Abstract
Corin is a transmembrane serine protease identified in the heart, where it converts natriuretic peptides from inactive precursors to mature active forms. Studies in animal models and patients with hypertension and heart disease demonstrate that corin is critical in maintaining normal blood pressure and cardiac function. Like many proteolytic enzymes, corin expression and activity are regulated. Cell biology experiments indicate that transcriptional control, intracellular protein trafficking, cell surface targeting, zymogen activation and ectodomain shedding are important mechanisms in regulating corin expression and activity in the heart. More recently, soluble corin was detected in human blood and its levels were found to be reduced in patients with heart failure (HF). These findings indicate that corin deficiency may be involved in the pathogenesis of HF and suggest that soluble corin may be used as a biomarker for the disease. In this review, we describe the function and regulation of corin and discuss recent studies of soluble corin in human blood and its potential use as a biomarker for HF.
1. Introduction
Proteolytic cleavage mediated by serine proteases plays an important role in many biological processes, including food digestion, inflammatory response, wound healing, hormone processing, blood coagulation, and fibrinolysis [1]. In the heart, for example, serine proteases such as tissue kallikrein, chymase and urokinase are involved in processing of many bioactive molecules, including bradykinin, angiotension II, interleukin-1β, transforming growth factor-β, stem cell factor, and matrix metalloproteases [2]. These protease-mediated activities are critical in regulating blood pressure and cardiac function, and may contribute to pathological conditions such as hypertension, cardiac hypertrophy and heart failure (HF).
Most trypsin-like serine proteases are secreted proteins. More recently, a new class of type II transmembrane serine proteases (TTSPs) has been identified [3–5], which includes enteropeptidase [6–8], hepsin [9–11] and matriptases [12–15]. All these proteases consist of an N-terminal cytoplasmic tail, a single-span transmembrane domain and an extracellular region with a C-terminal trypsin-like protease domain. Corin is a TTSP identified in the heart [16–18]. In this review, we describe the biology of corin and discuss recent findings of soluble corin in human blood and its potential use as a biomarker for the diagnosis of HF.
2. Corin protein and domain structure
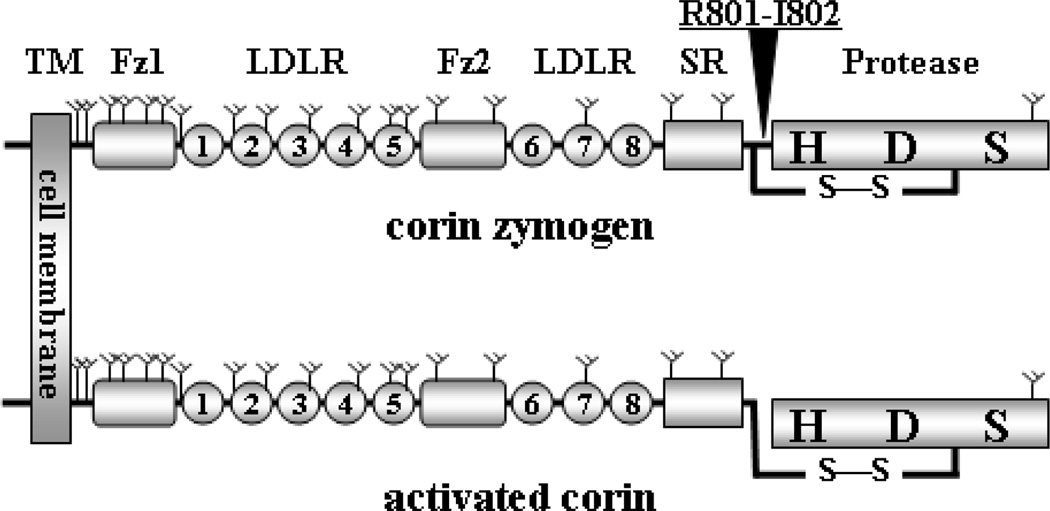
Human corin is a protein of 1042 amino acids [18]. It contains an N-terminal cytoplasmic tail of 45 amino acids followed by a single-span transmembrane domain of 21 amino acids. The rest molecule is extracellular and contains several types of domains, including two frizzled-like domains, eight LDL receptor (LDLR)-like repeats, one scavenger receptor-like domain, and a C-terminal trypsin-like protease domain (Fig. 1). These distinct domains serve for specific functions [19, 20]. The transmembrane domain anchors the protein on the cell surface, whereas the protease domain carries out the catalytic function. The other extracellular domains participate in interactions with corin substrates and possibly activator(s). Unlike many membrane receptors whose cytoplasmic tails transduce outside-in signals, the cytoplasmic tail of corin did not appear to have such a function. Instead, it has a role in intracellular trafficking and membrane targeting. A recent study identified a specific amino acid motif, DDNN, in human corin cytoplasmic tail that is important for cell surface expression [21].
Fig. 1.
Corin protein domain structure. The transmembrane domain (TM), frizzled-like domains (Fz), LDLR repeats, scavenger receptor-like domain (SR), and protease domain (Protease) with active site residues histidine (H), aspartate (D), and serine (S) are indicated. Y-shaped symbols indicate predicted N-glycosylation sites. An arrow head indicates the activation cleavage site between Arg801-Ile802. A disulfide bond (S-S) connects the protease domain and the rest of the molecule after corin zymogen (upper) is activated (lower).
Human corin contains 19 predicted N-linked glycosylation sites in its extracellular region [18] (Fig. 1). Most of these glycosylation sites are conserved among mammalian species [18, 22], indicating the importance of glycosylation in corin biosynthesis and/or function. Studies with tunicamycin-treated cells and glycosidase digestion have detected abundant N-glycans on human, rat and mouse corin, which are critical for corin cell membrane targeting and zymogen activation [23, 24]. To date, no O-linked glycans or sialic acids have been detected on corin [24].
Corin is made as a zymogen, which is activated by cleavage at a conserved site, Arg801-Ile802 (Fig. 1). The cleavage induces conformational changes in the protease domain, making it catalytically active [25]. Purified single-chain corin had no detectable enzymatic activity [20]. Substitution of Arg801 with Ala prevented corin activation, thereby abolishing its function [20, 26]. After the Arg801-Ile802 peptide bond is cleaved, the protease domain remains attached to the rest of molecule through a disulfide bond (Fig. 1). The disulfide bond can be broken by reducing agents such as β-mercaptoethanol and dithiothreitol. This method is used to distinguish corin zymogen from the activated form [20, 24]. Based on the Arg801-Ile802 activation sequence, corin activator is predicted to be a serine protease that favors basic residues. To date, however, the corin activator has not been defined.
3. Corin gene and expression
The human CORIN gene is located on the short arm of chromosome 4 at p12–13, a region adjacent to the centromere [18]. It has 22 exons and spans >200 kb [27]. The intron-exon junctions correspond to boundaries of corin protein domains. For example, frizzled-like domains are encoded by two exons each whereas each LDLR repeat is encoded by one exon [27]. Such a genomic structure supports that the CORIN gene arose from exon duplication and rearrangement during evolution.
The mouse corin gene is located on chromosome 5 with an overall structure similar to that of the human gene. One exception is that the first exon, which encodes the cytoplasmic tail, differs in both length and sequence between human and mouse, suggesting that alternatively spliced variants may exist [27]. Indeed, a recent study showed that both human and mouse corin genes have alternative first exons, encoding two versions of cytoplasmic tails with different membrane targeting efficiencies [21]. It remains to be determined if mechanisms exist to regulate the use of these alternative exons, which in turn regulate corin expression and activity on the cell surface.
Corin is highly expressed in the heart, primarily in cardiomyocytes [16, 18, 28, 29]. This tissue expression pattern is controlled by its promoter that contains conserved binding sites for TBX5, GATA, NKX2.5 and Krüppel-like transcription factors [27]. GATA-4 appears to be a major transcription factor for corin expression in the heart. Mutations at a major GATA-binding site and antibodies again GATA-4 markedly inhibited corin expression in cardiomyocytes [27]. A similar GATA-4-mediated mechanism also is involved in natriuretic peptide expression in the heart [30].
Corin mRNA expression has been detected in other tissues, including kidney [18, 29, 31], skin [32], bone [18], brain [33, 34] and pregnant uterus [18]. In general, expression levels in these tissues were lower than that in the heart. The significance of corin expression in these tissues is not well understood. For example, corin expression is detected in dopaminergic neurons but its function in the brain remains unknown [33, 34]. In mice, corin is expressed in the dermal papilla of the hair follicle [32, 35]. Mice lacking corin have a lighter coat color. It is unclear if the role of corin in regulating coat color provides an advantage for these animals in natural environments. In addition to its expression in normal tissues, corin mRNA is detected in cancer cells, including small cell lung cancer, osteosarcoma, endometrium carcinoma, and leiomyosarcoma [18, 36].
4. Corin in natriuretic peptide processing
Atrial and B-type or brain natriuretic peptides (ANP and BNP) are cardiac hormones that regulate body fluid balance and blood pressure [37, 38]. Upon binding to their receptor, these peptides stimulate intracellular cGMP production, thereby promoting natriuresis and diuresis in the kidney and muscle relaxation in the blood vessel. These peptide hormones are well conserved from primitive vertebrates to humans. In many migratory fish species such as salmon and eels, natriuretic peptides are critical for maintaining electrolyte homeostasis during their life cycles in fresh and salty water environments [39–41].
Like many peptide hormones, natriuretic peptides are made as inactive pro-forms that are converted to active forms by proteolysis. Corin has been identified as the physiological pro-ANP convertase (for reviews see refs. [42, 43]). When pro-ANP is secreted from cardiomyocytes, corin activates it on the cell surface. In mice, knockout the corin gene abolished ANP generation [44], indicating that no other enzymes act redundantly for this function in vivo. Apparently, the function of corin is not cell membrane-dependent. A soluble corin lacking the transmembrane domain cleaved pro-ANP as efficiently as the membrane-bound corin [20]. Similar findings of cell membrane-independence have been reported in other TTSPs, such as hepsin [45] and matriptase [15, 46]. These data suggest that the primary function of the transmembrane domain in TTSPs is to localize the enzymes at specific tissue sites but not to enhance their catalytic activities [3, 47].
In addition to pro-ANP processing, corin also cleaves pro-BNP [29, 48–50]. The reaction, however, is less sequence-specific and less efficient. To date, several other enzymes such as furin and dipeptidyl peptidase IV have been shown to process pro-BNP [48, 51–53]. Furin also cleaves pro-C-type natriuretic peptide (pro-CNP) but not pro-ANP [54]. Recent studies show that human pro-BNP contains abundant O-glycans that are terminally sialylated [55–59]. This posttranslational modification is unusual, because no N- or O-linked glycosylation was detected in human pro-ANP and pro-CNP [56]. The O-glycans were shown to increase pro-BNP stability [56]. In human pro-BNP produced from HEK293 cells, O-glycans near the processing site inhibited furin- and corin-mediated cleavage, indicating that glycosylation may regulate BNP production and activity [59–61]. Currently, pro-BNP and its derivatives are used as biomarkers for HF [62]. It will be important to determine if pro-BNP glycosylation is altered under pathological conditions.
5. Corin shedding from the cell membrane
Ectodomain shedding is an important mechanism in regulating the function of a variety of membrane proteins, including adhesion molecules, enzymes, cytokines, growth factors, and receptors [63, 64]. Many TTSPs have soluble forms. In fact, enteropeptidase was first found in the intestinal juice by Ivan Pavlov, who won a Nobel Prize in 1904 for his discovery of digestive enzymes [5, 47]. What Pavlov found was likely a soluble form of enteropeptidase, which was shown to be released into the small intestine lumen upon bile stimulation [65]. Ectodomain shedding is also a critical mechanism in controlling the activity of matriptases on the cell surface [15, 46, 66–68].
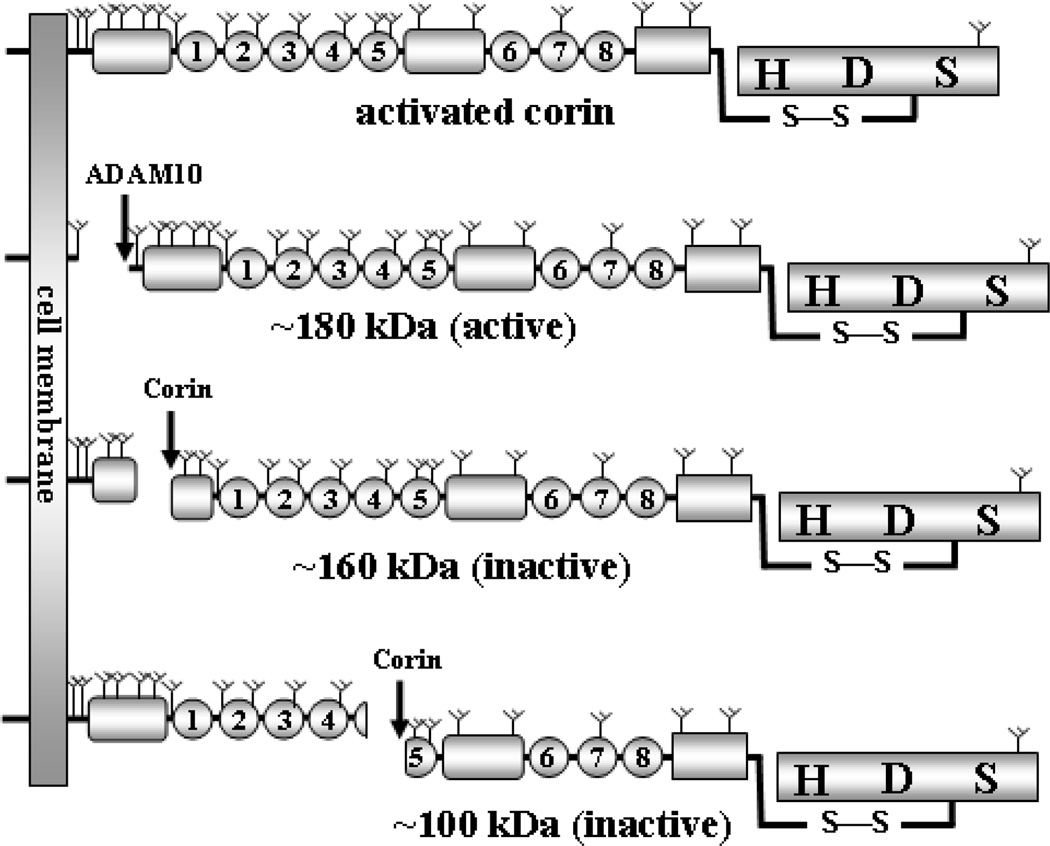
Several forms of soluble corin have been identified in cell culture. In the conditioned medium from transfected HEK293 cells, fragments of recombinant human corin were detected by immunoprecipitation and Western blotting [69]. Three major fragments were of ~180, ~160, and ~100 kDa, respectively. These fragments were generated from proteolytic cleavage but not from alternatively spliced mRNAs that lack the transmembrane domain coding sequence, because the production of these fragments was inhibited when the cells were incubated with protease inhibitors. Similar findings were confirmed in transfected HL-1 cardiomyocytes [69].
In experiments with protease inhibitors, small interfering RNA knockdown and site-directed mutagenesis, the metalloproteinase ADAM10 was found to be responsible for cleaving corin in its juxtamembrane region, producing the ~180-kDa fragment that corresponds to the near entire extracellular region [69] (Fig. 2). Corin also cleaved itself at Arg164 in frizzled 1 domain and Arg427 in LDLR 5 repeat, generating the ~160- and ~100-kDa fragments, respectively (Fig. 2). In functional studies, the ~180-kDa fragment, but not the ~160- and ~100-kDa fragments, was active in processing pro-ANP [69] (Fig. 2). The result was consistent with early structure-function studies, showing that frizzled 1 domain and LDLR repeats are required for corin to process pro-ANP [19].
Fig. 2.
Illustration of soluble corin fragments. Activated corin (top) on the cell surface is shed by ADAM10 to produce a near full-length extracellular fragment that is active in processing pro-ANP. Corin also cleaves itself in Fz1 domain and LDLR5 repeat, respectively, to produce two shorter but inactive fragments [69].
Physiologically, proteolytic enzymes are tightly regulated to avoid potential hazardous consequences. The ectodomain shedding and autocleavage of corin may represent an important mechanism to regulate its activity in the heart. It is likely that after corin is activated and cleaves natriuretic peptides, active corin molecules are removed to prevent excessive proteolytic activities on the surface of cardiomyocytes. This function appears to be carried out primarily by ADAM10 [69]. Corin inactivates the remaining molecules by autocleavage. This hypothesis was consistent with the finding that the majority of soluble fragments were from activated corin molecules [69]. Once corin fragments are detached from cardiomyocytes, these molecules may enter blood circulation if they are not degraded quickly in the tissue.
6. Detection of soluble corin in human blood
By ELISA-based assays, soluble corin has been detected in human blood [29, 70–73]. The levels in plasma and serum were similar [71, 73], indicating that soluble corin did not interact with activated platelets or clotting proteins. The reported values from five published studies are listed in Table 1. In addition to corin antigen, corin activity in human plasma was detected by pro-ANP or pro-BNP processing assays [29, 72]. To date, molecular forms of soluble corin in plasma or serum have not been well characterized. Most likely, fragments of various lengths are present, which were detected by antibodies used in the ELISA assays.
Table 1.
Serum and plasma corin concentrations in healthy individuals.
| Study | sample | total | male | female |
|---|---|---|---|---|
| Peleg et al. [73] | serum | 296–2590a (n=30) |
n/a | n/a |
| Dong et al. [70] | plasma | 690 ± 260b (n=198) |
798 ± 285b (n=104) |
551 ± 224b (n=94) |
| Dong et al. [71] | plasma | 216–1663a (n=348) |
842 ± 283b (n=182) |
569 ± 192b (n=166) |
| Ichiki et al. [29] | plasma | 889 (587 – 1477)c (n=55) |
1623 (1187–1827)c (n=19) |
810 (509–982)c (n=36) |
| Ibebuogu et al. [72] | plasma | 180d (n=16) |
n/a | n/a |
All concentrations were in pg/mL.
range;
mean ± S.D.;
median (25th–75th quartiles);
median;
n/a, not available.
Interestingly, plasma corin levels were significantly higher in males than females [29, 70, 71]. It is unclear if this difference reflects different corin expression levels or rates of corin shedding and/or degradation between males and females. Within the same gender group, plasma corin levels were similar among different age groups [71]. One report, however, suggested that plasma corin levels may be slightly higher among older (>60 years) individuals [29]. It was noticed that the levels reported in the Chinese were lower than those from other ethnic groups [29, 70, 71, 73]. Previously, lower levels of plasma pro-BNP were reported in a Chinese population when compared to those in European and American populations [74]. Because soluble corin assays in the reported studies were not standardized, it is unknown if the observed difference was due to specific ethnic backgrounds or simply due to different assay conditions. Values of plasma soluble corin concentration may vary if different types of anticoagulants are used. For example, the values in plasma samples with heparin were found to be significantly higher than that in samples with sodium citrate or EDTA [71].
Many plasma proteases such as blood coagulation factors are unstable in test tubes. In comparison, soluble corin was remarkably stable in plasma or serum. No apparent degradation was observed when plasma samples were left at room temperature for up to 12 hours [71]. If samples were kept at 4°C with or without protease inhibitors, no significant reduction in soluble corin levels was detected within 72 hours [71]. Similar results were obtained if recombinant corin was added to pooled human plasma. If samples were stored below −20°C, soluble corin remained stable for at least one year [73]. In serum or sodium citrate-containing plasma samples, levels of soluble corin remained unchanged after several cycles of freezing-and-thawing [71]. This remarkable protein stability may represent a significant advantage over other unstable plasma proteins or peptides if soluble corin is used as a diagnostic biomarker in clinical settings, where strict time or temperature controls may not be feasible.
7. Plasma soluble corin in patients with HF
Corin is essential for maintaining normal blood pressure. In mice, corin deficiency causes spontaneous hypertension and cardiac hypertrophy [44, 75]. In African Americans, who are known for their high prevalence of cardiovascular disease, corin variants with impaired natriuretic peptide processing activity have been associated with hypertension [49, 76]. Patients with these variants developed severe cardiac hypertrophy and had poor clinical outcomes [77, 78]. These data suggest that corin defects may be an important contributing factor in hypertension and heart disease.
Recently, plasma corin antigen levels were found to be significantly lower in patients with HF than that in normal individuals [70]. This finding was supported by another independent study, in which both plasma corin antigen and activity were measured [72]. The reduction of plasma corin levels appeared to correlate with the severity of HF, as indicated by lowest levels in patients with New York Heart Association classes III and IV [70, 79]. In contrast, no significant changes in plasma corin levels were found in patients with acute myocardial infarction (AMI) [70]. These results indicate that low plasma corin levels are associated closely with pathological changes in HF but not AMI.
Natriuretic peptide production is highly elevated in patients with hypertensive disease [62]. The function of this compensatory mechanism is to reduce blood volume and pressure. Many studies detected unprocessed pro-ANP and pro-BNP in patients with severe HF, suggesting that the function to process these peptides is compromised under the pathological condition [43, 80–82]. In animal models of HF and human failing hearts, corin expression was increased but the activity was not [83–85], indicating that corin zymogen activation may be a rate-limiting step in HF. As reported in cell-based studies, most soluble corin fragments were derived from activated corin molecules [69]. These data suggest that low plasma corin levels in patients with HF may reflect impaired corin activation in failing hearts. It is possible, therefore, that plasma corin may be used as a biomarker for the diagnosis of HF. Such a biomarker may also be tested in other hypertensive disease.
8. Perspectives
HF is a major disease. Effective managing this life-threatening disease depends on timely and accurate diagnosis. Currently, N-terminal pro-BNP and BNP are used as diagnostic markers to identify patients with HF [62, 86, 87]. The accuracy rates of these diagnostic tests are only ~75–85% in hospital emergency settings [88–91]. Similar results also were reported in patients with chronic HF [92]. Therefore, more sensitive and accurate tests are needed to improve the diagnosis and treatment of HF. Previously, soluble forms of several membrane proteins such as tumor necrosis factor-α and interleukin-1 receptors were found to be increased in patients with HF [93–96]. However, the levels of these proteins were also increased in patients with AMI, indicating that the shedding of these membrane receptors may represent a general inflammatory response in the heart, which is not specific for HF.
Discovery of corin as the long-sought natriuretic peptide convertase has extended our knowledge of the natriuretic peptide system [42, 43]. Many important questions remain regarding the role of corin in the cardiovascular biology and disease. Recent findings of soluble corin in human blood and the reduced levels in patient with HF are intriguing. Given its remarkable stability in plasma and serum, soluble corin could be used as a novel biomarker for HF. Current data are still limited. More prospective and comparative studies are needed with large patient populations to determine how soluble corin levels change in patients with HF and if the changes correlate with the underlying pathology. The results shall help us to understand the role of corin in HF or other heart disease and to determine diagnostic and prognostic values of soluble corin in clinical settings.
Highlights.
> Corin is a transmembrane protease that processes natriuretic peptides. > Corin is critical for maintaining normal blood pressure and cardiac function. > Proteolytic shedding is an important mechanism in regulating corin activity. > Soluble corin is detected in human blood and its levels are lower in patients with heart failure. > Soluble corin may be used as a novel biomarker for heart failure.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (31070716), the Natural Science Foundation of the Jiangsu Higher Education Institutions (10KJB320017), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China, and grants from the Bakken Heart-Brain Institute, the Research Project Committee of the Cleveland Clinic, and the NIH (HL089298).
Abbreviations
- AMI
acute myocardial infarction
- ANP
atrial natriuretic peptide
- BNP
B-type or brain natriuretic peptide
- CNP
C-type natriuretic peptide
- HF
heart failure
- LDLR
LDL receptor
- TTSP
type II transmembrane serine protease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neurath H. Evolution of proteolytic enzymes. Science. 1984;224:350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751:82–94. doi: 10.1016/j.bbapap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q. Type II transmembrane serine proteases. Curr Top Dev Biol. 2003;54:167–206. doi: 10.1016/s0070-2153(03)54009-1. [DOI] [PubMed] [Google Scholar]
- 6.Kitamoto Y, Yuan X, Wu Q, McCourt DW, Sadler JE. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proc Natl Acad Sci U S A. 1994;91:7588–7592. doi: 10.1073/pnas.91.16.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann NS, Mann SK. Enterokinase. Proc Soc Exp Biol Med. 1994;206:114–118. doi: 10.3181/00379727-206-43728. [DOI] [PubMed] [Google Scholar]
- 8.Zheng XL, Kitamoto Y, Sadler JE. Enteropeptidase, a type II transmembrane serine protease. Front Biosci (Elite Ed) 2009;1:242–249. doi: 10.2741/E23. [DOI] [PubMed] [Google Scholar]
- 9.Kurachi K, Torres-Rosado A, Tsuji A. Hepsin. Methods Enzymol. 1994;244:100–114. doi: 10.1016/0076-6879(94)44009-3. [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Parry G. Hepsin and prostate cancer. Front Biosci. 2007;12:5052–5059. doi: 10.2741/2447. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest. 1998;101:321–326. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugge TH, List K, Szabo R. Matriptase-dependent cell surface proteolysis in epithelial development and pathogenesis. Front Biosci. 2007;12:5060–5070. doi: 10.2741/2448. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Wu Q, Zhou Y. Iron-refractory iron deficiency anemia: new molecular mechanisms. Kidney Int. 2009;76:1137–1141. doi: 10.1038/ki.2009.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darragh MR, Bhatt AS, Craik CS. MT-SP1 proteolysis and regulation of cell-microenvironment interactions. Front Biosci. 2008;13:528–539. doi: 10.2741/2698. [DOI] [PubMed] [Google Scholar]
- 15.Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci. 2008;13:621–635. doi: 10.2741/2707. [DOI] [PubMed] [Google Scholar]
- 16.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–6937. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomita Y, Kim DH, Magoori K, Fujino T, Yamamoto TT. A novel low-density lipoprotein receptor-related protein with type II membrane protein-like structure is abundant in heart. J Biochem (Tokyo) 1998;124:784–789. doi: 10.1093/oxfordjournals.jbchem.a022180. [DOI] [PubMed] [Google Scholar]
- 18.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 19.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–34471. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 20.Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278:52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 21.Qi X, Jiang J, Zhu M, Wu Q. Human corin isoforms with different cytoplasmic tails that alter cell surface targeting. J Biol Chem. 2011;286:20963–20969. doi: 10.1074/jbc.M110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langenickel TH, Pagel I, Buttgereit J, Tenner K, Lindner M, Dietz R, Willenbrock R, Bader M. Rat corin gene: molecular cloning and reduced expression in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1516–H1521. doi: 10.1152/ajpheart.00947.2003. [DOI] [PubMed] [Google Scholar]
- 23.Gladysheva IP, King SM, Houng AK. N-glycosylation modulates the cell-surface expression and catalytic activity of corin. Biochem Biophys Res Commun. 2008;373:130–135. doi: 10.1016/j.bbrc.2008.05.181. [DOI] [PubMed] [Google Scholar]
- 24.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–27735. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 25.Huber R, Bode W. Structural basis of the activation and action of trypsin. Acc Chem Res. 1978;11:114–122. [Google Scholar]
- 26.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 28.Calderone A, Bel-Hadj S, Drapeau J, El-Helou V, Gosselin H, Clement R, Villeneuve L. Scar myofibroblasts of the infarcted rat heart express natriuretic peptides. J Cell Physiol. 2006;207:165–173. doi: 10.1002/jcp.20548. [DOI] [PubMed] [Google Scholar]
- 29.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC., Jr Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 30.McBride K, Nemer M. Regulation of the ANF and BNP promoters by GATA factors: lessons learned for cardiac transcription. Can J Physiol Pharmacol. 2001;79:673–681. [PubMed] [Google Scholar]
- 31.Polzin D, Kaminski HJ, Kastner C, Wang W, Kramer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–659. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y, Park S, Li Y, Bolshakov VY, Lamonerie T, Kim KS. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci U S A. 2011;108:9703–9708. doi: 10.1073/pnas.1016443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 35.Enshell-Seijffers D, Lindon C, Wu E, Taketo MM, Morgan BA. Beta-catenin activity in the dermal papilla of the hair follicle regulates pigment-type switching. Proc Natl Acad Sci U S A. 2010;107:21564–21569. doi: 10.1073/pnas.1007326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu F, Wu Q. Corin-mediated processing of pro-atrial natriuretic peptide in human small cell lung cancer cells. Cancer Res. 2003;63:8318–8322. [PubMed] [Google Scholar]
- 37.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 39.Tervonen V, Arjamaa O, Kokkonen K, Ruskoaho H, Vuolteenaho O. A novel cardiac hormone related to A-, B- and C-type natriuretic peptides. Endocrinology. 1998;139:4021–4025. doi: 10.1210/endo.139.9.6292. [DOI] [PubMed] [Google Scholar]
- 40.Tsukada T, Takei Y. Integrative approach to osmoregulatory action of atrial natriuretic peptide in seawater eels. Gen Comp Endocrinol. 2006;147:31–38. doi: 10.1016/j.ygcen.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Jiang J, Cui Y, Wu Q. Corin, atrial natriuretic peptide and hypertension. Nephrol Dial Transplant. 2009;24:1071–1073. doi: 10.1093/ndt/gfn727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 43.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, Light D, Parry R, Polokoff M, Whitlow M, Wu Q, Parry G. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res. 2006;66:3611–3619. doi: 10.1158/0008-5472.CAN-05-2983. [DOI] [PubMed] [Google Scholar]
- 46.Owen KA, Qiu D, Alves J, Schumacher AM, Kilpatrick LM, Li J, Harris JL, Ellis V. Pericellular activation of hepatocyte growth factor by the transmembrane serine proteases matriptase and hepsin, but not by the membrane-associated protease uPA. Biochem J. 2010;426:219–228. doi: 10.1042/BJ20091448. [DOI] [PubMed] [Google Scholar]
- 47.Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50. doi: 10.1016/B978-0-12-385504-6.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 52.Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 53.Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, Nagai R, Abe S, Takeuchi T. Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin. J Biol Chem. 1997;272:20545–20554. doi: 10.1074/jbc.272.33.20545. [DOI] [PubMed] [Google Scholar]
- 54.Wu C, Wu F, Pan J, Morser J, Wu Q. Furin-mediated processing of pro-C-type natriuretic peptide. J Biol Chem. 2003;278:25847–25852. doi: 10.1074/jbc.M301223200. [DOI] [PubMed] [Google Scholar]
- 55.Crimmins DL, Kao JL. A glycosylated form of the human cardiac hormone pro B-type natriuretic peptide is an intrinsically unstructured monomeric protein. Arch Biochem Biophys. 2008;475:36–41. doi: 10.1016/j.abb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J, Pristera N, Wang W, Zhang X, Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem. 2010;56:959–966. doi: 10.1373/clinchem.2009.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 58.Schellenberger U, O'Rear J, Guzzetta A, Jue RA, Protter AA, Pollitt NS. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys. 2006;451:160–166. doi: 10.1016/j.abb.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 59.Semenov AG, Postnikov AB, Tamm NN, Seferian KR, Karpova NS, Bloshchitsyna MN, Koshkina EV, Krasnoselsky MI, Serebryanaya DV, Katrukha AG. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–498. doi: 10.1373/clinchem.2008.113373. [DOI] [PubMed] [Google Scholar]
- 60.Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-B-type natriuretic peptide in cardiomyocytes. Biochem Biophys Res Commun. 2011;411:593–598. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semenov AG, Seferian KR. Biochemistry of the human B-type natriuretic peptide precursor and molecular aspects of its processing. Clin Chim Acta. 2011;412:850–860. doi: 10.1016/j.cca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 63.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 64.Reiss K, Saftig P. The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Hadorn B, Steiner N, Sumida C, Peters TJ. Intestinal enterokinase. Mechanisms of tts "secretion" into the lumen of the small intestine. Lancet. 1971;1:165–166. doi: 10.1016/s0140-6736(71)91936-2. [DOI] [PubMed] [Google Scholar]
- 66.Cho EG, Schwartz RH, Kim MG. Shedding of membrane epithin is blocked without LDLRA4 and its protease activation site. Biochem Biophys Res Commun. 2005;327:328–334. doi: 10.1016/j.bbrc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 67.Kiyomiya K, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol Cell Physiol. 2006;291:C40–C49. doi: 10.1152/ajpcell.00351.2005. [DOI] [PubMed] [Google Scholar]
- 68.Stirnberg M, Maurer E, Horstmeyer A, Kolp S, Frank S, Bald T, Arenz K, Janzer A, Prager K, Wunderlich P, Walter J, Gütschow M. Proteolytic processing of the serine protease matriptase-2: identification of the cleavage sites required for its autocatalytic release from the cell surface. Biochem J. 2010;430:87–95. doi: 10.1042/BJ20091565. [DOI] [PubMed] [Google Scholar]
- 69.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, Wu Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong N, Chen S, Yang J, He L, Liu P, Zhen D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong N, Dong J, Liu P, Xu L, Shi S, Wu Q. Effects of anticoagulants on human plasma soluble corin levels measured by ELISA. Clin Chim Acta. 2010;411:1998–2003. doi: 10.1016/j.cca.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;2011 doi: 10.1161/CIRCHEARTFAILURE.109.895581. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peleg A, Jaffe AS, Hasin Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin Chim Acta. 2009;409:85–89. doi: 10.1016/j.cca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Shi X, Xu G, Xia T, Song Y, Lin Q. N-terminal-pro-B-type natriuretic peptide (NT-proBNP): reference range for Chinese apparently healthy people and clinical performance in Chinese elderly patients with heart failure. Clin Chim Acta. 2005;360:122–127. doi: 10.1016/j.cccn.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 75.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 77.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 78.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shrestha K, Troughton RW, Borowski AG, Yandle TG, Richards AM, Klein AL, Tang WH. Plasma Corin Levels Provide Minimal Prognostic Utility Incremental to Natriuretic Peptides in Chronic Systolic Heart Failure. J Card Fail. 2010;16:621–627. doi: 10.1016/j.cardfail.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 80.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–1091. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 81.Dries DL. Process matters: Emerging concepts underlying impaired natriuretic peptide system function in heart failure. Circ Heart Fail. 2011;4:107–110. doi: 10.1161/CIRCHEARTFAILURE.111.960948. [DOI] [PubMed] [Google Scholar]
- 82.Xu-Cai YO, Wu Q. Molecular forms of natriuretic peptides in heart failure and their implications. Heart. 2010;96:419–424. doi: 10.1136/hrt.2008.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S, Sen S, Young D, Wang W, Moravec CS, Wu Q. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–H1692. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang W, Cai DY, Pan CS, Qi YF, Jiang HF, Geng B, Tang CS. Changes in production and metabolism of brain natriuretic peptide in rats with myocardial necrosis. Eur J Pharmacol. 2005;507:153–162. doi: 10.1016/j.ejphar.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 85.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–H1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 86.Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152:828–834. doi: 10.1016/j.ahj.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 87.Wu AH, Smith A. Biological variation of the natriuretic peptides and their role in monitoring patients with heart failure. Eur J Heart Fail. 2004;6:355–358. doi: 10.1016/j.ejheart.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 89.Lainchbury JG, Campbell E, Frampton CM, Yandle TG, Nicholls MG, Richards AM. Brain natriuretic peptide and n-terminal brain natriuretic peptide in the diagnosis of heart failure in patients with acute shortness of breath. J Am Coll Cardiol. 2003;42:728–735. doi: 10.1016/s0735-1097(03)00787-3. [DOI] [PubMed] [Google Scholar]
- 90.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 91.Wright SP, Doughty RN, Pearl A, Gamble GD, Whalley GA, Walsh HJ, Gordon G, Bagg W, Oxenham H, Yandle T, Richards M, Sharpe N. Plasma amino-terminal pro-brain natriuretic peptide and accuracy of heart-failure diagnosis in primary care: a randomized, controlled trial. J Am Coll Cardiol. 2003;42:1793–1800. doi: 10.1016/j.jacc.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 92.Tang WH, Girod JP, Lee MJ, Starling RC, Young JB, Van Lente F, Francis GS. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 93.Okuyama M, Yamaguchi S, Nozaki N, Yamaoka M, Shirakabe M, Tomoike H. Serum levels of soluble form of Fas molecule in patients with congestive heart failure. Am J Cardiol. 1997;79:1698–1701. doi: 10.1016/s0002-9149(97)00228-2. [DOI] [PubMed] [Google Scholar]
- 94.Shimizu M, Fukuo K, Nagata S, Suhara T, Okuro M, Fujii K, Higashino Y, Mogi M, Hatanaka Y, Ogihara T. Increased plasma levels of the soluble form of Fas ligand in patients with acute myocardial infarction and unstable angina pectoris. J Am Coll Cardiol. 2002;39:585–590. doi: 10.1016/s0735-1097(01)01800-9. [DOI] [PubMed] [Google Scholar]
- 95.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]