores or enhances Th17 cell priming in Myd88−/− mice. Our investigations of T cell differentiation in Myd88−/− mice revealed normal Th1 cell priming, but defective Th17 cell commitment in the absence of Treg cells. This is an important result since it suggests that, in the absence of MyD88 signaling, the lack of Treg cells is permissive for Th1 cell priming but not Th17 cell priming. Our results from transgenic mice that express MyD88 only in DCs and macrophages, but not in T cells, demonstrate that MyD88 in T cells is absolutely required for Th17 cell commitment. Requirement for MyD88 signaling in CD4+ T cells is explained by the critical role for IL-1 in priming of Th17 lineage cells. Several studies have demonstrated that IL-6 is a master inducer of Th17 cell differentiation (Acosta-Rodriguez et al., 2007; Bettelli et al., 2006; Ivanov et al., 2006; Veldhoen et al., 2006; Zhou et al., 2007). It has also been argued that IL-6 regulates the induction of Th17 lineage cells by enhancing the expression of IL-1R on CD4+ T cells (Chung et al., 2009). Since we observed normal Th17 cell priming in vitro while using Il6−/− DCs for priming of naïve T cells, we decided to further investigate the exact roles of IL-1 and IL-6 in Th17 cell lineage commitment in vivo. As mice age, they develop a substantial proportion of CD44hi CD62Llo cells in the spleen, representing antigen experienced T cells. As revealed in our results, CD44hiCD62Llo CD4+ T cells from WT, Myd88−/−, and Il6−/− mice secreted IFN-γ. However, CD44hiCD62Llo CD4+ T cells from Myd88−/− and Il1r1−/− mice failed to secrete appreciable quantities of IL-17. Surprisingly, CD44hiCD62Llo cells from Il6−/− mice made similar quantities of IL-17 as WT T cells. WT and Il6−/− mice also had similar proportions of IL-17 secreting cells, as measured by intracellular staining. More importantly, the memory cell pool from Il6−/− mice expressed RORγt. These results suggest induction of the transcription factor RORγt, and subsequent Th17 cell lineage commitment, is not dependent on IL-6 in CD4+ T cells of the spleen.
Several reports suggest that IL-1 and IL-6, along with TGF-β, play a major role in the induction of Th17 cells. It has also been shown previously that LPLs from Il6−/− mice make reduced IL-17 as assayed by intracellular staining as well as quantitative polymerase chain reaction (PCR) (Ivanov et al., 2006). In agreement with this, we found that CD4+ T cells from the LP of Il6−/− mice did not make detectable IL-17. We also found that CD4+ T cells from the LP of Myd88−/− and Il1r1−/− mice secreted substantially lower or non-detectable quantities of IL-17 when compared to WT mice. These results along with our infection experiments argue that both IL-1 and IL-6 are required for generation of Th17 cells in the LP of the gut while IL-1 is critical for Th17 cell lineage development in the spleen. It is also remarkable that when naïve T cells are transferred into Rag1−/− mice, cells that are defective for gp130 signaling secrete IL-17 when isolated from the spleen and liver, but fail to do so in the lungs and the LP of the intestines. This unique differentiation pattern suggests that sterile and commensal bacteria-rich microenvironments impose different rules for Th17 cell lineage commitment.
It is also important to note here that skin draining lymph nodes have Th17 lineage cells, but subcutaneous immunization fails to induce Th17 cell priming in Il6−/− mice. The presence of Th17 lineage cells in the lymph nodes can be explained by the possibility that following splenic priming, Th17 cells migrate from the spleen to these lymph nodes. However upon subcutaneous immunization, IL-6 was required for local priming of Th17 lineage cells in the lymph nodes. It has been recently proposed that IL-6 induces Th17 cell lineage commitment through upregulation of IL-1R. Since Il6−/− mice have defective Th17 cell lineage priming only in the lamina propria and skin draining lymph nodes, IL-6 could be controlling Th17 cell lineage commitment through regulation of the IL-1R only in the LP of the gut and skin draining lymph nodes but not in the spleen.
There are well known differences in the populations of the DCs residing in the gut and peripheral secondary lymphoid organs (Coombes and Powrie, 2008); however our results provide further insights into the priming abilities of LP DCs. While splenic DCs induce normal Th17 cell priming, LP DCs fail to do so in the absence of IL-6. CD103+ DCs are of interest because of some unique abilities that have been ascribed to them. CD103+ DCs in the intestine have been shown to imprint T cells with gut-homing molecules such as the chemokine receptor CCR9 (Annacker et al., 2005; Jaensson et al., 2008). They are also responsible for inducing regulatory T cells in the intestine through the production of TGF-β and retinoic acid (Coombes et al., 2007), thereby contributing to the control of colitis (Varol et al., 2009). Our findings have ascribed an additional function to CD103+ DCs, of regulating the requirement for Th17 cell priming in the gut and the skin draining lymph nodes. We have demonstrated that CD103− cells are responsible for IL-6 independent priming of Th17 lineage cells. This conclusion is strongly supported by the fact that splenic DCs are all CD103− and also by the fact that CD103− DC populations from the lamina propria could induce Th17 cell priming in the absence of IL-6. There was normal presence of Th17 lineage cells in the lymph nodes of Il6−/− mice and that lymph node resident DCs could prime Th17 lineage cells in vitro, however subcutaneous challenge with TLR ligands led to accumulation of CD103+ DCs in the draining lymph nodes and these DCs seemed to determine the requirement of IL-6 for Th17 cell priming. Interestingly, CD103+ DCs did not seem to play a role in regulating Th1 cell priming. CD103+ DCs are known to induce generation of Foxp3+ iTreg cells (Coombes et al., 2007) and our study supports the notion that these DCs impose a requirement of IL-6 for Th17 cell priming by CD103− DCs. Our data also demonstrate that TGF-β is absolutely essential for driving Th17 cell differentiation by DCs. However a combination of TGF-β and RA, which provides a more physiological microenvironment since they are both made by CD103+ DCs, had an inhibitory effect on Th17 cell priming in the absence of IL-6, both by splenic and LP DCs. These data are consistent with earlier studies showing that RA enhances phosphorylation of Smad3 in response to TGF-β treatment (Nolting et al., 2009) and that activation of STAT3 by IL-6 leads to desensitization of TGF-β signaling (Jenkins et al., 2005). Our data therefore suggest that when CD103+ DCs are part of the DC population involved in T cell priming, TGF-β and RA made by these DCs have a dominant role in determining the outcome of T cell differentiation. Consequently, in the absence of IL-6, CD103+ DCs play a dominant role leading to generation of Foxp3+ iTreg cells. An earlier study demonstrated that in mice with DCs incapable of making active TGF-β, the proportion of Foxp3 positive CD4+ T cells is reduced in the LP, but not in the spleen (Travis et al., 2007). Conversely, our results demonstrate that the absence of IL-6 enhanced the proportion of Foxp3 positive CD4+ T cells only in the LP but not in the spleen.
In the systemic immune system, which is generally sterile, IL-1 mediated signaling seems to be sufficient to support Th17 cell lineage differentiation. In contrast, the mucosal and cutaneous surfaces are constantly exposed to commensal microorganisms and appear to have stringent requirements to induce Th17 cell priming. Since Th17 cells are pathogenic and can cause autoimmunity, it is particularly important for the mucosal and cutaneous immune systems to maintain a balance between immunity and tolerance. As IL-6 is highly pro-inflammatory, the mucosal and cutaneous systems may interpret the presence of IL-6 as a critical signal implicating pathogen invasion, and differentiate active infection from steady-state sampling of the commensal microflora and self antigens from the tissues. IL-6 seems to act as an additional checkpoint for inducing Th17 cells in the gut as well as in the skin draining lymph nodes, guiding the lineage choice between inhibitory iTreg cells and inflammatory Th17 cells. It has been reported that CD103+ DCs change their suppressive behavior in colitic mice (Laffont et al., 2010) and it is possible that there could be transient Th17 cell priming in the guts of lL6 deficient mice when CD103 DCs are functionally shut down and it would be worthwhile to explore the role of IL-6 in survival and maintenance of Th17 cells generated under such conditions.
In summary, our study provides important insights into how priming microenvironments guide the cytokine requirements for the development of Th17 lineage cells. In commensal-bearing sites such as the intestine and the skin, IL-1 is critical, but IL-6 acts as a second checkpoint for Th17 cell lineage commitment. More importantly our study discovers that DC populations in the spleen, lymph nodes and LP of the intestines are responsible for determining the cytokine requirements for Th17 cell priming in respective tissues. These findings could have important implications for designing therapies for systemic and mucosal autoimmunity, as well as for choosing routes of vaccination for inducing protective Th17 cell responses.
Experimental Procedures
Mice
Myd88−/−Trif−/−, Tlr2−/−Tlr4−/−, Myd88−/−, Ccd11c-Myd88 Tg, Cd2DNIl6st Tg, Il6−/−, Il1r1−/− and Rag1−/− mice were bred and maintained at the animal facility of UT Southwestern Medical Center. Control C57BL/6 mice were obtained from UT Southwestern mouse breeding core facility. All mouse experiments were done as per protocols approved by Institutional Animal Care and Use Committee (IACUC) at UT Southwestern Medical Center.
Generation of Cd2-DNIl6Tg mice
A dominant negative Il6st described previously (Kumanogoh et al., 1997) was cloned into VA-CD2 plasmid, linearized, and injected into C57BL/6 blastocysts by UT Southwestern Transgenic and Knockout Core Facility.
T cell purification
Spleens were harvested from 8 to 12 week or 16 to 20 week old mice. CD4+ T cells were purified from the spleens by negative selection as previously described (Pasare and Medzhitov, 2004). CD4+CD62LhiCD44lo and CD4+CD62LloCD44hi T cells, were purified by using biotin anti-CD62L followed by anti-biotin microbeads and sorted using the AutoMACS sorter (Miltenyi Biotec, Auburn, CA).
Isolation of lamina propria lymphocytes
Lamina propria lymphocytes (LPL) were isolated as described previously (Ivanov et al., 2008). CD4+ T cells were enriched by negative selection and purity of CD4+ T cells was confirmed by staining with anti-CD4.
Staining and flow cytometry
Cells were stained with relevant antibodies for 30 minutes on ice and were washed. For intracellular staining of cytokines, cells were stimulated with 50ng/ml phorbol myristate acetate (PMA) and 1µM ionomycin in the presence of 1µg/ml brefeldin A for 5 hours, followed by surface staining, fixed with 4% paraformaldehyde, permeabilized with 0.3% saponin, and stained for intracellular cytokines. For transcription factor staining, the Foxp3 Staining Buffer Set from Biolegend was used for fixation and permeabilization of freshly isolated cells. The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, inc).
Quantitative reverse transcribed polymerase chain reaction (RT-PCR)
Freshly sorted cells were lysed immediately in TRIzol Reagent (Invitrogen) and RNA was extracted following manufacturer’s instructions. cDNA was synthesized using M-MLV Reverse Transcriptase (Invitrogen). Tbx21 and Rorc (specific for the RORγt isoform) transcripts were measured with DyNAmo SYBR Green qPCR Kit (Finnzymes). Primer sequences are listed in supplemental information.
Ex vivo T cell stimulation
Tissue culture plates were coated with 0.5µg/ml of anti-CD3 and 0.5µg/ml of anti-CD28 at 37°C for 2 to 3 hours and washed 3 times before use. Sorted CD4+ T cells were plated at 5×105 cells/well in 48 well plates and LP CD4+ T cells were plated at ~3×104 cells/well. Cells were stimulated for 48 hours and culture supernatants were assayed for cytokine production.
Cell transfer
5×107 mononuclear cells isolated from the spleen and lymph nodes from young naïve donors (6 weeks of age) were transferred into Rag1−/− recipients through the tail vein. Differentiation of the donor cells in multiple organs was analyzed on day 7 post-transfer.
Infections
Mice were infected with 1–2×109 colony forming units (CFU) Citrobacter rodentium (strain ICC168) suspended in 100µl PBS by oral gavage, or 1000 CFU of Salmonella typhimurium (strain SL1344) suspended in 500µl PBS by intraperitoneal injection.
Dendritic cell preparation
Mononuclear cells from the spleen, lymph nodes (inguinal, popliteal, brachial, and axillary), or the LP were labeled using anti-CD11c and MHC-II antibody and CD11c+MHC-II+ cells were sorted on MoFlo cell sorter.
In vitro T cell priming
Purified naïve CD62LhiCD44lo CD4+ T cells (3×105/well) were cultured with splenic DCs (6×104/well) and anti-CD3 (10ng/ml) in the presence or absence of TLR ligands. Alternatively, purified naïve CD62LhiCD44lo CD4+ T cells (1.5×105/well) were cultured with enriched LP DCs (1.5×105/well) or sorted LP DCs (3×104/well) and anti-CD3 (10ng/ml) in the presence or absence of TLR ligands. Supernatants were analyzed for cytokines after 5 days of culture.
Supplementary Material
Acknowledgements
We thank Drs. J. Forman, R. Medzhitov, S. Rath, A. Unni, and C. Wulfing for helpful comments on the manuscript. This work was supported by National Institutes of Health grant AI082265 to C.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing Thelper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Marukawa S, Kumanogoh T, Hirota H, Yoshida K, Lee IS, Yasui T, Taga T, Kishimoto T. Impairment of antigen-specific antibody production in transgenic mice expressing a dominant-negative form of gp130. Proc Natl Acad Sci U S A. 1997;94:2478–2482. doi: 10.1073/pnas.94.6.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Williams A, Flavell RA, Eisenbarth SC. The role of NOD-like Receptors in shaping adaptive immunity. Curr Opin Immunol. 2010;22:34–40. doi: 10.1016/j.coi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008a;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008b;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.