Abstract
The global sequence diversity of HIV-1 presents a daunting challenge for vaccine development. We investigated whether a heterologous insert prime-boost regimen could expand global coverage by selectively boosting cellular immune responses to conserved epitopes. Rhesus monkeys were primed and boosted with recombinant adenovirus vectors expressing homologous or heterologous HIV-1 Gag sequences that were optimized to focus responses on highly conserved epitopes. We observed comparable responses directed to specific regions of the Gag protein in all experimental groups without evidence of improved coverage or expanded breadth in the heterologous insert group. These data suggest that antigen-independent factors contribute to the immunodominance patterns of vaccine-elicited cellular immune responses.
1. Introduction
HIV-1 infects 40 million individuals worldwide, with 2 million new infections reported annually. Therefore, a prophylactic vaccine is urgently needed. HIV-1 vaccine strategies must contend with the enormous global sequence diversity of HIV-1. Both population level and experimental data suggest that cellular immune responses play a critical role in containing viral replication and determining the rate of HIV-1 disease progression [1–7]. However, HIV-1 vaccine candidates capable of eliciting broadly reactive cellular immunity remain a challenge. Multiple cellular and immunologic constraints may shape vaccine-elicited immunodominance hierarchies and limit the breadth and global epitope coverage of vaccine-elicited cellular immunity. These include restrictions on proteolytic processing, transport and MHC binding of antigen-derived peptides, and constraints on T-cell receptor-MHC interactions that shape thymic selection and antigen-specific T-cell responses [8–10]. To date, HIV-1 vaccine candidates have elicited primarily narrowly focused T-cell responses in clinical trials [11, 12]. However, recent nonhuman primate studies have suggested that both vector and antigen selection may impact the breadth of vaccine-elicited cellular immunity [13–15]. In this study, we assessed a novel “heterologous insert” prime-boost regimen that utilizes different antigen sequences in the prime and boost immunizations as a strategy to focus cellular immune responses on conserved regions in nonhuman primates.
2. Materials and methods
2.1 Animals, vectors and immunizations
Adult Indian-origin rhesus monkeys (n=18) were housed in the biosafety level 3 containment facility at BIOQUAL, Inc., and all studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC). Animals were vaccinated by the intramuscular route with 1011 viral particles of replication-incompetent, E1-/E3-deleted vectors in the quadriceps muscles. rAd26 and rAd35 vectors expressing GagConM and GagCZA were constructed using an E1/E3-deleted adenovirus cosmid and adapter plasmid system as described previously [16, 17]. The HIV-1 antigens were expressed in the adenovirus E1 region under control of a human cytomegalovirus promoter.
2.2 ELISPOT assays and epitope mapping
Comprehensive T lymphocyte epitope mapping was performed utilizing Gag PTE peptides (NIH AIDS Research and Reference Reagent Program). IFN-γ ELISPOT assays were conducted at week 4 following both the prime and the boost immunization initially with complete peptide pools as well as with subpools containing 10 PTE peptides that were pooled sequentially by peptide number. All peptide subpools with positive responses were then deconvoluted, and epitopes were confirmed with individual 15 amino acid PTE peptides. Cell-depleted IFN-γ ELISPOT assays were performed to determine if reactive peptides represented CD8+ or CD4+ T lymphocyte epitopes. Cell depletions were performed by negative bead selection and were >98% efficient.
Results
2.3 Heterologous insert sequence selection
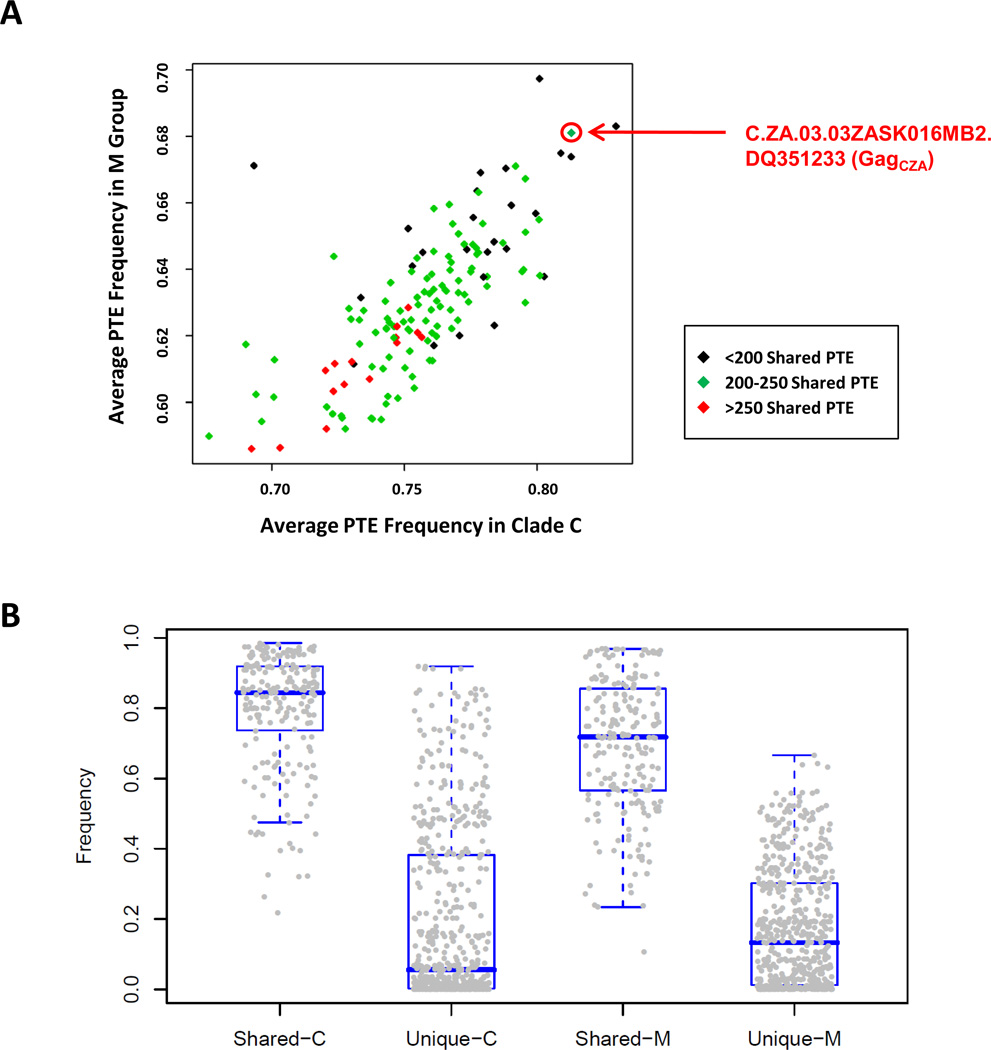
We employed the Los Alamos National Laboratory HIV-1 sequence database to select complementary HIV-1 Gag sequences that shared multiple highly conserved potential T-cell epitopes (PTEs) [18, 19] but were divergent at nonconserved sequences. Screening of clade C HIV-1 Gag sequences against the M consensus HIV-1 Gag sequence (GagConM) identified primary isolates that shared multiple high frequency PTEs (Fig. 1A). To optimize the sensitivity for detecting selective boosting of conserved epitopes, we selected the primary clade C isolate C.ZA.03.03ZASK016MB2.DQ351233 (GagCZA) that shared 247 high frequency PTEs representing 51% of all possible PTEs with GagConM but was divergent at other low frequency PTEs (Fig. 1A). The mean circulating frequency of shared PTEs in M group and clade C, as represented in the Los Alamos National Laboratory sequence database, was 0.72 and 0.86, respectively, while the mean circulating frequency of divergent PTEs was 0.16 and 0.09 (Fig. 1B). We therefore selected the combination of GagConM and GagCZA as heterologous antigen inserts that theoretically could optimize responses to conserved PTEs while also expanding PTE breadth. We hypothesized that a heterologous insert prime boost regimen that utilized GagConM and GagCZA would selectively focus cellular immune responses on conserved PTEs, leading to an increase in the frequency of circulating strain recognition relative to homologous insert prime boost regimens that utilized GagConM or GagCZA alone.
Fig. 1.
Selection of HIV-1 Gag antigens for heterologous insert prime-boost immunization. (A) Individual clade C sequences were compared against the M consesnsus sequence to determine the circulating frequency of shared PTEs among clade C isolates or in the M group as a whole. Isolates with a high circulating frequency by both criteria were evaluated for the total number of shared PTEs with GagConM in order to maximize the sensitivity for detecting selective boosting of conserved epitopes. (B) The mean circulating frequency of PTEs shared between GagConM and GagCZA was compared with the mean circulating frequency of divergent PTE among clade C isolates or in the M group as a whole.
3.2 Comparative immunogenicity of heterologous and homologous insert prime boost regimens in Rhesus monkeys
We utilized replication-incompetent recombinant adenovirus (rAd) vectors expressing GagConM and GagCZA to assess the breadth of cellular immune responses elicited by homologous and heterologous insert prime-boost regimens. We generated rAd serotype 26 (rAd26) and rAd serotype 35 (rAd35) vectors expressing GagConM and GagCZA as previously described [16, 17]. Rhesus monkeys (n=6/group) were primed IM at week 0 with 1011 viral particles (vp) rAd35 vectors expressing either GagConM or GagCZA and boosted at week 12 with 1011 vp rAd26 vectors expressing either homologous or heterologous Gag antigens. The magnitude and epitope specificity of vaccine-elicited cellular immune responses were determined at week 4 following both the prime and the boost immunizations by IFN-γ ELISPOT assays as previously described [14]. Individual epitope responses were mapped using HIV-1 Gag PTE peptides, which consist of 325 15-mer peptides optimized for global PTE coverage [19]. Positive responses were confirmed using CD4- and CD8-depleted IFN-γ ELISPOT assays [14].
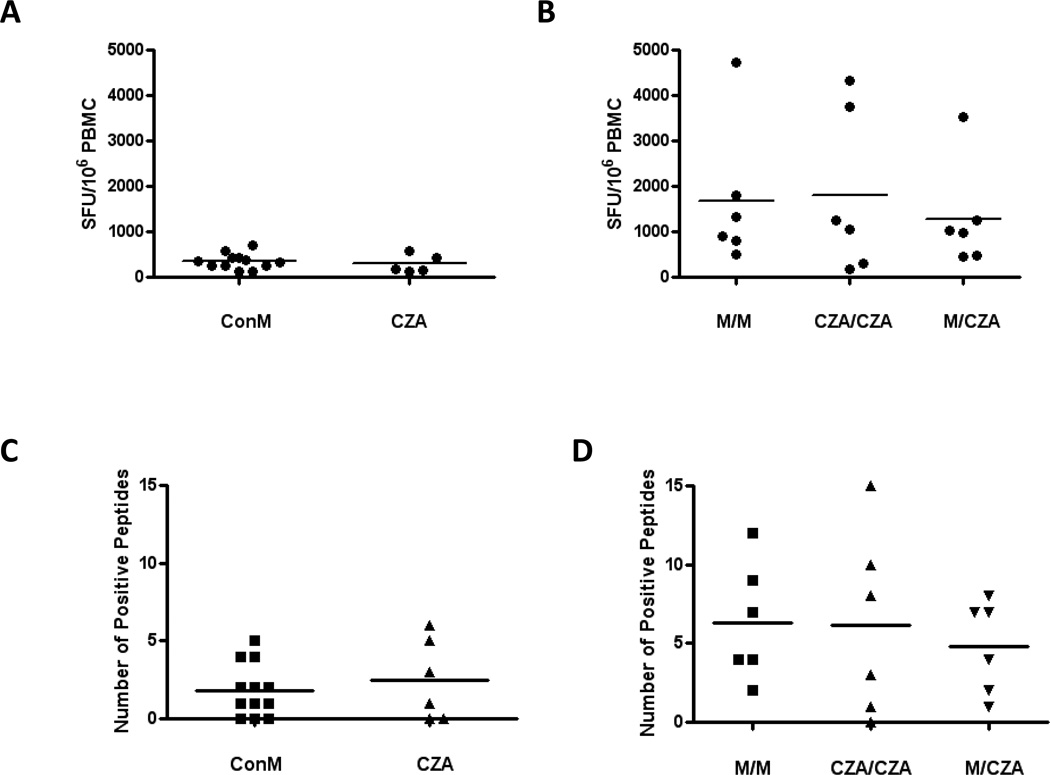
The overall magnitude of Gag-specific cellular immune responses was comparable at week 4 following priming with rAd35-GagConM and rAd35-GagCZA (Fig. 2A). The magnitude of Gag-specific cellular immune responses was also comparable following the boost immunization, with similar magnitude responses elicited by the homologous insert GagConM/GagConM and GagCZA/GagCZA regimens as well as the heterologous insert GagConM/GagCZA regimen (Fig. 2B). Additionally, the median number of individual epitope-specific responses proved comparable following both the prime (Fig. 2C) and the boost (Fig. 2D) immunizations in all groups. These data suggest that the heterologous insert prime-boost strategy did not detectably augment the magnitude or breadth of vaccine-elicited cellular immune responses as compared to traditional homologous insert prime-boost immunization strategies in this model. We cannot exclude, however, that minor differences could have been missed given the limited number of animals utilized in this study.
Fig. 2.
Magnitude and breadth of vaccine-elicited cellular immune responses following heterologous and homologous insert prime-boost immunization. The magnitude of vaccine-elicited cellular immune responses to HIV-1 Gag was determined by IFN-γ ELISPOT following the (A) prime and (B) boost immunizations. The breadth of vaccine-elicited cellular immune responses was determined by mapping all individual HIV-1 Gag PTE epitope responses following the (C) prime and (D) boost immunizations.
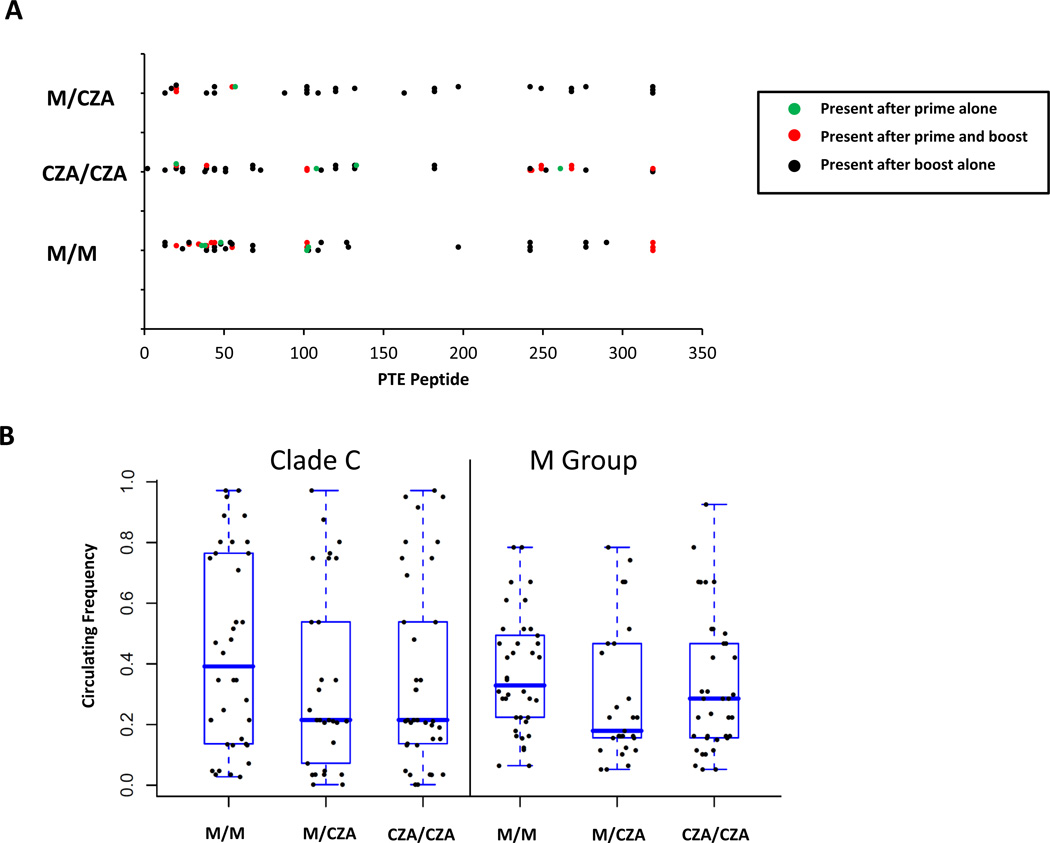
The epitope distribution of responses following the boost immunization was also comparable among all three experimental groups (Fig. 3A). Overall, 66% of epitopes detected following the prime immunization were also observed following the boost immunization, although additional epitope-specific responses were detected following the boost immunization (Fig. 3A). Overall, responses were not evenly distributed across all HIV-1 Gag PTE peptides but rather exhibited a stereotypical pattern of clustering that was independent of the specific Gag insert sequence utilized for the prime and boost immunizations (Fig. 3A). Moreover, the heterologous insert prime-boost regimen did not prove superior to the homologous insert prime-boost regimens for improving the global epitope coverage of vaccine-elicited cellular immune responses (Fig. 3B). The mean circulating frequency of M group epitopes elicited by the various vaccine regimens was 0.18 for the GagConM/GagCZA group, 0.31 for the GagConM/GagConM group and 0.25 for the GagCZA/GagCZA group (P = nonsignificant, 2-sided Mann-Whitney tests). These data demonstrate that the heterologous insert prime-boost strategy did not detectably alter the focus of vaccine-elicited cellular immune responses or improve immunologic coverage of HIV-1 global sequence diversity in this study.
Fig. 3.
Epitope distribution of vaccine-elicited cellular immune responses following heterologous and homologous insert prime-boost immunization. (A) Comparative distribution of vaccine-elicited, epitope-specific cellular immune responses following the prime and boost immunizations. PTE numbers on the X axis represent relative position within the HIV-1 Gag protein. (B) Mean circulating frequency of clade C and M group PTE-specific responses elicited by the homologous and heterologous insert prime-boost regimens.
3. Discussion
The protective efficacy of T-cell based HIV-1 vaccines may be critically dependent on the breadth of vaccine-elicited cellular immunity. Indeed, the breadth of vaccine-elicited cellular immune responses has been positively correlated with control of viral replication following SIV challenge of rhesus monkeys [13]. Previous studies have shown that both the choice of the vector and the choice of the insert may influence the breadth and global epitope coverage of vaccine-elicited cellular immune responses. For example, heterologous vector prime-boost regimens (i.e. rAd26/rAd5) have proven superior to homologous vector prime-boost regimens (i.e. rAd5/rAd5) for enhancing cellular immune breadth [13]. Moreover, a combination of mosaic antigens has been shown to augment the breadth of cellular immune responses as compared with individual consensus or natural sequences [14, 15]. In contrast, heterologous insert prime-boost immunization strategies have not previously been evaluated and represent a potential strategy to increase the breadth and global epitope coverage of vaccine-elicited cellular immunity. This is of particular interest, since heterologous inserts are currently being explored in phase 1 HIV-1 vaccine clinical trials.
We investigated whether different HIV-1 Gag sequences that shared highly conserved epitopes could improve the global epitope coverage of vaccine-elicited cellular immune responses when delivered as a heterologous insert prime-boost regimen. We observed that the heterologous insert regimen did not prove superior to the homologous insert regimens with respect to global epitope coverage or cellular immune breadth. Vaccine-elicited cellular immune responses in all groups were focused on stereotypical regions of HIV-1 Gag regardless of the insert used for the prime and boost immunization. These data indicate that this heterologous insert prime-boost regimen was unable to overcome immunodominance constraints, suggesting that antigen-independent constraints on antigen processing, presentation and T-cell development and/or vaccine-specific constraints established during the priming immunization may limit the utility of heterologous inserts to expand cellular immune breadth or coverage. It is certainly possible that heterologous insert prime-boost regimens may have more subtle effects on breadth, coverage, and qualitative features of the T-cell repertoire that we were not powered to detect in this study. Future studies should therefore be directed to address these questions in both nonhuman primates and humans.
Highlights.
-
-
A heterologous HIV-1 Gag prime-boost regimen was evaluated in rhesus monkeys
-
-
No improvement in cellular immune breadth or coverage was observed
-
-
Similar patterns of responses were induced by homologous and heterologous inserts
Acknowledgments
We thank S. Clark, Y. Sun, P. Abbink, D. Lynch, M. Collins, J. Yalley and M. Lewis for generous advice and assistance. This work was supported by an NIH CHAVI/HVTN Early Career Investigator Award (AI067854) (D.R.K.) as well as NIH grants AI078526 (D.H.B.), AI066924 (D.H.B.) and AI066305 (D.H.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283(5408):1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 2.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol. 2004;4(8):630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 3.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296(5572):1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 5.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415(6869):335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 7.Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3(2):212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 8.Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25(4):533–543. doi: 10.1016/j.immuni.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Venturi V, Price DA, Douek DC, Davenport MP. The molecular basis for public T-cell responses? Nat Rev Immunol. 2008;8(3):231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- 10.Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465(7296):350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F, McKenney DM, Malhotra U, Crimi C, Nolin J, Corey L, et al. Towards prediction of degenerate CTL epitope recognition. Hum Vaccin. 2008;4(2):115–121. doi: 10.4161/hv.4.2.5215. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2008;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, O'Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nature Med. 2010;16(3):319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santra S, Korber BT, Muldoon M, Barouch DH, Nabel GJ, Gao F, et al. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proc Natl Acad Sci USA. 2008;105(30):10489–10494. doi: 10.1073/pnas.0803352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Horton H, Gilbert PB, McElrath JM, Corey L, Self SG. HIV-1 CTL-based vaccine immunogen selection: antigen diversity and cellular response features. Curr HIV Res. 2007;5(1):97–107. doi: 10.2174/157016207779316260. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Malhotra U, Gilbert PB, Hawkins NR, Duerr AC, McElrath JM, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24(47–48):6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]