Abstract
The db/db mouse is one of the diabetes mellitus animal models and if the pathophysiological stages of diabetic changes in the mouse model could simulate the stages in human diabetes, the db/db mouse could be used to better evaluate drug candidates. Blood insulin, HbA1c levels and morphological features of pancreatic islets in db/db mice were evaluated to determine the pathophysiological stage. At 6 weeks of age, db/db mice showed the highest level of plasma insulin and lowest level of HbA1c, and histopathological examination revealed enlarged islets with a circular shape and hypertrophic islet cells. By 9 and 12 weeks of age, the plasma insulin levels had decreased to mid levels and HbA1c had increased to mid to high levels; histopathological examination at this time revealed two types of islets coexisting, enlarged circular islets and small irregular-shaped islets. By 15 and 22 weeks of age, plasma insulin had decreased further to low levels and HbA1c was at its highest level; the histopathological examination at this time revealed an increase in irregular-shaped and small islets. Based on blood insulin levels, HbA1c levels and histopathology findings in the db/db mice in this study, the clinical staging of diabetic changes were recognized. The pathophysiological stages of diabetes mellitus in this animal model were similar to the stages in humans.
Keywords: db/db mouse, pancreas, histopathology, diabetes mellitus, insulin, HbA1c
Introduction
Diabetes mellitus is clinically classified into three stages based on the degree of insulin dependence. The stages are non-insulin requiring (NIR), insulin requiring for control of blood glucose (IRC) and insulin requiring for survival (IRS)1–4. A patients’ pharmacologic treatment is selected based on these stages because combinations of different oral agents may be useful for controlling hyperglycemia before insulin therapy becomes necessary3,5. At the NIR stage, adequate glycemic control can be achieved through weight reduction, exercise and/or oral glucose-lowering agents, and so individuals at this stage do not require insulin. Individuals at the IRC stage have some residual insulin secretion, but require exogenous insulin for adequate glycemic control, and can also survive without taking insulin. Individuals at the IRS stage with extensive β-cell destruction and therefore no residual insulin secretion require insulin for survival.
The C57BL/KsL db/db mouse (db/db mouse) is a diabetes mellitus animal model that is a spontaneous mutant strain of the C57BL/KsJ db/db mouse resulting from a point mutation of the downstream intron of the leptin receptor gene rendering it unresponsive to leptin6–9. Leptin is a peptide hormone secreted by adipocytes and is involved in eating behavior and energy homeostasis. So, this animal models expresses unrepressed eating behavior, becomes obese and develops severe insulin resistance associated with hyperinsulinemia and hypertriglyceridemia, followed by hyperglycemia peaking at 3–4 months of age10. Pancreatic islet β-cell mass is reduced as disease progresses, resulting in severe insufficiency of insulin secretion11–14. It has also been well shown by immunohistochemistry that a decrease in insulin levels of islets of db/db mice occurs at 18 weeks of age, without referreing to the blood insulin levels, which is one of the most important biomarkers6. In spite of the extensive use of the db/db mouse in this field, there are no reports on the three clinical stages in the db/db mouse. However, if pathophysiological staging were possible in the db/db mouse, drug candidates for diabetes mellitus could be better evaluated in preclinical studies to selectively target a specific pathophysiological stage.
In this study, time course blood insulin and glycosylated hemoglobin (HbA1c) levels, the clinical parameters for evaluation of the pathophysiological stages of diabetes mellitus in humans3,15, and morphological features of pancreatic islets in db/db mice were examined in order to determine the pathophysiological stage of the disease in the diabetic mouse model. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) recommend monitoring glycemic control using HbA1c as the parameter15–18. The major advantage of measuring HbA1c is that a specimen can be collected without regard to when the patient last ate19.
Materials and Methods
Animals
Twenty five male db/db mice were purchased from Charles River Laboratories (Japan) and subjected to experimentation at 5 weeks of age. The animals were housed in cages in an animal room maintained at a temperature of 23 ± 2°C and a humidity of 55 ± 10%, with 14 to 16 air changes per hour and a 14-hour light and 10-hour dark cycle. The animals were given pelleted chow (CE-2; Clea Japan, Inc., Tokyo, Japan) and tap water ad libitum. All animal procedures were conducted in accordance with Chugai Pharmaceutical’s Guide for the Care and Use of Laboratory Animals, and all experimental protocols were approved by the Institutional Animal Care and Use Committee.
Experimental design
The db/db mice were divided into 5 groups (n=5 per group), and the animals of each group were sacrificed by exsanguination under ether anesthesia at the age of 6, 9, 12, 15 or 22 weeks after their body weights were measured and blood samples were collected. Blood samples were obtained from the caudal vena cava for measurement of plasma insulin and HbA1c.
Plasma insulin levels were measured using ELISA (Institute of Biological Science, Inc., Yokohama, Japan), and the percentage of HbA1c was measured using an auto analyzer (Type 7170, Hitachi High-Technologies Corporation, Tokyo, Japan). The pancreas was removed from all necropsied animals, fixed in 20% neutral buffer formalin solution, embedded in paraffin wholly, sectioned longitudinally and stained with hematoxylin and eosin. Histopathological evaluation of pancreatic islets was performed under light microscopy.
Results
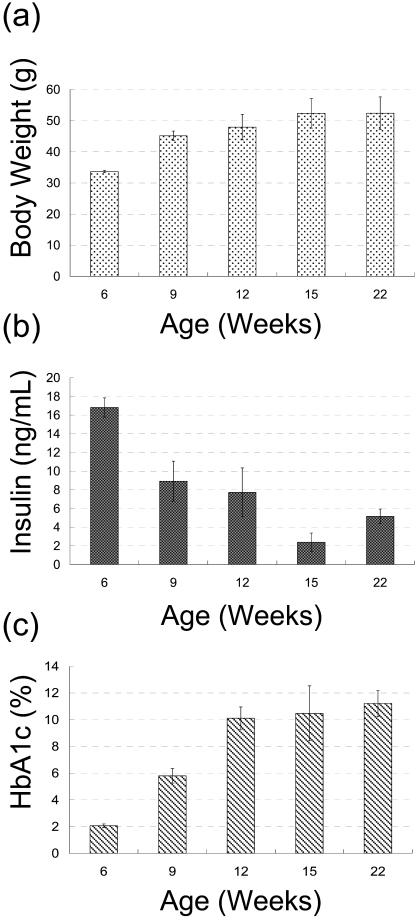
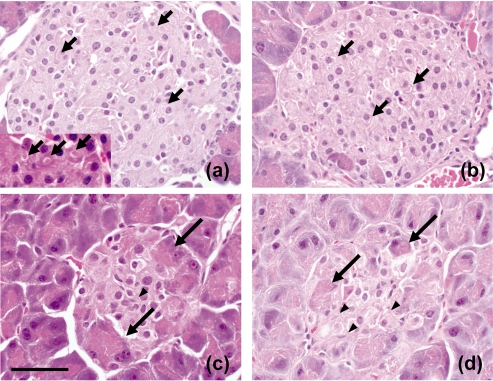
At 6 weeks of age, the mean body weight was 33.67 ± 0.39 g, the level of the plasma insulin was at its highest (16.82 ± 1.03 ng/mL) and the level of HbA1c was at its lowest (2.06 ± 0.14%) during the observation period (Fig. 1). Histopathological examination revealed enlarged, circular-shaped islets, which were consistent with hypertrophic islet cells having abundant cytoplasm and a large vacuole with a curved lucent region considered to be the Golgi apparatus20 (Fig. 2a).
Fig. 1.
Time course of changes of body weight (a), plasma insulin level (b) and HbA1c level (c) in the db/db mice. Each value represents the mean ± standard deviation.
Fig. 2.
The morphological features of pancreatic islets in db/db mice. Enlarged, circular islet at 6 weeks of age and islet cells with vacuoles (insert, × 600) (a), enlarged islet at 12 weeks of age (b), small irregular-shaped islet at 12 weeks of age (c) and a smaller and irregular-shaped islet at 22 weeks of age (d). Short arrows: large vacuoles considered to be Golgi apparatus. Long arrows: acinar cells, Arrowheads: spindle cells. Magnification: × 400. HE stain. Bar: 50 μm.
At 9 and 12 weeks of age, the mean body weights were 45.13 g ± 1.50 and 47.93 g ± 4.14, respectively. The level of plasma insulin had decreased to mid levels (8.92 ± 2.14 ng/ mL and 7.73 ± 2.61 ng/mL, respectively), and the level of HbA1c had increased to middle to high mean levels (5.79 ± 0.54% and 10.11 ± 0.84%; Fig. 1). In the histopathological examination, two types of islets were coexisting: enlarged islets similar to those observed in the 6-week-old animals (Fig. 2b) and small, irregular-shaped islets consisting of atrophic islet cells with acinar cells and spindle cells thought to be myofibroblasts21 (Fig. 2c).
At 15 and 22 weeks of age, the mean body weights were 52.35 ± 4.75 g and 52.37 ± 5.24 g, respectively, the level of plasma insulin had decreased further to low mean levels (2.38 ± 0.99 ng/mL and 5.15 ± 0.78 ng/mL, respectively) and the level of HbA1c had increased to high mean levels (10.47 ± 2.06% and 11.21 ± 0.97%; Fig. 1). Histopathological examination revealed that the irregular-shaped islets had decreased further in size. Islet cells were even more atrophic than in the 12-week-old animals, and the presence of acinar cells and spindle cells was obvious (Fig. 2d).
Discussion
Staging of diabetes mellitus is clinically based on insulin dependence, namely non-insulin requiring (NIR), insulin requiring for control (IRC) of blood glucose and insulin requiring for survival (IRS)1–4. The purpose of this study was to determine the pathophysiological stage of diabetes mellitus in db/db mouse at various ages and discuss the relevance of the stages in the animal model to those in humans.
In patients at the NIR stage of diabetes mellitus, adequate glycemic control can be achieved with weight reduction, exercise and/or oral glucose-lowering agents. The patients in this stage have insulin secretion ability, and the glucose level is occasionally high but controllable. In this study, the 6-week-old db/db mice can be considered equivalent to being in the NIR stage of human diabetes mellitus patients according to the following results: the animals showed obesity, unlike the nonobese heterozygote (db/+) mouse6, hypertrophic islet cells were observed in the histopathological examination and the findings well reflected blood parameters. These findings are considered to be an insulin secretion reaction of islet cells caused by hyperphagia, a characteristic of the db/db mouse6–14. Based on the blood parameters of the animals at this stage, sufficient insulin secreting ability (the highest insulin level) was preserved to counteract high blood glucose levels.
At the IRC stage in human diabetes mellitus, patients have some residual insulin secretion but require exogenous insulin for adequate glycemic control. The findings in the db/db models at 9 and 12 weeks of age were equivalent to the IRC stage. The animals showed mid-level residual insulin secretion and mildly increased blood glucose level (mid levels of HbA1c). The histopathological findings showed some irregular-shaped islets, which correlates to the existence of acinar cells and spindle cells. It is known that the fibrotic distraction of islets is commonly observed in humans and several animal models of type 2 diabetes21.
At 15 and 22 weeks, insulin secretion in the mice was very low (low levels of insulin), because islets were destructed (irregular-shaped and small), and the blood glucose levels had increased (high levels of HbA1c). In addition, the decrease of insulin in the islets of db/db mice at 18 weeks of age was shown well in a previous report6, and the morphology of islet is similar to that in this study. Our results indicate that the sustained secretion of insulin induced β-cell destruction and that the ability to secrete insulin had almost disappeared. We consider these findings to be equivalent to the IRS stage in humans21.
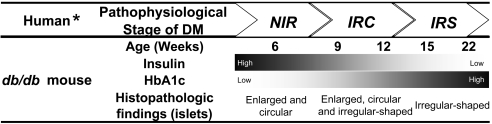
In the db/db mouse used in this study, three distinctive pathophysiological stages of diabetic change were identified as shown in Fig. 3. The results elucidate a time course relationship between the blood parameters and the morphology of pancreatic islets in the db/db mouse, where pathophysiological stages based on plasma insulin levels, HbA1c levels and histopathological findings are clearly distinguishable and characteristically reflect the stages of human diabetes mellitus. To our knowledge, this is the first report of a db/db mouse exhibiting pathophysiological stages similar to those in human diabetes mellitus. In the evaluation of new drug candidates for diabetes mellitus, indicating the time point in the life cycle of the disease would be very important, as the pharmacologic effects of a drug must be selected to match patient status3,5. In addition, there is evidence that environmental factors contribute to the development of obesity and type 2 diabetes22. Being able to understand the pathophysiological stage of an animal model based on blood glucose and insulin would be beneficial.
Fig. 3.
Pathophysiological stages of DM in humans and as represented in the db/db mouse. DM: Diabetes Mellitus. *The human column is quoted from reference 1–4.
Acknowledgments
We thank Ms. Yoshimi Otani at Chugai Research Institute for Medical Science, Inc. for her skillful technical assistance.
References
- 1.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 26(Suppl 1): S5–S20 2003 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 27(Suppl 1): S5–S10 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T. Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Research and Clinical Practice. 55: 65–85 2002 [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med. 15: 539–553 1998 [DOI] [PubMed] [Google Scholar]
- 5.Florence JA, Yeager BF. Treatment of type 2 diabetes mellitus. Am Fam Physician. 59: 2835–2844, 2849–2850 1999 [PubMed] [Google Scholar]
- 6.Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab. 288: 510–518 2005 [DOI] [PubMed] [Google Scholar]
- 7.Coleman DL. Obese and diabetes: two mutant genes causing diabetesobesity syndromes in mice. Diabetologia. 14: 141–148 1978 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 49: 22–31 2000 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 84: 491–495 1996 [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 125: 451–472 2007 [PubMed] [Google Scholar]
- 11.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 153: 1127–1128 1966 [DOI] [PubMed] [Google Scholar]
- 12.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 379: 632–635 1996 [DOI] [PubMed] [Google Scholar]
- 13.Orland MJ, Permutt MA. Quantitative analysis of pancreatic proinsulin mRNA in genetically diabetic (db/db) mice. Diabetes. 37: 341–347 1987 [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Wada M, Fukuda K, Fukami M, Yoshioka S, Yoshioka T, Horikoshi H. Characterization of CS–045, a new oral antidiabetic agent, II. Effects on glycemic control and pancreatic islet structure at a late stage of the diabetic syndrome in C57BL/KsJ-db/db mice. Metabolism. 40: 1213–1218 1991 [DOI] [PubMed] [Google Scholar]
- 15.Unger J. Current strategies for evaluating, monitoring, and treating type 2 diabetes mellitus. Am J Med. 121(6Suppl): S3–S8 2008 [DOI] [PubMed] [Google Scholar]
- 16.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 291: 335–342 2004 [DOI] [PubMed] [Google Scholar]
- 17.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 27: 17–20 2004 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care for patients with diabetes mellitus. Diabetes Care. 26(suppl 1): S33–S50 2003 [DOI] [PubMed] [Google Scholar]
- 19.Mayfield J. Diagnosis and classification of diabetes mellitus: new criteria. Am Fam Physician. 58: 1355–1362, 1369–1370 1998 [PubMed] [Google Scholar]
- 20.Ravazzola M, Perrelet A, Roth J, Orci L. Insulin immunoreactive sites demonstrated in the Golgi apparatus of pancreatic B cells. Proc Natl Acad Sci. 78: 5661–5664 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JW, Ko SH, Cho JH, Sun C, Hong OK, Lee SH, Kim JH, Lee KW, Kwon HS, Lee JM, Song KH, Son HY, Yoon KH. Loss of beta-cells with fibrotic islet destruction in type 2 diabetes mellitus. Front Biosci. 13: 6022–6033 2008 [DOI] [PubMed] [Google Scholar]
- 22.Nonogaki K, Nozue K, Oka Y. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology. 148: 4658–4666 2007 [DOI] [PubMed] [Google Scholar]