Abstract
Introduction
When employing tissue engineering approaches to clinical problems, cells are often transplanted to a distant site on a scaffold into an environment different from their original niche. Previous studies have demonstrated the role of Noggin, a BMP inhibitor in vascular development and angiogenesis. We hypothesized that noggin suppression in human adipose derived stromal cells (hASCs) would enhance VEGF secretion and angiogenesis in vitro and in vivo to a greater extent than BMP-2 alone.
Methods
hASCs were isolated from human lipoaspirate (n=6) and transfected with a Noggin shRNA construct. Knockdown was confirmed and angiogenesis was assessed by tubule formation and qRT-PCR. Cells were seeded on scaffolds with or without BMP-2 and implanted into a 4mm critical size calvarial defect. In vivo angiogenic signaling was assessed by immunofluoresence and immunohistochemistry.
Results
hASCs with noggin suppression secreted significantly higher amounts of VEGF protein on ELISA (*p<0.05). hASCs with noggin knockdown expressed higher levels of angionegic gene markers by qRT-PCR (VE-cadherein, VEGFA, and HIF1A), and displayed enhanced vascular tubule formation in vitro. In vivo, calvarial defects seeded with noggin shRNA hASCs exhibited a significantly higher number of vessels in the defect site than controls by immunohistochemistry (*p<0.05). Additionally, BMP-2 releasing scaffolds significantly enhanced VEGF and PECAM protein levels in the defect site.
Conclusion
hASCs demonstrate significant increases in angiogenesis both in vitro and in vivo both with noggin suppression and BMP-2 supplementation. By creating a cell with noggin suppressed and by using a scaffold with increased BMP-2, we can create a more angiogenic niche.
Keywords: Angiogenesis, vasculogenesis, Skeletal tissue engineering, Tissue regeneration, Multipotent stromal cells, Calvarial defect, Noggin, Bone morphogenetic protein, scaffold, adipose-derived stromal cells
Introduction
Despite significant strides in the understanding of vascularization and neovascularization in cutaneous wounds and flaps, there is a paucity of research on strategies to improve vascularization of an osseous defect microenvironment. Current techniques to reconstruct large calvarial defects use non-vascularized bone grafts, which depend on creeping substitution of osteocytes. Large osseous vascularized tissue, such as a microvascular fibula or scapular flaps, do provide osteoblasts that can effect osteogenesis, however, these tissues are limited by both in size and availability.
Tissue engineering employs the utilization of cells, and three-dimensional scaffolds. Once a scaffold exceeds 5–8mm in thickness, cells within the scaffold require vascularization to survive and differentiate. (1) Although placing cell seeded scaffolds in vascular regions will aid in repair, several groups have attempted to vascularize these constructs prior to transplantation. (2–4) However, such manipulations often require prolonged time ex-vivo while the cells are incorporated into de novo vascular structures. With regard to bone tissue engineering, an ideal scaffold would stimulate both robust osteogenesis and angiogenesis. Though osteoblasts and periosteal cells are both osteogenic, neither are readily available in large quantities. Adipose derived stromal cells are attractive candidates for tissue engineering due to their osteogenic and vasculogenic potential, as well as their relative abundance. (5–14) Priming these cells to secrete more Vascular Endothelial Growth Factor A (VEGFA) to stimulate angiogenesis would make these cells even more attractive candidates for tissue engineering.
Bone Morphogenetic Protein 2 (BMP-2) specifically has also been shown to play a key role in vasculogenesis as BMP-2 antagonism leads to depressed angiogenesis in cancer cells in vivo.(15) Noggin is a glycosylated cystein-knot chemokine protein that inhibits BMP-2 and BMP-4 from binding to its receptor BMPR1B.(16–18) In our laboratory we have demonstrated that inhibition of noggin enhances the osteogenic capability of osteoblasts and hASCs.(19, 20) Studies have also shown that differentiation of human embroyonic cells to endothelial cells is inhibited by noggin.(21) Along with differentiation, noggin has also been shown to play a role in vascular patterning through its effect on BMP-4. Specifically, BMP-4 has been shown to be required for vascular formation and outgrowth of the vascular system.(22) Conversely, BMP inhibition through exogenous noggin supplementation has been shown to interfere with endothelial migration.(23, 24) Dosomrphin, is another BMP inhibitory ligand that plays a role in dorso-ventral patterning, and prevents the phosphorylation or activation of the receptor Smads 1/5 thus inhibiting BMP-2 signaling.(25)
Mechanistically, it is believed that BMP inhibitors such as dosomorphin and noggin lead to the interruption of the BMP-initiated VEGF promoter activation.(26) Though direct supplementation of rhBMP-2 is an option, this cytokine is extremely expensive and along with increased costs, clinical outcome studies have found that high doses of BMP-2 are accompanied by a concerning side effect profile, including ectopic bone formation, which in spinal surgery can cause neural compression.(27, 28) Thus, an approach to lower the amount of recombinant BMP-2 required while maintaining BMP signaling through modulation of an inhibitor may be safer, and more cost-effective. Recent studies have also demonstrated that HUVEC cells over expressing noggin had a decreased capacity to form tubules, leading us to hypothesize about the antagonistic role of noggin and BMP-2 in angiogenesis.(29) We propose that by knocking down noggin, we can increase the angiogenic capability of hASCs, and that, along with a BMP-loaded biomimetic scaffold, we can greatly augment the vascularization of a calvarial defect.
Methods
Chemicals, supplies and animals
Medium, fetal bovine serum (FBS), and penicillin/streptomycin were purchased from GIBCO Life Technologies, (Carlsbad, CA). Cell culture wares were purchased from Corning Inc. (San Mateo, CA). Unless otherwise specified, all other chemicals were purchased from Sigma-Aldrich. CD-1 nude mice (Crl:CD-1 Foxn1nu), were obtained from Charles Rivers, (Wilmington, MA).
Cell Harvest
Human ASCs were harvested from human lipoaspirate derived from the abdominal and flank regions of six women between the ages of 33 and 55 with no major medical co-morbidities. Lipoaspirate was digested with a Type II collagenase solution at 37°C. Cells were pelleted via centrifugation, filtered at 100 micrometer pore size, and primary cultures were established at 37°C, 5% CO2 in DMEM with 10% FBS. Only passage 1–3 hASCs were used for shRNA transfection. After transfection and expansion, hASCs between passage 3 and 6 were used for in vitro and in vivo assays. As this study took place over several months and cells were only used up to passage 3, a total of 6 lines were derived. In each assay, 3 cell lines from 3 patients were used. Control and noggin shRNA transfected cells were always compared between the same patient for internal consistency.
Human ASC labeling
To verify viability, for select in vivo experiments, hASCs were stably transduced with the lentivirus carrying the triple fusion reporter genes, firefly luciferase (Fluc), red fluorescence protein (GFP), and herpes simplex virus truncated thymidine kinase (HSV-ttk) genes. Stably expressed hASCs were purified by fluorescence activated cell sorting based on GFP expression as previously described.(30)
In vitro culture assays
For experiments involving isolation of RNA, hASCs were seeded in 6-well plates at a density of 80,000 cells per well. All assays were performed in triplicate wells. Knockdown and scramble transfected cell lines were developed from an individual patient. Thus, when assays were performed, we compared a scramble shRNA and a knockdown shRNA line from the same patient. We then run 3 separate lines per assay (thus 3 patients).
After attachment, cells were treated with standard growth medium (SGM) (Dulbecco’s Modified Eagle Medium, 10% FBS), 1% penicillin/streptomycin or osteogenic differentiation medium (ODM) (Dulbecco’s Modified Eagle Medium, 10% FBS, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate), 1% penicillin/streptomycin. Cells were maintained for 7 days in ODM. For select experiments, rhNoggin (400ng/ml) or dorsomorphin (10uM) were added to SGM or ODM.(31, 32) Concentrations used were based on data obtained from mouse ASCs and from previous studies.(33, 34) The vehicle control used for rhNoggin and Dorsomorphin was 0.01% BSA.
Matrigel Tubule Assay
Matrigel (BD Biosciences) was thawed and placed in four-well chamber slides at 37°C for 30 minutes to allow solidification. 50,000 shRNA control or noggin shRNA transfected hASCs were plated alone on Matrigel and incubated at 37°C under 1% for 12 hours. Tubule formation was defined as a structure exhibiting a length four times its width. Experiments were performed with n = 6. Tubule counts were determined in 10 random fields per well using an inverted Leica DMIL light microscope (Leica Microsystems GmbH, Wetzlar, Germany) at 100× magnification as previously described.(5)
Preparation of scaffolds
Apatite-coated PLGA scaffolds were fabricated from 85/15 poly(lactic-co-glycolic acid) by solvent casting and a particulate leaching process as previously described (6).
For BMP-2 loaded scaffolds, recombinant human BMP-2 (rhBMP-2; Medtronic, Minneapolis, MN) was adsorbed onto fabricated scaffolds by dropping the protein solution onto the scaffolds for 20 min and further lyophilized on a freeze drier (Labconco, Kansas City, MO) overnight. 1.25ug of BMP-2 was applied to our scaffold, which had a volume of 6.28ul, resulting in final concentrations of 200ug/ml.
Creation of calvarial defects
Non-healing, critical-sized (4mm) calvarial defects were created in the right parietal bone of adult (60 day-old) male CD-1 nude mice as previously described.(6)
In preparation for cell engraftment, scaffolds were seeded with hASCs 24 hours prior to implantation. Cells (150,000) were placed on scaffolds in 125 μl of medium in 96-well culture plates and incubated for 24 hours. Before implantation, scaffolds were copiously rinsed with PBS. Animals were divided equally into four treatment groups: 1) Noggin knockdown hASCs on a scaffold, in which hASCs were impregnated in a HA coated PLGA scaffold, which was then placed in the defect site 2) Scramble shRNA and control (non-transfected) hASCs on a scaffold, in which hASCs were impregnated in a HA coated PLGA scaffold and then placed in the defect site. 3) hASCs with noggin knockdown placed on a BMP-2 loaded scaffold (200ug/ml rhBMP-2), and 4) hASCs transduced with a Scramble GFP construct, placed on a BMP-2 loaded scaffold (200ug/ml rhBMP-2). (n=4 for each subgroup) Finally, the skin was sutured and animal monitored per established post-operative protocols.
Histologic analyses
One week postoperatively, animals were sacrificed for histology. Calvaria were harvested, formalin-fixed, decalcified in 19% EDTA, paraffin-embedded and sectioned at 8 um thickness.
Immunohistochemistry of angiogenic proteins
Immunohistochemistry was performed on select slides for VEGF (Abcam, Cambridge, MA), and PECAM (Santa Cruz Laboratories, Santa Cruz, CA). Slides were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide; slides were blocked with 5% goat serum in PBS. Antibodies used included anti-rabbit polyclonal anti-VEGFA and anti-PECAM (1[ratio]80 in dilution, Santa Cruz Laboratories, Santa Cruz, CA). Appropriate biotinylated secondary antibodies were used in 1[ratio]1000 dilution (Vector Laboratories, Burlingame, CA). The Vectastain ABC system (Vector Laboratories, Burlingame, CA) was used according to the manufacturer’s instructions. Visualization was achieved with diaminobenzidine solution (Zymed Laboratories, South San Francisco, CA). Confocal microscopy was then performed. Vessels were quantified on 7 stained slides per defect by three blinded independent examiners at 5× magnification. Vessels were defined by their positive PECAM-1 stain and their typical round or oval structure containing a lumen. Vessel surface was histomorphometrically quantified as previously described.(7)
Western Blot analysis of Vascular Signaling
The endogenous activation of the VEGF signaling pathway in hASCs was investigated by immunoblotting analysis of VEGFA as previously described.(35) Subconfluent hASCs were washed twice with 1X PBS and starved in serum-free medium overnight. Then the cells were washed twice with ice-cold PBS and lysed with cold lysis buffer (50 mmol/L of HEPES, pH 7.5, 150 mmol/L of NaCl, 1 mmol/of EDTA, 10% glycerol, 1% Triton-X-100, 25 mmol/L of sodium fluoride) containing 1 mmol/L of sodium orthovanadate and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO). Cell lysates were assayed for protein concentration by BCA assay. Aliquots (50–100 μg) of cell lysate were electrophoresed on 12% Tris-HCl sodium dodecyl sulfate (SDS)-PAGE gels (Precast Nupage gels, Invitrogen, Life technologies) and transferred onto Immobilon-P membrane (Millipore Corporation, Bedford, MA). Antibodies against the VEGFA were used (Abcam, Cambridge, MA). A horseradish peroxidase-conjugated anti-rabbit antibody (HRP) 1:8000 was used as secondary antibody. α-tubulin antibody was used to control for equal loading and transfer of the samples. All bands in the immunoblots were normalized with the loading controls (α-tubulin) and quantified by densitometry.
Polymerase chain reaction
Total RNA was isolated from cells and tissue as previously described.(36, 37) Reverse transcription was performed with 1 ug RNA using Taqman Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System and Sybr Green PCR Master Mix (Applied Biosystems). Specific primers for the genes examined were based on their PrimerBank sequences (Table 1). Gene expression was normalized to values of mouse or human GAPDH and performed in triplicate. The PCR product was run out on a 2% agarose gel to verify appropriate product size.
Statistical analysis
All statistics were performed with the assistance of the expert assistance of the Stanford Department of Statistics. Means and standard deviations were calculated from numerical data, as presented in figures, figure legends and Table 2. In figures, bar graphs represent means, whereas error bars represent one standard deviation. Statistical analyses were performed using a Student’s two-sample t-test when comparing two groups. When more than 2 groups are used as in Fig. 1C, a Student’s two sample t-test was used first to compare the control to the Noggin treatment group and subsequently to compare the control to the Dosomorphin treated group. Though a one way anova could be used in Fig. 1C, the Bonferonni corrections are equivalent and a Student t-test allows us to pick out all combinations of groups 2 at a time to compare. A Bonferonni correction was used to handle multiple hypothesis testing. The exact statistical analysis for each dataset is described in the figure legends.
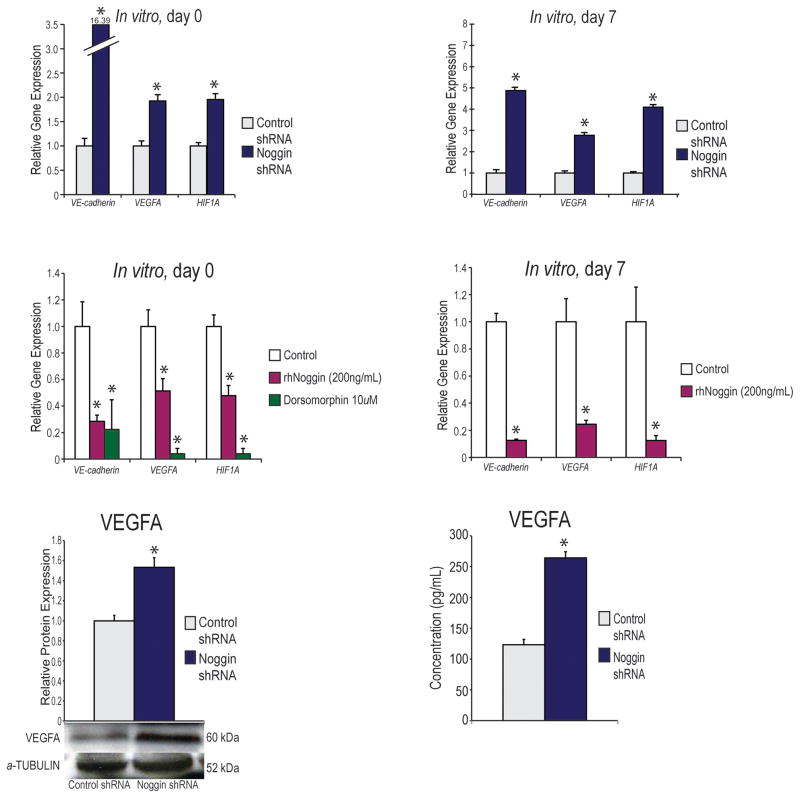
Figure 1. Effect of noggin suppression on angiogenesis in vitro.
(A) Angiogenic gene expression profile of hASCs cultured in Standard Growth Medium (SGM). Markers examined include angiogenic specific genes, VE-CADHEREIN, VEGFA and HIF1A. An up regulation of all genes was noted in noggin-suppressed cells. A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05) (B) Gene expression profile of hASCs after seven days of osteogenic differentiation. Markers examined include angiogenic specific genes, VE-CADHEREIN, VEGFA, and HIF1A. An up regulation of all genes was noted in noggin-suppressed cells (mean expression +/− S.D. *p<0.05). All transcript levels were expressed relative to shRNA control. A student’s two sample t-test was used to compare groups. (C) Angiogenic gene expression profile of hASCs cultured in SGM with or without addition of rhNoggin (200ng/ml) or dorsomorphin (10uM). Markers examined include angiogenic specific genes, VE-CADHEREIN, VEGFA, and HIF1A. A down regulation of all genes was noted in rhNoggin treated and dorsomorphin treated hASCs (mean expression +/− S.D. *p<0.05) A two sample t-test was used to compare the control group to each treatment groups separately and then a Bonferonni correction was performed to handle multiple hypothesis testing. (D) Gene expression profile of hASCs after seven days of osteogenic differentiation with or without addition of rhNoggin (400ng/ml). Markers examined include angiogenic specific genes, VE-CADHEREIN, VEGFA, and HIF1A. A down regulation of all genes was noted in rhNoggin treated hASCs. A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05) (E) VEGFA protein levels of control shRNA and Noggin shRNA transfected cells by Western Blot analysis. A student’s two tailed t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (F) VEGFA protein secretion by control shRNA and Noggin shRNA transfected cells measured by VEGF ELISA. A student’s two tailed t-test was used to compare groups (mean expression +/− S.D. *p<0.05).
Results
Evaluation of Noggin Suppression
In order to reduce the level of noggin transcript and protein, RNA interference was performed using a noggin-directed shRNA construct and delivered through lentiviral particles (Santa Cruz Biotech, CA). Transfection efficiency was evaluated using GFP-targeted shRNA control lentiviral particles, demonstrating over 95% transfection efficiency using our lenitviral construct based on FACS analysis (data not shown). qRT-PCR analysis of noggin after lentiviral transfection was performed and displayed a significant reduction in transcript level (*p<0.001) when compared to shRNA scramble control (See Figure, Supplemental Digital Content 1A, which demonstrates Validation of transfection and noggin-directed shRNA construct. (A) Evaluation of mean transcripts using QRT-PCR analysis demonstrated significant suppression with noggin directed shRNA (*p<0.001) by lentiviral transfection methodologies. A student’s two tailed t-test was used to compare groups. INSERT LINK HERE). hASCs transfected with control GFP-targeted shRNA construct demonstrated similar noggin levels compared to non-transfected hASCs (data not shown). Likewise, noggin protein showed complete knockdown of detectable noggin protein, as assessed by Western blot using lentiviral knockdown (See Figure, Supplemental Digital Content 1B, which demonstrates Validation of transfection and noggin-directed shRNA construct. (B) Western blot quantification of noggin protein knockdown after lentiviral noggin knockdown. INSERT LINK HERE). We next examined the consequence of noggin knockdown in angiogenic differentiation of hASCs.
Noggin Suppression Enhances hASC Angiogenic Signaling
We first examined the in vitro differences between our noggin shRNA and control shRNA with regard to angiogenic gene expression. We found that in standard growth medium, noggin shRNA hASCs had significantly higher expression levels of angiogenic genes, including VE-cadherein (VCAD), VEGFA, and hypoxia inducing factor A (HIF1A) (Fig. 1A). Similarly, when treated with osteogenic differentiation medium (ODM), all of these angiogenic genes were more highly expressed in the noggin shRNA hASCs than in the control shRNA cells at 7 days, and this difference was statistically significant (Fig. 1B). To assess the converse relationship, we subsequently treated hASCs with rhNoggin (200ng/ml), and, as expected, we noted a significant decrease in vasculogenic gene expression (Fig. 1C,D). In order to further analyze the BMP-2 pathway effect on hASC angiogenesis, we also treated hASCs with dorsomorphin (10uM), which has been shown to inhibit the BMPR1B receptor. Similar to our findings with blocking the BMP-2 ligand with noggin, dorsomorphin significantly down regulated angiogenic gene expression (Fig. 1C).
In addition to increased transcript, we set out to explore how noggin suppression would enhance VEGFA ligand production, as this would ultimately determine how these cells would influence surrounding cells if placed in vivo. On a protein level, Noggin shRNA transfected cells had an increased amount of VEGFA (Fig. 1E). Similarly, when analyzing secreted VEGF protein from hASCs using a protein ELISA, those cells with lower Noggin expression demonstrated higher levels of VEGFA (Fig. 1F). Next, to assess if simply adding more BMP-2 would be sufficient to enhance angiogenesis, we compared vascular gene expression in our control shRNA and Noggin shRNA transfected cell lines. We demonstrated, that the BMP-2 treated Noggin shRNA transfected cells had a higher up-regulation of angiogenic genes than the BMP-2 treated, control shRNA transfected cells indicating that both supplementing BMP-2 and removing a BMP-2 inhibitor maximally drives vascular gene expression (See Figure, Supplemental Digital Content 2 A–C, which demonstrates Vascular signaling is greatly enhanced with noggin knockdown and BMP-2 over BMP-2 alone. (A) VEGFA gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups. (B) VEGFB gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (C) HIF-1alpha gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). An up regulation of all genes was noted in noggin-suppressed cells. A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). INSERT LINK HERE).
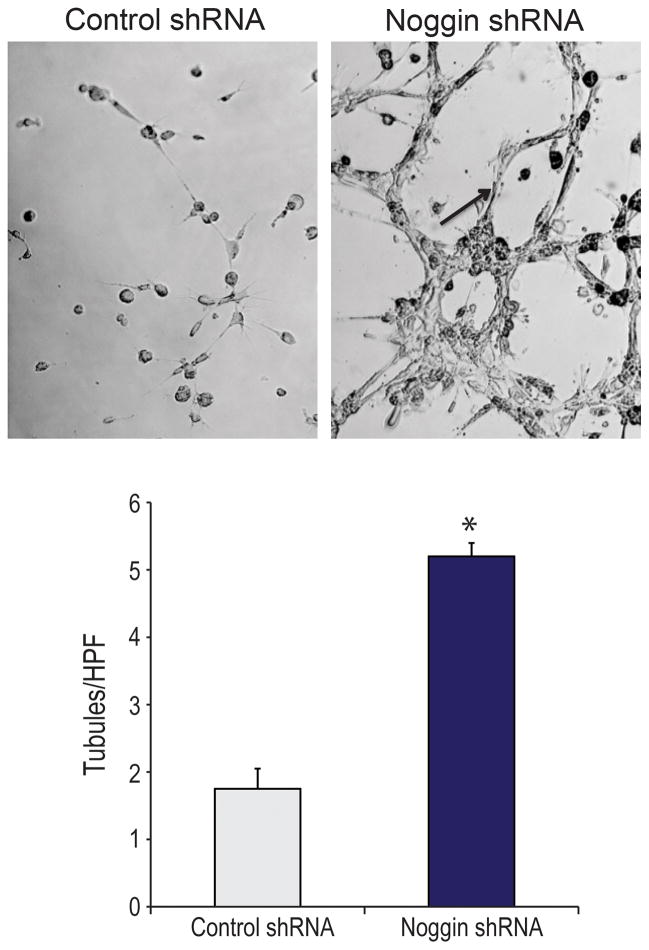
Tubulogenesis is Enhanced by Noggin Knockdown
To demonstrate that those cells with noggin suppression had increased angiogenic gene expression as well as tubule formation, we next performed a matrigel vascular tubule assay. After 12 hours in hypoxia (1% O2), those hASCs with suppressed noggin demonstrated significantly higher tubule formation in matrigel compared to control hASC (Fig. 2A,B). Similarly, when using our tubule assay, we noted significantly more tubules in our Noggin shRNA treated group than our control shRNA and control shRNA+BMP-2 groups indicating that hASCs with Noggin knockdown perhaps have a superior angiogenic capacity to just hASCs treated with BMP-2 alone (See Figure, Supplemental Digital Content 2 D, which demonstrates Vascular signaling is greatly enhanced with noggin knockdown and BMP-2 over BMP-2 alone. (D) Quantification of tubules of control shRNA, control shRNA with BMP-2 (200ng/ml) and Noggin shRNA cells as counted by 5 blinded, independent observers. A student’s two tailed t-test was used to compare groups. A Bonferonni correction was used to handle multiple hypothesis testing (mean expression +/− S.D. *p<0.05). INSERT LINK HERE). Such findings are potentially significant as we look to enhance angiogenesis in vivo by suppressing noggin.
Figure 2.
(A) In vitro tubule assay of control shRNA and Noggin shRNA cells in matrigel after 12 hours of hypoxia. (B) Quantification of tubules of control shRNA and Noggin shRNA cells as counted by 5 blinded, independent observers. A student’s two tailed t-test was used to compare groups (mean expression +/− S.D. *p<0.05).
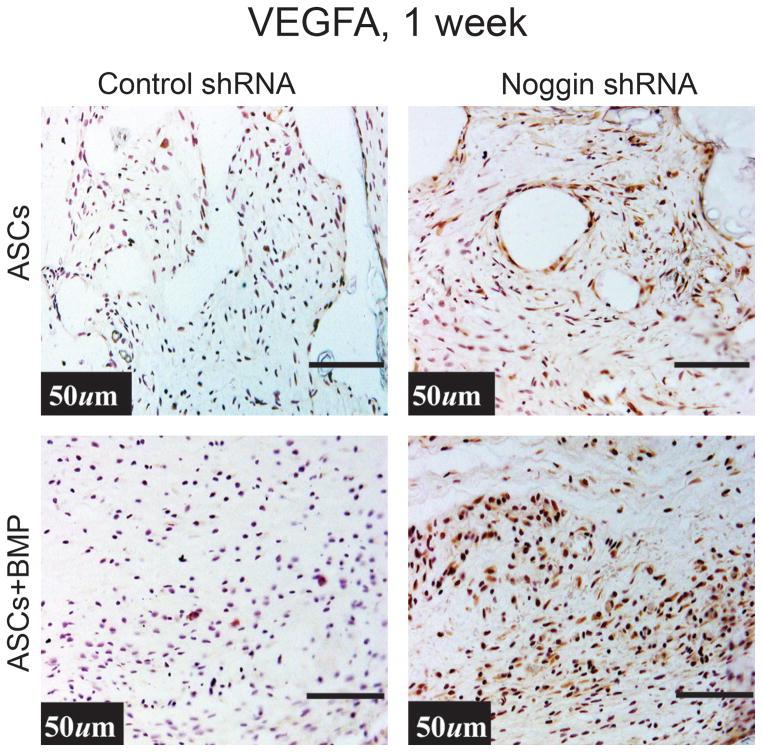
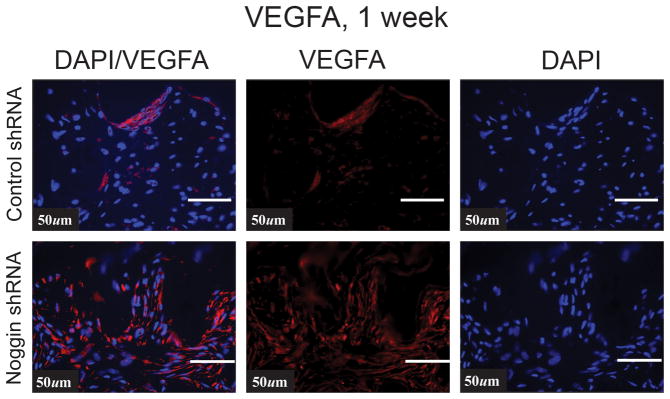
Noggin Knockdown Enhances Vascular Signaling In Vivo
Next we set out to determine if this difference carried over to in vivo angiogenesis using our calvarial defect model. To this end, 4 mm calvarial defects were made in the parietal bone of adult nude mice. These defects were next treated with hydroxyapatite (HA) coated PLGA scaffolds seeded with 150,000 hASCs with either the noggin knockdown construct or GFP control construct, transfected as previously described (6, 38). The specimens were examined on postoperative day 7, as this time point allows for assessment of the early wound environment as previously described.(39) First we examined VEGF signaling in the region of the calvarial defect where hASCS with control or noggin shRNA transfected hASCs. VEGFA protein immunoreactivity was more intense in those defects treated with hASCs transfected with Noggin shRNA by immunohistochemistry (Fig. 3A). Interestingly, when placing both control and noggin shRNA hASCs on a BMP-2 loaded scaffold, both groups of cells demonstrated an enhancement in VEGFA immunoreactivity, and the difference between the groups became even more striking, suggesting that removing a BMP-2 inhibitor and adding BMP-2 ligand further stimulated VEGF production. Similarly, by immunofluoresence, there was enhanced VEGF immunoreactivity in the noggin shRNA transfected hASCs, especially around tubular like structures (Fig. 3B).
Figure 3.
(A) Human VEGF immunohistochemistry in a calvarial defect (brown color) with control shRNA or Noggin shRNA hASCs on a HA coated PLGA scaffold with or without BMP-2 (200ng/ml). (B) VEGFA expression by immunofluorsence (red color) in the region of the calvarial defect treated either with control shRNA treated or Noggin shRNA treated hASCs.
Calvarial Defects treated with hASCs with Noggin ShRNA increased angiogenesis
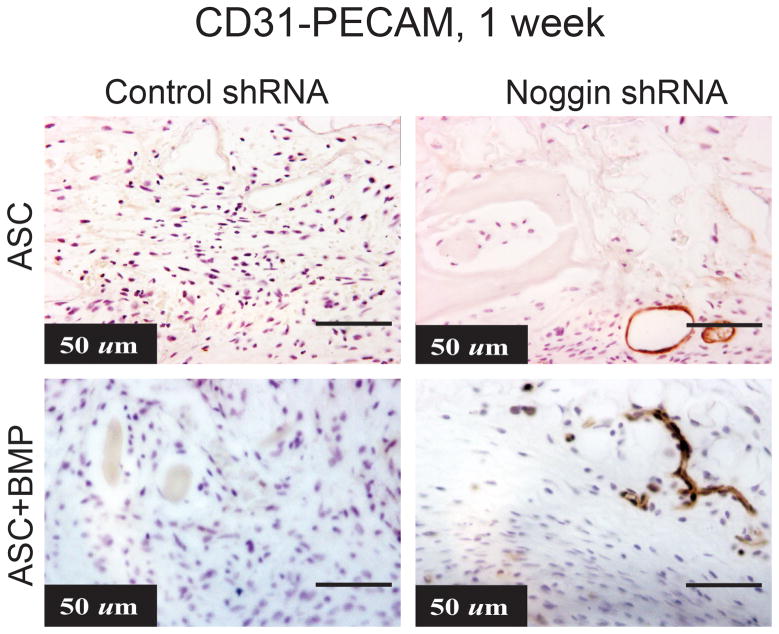
After demonstrating enhanced tubule formation in vitro and enhanced VEGF localization in vivo, we next set out to explore blood vessel formation in vivo. To detect endothelial cells, we first performed immunohistochemistry using mouse-CD31/PECAM-1. After 7 days in vivo, we noted increased mouse specific PECAM staining in our Noggin shRNA group when compared to control shRNA group (Fig. 4A,B). We then quantified the number of vessels in the calvarial defect and found significantly higher number of vessels in the defect site of mice receiving noggin shRNA treated cells than those receiving control hASCs. Furthermore, a BMP-2 loaded scaffold appears to even further enhance tubule formation in both groups (Fig. 4A,B). Thus, it appears that there is an increase in vascular structures in vivo in an osseous defect when treated with hASCs with noggin suppression or supplemented with BMP-2.
Figure 4.
(A) CD31-Pecam stain (brown color) of calvarial defect one week after injury treated with either control shRNA or Noggin shRNA hASCs on an HA coated PLGA scaffold with or without BMP-2 (200ng/ml) (B) Average vessel count per 5× field. Statistical analysis was conducted utilizing the Mann-Whitney Test. A student’s two tailed t-test was used to compare groups. P-values (mean +/− S.D. *p<0.05).
Lastly, we performed additional immunohistochemistry analyses comparing control hASCs on a BMP-2 releasing scaffold to our Noggin shRNA transfected hASCs on a BMP-2 releasing scaffold. The Noggin shRNA transfected cells on the BMP-2 releasing scaffold demonstrated increased immunostain for VEGFA and significantly more tubule like structures with PECAM (CD-31) stain (See Figure, Supplemental Digital Content 2 E, F, which demonstrates Vascular signaling is greatly enhanced with noggin knockdown and BMP-2 over BMP-2 alone. (E) Human VEGF immunohistochemistry in a calvarial defect (brown color) with control non-transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). (F) CD31-Pecam stain (brown color) of calvarial defect one week after injury treated with control nontransfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). INSERT LINK HERE). These data indicate that vascular signaling is maximally enhanced with both noggin knockdown and BMP-2 supplementation.
hASCs Participate in Angiogenesis
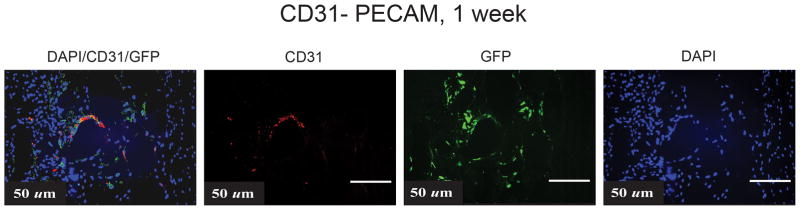
Though we believe that the enhanced angiogenesis comes primarily from host or surrounding mouse tissue, we set out to determine if the hASCs participated in vessel formation. To make this determination, we co-stained hASCs with control GFP shRNA as well as human specific PECAM-1 and showed, indeed, that the implanted hASCs (GFP+) express CD31 and are clustered around tubule-like structures, indicating their participation in vessel formation (Fig. 5). Thus, along with increased BMP-2 signaling, noggin suppression does appear to enhance VEGF secretion, angiogenic gene expression and angiogenesis.
Figure 5.
Co-localization of GFP+ hASCs, human specific PECAM-1 and DAPI to assess for vessel formation in a calvarial defect.
Discussion
When employing tissue engineering approaches to clinical problems, cells are often transplanted to a distant site on a scaffold into an environment different from their original niche. Tissue engineered constructs larger than 5mm require vascularization to allow for their survival after transplantation. Regulation of BMP signaling has been shown to stimulate differentiation of precursor cells towards an osteoblast lineage and to produce an overall greater amount of calcified matrix. Though we have previously demonstrated the effect of noggin suppression on osteogenesis, in this study we set out to examine its role in angiogenesis in a calvarial defect.(19) Previous studies have demonstrated that over-expression of noggin in HUVECs inhibits angiogenic capacity in vitro and in vivo.(29) In addition, BMP has been shown to stimulate angiogenesis through production of VEGF-A by osteoblasts.(40) Thus, we hypothesized that noggin suppressed hASCs on a BMP-2 releasing scaffold could create an angiogenic niche that would be ideal for tissue engineering technology.
By transplanting a cell type that has the capability of secreting VEGFA and undergoing differentiation into endothelial cells, we believe angiogenesis can be further enhanced. Interestingly, recent studies have also demonstrated that in addition to angiogenesis, new vessel formation is also created by vasculogenesis, which describes ischemia induced recruitment of vascular progenitor cells. (41–45). Bone marrow is generally thought as the main location of these vascular progenitor cells, though other studies have demonstrated the participation of perivascular cells and adipose tissue. (46, 47) In our model, we are directly applying a mesenchymal cell type (hASCs) to the region of hypoxia (calvarial defect). We do, believe, however, that our calvarial defect model does not necessarily mimic appendicular skeletal models. Recent work by Zuk et al failed to demonstrate improved healing of a femoral defect when a BMP-2 scaffold was used with or without adipose derived stromal cells, however angiogenesis as not assessed.(48) A calvarial defect benefits by being very vascular at baseline and receiving stimulation by surrounding periosteum as well as underlying dura mater which has been shown to enhance the osteogenic capacity of overlying hASCs.(49) Though other laboratories have failed to demonstrate ASC response to BMP-2,(50) our laboratory and others have demonstrated through multiple studies, the response of ASCs and bone marrow derived stromal cells to BMP-2 in vivo and in vitro.(34, 49, 51–53) Thus, we believe that by altering BMP signaling in hASCs, we have improved their angiogenic capacity in vitro as well the angiogenic niche in vivo. Future studies are underway to further explore the effect of enhanced BMP signaling on hASCs with regards to their osteogenic and chondrogenic capacity.
By suppressing a BMP inhibitor in hASCs, while supplementing BMP-2, we have created a niche that possesses enhanced angiogenic potential in vitro and in vivo. We believe that BMP-2 mediated angiogenesis up-regulated by BMP-2 supplementation, however, this signaling is further augmented through concomitant BMP-2 supplementation and noggin suppression. Previous studies have demonstrated that both BMP-2 and BMP-4 enhance VEGF-A protein levels in osteoblasts and we believe we are seeing a similar effect on hASCs.(40) This augmented angiogenesis is likely due to noggin suppression leading to increased BMP signaling and thus increased VEGF production and secretion by hASCs.
Conclusion
When suppressing noggin, and supplementing BMP-2, hASCs demonstrate significant increases in angiogenesis both in vitro and in vivo. By creating a cell with noggin suppressed and by using a scaffold with increased BMP-2, we can create a more angiogenic niche.
Supplementary Material
See Figure, Supplemental Digital Content 1, which demonstrates Validation of transfection and noggin-directed shRNA construct. (A) Evaluation of mean transcripts using QRT-PCR analysis demonstrated significant suppression with noggin directed shRNA (*p<0.001) by lentiviral transfection methodologies. A student’s two tailed t-test was used to compare groups. (B) Western blot quantification of noggin protein knockdown after lentiviral noggin knockdown. INSERT LINK HERE.
See Figure, Supplemental Digital content 2, which demonstrates Vascular signaling is greatly enhanced with noggin knockdown and BMP-2 over BMP-2 alone. (A) VEGFA gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups. (B) VEGFB gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (C) HIF-1alpha gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). An up regulation of all genes was noted in noggin-suppressed cells. A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (D) Quantification of tubules of control shRNA, control shRNA with BMP-2 (200ng/ml) and Noggin shRNA cells as counted by 5 blinded, independent observers. A student’s two tailed t-test was used to compare groups. A Bonferonni correction was used to handle multiple hypothesis testing (mean expression +/− S.D. *p<0.05). (E) Human VEGF immunohistochemistry in a calvarial defect (brown color) with control non-transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). (F) CD31-Pecam stain (brown color) of calvarial defect one week after injury treated with control nontransfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). INSERT LINK HERE.
SUPPLEMENTAL TABLE 1: Quantitative PCR Genes and Primer Sequences
SUPPLEMENTAL TABLE 2: Means and Standard Deviations
Acknowledgments
Sources of Support: This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grants 1 R21 DE019274-01 and RC2 DE020771-01, the Oak Foundation and Hagey Laboratory for Pediatric Regenerative Medicine to M.T.L. National Institutes of Health, National Institute of Diabetes, Digestive Disease Kidney Disease Grant 2 RO1 DK074095-07 to G.C.G. J.C.W. was supported by R01EB009689 and RC1HL099117. B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302. National Endowment for Plastic Surgery
We would like to thank Alex McMillan, PhD statistician and Jeffrey Wong, BS and MS student in Statistics at Stanford University for their assistance in Statistical Computations.
Footnotes
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Wu B, Dunn J. Accelerating Vascularization in Polycaprolactone Scaffolds by Endothelial Progenitor Cells. Tissue Eng Part A. doi: 10.1089/ten.tea.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao M, Zhou J, Li X, et al. Repair of bone defect with vascularized tissue engineered bone graft seeded with mesenchymal stem cells in rabbits. Microsurgery. 31:130–137. doi: 10.1002/micr.20854. [DOI] [PubMed] [Google Scholar]
- 4.Hegen A, Blois A, Tiron CE, et al. Efficient in vivo vascularization of tissue-engineering scaffolds. Journal of tissue engineering and regenerative medicine. 5:e52–62. doi: 10.1002/term.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thangarajah H, Vial IN, Chang E, et al. IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells. 2009;27:266–274. doi: 10.1634/stemcells.2008-0276. [DOI] [PubMed] [Google Scholar]
- 6.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behr B, Tang C, Germann G, et al. Locally Applied VEGFA Increases the Osteogenic Healing Capacity of Human Adipose Derived Stem Cells by Promoting Osteogenic and Endothelial Differentiation. Stem Cells. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhang SH, Cho SW, La WG, et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen JG, Frobert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Park C, Kim D, et al. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc Res. 80:310–316. doi: 10.1016/j.mvr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Rubina K, Kalinina N, Efimenko A, et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A. 2009;15:2039–2050. doi: 10.1089/ten.tea.2008.0359. [DOI] [PubMed] [Google Scholar]
- 12.Cai L, Johnstone BH, Cook TG, et al. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroeze RJ, Knippenberg M, Helder MN. Osteogenic differentiation strategies for adipose-derived mesenchymal stem cells. Methods Mol Biol. 702:233–248. doi: 10.1007/978-1-61737-960-4_17. [DOI] [PubMed] [Google Scholar]
- 14.Zou J, Wang G, Geng D, et al. A Novel Cell-Based Therapy in Segmental Bone Defect: Using Adipose Derived Stromal Cells. J Surg Res. 2009 doi: 10.1016/j.jss.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- 16.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 17.Nimmagadda S, Geetha Loganathan P, Huang R, et al. BMP4 and noggin control embryonic blood vessel formation by antagonistic regulation of VEGFR-2 (Quek1) expression. Dev Biol. 2005;280:100–110. doi: 10.1016/j.ydbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 19.Wan DC, Pomerantz JH, Brunet LJ, et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–26459. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 20.Levi BNE, Li S, Hyun JS, Jia FJ, Glotzbach JC, James AW, Montoro DT, Lee M, Quarto N, Huang M, Gurter GC, Wu J, Longaker MT. Novel Regulation of Human Adipose-Derived Stromal Cell Osteogenesis through Noggin Signaling. Under Review: Lancet. 2011 [Google Scholar]
- 21.Kelly MA, Hirschi KK. Signaling hierarchy regulating human endothelial cell development. Arterioscler Thromb Vasc Biol. 2009;29:718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd NL, Dhara SK, Rekaya R, et al. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2007;232:833–843. [PubMed] [Google Scholar]
- 23.Bressan M, Davis P, Timmer J, et al. Notochord-derived BMP antagonists inhibit endothelial cell generation and network formation. Dev Biol. 2009;326:101–111. doi: 10.1016/j.ydbio.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GJ, Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol. 2008;4:15–16. doi: 10.1038/nchembio0108-15. [DOI] [PubMed] [Google Scholar]
- 26.Dai J, Kitagawa Y, Zhang J, et al. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–999. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- 27.Chen NF, Smith ZA, Stiner E, et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 12:40–46. doi: 10.3171/2009.4.SPINE0876. [DOI] [PubMed] [Google Scholar]
- 28.Cahill KS, Chi JH, Day A, et al. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. Jama. 2009;302:58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 29.Kang HW, Walvick R, Bogdanov A., Jr In vitro and In vivo imaging of antivasculogenesis induced by Noggin protein expression in human venous endothelial cells. FASEB J. 2009;23:4126–4134. doi: 10.1096/fj.08-127795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levi B, James AW, Nelson ER, et al. Studies in adipose-derived stromal cells: migration and participation in repair of cranial injury after systemic injection. Plast Reconstr Surg. 127:1130–1140. doi: 10.1097/PRS.0b013e3182043712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James AW, Levi B, Commons GW, et al. Paracrine interaction between adipose-derived stromal cells and cranial suture-derived mesenchymal cells. Plast Reconstr Surg. 2010;126:806–821. doi: 10.1097/PRS.0b013e3181e5f81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao J, Ho JN, Lewis JA, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James AW, Leucht P, Levi B, et al. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16:2605–2616. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panetta NJ, Gupta DM, Lee JK, et al. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg. 2010;125:483–493. doi: 10.1097/PRS.0b013e3181c82d75. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Quarto N, Longaker MT. Activation of FGF signaling mediates proliferative and osteogenic differences between neural crest derived frontal and mesoderm parietal derived bone. PLoS One. 2011;5:e14033. doi: 10.1371/journal.pone.0014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James AW, Xu Y, Wang R, et al. Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- 37.James AW, Theologis AA, Brugmann SA, et al. Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS ONE. 2009;4:e7120. doi: 10.1371/journal.pone.0007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 39.Levi B, James AW, Nelson ER, et al. Human Adipose-Derived Stromal Cells Stimulate Autogenous Skeletal Repair via Paracrine Hedgehog Signaling with Calvarial Osteoblasts. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deckers MM, van Bezooijen RL, van der Horst G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 41.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 43.Rafii DC, Psaila B, Butler J, et al. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 44.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45 (Suppl A):A39–47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 47.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Chou YF, Zuk PA, Chang TL, et al. Adipose-derived stem cells and BMP2: Part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect Tissue Res. doi: 10.3109/03008207.2010.484514. [DOI] [PubMed] [Google Scholar]
- 49.Levi B, Nelson ER, Li S, et al. Dura Mater Stimulates Human Adipose-Derived Stromal to Undergo Bone Formation in Mouse Calvarial Defects. Stem Cells. 2011 doi: 10.1002/stem.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuk P, Chou YF, Mussano F, et al. Adipose-derived stem cells and BMP2: part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connect Tissue Res. 52:119–132. doi: 10.3109/03008207.2010.484515. [DOI] [PubMed] [Google Scholar]
- 51.Wan DC, Shi YY, Nacamuli RP, et al. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SJ, Kang SW, Do HJ, et al. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials. 2010;31:5652–5659. doi: 10.1016/j.biomaterials.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Tang Y, Tang W, Lin Y, et al. Combination of bone tissue engineering and BMP-2 gene transfection promotes bone healing in osteoporotic rats. Cell Biol Int. 2008;32:1150–1157. doi: 10.1016/j.cellbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See Figure, Supplemental Digital Content 1, which demonstrates Validation of transfection and noggin-directed shRNA construct. (A) Evaluation of mean transcripts using QRT-PCR analysis demonstrated significant suppression with noggin directed shRNA (*p<0.001) by lentiviral transfection methodologies. A student’s two tailed t-test was used to compare groups. (B) Western blot quantification of noggin protein knockdown after lentiviral noggin knockdown. INSERT LINK HERE.
See Figure, Supplemental Digital content 2, which demonstrates Vascular signaling is greatly enhanced with noggin knockdown and BMP-2 over BMP-2 alone. (A) VEGFA gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups. (B) VEGFB gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (C) HIF-1alpha gene expression profile of hASCs cultured in ODM or ODM with BMP-2 (200ng/ml). An up regulation of all genes was noted in noggin-suppressed cells. A student’s two sample t-test was used to compare groups (mean expression +/− S.D. *p<0.05). (D) Quantification of tubules of control shRNA, control shRNA with BMP-2 (200ng/ml) and Noggin shRNA cells as counted by 5 blinded, independent observers. A student’s two tailed t-test was used to compare groups. A Bonferonni correction was used to handle multiple hypothesis testing (mean expression +/− S.D. *p<0.05). (E) Human VEGF immunohistochemistry in a calvarial defect (brown color) with control non-transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). (F) CD31-Pecam stain (brown color) of calvarial defect one week after injury treated with control nontransfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml) or Noggin shRNA transfected hASCs on a HA coated BMP-2 releasing scaffold (200ug/ml). INSERT LINK HERE.
SUPPLEMENTAL TABLE 1: Quantitative PCR Genes and Primer Sequences
SUPPLEMENTAL TABLE 2: Means and Standard Deviations