Abstract
The vitamin A (VA) metabolite all-trans retinoic acid (RA) plays a key role in mucosal immune responses. RA is produced by gut-associated dendritic cells (DC), on which it also acts in a positive feedback loop to induce enzymes involved in its own synthesis. RA is required for generating gut-tropic lymphocytes and IgA-antibody-secreting cells (IgA-ASC). Moreover, RA modulates Foxp3+ TREG and Th17 differentiation. Thus, although recent evidence indicates that RA could be used as an effective “mucosal adjuvant” in vaccines, it also appears to be required for establishing intestinal immune tolerance. Here we discuss the roles proposed for RA in shaping intestinal immune responses and tolerance at the gut mucosal interface. We also focus on recent data exploring the mechanisms by which gut-associated DC acquire RA-producing capacity.
Keywords: vitamin A, retinoic acid, homing, dendritic cells, tolerance
1. Introduction
VA deficiency is an important public health problem, particularly in developing countries where it is associated with increased susceptibility to gastrointestinal and lung infectious diseases, poor response to vaccination, increased HIV pathogenesis, and overall increased mortality, especially in children (Sommer et al., 1983; Villamor and Fawzi, 2005). Accordingly, VA supplementation can dramatically reduce child mortality in these settings (Sommer et al., 1986; Villamor and Fawzi, 2005; West et al., 1991).
The VA metabolite RA is responsible for most of the biological effects of VA (Theodosiou et al., 2010). RA plays essential and pleiotropic roles in bone formation, reproduction, and organogenesis during embryonic development (Mark et al., 2006). Moreover, RA fulfills important functions in the formation of epithelial linings of the skin and mucosal tissues, which act as barriers to the external environment (McCullough et al., 1999; Wang et al., 1997). In addition, as we will discuss below, RA affects the innate and adaptive immune system in a number of ways (Iwata, 2009; Mora et al., 2008; Stephensen, 2001).
A seminal paper published by Iwata and collaborators in 2004 showed that RA is critical for inducing lymphocyte trafficking to the intestinal mucosa and that DC from Peyer’s patches (PP) and mesenteric lymph nodes (MLN) (gut-associated DC), but not from extra-intestinal tissues, can metabolize VA into RA (Iwata et al., 2004). Subsequent work showed that RA was also required to induce gut tropic B cells and to promote the differentiation of IgA-ASC in mice and humans (Mora et al., 2006). Furthermore, xRA was also shown to modulate Foxp3+ regulatory T cell (TREG) and Th17 cell differentiation (Benson et al., 2007; Coombes et al., 2007; Kang et al., 2007; Mucida et al., 2007; Schambach et al., 2007; Sun et al., 2007; Wang et al., 2010). Thus, vitamin A is important for both intestinal immune responses to pathogens and tolerance to food antigens and commensals. Disrupted RA signals, causing altered homing or impaired functional differentiation of lymphocytes, might be implicated in diseases such as inflammatory bowel diseases, type I diabetes, food allergy, and some infectious diarrhea.
Here we focus on the mechanisms regulating RA production by gut-associated DC and on the role of RA and gut-tropic lymphocytes in intestinal immune homeostasis and tolerance.
2. Vitamin A metabolism
VA is usually acquired from the diet either as all-trans retinol, retinyl esters, or β-carotene (Napoli, 2011; Theodosiou et al., 2010). All-trans retinol is esterified to retinyl esters and stored in the liver or it can associate to retinol binding protein (RBP), which transports retinol to target tissues (Napoli, 2011; Theodosiou et al., 2010). All-trans retinol is then oxidized intracellularly to all-trans retinal by ubiquitously expressed retinol dehydrogenases (RDH), which belong to the short chain dehydrogenase reductase (SDR) gene family. At least three RDH seem to be physiologically involved in this rate-limiting step: RDH1, RDH10 and DHRS9 (Napoli, 2011). Then, cytosolic retinal dehydrogenase enzymes (RALDH) catalyze the irreversible oxidation of all-trans retinal to RA (Napoli, 2011; Theodosiou et al., 2010).
At least four RALDH enzyme isoforms (RALDH1, RALDH2, RALDH3, and RALDH4) have been identified in mice, and highly homologous enzymes are present in humans and other chordates, indicative of the physiological importance of RA metabolism for many organisms. Genetic deletion experiments allowed to analyze the respective physiological contribution of the various RALDH to RA production (Penzes et al., 1997). Whereas RALDH1−/− mice are viable (Fan et al., 2003), RALDH2−/− and RALDH3−/− mice show early lethality, suggesting that these enzymes play essential roles in RA production during development (Dupe et al., 2003; Niederreither et al., 2003). RALDH4 has been cloned in mice, but its physiological contribution to retinoid metabolism remains to be determined (Lin et al., 2003).
RALDH expression is restricted to limited cell types. In adult mammals, three RALDH isoforms have been described in gut-associated cells, including small- and large-intestinal epithelial cells (IECs), mesenteric lymph nodes (MLN) stromal cells, and gut-associated DC (DC from Peyer’s patches, small-intestinal LP and MLN). IEC express RALDH1 (Bhat, 1998; Frota-Ruchon et al., 2000; Iwata et al., 2004; Lampen et al., 2000), whereas stromal cells in MLN express RALDH2 and probably RALDH1 and RALDH3 (Hammerschmidt et al., 2008; Molenaar et al., 2011). Among gut-associated DC, PP-DC express RALDH1 and to a lower extent RALDH2, whereas MLN-DC only express RALDH2 (Coombes et al., 2007; Iwata et al., 2004; Jaensson et al., 2008; Yokota et al., 2009). However, as we will discuss below, the relative in vivo relevance of RA production by different types of gut-associated cells, as well as the functional implications of expressing different RALDH isoforms, remain to be fully determined.
RA exerts its effects mostly through binding to heterodimers of nuclear RA receptors (RARα, β, γ) and retinoid X receptors (RXRα, β, γ) (Samarut and Rochette-Egly, 2011), although some specific effects can be mediated via PPARβ/γ (Mora et al., 2008; Schug et al., 2007). RAR-RXR heterodimers are ligand-dependent transcription factors that bind to cis-acting DNA sequences, called RA response elements (RARE), located in the promoter region of RA target genes. Although RAR receptors are ubiquitously expressed, RARβ expression is markedly enhanced by RA (Samarut and Rochette-Egly, 2011).
Termination of RA signaling is achieved through its catabolism into oxidized metabolites, such as 4-hydroxy RA and 4-oxo RA, by enzymes of the CYP26 family. Among these enzymes, CYP26A1 is directly upregulated by RA (Samarut and Rochette-Egly, 2011). While RA metabolites have been mostly considered to be biologically inert, there is evidence indicating that some of these metabolites retain the ability to signal through RAR (Sorg et al., 2008; Theodosiou et al., 2010). However, potential roles of RA metabolites in immune responses have not been described.
3. Role of RA in the regulation of T and B cell immune responses
3.1 RA reciprocally regulates the induction of gut- and skin-tropic lymphocytes
Lymphocyte migration to different lymphoid and extra-lymphoid tissues requires the expression of specific receptors on lymphocytes (homing receptors) and their corresponding ligands (addressins) on endothelial cells from tissue postcapillary venules (Mora, 2008). Naïve T and B cells recirculate among different lymphoid compartments and once they are activated by their cognate antigen they acquire the capacity to migrate to extra-lymphoid tissues (Mora, 2008). The skin and the gastrointestinal (GI) mucosa are the largest surfaces in the body exposed to the external environment and are also the extra-lymphoid tissues with the best-characterized migration requirements. Homing to the skin requires the expression of P-/E-selectin ligands, chemokine receptor CCR4, and integrin αLβ2 (LFA-1) on T cells as well as their respective ligands P-/E-selectin, CCL17/TARC, and ICAM-1 expressed in skin postcapillary venules (Mora, 2008). In contrast, migration to the small intestine lamina propria (LP) relies on integrin α4β7 (LPAM-1) and chemokine receptor CCR9 on T and B cells and their respective ligands, i.e., mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and CCL25/TECK in small bowel postcapillary venules. Of note, homing to the large bowel LP requires the integrins α4β7 and α4β7, but not CCR9 (Mora, 2008). In addition, it should be kept in mind that alternative pathways of lymphocyte recruitment to the intestine may occur in steady state as well as during inflammatory conditions (Villablanca et al., 2011a).
Regarding how lymphocytes acquire tissue-specific migratory capacity upon activation, an important observation was that gut-associated DC induce gut-homing receptors α4β7 and CCR9 on T cells upon activation, thus endowing activated T cells with gut-tropism (Johansson-Lindbom et al., 2003; Mora et al., 2003; Stagg et al., 2002). In addition to demonstrating for the first time a tissue-specific factor responsible for directing T cell migration, these studies highlighted a completely new function for DC, aside from their well-known role in T cell activation.
From a mechanistic standpoint, a groundbreaking advance in the field was the discovery that RA is sufficient and also necessary to induce gut-homing receptors on T cells (Iwata et al., 2004). RA is also required, and sufficient, to induce gut-tropic IgA-ASC in mice and humans (Hammerschmidt et al., 2011; Mora et al., 2006; Uematsu et al., 2008) as well as gut-homing human T cells (Eksteen et al., 2009) (Figure 1). Accordingly, some gut-associated DC, but not extra-intestinal DC, express high levels of RALDH enzymes and can synthesize RA, which is required for their gut-homing imprinting capacity. Indeed, induction of gut homing receptors on activated T cells occurred only when retinol, the substrate to synthesize RA, was provided to gut-associated DC (Coombes et al., 2007; Iwata et al., 2004; Yokota et al., 2009).
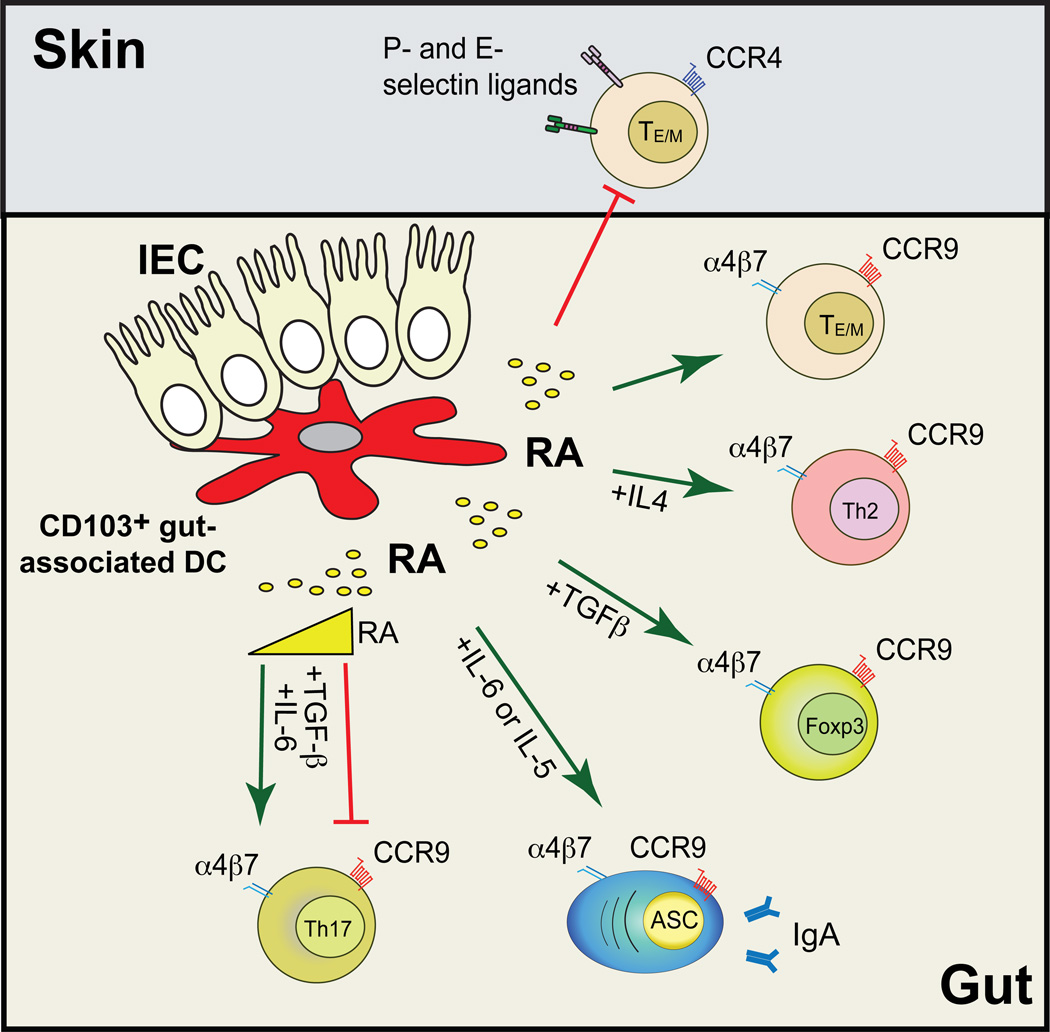
Figure 1. Retinoic acid orchestrates intestinal immunity.
CD103+ gut-associated-DC synthesize all-trans retinoic acid (RA), which is necessary and sufficient to induce gut homing receptors α4β7 and CCR9 on effector T and B cells, while reciprocally inhibiting the expression of skin homing receptors (P- and E-selectin ligands and CCR4). RA also potentiates the differentiation of Foxp3+ regulatory T cells (requiring TGF-β) and Th2 cells (requiring Th2 polarizing cytokines). In addition, RA promotes the induction of IgA-ASC (also needing IL-5 and/or IL-6). Of note, RA seems to be required for the induction of intestinal Th17 cells, although medium to high RA concentrations have been shown to inhibit ex vivo Th17 differentiation. It is also unclear whether RA is needed for extra-intestinal Th17 responses. Green and red lines indicate induction or inhibition, respectively.
The in vivo evidence demonstrating the importance of RA for inducing gut-tropic lymphocytes was gained from studies performed in vitamin A (VA)-depleted (VAD) mice. In order to generate VAD mice, pregnant females received a purified diet that lacked VA starting from days 7–10 of gestation. The pups were weaned at 4 weeks and were maintained on a VAD diet until 12 weeks of age before analysis was performed (Iwata et al., 2004; Mora et al., 2006). Mice deficient in lecithin:retinol acyltransferase (LRAT), which cannot store retinol in the liver, provide a convenient alternative to achieve VA depletion in a shorter time-frame and hence avoid potential unwanted effects of chronic VA depletion (O'Byrne et al., 2005). LRAT−/− mice develop normally when maintained on a VA sufficient diet, but they become VAD after only 2–4 weeks on a VAD diet (O'Byrne et al., 2005). VA depletion can be readily attested by the sharp reduction in the levels of the free retinol and retinyl esters in adipose tissue (O'Byrne et al., 2005).
Importantly, VA-deficient (VAD) mice lack T cells and IgA-ASC in the small intestine but not elsewhere (Iwata et al., 2004; Mora et al., 2006) and the induction of gut-tropic effector T cells is abrogated in VAD mice (Hall et al., 2011; Jaensson-Gyllenback et al., 2011; Villablanca et al., 2011b). The mechanism by which RA induces α4β7 and CCR9 in T cells is mediated, at least in part, by a direct effect of RA/RARα on Itga4 (encoding α4 integrin chain) and Ccr9 gene promoters (Hill et al., 2008; Kang et al., 2011; Ohoka et al., 2011). Interestingly, in addition to inducing gut-homing receptors, gut-associated DC and RA block the upregulation of skin-homing receptors on T lymphocytes (Iwata et al., 2004; Mora, 2008). Thus, RA reciprocally regulates the differentiation of gut- and skin-homing lymphocytes.
In addition to gut-associated DC, murine MLN stromal cells (CD45NegMHCIINegCD11cNeggp38+), can also produce RA and are sufficient to imprint gut-tropic T and/or B cells ex vivo (Hammerschmidt et al., 2008; Molenaar et al., 2011). Nonetheless, whether RA production by DC and/or stromal cells is required in vivo for imprinting gut-homing lymphocytes has not been determined. Given that depletion of these cell types will likely affect lymphoid architecture and/or lymphocyte activation, cell-specific knockouts for RALDH enzymes will be required to clarify this issue.
3.2 Role of RA in IgA production
Secretory IgA (SIgA) contributes to the intestinal barrier function and recent evidence supports the notion that such antibodies are involved in immunological homeostasis (Mantis et al., Muc Immunol 2011). Production of SIgA depends on IgA-antibody secreting plasma cells (ASC) and their immediate precursors (plasmablasts), which accumulate in the mucosa by selective homing mechanisms among which RA play an important role.
VAD rats or mice have decreased levels of total SIgA in intestinal lavages and decreased mucosal antigen-specific IgA responses, which have been correlated with impaired protection against oral infections and bacterial toxins (Mora et al., 2008; Sirisinha et al., 1980; Wiedermann et al., 1993). Of note, although VAD animals exhibited decreased numbers of IgA-ASC in the small bowel (Bjersing et al., 2002; Mora et al., 2006), their serum IgA levels were normal (Mora et al., 2006), suggesting that RA is not required for IgA-ASC differentiation in other mucosal compartments.
In addition to its role in physiological IgA-ASC differentiation, RA is sufficient to induce IgA production in ex vivo LPS-activated splenocytes (Tokuyama and Tokuyama, 1993; Tokuyama and Tokuyama, 1996). In this line, gut-associated DC induce IgA-ASCs by a mechanism depending, at least in part, on RA (Chang et al., 2008; Massacand et al., 2008; Mora et al., 2006; Uematsu et al., 2008), however other factors, including IL-5, IL-6 and TGF-β, are required for IgA class-switching and plasma cell differentiation (Sato et al., 2003; Tokuyama and Tokuyama, 1999; Watanabe et al., 2010) (Figure 1).
Interestingly, a recent report proposed that follicular dendritic cells (FDC), a subset of stromal cells found in lymphoid follicles, are necessary for IgA production in PP and that RA and TLR signals are required to confer FDC with IgA-inducing capacity (Fagarasan et al., 2010). In addition, plasmacytoid DC (pDC; CD11cintB220+PDCA1+) were proposed to be the main DC subset in charge of inducing thymus-independent IgA in PP and MLN via a mechanism involving APRIL, but not RA (Tezuka et al., 2011). However, although pDC were sufficient to induce IgA in vitro, whether these cells are necessary in vivo for IgA production remains to be determined. It is also unclear whether FDC and DC might interact or complement each other for IgA production.
3.3 RA modulates TREG and Th17 differentiation
While the intestinal mucosa needs to elicit protective immune responses against pathogenic microorganisms, inflammatory immune responses to food and harmless commensal microbiota should be prevented. In fact, T cell activation by oral antigens in the absence of inflammation favors naïve T cell differentiation to Foxp3+ regulatory T cells (TREG), which are crucial to prevent deleterious immune responses in the gut mucosa.
Initial studies showed that RA potentiates TGF-β-dependent TREG induction, while it reciprocally inhibits pro-inflammatory Th17 differentiation ex vivo (Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007). In addition, RA confers TREG with gut-homing capacity by inducing α4β7 and CCR9 (Benson et al., 2007; Kang et al., 2007; Moore et al., 2009; Siewert et al., 2007). Although it is possible that RA directly acts on T cells for TREG differentiation (Nolting et al., 2009; Xiao et al., 2008a), RA might also contribute indirectly by blocking suppressive signals provided by memory T cells and/or antigen-presenting cells (e.g., pro-inflammatory or Th2 cytokines that inhibit TREG induction) (Hill et al., 2008; Takaki et al., 2008).
While the in vitro effects of RA on TREG/Th17 are consistently reported in different studies, the in vivo effects and physiological roles of RA on TREG/Th17 differentiation are much less clear and seem to vary significantly depending on the experimental system. For instance, one study showed that RA supplementation did not affect TREG induction, while it blocked pathogenic Th17 cells in experimental allergic encephalomyelitis (EAE) (Xiao et al., 2008b). Conversely, data in a diabetes model showed that RA increased TREG without affecting Th17 cell frequencies (Van et al., 2009).
Interestingly, although RA enhances TREG differentiation in vitro, VAD mice did not show a decrease in TREG frequencies (Cha et al., 2010; Kang et al., 2009) and TREG from VAD mice efficiently suppressed inflammation in a mouse model of ileitis (Kang et al., 2009). However, the latter study did not assess whether RA was required in the recipient mice to suppress inflammation upon donor TREG transfer. Similarly, although in vitro data show that RA acts via RARα to induce TREG (Hill et al., 2008; Schambach et al., 2007), RARα−/− mice showed normal TREG levels in the lamina propria (Hall et al., 2011; Hill et al., 2008).
A caveat in the interpretation of the results described above is that it was not systematically discriminated between naturally occurring/thymus-derived TREG (nTREG, CD4+Foxp3+Helios+) and adaptive/inducible antigen-specific TREG (iTREG, CD4+Foxp3+HeliosNeg). In fact, whereas nTREG were not affected, de novo iTREG induction upon oral immunization was significantly abrogated in VAD mice (Hall et al., 2011).
On the other hand, despite the effect of RA blocking in vitro Th17 differentiation, RA induced gut-homing receptors on Th17 cells and VAD mice exhibited a marked decrease in Th17 numbers in the small bowel (Cha et al., 2010; Wang et al., 2010). Moreover, RA was required for Th1 and Th17 differentiation during intestinal Toxoplama gondii infection (Hall et al., 2011) and during an experimental model of celiac disease (DePaolo et al., 2011). Thus, RA appears to be critical in vivo for inducing and/or maintaining Th17 cells in the intestinal mucosa under steady-state and at least during some inflammatory settings. Alternatively, RA might be required to induce gut-homing Th17 cells, but not extra-intestinal pro-inflammatory Th17 responses. In fact, whereas high RA concentrations block Th17 induction, low RA levels seem to be required to induce Th17 cells in vitro (Uematsu et al., 2008), Analogously, low RA concentrations might allow the in vivo induction of gut-homing Th17 cells (Cha et al., 2010) (Figure 1).
Given the discrepancies mentioned above between in vitro and in vivo results regarding the effects of RA on TREG/Th17 responses, it will be important that future studies routinely analyze antigen-specific iTREG, nTREG, and Th17 responses, ideally in intestinal and extra-intestinal compartments. In addition, it should be kept in mind that RA deficiency might also affect epithelial integrity and the gut microbiota. In this regard, Segmented filamentous bacteria (SFB) were significantly reduced in the ileum of VAD mice (Cha et al., 2010). Since SFB induce intestinal Th17 differentiation (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009), decreased SFB might also partially explain the decrease in intestinal Th17 cells in VAD mice.
Among RA sources in the intestinal mucosa are gut-associated DC (Iwata et al., 2004), LP macrophages (Denning et al., 2007), IEC (Bhat, 1998; Frota-Ruchon et al., 2000; Lampen et al., 2000), and stromal cells (Hammerschmidt et al., 2008; Molenaar et al., 2009). Although all these cell types might potentially affect TREG/Th17 balance in the gut, DC are likely to play a determinant role in these responses. In fact, in addition to synthesizing RA, CD103+ DC express αvβ8 integrin (critical to activate latent TGF-β), and specific deletion of αv or β8 integrin chains in DC significantly impaired TREG (Paidassi et al., 2011; Worthington et al., 2011) and Th17 (Acharya et al., 2010; Melton et al., 2010) responses.
3.4 Role of RA in Th1 and Th2 polarization
VA deficiency correlates with decreased Th2 responses (Wiedermann et al., 1993), while VA supplementation blocks Th1 and promotes Th2 differentiation in vitro and in vivo (Iwata et al., 2003; Nikawa et al., 1999). While this Th2-promoting effect might favor protection in the gut mucosa by increasing humoral immune responses (Nikawa et al., 1999), it might also cause a predisposition to Th2-associated pathologies, such as asthma (Schuster et al., 2008). Mechanistically, RA promotes Th2 differentiation by inducing Gata3, Maf, Stat6, and Il4 genes, while reciprocally inhibiting Tbet expression (Dawson et al., 2006; Iwata et al., 2003; Lovett-Racke and Racke, 2002) (Figure 1). RA might also indirectly exert a Th2-promoting effect through modulation of DC (Hoag et al., 2002). It will be interesting to determine whether RA interacts with other DC-modulating factors for inducing Th2 responses, such as thymic stromal lymphopoietin (TSLP) (Ito et al., 2005; Rimoldi et al., 2005), IL-33 (Rank et al., 2009), or Notch ligands (Amsen et al., 2004; Maekawa et al., 2003).
4. Origin and differentiation of RA-producing DC
During the past years, several studies highlighted the presence of phenotypically distinct gut-specific APC subsets, which express macrophage and DC markers. Emerging evidence suggest that RA plays a significant role in regulating the functions of APC in intestinal immune compartments. Moreover, RA is produced by many APC subsets and might differentially modulate the induction of Th1, Th2, Th17, and TREG responses.
4.1 DC subsets in intestinal LP
Two major populations of murine phagocytic mononuclear subsets have been identified in the steady-state intestinal mucosa: CD11c+CD103+CX3CR1Neg (also CD11b+/NegCD8α+/NegF4/80Neg) DC and CD11c+/Neg CD103NegCX3CR1+ (also CD11b+CD8αNegF4/80+) macrophage-like cells, hereafter referred to as CD103+ and CX3CR1+ subsets, respectively (Denning et al., 2011; Yokota et al., 2009). While macrophage/DC precursors (MDP) give raise to both CD103+ DC and CX3CR1+ macrophages, common dendritic cell precursors (CDP) and pre-DC give raise only to CD103+ DC. On the other hand, LyC6high monocytes only produce CX3CR1+ macrophages (Bogunovic et al., 2009; Varol et al., 2007; Varol et al., 2009). In addition, CD103+ DC development depends on Flt3L (Bogunovic et al., 2009; Varol et al., 2007; Varol et al., 2009) whereas CX3CR1+ macrophages depend on M-CSF (Bogunovic et al., 2009). Whether all murine DC/macrophage subsets have phenotypic and functional counterparts in humans remains to be elucidated. Nonetheless, most CD103+ gut-associated DC express high levels of Aldh1a2 mRNA (encoding RALDH2) in mice and humans, hence they are able to synthesize RA, induce gut-tropic T cells, and promote TGF-β-dependent TREG differentiation (Coombes et al., 2007; Jaensson et al., 2008; Sun et al., 2007). Moreover, only CD103+ DC transport antigens from intestinal LP to the MLN, where they activate cognate antigen-specific naïve T cells (Schulz et al., 2009). In contrast, LP CX3CR1+ macrophages express Aldh1a1 (encoding RALDH1) (Denning et al., 2011) and exhibit significantly lower RA producing capacity than CD103+ LP DC in the small intestine, which correlates with a decreased gut-homing-inducing ability (Denning et al., 2011; Schulz et al., 2009). Interestingly, a subset of human lamina propria macrophages expressing CD14 and CD209 and which have been proposed to play a pro-inflammatory role in Crohn’s disease (CD), also express ALDH1A2 (Kamada et al., JI 2009).
CX3CR1+ macrophages are characterized by their close association with IEC, with some of them sending dendrites through the epithelial layer to sample antigens from the intestinal lumen (Niess et al., 2005). However, despite the fact that CX3CR1+ macrophages can capture gut-borne antigens, these cells exhibit slow turnover and remain stationary in the LP, without migrating to the MLN (Schulz et al., 2009). How CD103+ DC acquire intestinal antigens to activate T cells in MLN remains to be clarified, although it is possible that these cells get antigens indirectly via IEC and/or from CX3CR1+ macrophages.
4.2 How are gut-associated DC educated to produce RA?
Although it is well demonstrated that some gut-associated DC express RALDH enzymes and therefore are specialized in producing RA, it was important to understand how DC are “educated” in this tissue-specific functional property. Are bone marrow (BM) DC precursors pre-committed to become RA-producers or are they educated locally in the intestinal mucosal environment?
Since some DC are in close contact with IEC (Niess et al., 2005), it was proposed that these cells educate DC with gut-associated functional properties. In this regard, extra-intestinal DC co-cultured with IEC, or IEC lines, induced TREG (Iliev et al., 2009a; Iliev et al., 2009b), Th2 cells (Rimoldi et al., 2005) and gut-tropic T cells (Edele et al., 2008). Such ex vivo DC education required RA, TGF-β, and/or TSLP production by IEC (Edele et al., 2008; Iliev et al., 2009a; Rimoldi et al., 2005), both in mice and humans. However, whether, and how, IEC condition gut-associated DC in vivo remains to be demonstrated. In fact, CX3CR1+ DC do not acquire RA-producing capacity despite being in close contact to IEC (Schulz et al., 2009), suggesting that contact with IEC is not sufficient to educate gut-associated DC or that different DC subsets have different conditioning requirements.
Of interest, GM-CSF was shown to be sufficient to induce Aldh1a2 in extra-intestinal murine DC and mice lacking the common β subunit of GM-CSF/IL-3/IL-5 receptor exhibited impaired gut-associated DC education (Yokota et al., 2009). However, gut-associated DC from GM-CSF−/− mice were not impaired in their RA-synthesizing or gut-homing inducing potential (Wang et al., 2011), suggesting that this cytokine might not be essential for gut-associated DC education.
Among other possible candidates for DC education, PPARγ−agonists induced RA-synthesizing enzymes in human DC (Szatmari et al., 2006), but the results in murine DC are less clear (Housley et al., 2009; Villablanca et al., 2011b). In addition, LXR-ligands induced Aldh1a1 and Aldh1a2 in liver cells but not in DC (Huq et al., 2006; Villablanca et al., 2011b), suggesting that the expression of RALDH enzymes is differentially regulated among different tissues. On the other hand, PGE2, which is produced by skin stromal cells, inhibits Aldh1a2 expression in skin-derived DC (Stock et al., 2011). However, blocking prostaglandin synthesis by using cyclooxygenase (COX)-inhibitors markedly decreased Aldh1a2 expression in MLN-DC in the context of ileitis (Samson et al., 2011), suggesting that the effect of prostaglandins on RA production by DC might vary depending on the tissue analyzed and/or inflammatory status.
Interestingly, a recent report proposed that the Wnt/β-catenin pathway is involved in gut-associated DC education to induce tolerogenic responses in the gut mucosa. Several Wnt signaling proteins are expressed in LP-DC and specific deletion of β-catenin in DC resulted in markedly decreased expression of Aldh1a2 and RA-synthesizing capacity by gut-associated DC, which correlated with decreased induction of Treg and exacerbated inflammation in DSS colitis (Manicassamy et al., 2010).
4.3 Role of RA and TLR signals in gut-associated DC differentiation
Gut-associated DC are exposed to RA in the small intestine LP, and RA levels correlate with the ability of DC to induce gut tropic T cells and Treg (Villablanca et al., 2011b). In line with these observations, recent work by several groups supports the hypothesis that RA induces its own synthesis in DC in a positive feedback loop. RA is sufficient in vitro and in vivo to induce Aldh1a2 in extra-intestinal DC (e.g., PLN-DC), conferring them with RA-producing and gut-homing imprinting capacity, both in mice and humans cells (Hammerschmidt et al., 2011; Villablanca et al., 2011b) (Figure 2). Yokota et al. initially showed that MLN-DC from VAD mice exhibited markedly impaired RA-producing capacity (Yokota et al., 2009). These results were reproduced and extended by showing that gut-associated DC from VAD mice induced lower levels of gut-tropic lymphocytes, TREG and IgA-ASC compared with their counterparts from mice on a VA-sufficient diet (Feng et al., 2010; Jaensson-Gyllenback et al., 2011; Molenaar et al., 2011; Villablanca et al., 2011b; Yokota et al., 2009). Of note, MLN stromal cells from VAD mice also expressed low Aldh1a2 mRNA levels, suggesting that RA is also required for MLN stromal cell education (Molenaar et al., 2011). Although clear evidence demonstrates the physiological importance of RA for in vivo DC and stromal cell education, the molecular mechanism by which RA induces its own synthesis in the target cells is not fully understood. However, the lack of retinoic acid response elements (RARE) in the Aldh1a2 gene promoter, the requirement for de novo protein synthesis, and the delayed kinetic of protein transcription compared to known RA-target genes (Villablanca et al., 2011b) suggest that RA might induce Aldh1a2 transcription indirectly.
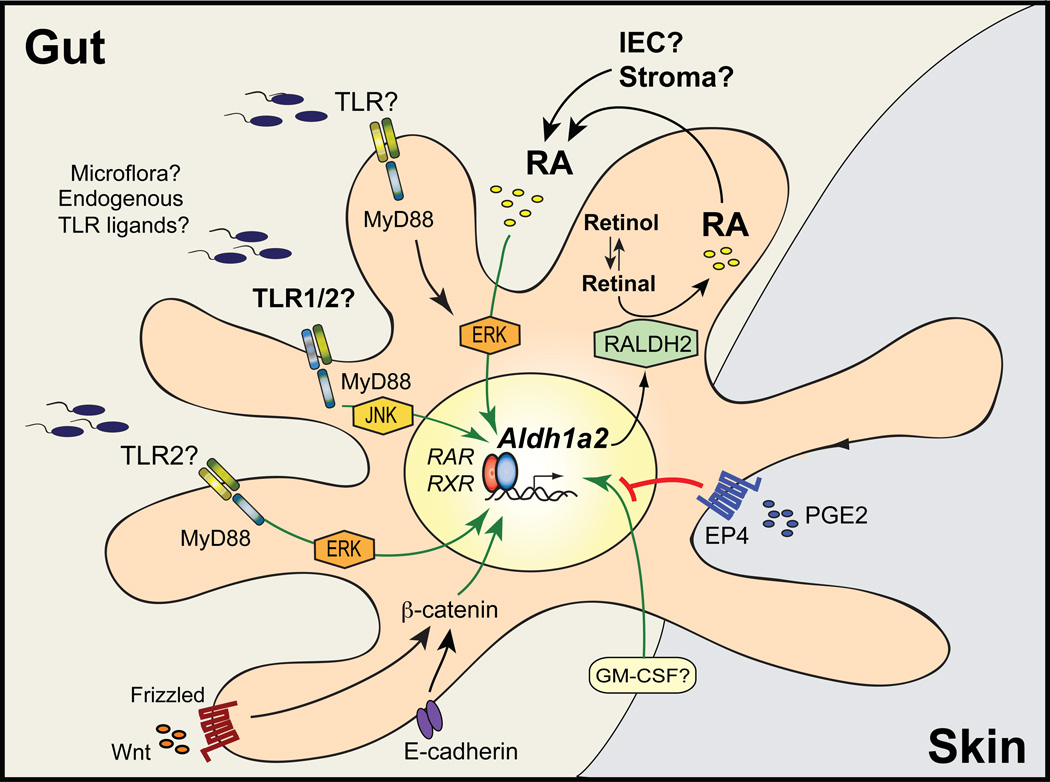
Figure 2. Mechanisms proposed to induce Aldh1a2 in gut-associated DC.
Aldh1a2 is the main RALDH isoform expressed in gut-associated DC, although Aldh1a1 and Aldh1a3 have also been described in other gut-associated cell types, such as IEC and stromal cells. Whereas the mechanisms controlling the expression of the latter RALDH isoforms remain to be determined, recent literature show that the expression of Aldh1a2 in DC can be modulated by several microenvironmental stimuli. In particular, recent evidence indicates that RA itself is necessary and sufficient to induce its own synthesis by upregulating Aldh1a2 in DC as a positive feedback loop. IEC are among the potential sources of RA to educate DC in the gut, although this remains to be demonstrated. In addition, several other factors have been proposed to induce Aldh1a2 in DC, including MyD88-dependent TLR2 signals, Wnt/β-catenin pathway, GM-CSF, and PPARγ-agonists (the latter not shown in the figure). In contrast, PGE2 acting on EP4 receptor inhibits Aldh1a2 induction in skin-associated DC. However, although the critical role of RA in Aldh1a2 induction in gut-associated DC has been consistently shown by several groups, the contribution of other factors in physiological gut-associated DC education and the intracellular signaling pathways mediating these effects (including MyD88, ERK and JNK) are currently less clear. Green and red lines indicate induction or inhibition of Aldh1a2 expression, respectively.
If RA is necessary in vivo to induce Aldh1a2 expression in gut-associated DC, hence conferring them with RA-synthesizing capacity, it raises the “chicken-and-egg” question of which cells are the initial source of RA which then educates DC in the gut. IEC express Aldh1a1 (Iliev et al., 2009a; Lampen et al., 2000) and can produce RA (Frota-Ruchon et al., 2000; Iwata et al., 2004). Moreover, Aldh1a1 expression in IEC does not decrease in VAD mice (rather it increases) (Bhat, 1998; Frota-Ruchon et al., 2000), suggesting that IEC might be hard-wired to synthesize RA, or that their RA-producing capacity depends on other environmental factors (e.g., TLR signals, Wnt/β-catenin pathway, or microbiota). Thus, IEC might provide, at least in part, a primary supply of RA in the gut mucosa, which might act like a “spark-plug” in gut-associated DC to induce in these cells a positive feedback loop driving Aldh1a2 expression. Experiments using in vivo cell-specific Aldh1 and/or Aldh2 deletion might be needed to answer these questions.
Interestingly, a subset of BM cells also express Aldh1a2 (Feng et al., 2010), suggesting that RA might also be produced in the BM microenvironment, perhaps pre-conditioning DC precursors with RA synthesizing capacity prior to their arrival in the gut mucosa. Of note, RA induced CCR9 on in vitro-generated BM-DC, while CD103 was not upregulated in BM-DC by RA treatment (Feng et al., 2010). This is in agreement with a lack of RA requirement in vivo for CD103 expression in gut-associated DC (Villablanca et al., 2011b). Moreover, MLN-DC from CD103−/− mice were not impaired in inducing gut-tropic T cells (Jaensson et al., 2008), suggesting that although CD103 is a useful marker for identifying RA-producing DC, it is not required for DC intestinal education and/or activity.
In the steady state, Aldh1a2 is induced in MLN-DC as well as in MLN stromal cells after birth and its expression increases throughout life (Molenaar et al., 2011), suggesting that postnatal exposure to the external environment might be required for conferring gut-associated DC with RA-synthesizing capacity. In this regard, the intestinal microflora plays a major role in maintaining normal immune homeostasis in the gut (Hooper and Macpherson, 2010), and microbial-associated molecular patterns, in particular TLR-agonists, modulate DC function in a number of ways. The TLR5-ligand flagellin increased Aldh1a2 expression in LP-DC and induces IgA-ASC in a RA-dependent manner (Uematsu et al., 2008). Moreover, the TLR2-ligand zymosan induced de novo Aldh1a2 mRNA and IL-10 production by extra-intestinal DC (Manicassamy et al., 2009). Similarly, the TLR1/2-agonist Pam3CSK4 efficiently induced Aldh1a2 in spleen-DC (Wang et al., 2011) (Figure 2).
In line with the previous data, MLN-DC from MyD88−/− mice exhibited decreased expression of Aldh1a2 mRNA and RALDH activity (Guilliams et al., 2010; Wang et al., 2011) and an impaired capacity to induce gut-tropic T cells (Wang et al., 2011). However, a recent report did not find differences in RALDH activity between wild type and MyD88−/− MLN-DC (Molenaar et al., 2011). Although the reasons for these discrepant results remain to be determined, MLN-DC from mice lacking TLR2 (MyD88-dependent TLR) also showed decreased RALDH activity and impaired capacity for gut-homing induction (Wang et al., 2011).
Interestingly, RA did not efficiently induce Aldh1a2 in MyD88−/− spleen-DC, which was correlated with a lack of RARβ in MyD88−/− DC, suggesting that MyD88 expression is also required for RA-mediated DC education (Villablanca et al., 2011b). Nonetheless, another study showed that daily RA treatment during ex vivo DC differentiation induced Aldh1a2 mRNA in MyD88−/− BM-DC (Feng et al., 2010). Since RA induces RARβ (Villablanca et al., 2011b), it is possible that prolonged RA treatment during BM-DC differentiation might induce RARβ in MyD88−/− DC, hence restoring their responsiveness to RA.
Although TLR signals are sufficient in vitro, and partially required in vivo, for gut-associated DC education, it is interesting to mention that MLN-DC from germ-free mice exhibited only a mild reduction in RALDH activity (Guilliams et al., 2010) and were able to induce α4β7 on T cells (Stagg et al., 2007). Although the latter study did not examine CCR9, it is possible that in the absence of gut microflora, food-derived and/or endogenous TLR agonists (Erridge, 2010) might still contribute to gut-associated DC conditioning.
5. Role of RA and gut-homing T cells in intestinal immune tolerance
5.1 Oral Immune Tolerance
Oral immune tolerance (OT) prevents pro-inflammatory responses to innocuous orally administered antigens, such as food and commensal microbiota, and it has been investigated as a potential treatment for diverse autoimmune conditions (Mayer and Shao, 2004; Weiner et al., 2011). Since patients with inflammatory bowel diseases (IBD) exhibit markedly decreased OT (Kraus et al., 2006; Kraus et al., 2004), impaired OT is believed to contribute importantly to the pathogenesis of IBD. However, despite being known for over 50 years (Chase, 1946), the mechanisms responsible for OT remain unclear (Weiner et al., 2011).
Administering high doses of oral antigen typically results in T cell clonal deletion and/or anergy (Chen et al., 1995; Van Houten and Blake, 1996), while low doses and repeating regimes of oral antigen administration lead to active immunosuppression by inducing Treg (Dubois et al., 2009; Hauet-Broere et al., 2003; Zhang et al., 2001). However, these two mechanisms are not mutually exclusive and some Treg also display characteristics of anergic T cells (von Boehmer, 2005).
Although orally administered antigens can be presented to T cells by DC in PP and MLN, it has been proposed that OT requires MLN (Spahn et al., 2002; Worbs et al., 2006) but not PP (Kraus et al., 2005; Spahn et al., 2001). In this setting, intestinal CD103+ DC take up antigens in the LP and, in a CCR7-dependent manner, migrate to MLN to activate naïve T cells (Worbs et al., 2006). Nevertheless, it is possible that the site for OT induction might also be determined by the nature of the antigen or its site of entry into the intestinal mucosa (Niedergang and Kweon, 2005). For instance, a prominent role for the liver has been proposed in OT models using haptens as a tolerizing agent (Dubois et al., 2009; Goubier et al., 2008; Yang et al., 1994), while tolerance to gliadin seems to rely on the spleen (Pre et al., 2011).
5.2 Immunological cellular network orchestrating OT
Antigen-specific TREG can be readily observed in PP and MLN as early as 24–48h post oral feeding (Hauet-Broere et al., 2003; Zinselmeyer et al., 2005). Previous work indicated that nTREG are not required for OT (Mucida et al., 2005), whereas iTREG are critical for OT generation (Curotto de Lafaille et al., 2008; Hadis et al., 2011).
Other studies have highlighted a contribution of pDC in OT. PDCA-1+ pDC are recruited to the LP early after exposure to food proteins (Ohue et al., 2011). In fact, a subset of immature pDC expresses CCR9 (Hadeiba et al., 2008), which has been proposed to play a role in pDC homing to the gut (Wendland et al., 2007). Moreover, pDC carrying dietary antigens to the liver were required for OT by inducing anergy/deletion of effector T cells (Dubois et al., 2009; Goubier et al., 2008). In addition, although adoptive transfer of TREG isolated from orally tolerized mice is sufficient to confer tolerance to non-tolerized mice (Broere et al., 2008; Hauet-Broere et al., 2003), a recent study showed that pDC were also sufficient to transfer tolerance (Goubier et al., 2008). Thus, it will be important to determine whether the involvement of TREG and/or pDC in OT might depend on the specific experimental system.
Intestinal macrophages also contribute to TREG induction in an IL-10-dependent manner (Denning et al., 2007; Murai et al., 2009). Moreover, mice lacking F4/80 or CX3CR1 (the latter expressed in intestinal macrophages), exhibited impaired OT (Hadis et al., 2011; Lin et al., 2005). Given that CX3CR1+ cells do not migrate to MLN (Schulz et al., 2009), these cells are likely to play a role in OT by contributing to IL-10 production and/or by promoting TREG proliferation in the intestinal LP (Hadis et al., 2011). Interestingly, IL-10 is required for OT and for proper TREG function in the gut mucosa (Cassani et al., 2011; Chaudhry et al., 2011; Murai et al., 2009).
5.3 Critical role of RA-dependent gut-homing T cells in OT
RA promotes TREG differentiation and upregulates gut-homing receptors on T cells (Mora, 2008; Siewert et al., 2007), suggesting that RA and/or gut-homing receptors might be required for OT generation. In agreement with this possibility, we have recently found that OT is abrogated in VAD mice, in mice lacking CCR9, or upon MAdCAM-1 blockade (Cassani et al., 2011). Our data are in agreement with recently published work showing that OT is significantly impaired in mice lacking α4β7 or its endothelial ligand MAdCAM-1 (Hadis et al., 2011). It is possible that TREG need to home to the small intestine LP to undergo further antigen-dependent expansion (Hadis et al., 2011). In addition, our data suggest that TREG need to home to the small intestine LP to complete their functional differentiation and acquire IL-10-producing capacity, a process that requires IL-10 signaling as well as IL-10 production by gut-homing TREG (Cassani et al., 2011).In fact, IL-10+ TREG are critical for immune tolerance at mucosal surfaces (Rubtsov et al., 2008) and IL-10 is needed for OT induction in different experimental models (Cong et al., 2004; Kraus et al., 2005; Navarro et al., 2011; Rizzo et al., 1999).
In line with our hypothesis that TREG need gut-homing receptors to become IL-10-producing cells, OT induction in an asthma model was correlated with the presence of CCR9+ IL-10+ Treg in the lung (Navarro et al., 2011). In addition, IL-10+ cells were found among circulating memory CCR9+ T cells in healthy human volunteers, whereas memory CCR9Neg T cells did not produce IL-10 (Papadakis et al., 2003), suggesting that human T cells might also need to express gut-homing receptors to acquire tolerogenic IL-10-producing capacity.
Interestingly, a recent report showed that Th17 cells also need to home to the small bowel to become tolerogenic, a process that is mediated by the chemokine receptor CCR6 (Esplugues et al., 2011). Although this study did not assess the role of RA or α4β7/CCR9 in Th17 homing and/or tolerogenicity, both RA and α4β7/CCR9 also appear to be required for efficient Th17 location/differentiation in the gut (Cha et al., 2010; Wang et al., 2010). Nonetheless, it is also possible that different types of immune responses or inflammation might induce alternative homing mechanisms for T cell migration to the gut mucosa (Villablanca et al., 2011a).
Even though the data discussed above suggest that RA plays a critical role in mucosal immune tolerance, it was recently shown that RA and IL-15 cooperated to induce proinflammatory cytokines and pathogenic effector T cells in an experimental model of gluten-related enteropathy (DePaolo et al., 2011). Given that these effects were observed in mice overexpressing IL-15, the physiological implications of these observations remain to be determined. Nevertheless, since RA is also required for homing and/or differentiation of potentially proinflammatory Th17 cells in the gut mucosa (Cha et al., 2010; Wang et al., 2010), it is possible that RA will have either a tolerogenic or pro-inflammatory role depending on the immunological context.
Moreover, some inflammatory conditions can affect gut-associated DC imprinting capacity. Induction of chronic experimental colitis decreased Tgfb2 and Aldh1a2 mRNA expression in CD103+ MLN-DC, which correlated with impaired RALDH activity and decreased TREG induction (Laffont et al., 2010). On the other hand, CD103+ MLN-DC from patients with active Crohn’s disease efficiently induced gut-tropic T cells (Jaensson et al., 2008), suggesting that intestinal inflammation does not necessarily lead to decreased RA production by DC. However, the latter study did not look at TREG induction by MLN-DC from Crohn’s patients and it could be that in a given inflammatory context induction of RA-dependent gut-tropic effector T cells is maintained, but TREG generation is not.
6. RA as a “mucosal adjuvant”
Transcutaneous immunization (TCI) requires RA to generate efficient IgA immune responses in the gut, an effect that was ascribed to the induction of gut-tropic IgA-ASC (Chang et al., 2008). Moreover, gut-immune responses induced upon vaccination with adenovirus (Ad)-based vectors were impaired in β7−/− mice (lacking α4β7) or in VAD mice (Kaufman et al., 2011). In fact, even mild to moderate VA deficiency significantly impaired vaccine-induced protection in the intestinal mucosa, a defect that was completely reversed by short-term VA supplementation (Kaufman et al., 2011). Thus, RA appears to be a critical factor for inducing efficient humoral and T cell immune responses in the intestinal mucosa.
It should be kept in mind that, besides inducing gut-tropic T cells, RA might have additional homing-independent effects on immune cells (e.g., on DC migration/maturation), which might contribute to boost immune responses in extra-intestinal compartments, such as lungs and vaginal mucosa (Martin Mdel et al., 2010; Tan et al., 2011).
Of interest, Ad-based vectors can also induce extra-intestinal expression of Aldh1a1 and Aldh1a2 enzymes, raising the possibility that ectopic RA production might contribute to induce gut-homing T cells at the site of immunization (Ganguly et al., 2011; Kaufman et al., 2011). However, analogous to subcutaneous immunization with vaccinia virus (Liu et al., 2006), it is likely that intramuscular immunization with Ad-based vectors also requires gut-associated lymphoid tissues (e.g., MLN) to imprint gut-tropic T cells.
Remarkably, oral or subcutaneous RA supplementation was sufficient to condition DC in skin-draining PLN to produce RA and to induce gut-tropic T and B cells in this extra-intestinal lymphoid compartment (Hammerschmidt et al., 2011; Villablanca et al., 2011b). Moreover, subcutaneous RA supplementation at the time of immunization elicited a potent IgA response in the small intestine, which protected mice from cholera toxin-induced diarrhea (Hammerschmidt et al., 2011). Therefore, given its capacity to efficiently induce gut-homing lymphocytes in extra-intestinal tissue, RA, or other pharmacological RAR-agonists, could potentially be used as “mucosal adjuvants” in the context of vaccination to enhance protective immune responses in the gut (Mora et al., 2008).
7. Concluding remarks
After the seminal observation identifying RA as the key factor to induce gut-homing T cells (Iwata et al., 2004), it was determined that RA modulates several other immunological properties in the intestinal mucosa, such as the induction of gut-tropic B cells, IgA-ASC, TREG, and Th17 responses. It will be important to further define in which contexts RA promotes tolerogenic versus pro-inflammatory immune responses in the gut mucosa.
Regarding gut-associated DC education, several microenvironmental factors have been proposed to be important to confer DC with RA-producing capacity, and recent evidence suggests that GM-CSF, Wnt/β-catenin pathway, TLR signals, and RA itself might have a role in gut-associated DC education. Whether and how these conditioning factors interact with each other to educate intestinal DC remains to be determined.
In addition to enhance protective immune responses in the gut, recent evidence indicates that RA-dependent gut-homing T cells also play a key role in establishing, and probably maintaining, immune tolerance in the intestinal mucosa. Given that therapies blocking gut-homing receptors are currently in clinical use or in advanced clinical trial for IBD, it will be important to assess whether a chronic gut-homing blockade might paradoxically interfere with normal intestinal immune homeostasis.
Acknowledgements
We thank Ms. Allison McNulty for excellent editorial assistance. JRM is indebted to Ingrid Ramos for constant support. BC was supported by a postdoctoral fellowship from EMBO. EJV was supported by a postdoctoral fellowship from Crohn’s & Colitis Foundation of America (CCFA). JRM was supported by grants from CCFA, Cancer Research Institute (CRI), Massachusetts Life Science Center (MLSC), Pilot Feasibility Grant NIH/NIAMS P30 AR042689 (HSDRC), and NIH New Director’s Innovator Award DP2 2009A054301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no competing financial interests.
References
- Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, Lacy-Hulbert A. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. The Journal of clinical investigation. 2010;120(12):4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117(4):515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007 doi: 10.1084/jem.20070719. 1775-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat PV. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett. 1998;426(2):260–262. doi: 10.1016/s0014-5793(98)00355-x. [DOI] [PubMed] [Google Scholar]
- Bjersing JL, Telemo E, Dahlgren U, Hanson LA. Loss of ileal IgA+ plasma cells and of CD4+ lymphocytes in ileal Peyer's patches of vitamin A deficient rats. Clin Exp Immunol. 2002;130(3):404–408. doi: 10.1046/j.1365-2249.2002.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere F, Wieten L, Klein Koerkamp EI, van Roon JA, Guichelaar T, Lafeber FP, van Eden W. Oral or nasal antigen induces regulatory T cells that suppress arthritis and proliferation of arthritogenic T cells in joint draining lymph nodes. J Immunol. 2008;181(2):899–906. doi: 10.4049/jimmunol.181.2.899. [DOI] [PubMed] [Google Scholar]
- Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, Sparwasser T, Snapper SB, Weiner HL, Mora JR. Gut-Tropic T Cells That Express Integrin alpha4beta7 and CCR9 Are Required for Induction of Oral Immune Tolerance in Mice. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184(12):6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- Chang SY, Cha HR, Igarashi O, Rennert PD, Kissenpfennig A, Malissen B, Nanno M, Kiyono H, Kweon MN. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180(7):4361–4365. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]
- Chase MW. Inhibition of experimental drug allergy by prior feeding of the sensitizing agent. Proc Soc Exp Biol Med. 1946;61:257–259. doi: 10.3181/00379727-61-15294p. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376(6536):177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Cong Y, Liu C, Weaver CT, Elson CO. Early upregulation of T cell IL-10 production plays an important role in oral tolerance induction. Ann N Y Acad Sci. 2004;1029:319–320. doi: 10.1196/annals.1309.037. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional Specializations of Intestinal Dendritic Cell and Macrophage Subsets That Control Th17 and Regulatory T Cell Responses Are Dependent on the T Cell/APC Ratio, Source of Mouse Strain, and Regional Localization. J Immunol. 2011 doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471(7337):220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137(3):1019–1028. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181(6):3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- Eksteen B, Mora JR, Haughton EL, Henderson NC, Turner LL, Villablanca EJ, Curbishley SM, Aspinall AI, von Andrian UH, Adams DH. Gut Homing Receptors on CD8 T Cells Are Retinoic Acid Dependent and Not Maintained by Liver Dendritic or Stellate Cells. Gastroenterology. 2009;137(1):320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? Journal of leukocyte biology. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O'Connor W, Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011;475(7357):514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23(13):4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. Journal of immunology. 2010;185(10):5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frota-Ruchon A, Marcinkiewicz M, Bhat PV. Localization of retinal dehydrogenase type 1 in the stomach and intestine. Cell Tissue Res. 2000;302(3):397–400. doi: 10.1007/s004410000281. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. Adenovirus type induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of gut-homing CD8 T cells. Mucosal immunology. 2011 doi: 10.1038/mi.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115(10):1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9(11):1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3(+) Regulatory T Cells in the Lamina Propria. Immunity. 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205(11):2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, Forster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. The Journal of clinical investigation. 2011 doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauet-Broere F, Unger WW, Garssen J, Hoijer MA, Kraal G, Samsom JN. Functional CD25− and CD25+ mucosal regulatory T cells are induced in gut-draining lymphoid tissue within 48 h after oral antigen application. Eur J Immunol. 2003;33(10):2801–2810. doi: 10.1002/eji.200324115. [DOI] [PubMed] [Google Scholar]
- Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29(5):758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132(12):3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews. Immunology. 2010;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- Housley WJ, O'Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, Clark RB. PPARgamma regulates retinoic acid-mediated DC induction of Tregs. Journal of leukocyte biology. 2009;86(2):293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq MD, Tsai NP, Gupta P, Wei LN. Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. EMBO J. 2006;25(13):3203–3213. doi: 10.1038/sj.emboj.7601181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009a doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009b;58(11):1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21(1):8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15(8):1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205(9):2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaensson-Gyllenback E, Kotarsky K, Zapata F, Persson EK, Gundersen TE, Blomhoff R, Agace WW. Bile retinoids imprint intestinal CD103(+) dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011;4(4):438–447. doi: 10.1038/mi.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003;198(6):963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179(6):3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- Kang SG, Park J, Cho JY, Ulrich B, Kim CH. Complementary roles of retinoic acid and TGF-beta1 in coordinated expression of mucosal integrins by T cells. Mucosal immunology. 2011;4(1):66–82. doi: 10.1038/mi.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137(4):1391–1402. e1391–e1396. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DR, De Calisto J, Simmons NL, Cruz AN, Villablanca EJ, Mora JR, Barouch DH. Vitamin A Deficiency Impairs Vaccine-Elicited Gastrointestinal Immunity. Journal of immunology. 2011;187(4):1877–1883. doi: 10.4049/jimmunol.1101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115(8):2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Cheifetz A, Toy L, Meddings JB, Mayer L. Evidence for a genetic defect in oral tolerance induction in inflammatory bowel disease. Inflammatory bowel diseases. 2006;12(2):82–88. doi: 10.1097/01.MIB.0000200343.61707.52. discussion 81. [DOI] [PubMed] [Google Scholar]
- Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126(7):1771–1778. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40(7):1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen A, Meyer S, Arnhold T, Nau H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther. 2000;295(3):979–985. [PubMed] [Google Scholar]
- Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, Kerley M, Mucenski ML, Gordon S, Stein-Streilein J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. 2005;201(10):1615–1625. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Zhang M, Abraham M, Smith SM, Napoli JL. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem. 2003;278(11):9856–9861. doi: 10.1074/jbc.M211417200. [DOI] [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25(3):511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Racke MK. Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell Immunol. 2002;215(1):54–60. doi: 10.1016/s0008-8749(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19(4):549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15(4):401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Martin Mdel P, Seth S, Koutsonanos DG, Jacob J, Compans RW, Skountzou I. Adjuvanted influenza vaccine administered intradermally elicits robust long-term immune responses that confer protection from lethal challenge. PLoS One. 2010;5(5):e10897. doi: 10.1371/journal.pone.0010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massacand JC, Kaiser P, Ernst B, Tardivel A, Burki K, Schneider P, Harris NL. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS ONE. 2008;3(7):e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L, Shao L. Therapeutic potential of oral tolerance. Nature reviews. Immunology. 2004;4(6):407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- McCullough FS, Northrop-Clewes CA, Thurnham DI. The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58(2):289–293. doi: 10.1017/s0029665199000403. [DOI] [PubMed] [Google Scholar]
- Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. The Journal of clinical investigation. 2010;120(12):4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, Huehn J, Forster R, O'Toole T, Jansen W, Eestermans IL, Kraal G, Mebius RE. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183(10):6395–6402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O'Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. Journal of immunology. 2011;186(4):1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- Moore C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Transforming growth factor-beta and all-trans retinoic acid generate ex vivo transgenic regulatory T cells with intestinal homing receptors. Transplant Proc. 2009;41(6):2670–2672. doi: 10.1016/j.transproceed.2009.06.130. [DOI] [PubMed] [Google Scholar]
- Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14(2):275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115(7):1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10(11):1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, Cazareth J, Sparwasser T, Dombrowicz D, Glaichenhaus N, Julia V. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 2011;4(1):53–65. doi: 10.1038/mi.2010.51. [DOI] [PubMed] [Google Scholar]
- Niedergang F, Kweon MN. New trends in antigen uptake in the gut mucosa. Trends Microbiol. 2005;13(10):485–490. doi: 10.1016/j.tim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130(11):2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Nikawa T, Odahara K, Koizumi H, Kido Y, Teshima S, Rokutan K, Kishi K. Vitamin A prevents the decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J Nutr. 1999;129(5):934–941. doi: 10.1093/jn/129.5.934. [DOI] [PubMed] [Google Scholar]
- Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. The Journal of experimental medicine. 2009;206(10):2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280(42):35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex. Journal of immunology. 2011;186(2):733–744. doi: 10.4049/jimmunol.1000913. [DOI] [PubMed] [Google Scholar]
- Ohue R, Nakamoto M, Kitabatake N, Tani F. Changes in lamina propria dendritic cells on the oral administration of exogenous protein antigens during weaning. Cytotechnology. 2011 doi: 10.1007/s10616-011-9351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, Stuart LM, Savill J, Lacy-Hulbert A. Preferential Expression of Integrin alphavbeta8 Promotes Generation of Regulatory T Cells by Mouse CD103 + Dendritic Cells. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA, Landers C, Prehn J, Kouroumalis EA, Moreno ST, Gutierrez-Ramos JC, Hodge MR, Targan SR. CC chemokine receptor 9 expression defines a subset of peripheral blood lymphocytes with mucosal T cell phenotype and Th1 or T-regulatory 1 cytokine profile. J Immunol. 2003;171(1):159–165. doi: 10.4049/jimmunol.171.1.159. [DOI] [PubMed] [Google Scholar]
- Penzes P, Wang X, Napoli JL. Enzymatic characteristics of retinal dehydrogenase type I expressed in Escherichia coli. Biochim Biophys Acta. 1997;1342(2):175–181. doi: 10.1016/s0167-4838(97)00102-7. [DOI] [PubMed] [Google Scholar]
- Pre MF, Kozijn AE, van Berkel LA, Borg MN, Lindenbergh-Kortleve D, Jensen LT, Kooy-Winkelaar Y, Koning F, Boon L, Nieuwenhuis EE, Sollid LM, Fugger L, Samsom JN. Tolerance to Ingested Deamidated Gliadin in Mice is Maintained by Splenic, Type 1 Regulatory T Cells. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.04.048. [DOI] [PubMed] [Google Scholar]
- Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6(5):507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- Rizzo LV, Morawetz RA, Miller-Rivero NE, Choi R, Wiggert B, Chan CC, Morse HC, 3rd, Nussenblatt RB, Caspi RR. IL-4 and IL-10 are both required for the induction of oral tolerance. J Immunol. 1999;162(5):2613–2622. [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Samarut E, Rochette-Egly C. Nuclear retinoic acid receptors: Conductors of the retinoic acid symphony during development. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Samson CM, Jurickova I, Molden E, Schreiner W, Colliver J, Bonkowski E, Han X, Trapnell BC, Denson LA. Granulocyte-macrophage colony stimulating factor blockade promotes ccr9(+) lymphocyte expansion in Nod2 deficient mice. Inflammatory bowel diseases. 2011 doi: 10.1002/ibd.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171(7):3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37(9):2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129(4):723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. The Journal of experimental medicine. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180(3):1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Floess S, Campbell DJ, Hamann A, Huehn J. Induction of organ-selective CD4+ regulatory T cell homing. Eur J Immunol. 2007;37(4):978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- Sirisinha S, Darip MD, Moongkarndi P, Ongsakul M, Lamb AJ. Impaired local immune response in vitamin A-deficient rats. Clin Exp Immunol. 1980;40(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Tarwotjo I, Djunaedi E, West KP, Jr, Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet. 1986;1(8491):1169–1173. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983;2(8350):585–588. doi: 10.1016/s0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- Sorg O, Tran C, Carraux P, Grand D, Barraclough C, Arrighi JF, Descombes P, Piguet V, Saurat JH. Metabolism and biological activities of topical 4-oxoretinoids in mouse skin. J Invest Dermatol. 2008;128(4):999–1008. doi: 10.1038/sj.jid.5701106. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31(4):1278–1287. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32(4):1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32(5):1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Stagg AJ, Norin KE, Midtvedt T, Kamm MA, Knight SC, Bjorksten B. Mesenteric dendritic cells from germ-free mice cause less T-cell stimulation but still induce a4b7 integrin. Microbial Ecology in Health and Disease. 2007;19(3):171–183. [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Stock A, Booth S, Cerundolo V. Prostaglandin E2 suppresses the differentiation of retinoic acid-producing dendritic cells in mice and humans. The Journal of experimental medicine. 2011;208(4):761–773. doi: 10.1084/jem.20101967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203(10):2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. The Journal of biological chemistry. 2008;283(22):14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol. 2011 doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34(2):247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cellular and molecular life sciences : CMLS. 2010;67(9):1423–1445. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama H, Tokuyama Y. Retinoids enhance IgA production by lipopolysaccharide-stimulated murine spleen cells. Cell Immunol. 1993;150(2):353–363. doi: 10.1006/cimm.1993.1203. [DOI] [PubMed] [Google Scholar]
- Tokuyama H, Tokuyama Y. The regulatory effects of all-trans-retinoic acid on isotype switching: retinoic acid induces IgA switch rearrangement in cooperation with IL-5 and inhibits IgG1 switching. Cell Immunol. 1999;192(1):41–47. doi: 10.1006/cimm.1998.1438. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Tokuyama H. Retinoids as Ig isotype-switch modulators. Cell Immunol. 1996;170(2):230–234. [PubMed] [Google Scholar]
- Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- Van Houten N, Blake SF. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J Immunol. 1996;157(4):1337–1341. [PubMed] [Google Scholar]
- Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58(1):146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204(1):171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Villablanca EJ, Cassani B, von Andrian UH, Mora JR. Blocking lymphocyte localization to the gastrointestinal mucosa as a therapeutic strategy for inflammatory bowel diseases. Gastroenterology. 2011a;140(6):1776–1784. doi: 10.1053/j.gastro.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca EJ, Wang S, de Calisto J, Gomes DC, Kane MA, Napoli JL, Blaner WS, Kagechika H, Blomhoff R, Rosemblatt M, Bono MR, von Andrian UH, Mora JR. MyD88 and Retinoic Acid Signaling Pathways Interact to Modulate Gastrointestinal Activities of Dendritic Cells. Gastroenterology. 2011b;141(1):176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18(3):446–464. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol. 2010;184(10):5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Swartz-Basile DA, Rubin DC, Levin MS. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J Nutr. 1997;127(7):1297–1303. doi: 10.1093/jn/127.7.1297. [DOI] [PubMed] [Google Scholar]
- Wang S, Villablanca EJ, De Calisto J, Gomes DC, Nguyen DD, Mizoguchi E, Kagan JC, Reinecker HC, Hacohen N, Nagler C, Xavier RJ, Rossi-Bergmann B, Chen YB, Blomhoff R, Snapper SB, Mora JR. MyD88-Dependent TLR1/2 Signals Educate Dendritic Cells with Gut-Specific Imprinting Properties. Journal of immunology. 2011;187:141–150. doi: 10.4049/jimmunol.1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M, Komori T, Ito Y, Shimizu A. Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol. 2010;184(6):2785–2792. doi: 10.4049/jimmunol.0901823. [DOI] [PubMed] [Google Scholar]
- Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunological reviews. 2011;241(1):241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104(15):6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KP, Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. 1991;338(8759):67–71. doi: 10.1016/0140-6736(91)90070-6. [DOI] [PubMed] [Google Scholar]
- Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203(3):519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal Dendritic Cells Activate Transforming Growth Factor-beta and Induce Foxp3+ T Regulatory Cells via Integrin alphavbeta8. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008a;181(4):2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. Journal of immunology. 2008b;181(4):2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-D, Michie SA, Tisch R, Karin N, Steinman L, McDevitt HO. A predominant role of integrin α4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12604–12608. doi: 10.1073/pnas.91.26.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song SY, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21(4):361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167(8):4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, Millington OR, Smith KM, Rush CM, Parker I, Cahalan M, Brewer JM, Garside P. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201(11):1815–1823. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]