Abstract
Background
The risk of post-operative bleeding is the chief concern expressed by plastic surgeons who do not use pharmacologic prophylaxis against venous thromboembolism (VTE). The Plastic Surgery Foundation-funded Venous Thromboembolism Prevention Study (VTEPS) examined whether receipt of post-operative enoxaparin prophylaxis changed rates of 60-day re-operative hematoma.
Methods
In 2009, VTEPS Network sites uniformly adopted a “best practice” clinical protocol to provide post-operative enoxaparin prophylaxis to adult plastic surgery patients at risk for peri-operative VTE. VTEPS historic control patients (2006–2008) received no chemoprophylaxis for 60 days after surgery. Retrospective chart review identified demographic and surgery-specific risk factors that potentially contributed to bleeding risk. The primary study outcome was 60-day re-operative hematoma. Stratified analyses examined re-operative hematoma in the overall population and among high-risk patients. Multivariable logistic regression controlled for identified confounders.
Results
Complete data were available for 3,681 patients (2,114 controls and 1,567 enoxaparin patients). Overall, post-operative enoxaparin did not change the rate of re-operative hematoma when compared to controls (3.38% vs. 2.65%, p=0.169). Similar results were seen in subgroup analyses for breast reconstruction (5.25% vs. 4.21%, p=0.737), breast reduction (7.04% vs. 8.29%, p=0.194), or non-breast plastic surgery (2.20% vs. 1.46%, p=0.465). In the regression model, independent predictors of re-operative hematoma included breast surgery, microsurgical procedure, and post-bariatric body contouring. Receipt of post-operative enoxaparin was not an independent predictor (OR 1.16, 95% CI 0.77–1.76).
Conclusions
Post-operative enoxaparin does not produce a clinically relevant or statistically significant increase in observed rates of re-operative hematoma. Independent predictors for re-operative hematoma include breast surgery, post-bariatric body contouring, and microsurgical procedure.
Clinical Question
Risk
Level of Evidence
III (retrospective cohort study)
INTRODUCTION
Venous thromboembolic disease (VTE) includes deep venous thrombosis (DVT) and pulmonary embolus (PE). One in ten patients with symptomatic PE will be dead within 60 minutes, even when the event occurs in the hospital 1. Patients with DVT and patients who survive a PE event have potentially devastating consequences, including right ventricular strain, right heart failure, and/or the post-thrombotic syndrome 1–3. This underscores the importance of VTE prevention through risk stratification and prophylaxis 2, 4–8. Many VTE events are considered to be potentially preventable 9–12. VTE has been identified as an important patient safety issue by major policymakers and payers 12–14.
Multiple randomized controlled trials in general and orthopaedic surgery patients have demonstrated significant VTE risk reduction with chemoprophylaxis 15–22. Despite these high-quality studies, chemoprophylaxis remains under-utilized among surgical patients. In 2001, Arnold and colleagues reviewed VTE episodes diagnosed at a single institution over a one year period. Among patients for whom VTE prophylaxis was indicated based on guidelines, 67% received inadequate prophylaxis. The authors concluded that many VTE events are potentially preventable 4. Stratton and colleagues 23 examined VTE prophylaxis patterns in ten acute care hospitals one year after the “gold standard” American College of Chest Physicians (ACCP) guidelines were published 24. Compliance with ACCP recommendations ranged from a minimum of 50% for patients after major abdominal surgery to 84% for total hip replacement patients.
General and orthopaedic surgery patients are systematically different from plastic and reconstructive surgery patients. Thus, existing high-quality trials cannot necessarily be generalized to plastic surgery patients. Two surveys that examined VTE prophylaxis patterns among board-certified plastic surgeons have recently been published. 40% of surgeons who perform post-bariatric body contouring and 75% of surgeons who perform autologous tissue breast reconstruction do not routinely provide post-operative chemoprophylaxis 25, 26. Among surgeons who do not provide chemoprophylaxis, 84% cited concern related to the risk of re-operative hematoma and 50% cited lack of evidence specific to the plastic surgery population as reasons. Hematoma risk and lack of evidence appear to be the major drivers of plastic surgeons’ decisions not to use chemoprophylaxis.
The Plastic Surgery Foundation funded the Venous Thromboembolism Prevention Study (VTEPS) in 2008. VTEPS was conducted over a three year period by the VTEPS Network, a consortium of four high-volume plastic surgery groups at tertiary care institutions. The study examined the effectiveness and safety of post-operative enoxaparin prophylaxis in plastic surgery patients. This manuscript examines the effect of post-operative enoxaparin, a low-molecular weight heparin (LMWH), on 60-day rates of hematoma requiring surgical drainage in adult plastic surgery patients. VTEPS data on prevention of symptomatic VTE with postoperative enoxaparin are being published separately.
METHODS
Study inclusion and exclusion criteria
The VTEPS Network consisted of four high-volume plastic surgery groups, including University of Pittsburgh (Pittsburgh, Pennsylvania), University of Texas-Southwestern (Dallas, Texas), Regions Hospital (St. Paul, Minnesota), and University of Michigan (Ann Arbor, Michigan). VTEPS’ study protocol was established and agreed upon by Network members after a comprehensive literature review. The study protocol was implemented between March, 2009 and September, 2009 at each VTEPS site. Data collection was stopped on December 31, 2010. All data were acquired retrospectively.
Each VTEPS site implemented an identical “best practice” clinical protocol to risk-stratify and provide VTE prophylaxis to adult (age ≥ 18) plastic surgery patients. Study inclusion criteria included moderate to high risk for peri-operative VTE (Caprini score ≥ 3) 5, surgery under general anesthesia, and post-operative hospital admission. Patients who met inclusion criteria received daily enoxaparin prophylaxis (40mg subcutaneous once daily or 30mg subcutaneous twice daily for patients with body mass index (BMI) >40 kg/m2). The initial enoxaparin dose was provided between 6 and 8 hours after surgery. Prophylaxis was subsequently administered on a daily basis for the duration of the patient’s inpatient stay.
Patients who received intra-operative intravenous heparin during a microsurgical procedure and patients who received intra- or post-operative aspirin were included in the study. Patients who received any non-protocol chemoprophylaxis, including but not limited to unfractionated subcutaneous heparin, non-enoxaparin LMWH, anti-Xa inhibitors, or warfarin were excluded, except when these medications were used to treat a newly diagnosed VTE event. Patients who received pre- or intra-operative enoxaparin prophylaxis, who had a non-protocol enoxaparin dosage, who had missed enoxaparin prophylaxis doses, or who received post-discharge enoxaparin prophylaxis were excluded. Utilization of intra- and post-operative lower extremity sequential compression devices was the standard of care during the entire study period at all VTEPS sites.
The VTEPS protocol reflected the perceived “best practice” at the time of protocol development, as supported by published manuscripts in the general surgery and plastic surgery literature through 2008, or by data which were accessible to us pre-publication 25–35. Prior to 2008, post-operative chemoprophylaxis was not the standard of care at VTEPS sites. This allowed us to ethically identify a cohort of historic controls who did not receive chemoprophylaxis. Inclusion criteria for the historic control cohort were identical to the above inclusion criteria, with one exception. Patients who received any form of pre- or post-operative chemoprophylaxis, including but not limited to unfractionated heparin, LMWH, anti-Xa inhibitors, or warfarin, for any reason within 60 days after surgery were excluded from the historic control cohort.
In-progress review indicated that lower extremity trauma reconstruction patients were systematically excluded based on VTEPS’ criteria (>90% exclusion rate). Patients often had one or multiple operative procedures prior to plastic surgery consultation and had routinely received chemoprophylaxis. As receipt of pre- or intra-operative chemoprophylaxis represented a major confounder for our clinical question, lower extremity trauma reconstruction patients were dropped from the final analysis.
Independent variables
Independent variables included factors which had previously been shown to be associated with increased risk for re-operative hematoma. These included age, BMI, operative time, type of surgical procedure, receipt of intravenous heparin during microsurgery, receipt of intra- or postoperative aspirin, and receipt of post-operative enoxaparin prophylaxis.
Dependent variables
The dependent variable was a hematoma requiring surgical drainage (re-operative hematoma) that occurred within 60 days of the initial surgical procedure. Patients were considered to have a re-operative hematoma when three of three criteria were met, including: 1) no hematoma was present on initial transport from the operating room to the post-anesthesia care unit, 2) a hematoma was diagnosed clinically at any time between the post-anesthesia care unit and post-operative day 60, and 3) per surgeon discretion, the hematoma required a distinct surgical procedure performed in the operating room for hemostasis and/or evacuation. Procedures performed for hemostasis and/or hematoma evacuation on the patient care ward or in the clinic were not counted as re-operative hematomas. Patients who received therapeutic anticoagulation for objectively confirmed VTE and subsequently developed a hematoma were not counted as re-operative hematomas.
Data acquisition and storage
Each team leader participated in a mandatory, standardized training session administered by VTEPS study coordinators. This session addressed VTEPS eligibility criteria, defined variables, and discussed appropriate use of the web-based data collection system. Variables were identified using retrospective medical record review performed by physician-led teams at each VTEPS site. Patients who lacked 60 days of follow-up were not entered into the database. De-identified data were uploaded to a modified version of the American Society of Plastic Surgery’s web-based “Tracking Operations and Outcomes for Plastic Surgeons” (TOPS) data repository.
Statistical analysis
Data were analyzed using Stata11 (StataCorp LP, College Station, Texas). Bivariate statistics examined re-operative hematoma stratified by individual risk factors, including procedure type, using chi-squared test or Fisher’s exact test as appropriate. Stratified analysis of re-operative hematoma by procedure type and receipt of post-operative enoxaparin was then performed. Multivariable logistic regression controlled for confounding by identified confounders. Re-operative hematoma was the dependent variable for the regression model. For ease of usability, the continuous data elements age, BMI, and operative time were transformed into categorical data. A value of p<0.05 was considered significant.
Sample size calculation
Sample size calculation was performed for a 50% reduction in symptomatic, 60-day VTE events 31, 36. Pilot data from 634 historic control patients indicated that the baseline, 60-day VTE rate was 2.52%. VTEPS would have 80% power to detect the expected 50% risk reduction (assuming alpha equal to 0.05, beta equal to 0.20, n1:n2 of 1:1) if 1988 patients were included in each cohort. VTEPS original enrollment goals were 1988 patients per cohort.
Pilot data from 634 historic control patients demonstrated a re-operative hematoma rate of 2.4%. We assumed that an increase in re-operative hematoma rate of 1.5% (to 3.9%) would be clinically relevant. Our assumptions included alpha equal to 0.05 and n1:n2 of 1:1. Given a fixed sample size of 1988 patients per cohort, VTEPS would have a 74.5% power to detect this 1.5% difference if it were present.
This study was approved by the Institutional Review Board at each VTEPS site.
RESULTS
Complete data were available for 3,681 patients, including 2,114 control patients and 1,567 patients who received post-operative enoxaparin. Stratified analysis of 60-day re-operative hematoma rate by procedure type (Table 1) demonstrated that breast surgery patients were at increased risk for re-operative hematoma. Breast surgery patients also had a large number of re-operative hematoma events (43 in the reconstruction group and 22 in the reduction group), which allowed for an analysis stratified by procedure type to be performed.
Table 1.
Observed rates of hematoma stratified by procedure type.
| Procedure Type | Number of patients | Rate of re-operative hematoma (N) |
|---|---|---|
| Upper extremity reconstruction | 534 | 0.94% (5 patients) |
| Breast reconstruction (expander, implant or autologous tissue) | 899 | 4.78% (43 patients) |
| Breast reduction | 276 | 7.97% (22 patients) |
| Cosmetic breast surgery | 22 | 13.64% (3 patients) |
| Body contouring (non post-bariatric) | 151 | 0 (0 patients) |
| Body contouring (post-bariatric) | 247 | 3.64% (9 patients) |
| Non-trauma lower extremity reconstruction | 266 | 0.75% (2 patients) |
| Head and neck reconstruction | 449 | 1.79% (8 patients) |
| Chest/abdominal wall/back reconstruction | 324 | 1.54% (5 patients) |
| Burn reconstruction | 32 | 0 (0 patients) |
| Decubitus ulcers (debridement or reconstruction) | 251 | 2.79% (7 patients) |
| Facial cosmetic surgery | 83 | 2.41% (2 patients) |
| Genitourinary reconstruction | 63 | 3.17% (2 patients) |
Analysis was initially limited to historic control patients who received no chemoprophylaxis for 60 days after surgery. When compared to patients having non-breast surgery, breast reconstruction patients (1.32% vs. 4.21%, p<0.001) and breast reduction patients (1.32% vs. 8.39%, p<0.001) were significantly more likely to have re-operative hematoma. Additionally, breast reduction patients were significantly more likely to have re-operative hematoma when compared to breast reconstruction patients (8.39% vs. 4.21%, p=0.038).
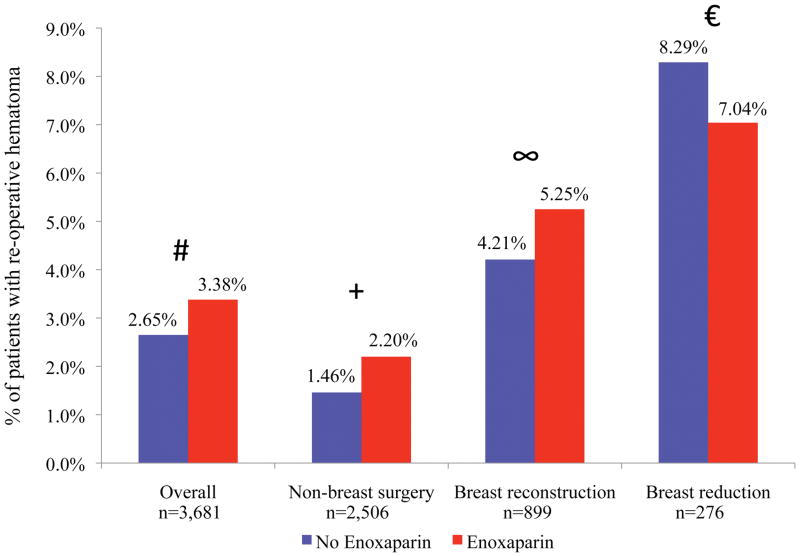
We compared 2,114 historic control patients with 1,567 patients who received postoperative enoxaparin. Overall, post-operative enoxaparin did not change the rate of re-operative hematoma when compared to controls (3.38% vs. 2.65%, p=0.169). Similar results were seen in subgroup analyses for breast reconstruction (5.25% vs. 4.21%, p=0.737), breast reduction (7.04% vs. 8.29%, p=0.194), or non-breast plastic surgery (2.20% vs. 1.46%, p=0.465) (Figure 1).
Figure 1.
Comparison of re-operative hematoma rate stratified by receipt of post-operative enoxaparin. No difference was statistically significant. # p=0.169, + p=0.465, ∞ p=0.737, €p=0.194
Stratified analysis (Figure 1) did not indicate that receipt of post-operative enoxaparin was a major driver of re-operative hematoma rates. Additional stratified analyses (Table 2) identified several associations between independent risk factors and re-operative hematomas. Confounding was potentially present between multiple risk factors (e.g. multiple site surgery, microsurgical procedure, receipt of intravenous heparin during surgery, and receipt of intra- or post-operative aspirin). Multivariable logistic regression was performed to identify independent predictors of re-operative hematoma. Independent variables included age, BMI, operative time, breast surgery, microsurgical procedure, multiple-site surgery, post-bariatric body contouring, receipt of IV heparin during surgery, receipt of intra- or post-operative aspirin, and receipt of post-operative enoxaparin.
Table 2.
Bivariate statistics examining rates of hematoma stratified by risk factors.
| Risk factor (N) | Hematoma rate, % | p value |
|---|---|---|
| Age | ||
| <40 years (915) | 2.30% | Reference |
| ≥40 years (2,757) | 3.16% | 0.182 |
|
| ||
| Body mass index | ||
| 0–25 kg/m2 (861) | 3.02% | Reference |
| 25–40 kg/m2 (2,219) | 3.06% | 0.948 |
| >40 kg/m2 (254) | 3.15% | 0.916 |
|
| ||
| Operative time | ||
| 0–1.5 hours (914) | 1.64% | Reference |
| 1.5–3 hours (1,218) | 4.35% | <0.001 |
| >3 hours (1,549) | 2.65% | 0.106 |
|
| ||
| Multiple site surgery | ||
| No (3,291) | 2.95% | Reference |
| Yes (390) | 3.08% | 0.887 |
|
| ||
| Microsurgical procedure | ||
| No (3,433) | 2.71% | Reference |
| Yes (248) | 6.45% | 0.001 |
|
| ||
| Breast surgery | ||
| No (2,484) | 1.65% | Reference |
| Yes (1,197) | 5.68% | <0.001 |
|
| ||
| Post-bariatric body contouring surgery | ||
| No (3,434) | 2.91% | Reference |
| Yes (247) | 3.64% | 0.512 |
|
| ||
| Intravenous heparin during surgery | ||
| No (3,624) | 3.01% | Reference |
| Yes (57) | 0% | 0.184 |
|
| ||
| Intra- or post-operative aspirin | ||
| No (3.385) | 2.95% | Reference |
| Yes (296) | 3.04% | 0.933 |
|
| ||
| Post-operative enoxaparin per protocol | ||
| No (2,114) | 2.65% | Reference |
| Yes (1,567) | 3.38% | 0.194 |
Multivariable logistic regression identified multiple independent predictors of 60-day re-operative hematoma. Independent predictors included breast surgery (adjusted odds ratio (OR) 4.49, 95% CI 2.80–7.20), microsurgical procedure (OR 3.20, 95% CI 1.59–6.43), and post-bariatric body contouring (OR 2.92, 95% CI 1.21–7.09) When controlling for all other factors, receipt of post-operative enoxaparin was not an independent predictor of re-operative hematoma (OR 1.16, 95% CI 0.77–1.76) (Table 3).
Table 3.
Multivariable logistic regression results to identify independent predictors of reoperative hematoma. No re-operative hematoma events were noted in patients who received intra-operative IV heparin. This variable was dropped from the model.
| Risk factor | Adjusted odds ratio (95% CI) | p value |
|---|---|---|
| Age | ||
| <40 years | Reference | ----- |
| ≥40 years | 1.34 (0.81–2.23) | 0.260 |
|
| ||
| Body mass index | ||
| <25 kg/m2 | Reference | ----- |
| 25–40 kg/m2 | 1.00 (0.63–1.59) | 1.00 |
| >40 kg/m2 | 0.99 (0.43–2.25) | 0.978 |
|
| ||
| Operative time | ||
| <1.5 hours | Reference | ----- |
| 1.5–3 hours | 1.85 (0.99–3.44) | 0.051 |
| >3 hours | 0.67 (0.34–1.34) | 0.260 |
|
| ||
| Breast surgery | 4.49 (2.80–7.20) | <0.001 |
|
| ||
| Microsurgical procedure | 3.20 (1.59–6.43) | 0.001 |
|
| ||
| Post-bariatric body contouring | 2.92 (1.21–7.09) | 0.017 |
|
| ||
| Multiple site surgery | 0.97 (0.45–2.09) | 0.940 |
|
| ||
| Receipt of intra- or post-operative aspirin | 1.42 (0.69–2.93) | 0.339 |
|
| ||
| Receipt of post-operative enoxaparin | 1.16 (0.77–1.76) | 0.471 |
DISCUSSION
VTEPS data indicates that receipt of post-operative enoxaparin does not produce a clinically or statistically significant increase in observed rates of 60-day re-operative hematoma. Multivariable logistic regression demonstrated that surgical procedures with large areas of dissection (breast surgery and post-bariatric body contouring) or low tolerance for post-operative bleeding (microsurgical procedure) were the major drivers of re-operative hematoma risk. Receipt of post-operative enoxaparin was not an independent predictor of re-operative hematoma in the regression model.
Several retrospective cohort studies have demonstrated that post-operative chemoprophylaxis may increase bleeding risk when compared to no prophylaxis, though differences were not significant 27, 35, 37, 38. Conversely, a retrospective cohort study that included 679 free TRAM patients showed the opposite relationship, with re-operative hematoma rates of 0.5% in patients who received chemoprophylaxis and 1.0% in patients who received mechanical prophylaxis alone 31. VTEPS data indicated that breast reduction patients were at substantially increased risk for re-operative hematoma. However, VTEPS’ breast reduction patients are not representative of the overall reduction population due to a severe selection bias. VTEPS’ reduction patients represent a group of reduction patients who were expected to require admission after what is typically outpatient surgery, likely due to multiple medical comorbidities. These comorbidities (e.g. poorly controlled blood pressure) may have contributed to an increased risk for re-operative hematoma 39.
Existing studies of re-operative hematoma and chemoprophylaxis are often confounded by several factors. Deleyiannis and colleagues noted four re-operative hematomas in a series of 114 free fibula patients who received twice-daily, post-operative unfractionated heparin prophylaxis after mandible reconstruction, for an overall rate of 3.5%. In each of the four cases, re-operative bleeding was attributed to salivary fistula or a clear technical error at the anastomosis 40. Lemaine and colleagues studied women who had free-flap autologous breast reconstruction and received LMWH prophylaxis. Overall, 5.3% (12 of 225 patients) had a re-operative hematoma. The majority of re-operative hematomas were attributed to flap venous congestion, and the authors note that “considering that the procedures were all microsurgical breast reconstructions, the threshold for re-operating on suspected hematomas was very low” 37. VTEPS demonstrated that microsurgical procedure was an independent predictor of re-operative hematoma. This finding may reflect surgeon’s low tolerance to explore a potentially compromised vascular pedicle, as opposed to an expanding hematoma. Of note, the multivariable regression performed in the analysis controls for this identified confounder.
In 2008, Hatef and colleagues at UT Southwestern published a retrospective cohort study of 347 excisional body contouring patients. Of 137 patients who received chemoprophylaxis, 36% received chemoprophylaxis prior to the operating room. The remaining patients received their first dose either during operation or within two hours of operation. This aggressive regimen was associated with significantly increased hematoma, transfusion requirements, and estimated blood loss 28. The UT Southwestern group also performed a separate matched case-control study of excisional body contouring patients who did or did not have re-operative hematoma. This study appears to have been done in the same cohort of patients used for Hatef’s 2008 study. Patients with hematoma had significantly decreased intraoperative mean arterial pressure (MAP) and significantly increased post-operative MAP when compared to controls 39. Thus, poorly controlled peri-operative blood pressure represents a significant confounder with enoxaparin administration and makes the subsequent 2008 paper by Hatef and colleagues difficult to interpret.
Durnig and Jungwirth 41 performed a retrospective cohort study of facial rhytidectomy patients who received LMWH both two hours prior to surgery and for 48 hours post-operatively. The first dose of post-operative LMWH was provided 24 hours after surgery. LMWH patients had significantly increased risk for re-operative hematoma(16.2% vs. 1.1%). Interestingly, all re-operative hematomas occurred between 1 and 10 hours after surgery, which was prior to the first dose of post-operative LMWH.
Data from Hatef and colleagues and Durnig and Jungwirth support the VTEPS finding that plastic surgery procedures with large areas of dissection, such as excisional body contouring or breast surgery, are predisposed to re-operative hematoma. They suggested that for procedures with extensive dissection, pre- or intra-operative chemoprophylaxis may increase risk of hematoma, and thus should be used with caution. Similarly, chemoprophylaxis should be used with extreme caution in patients with risk of bleeding into a critical space (e.g. intraocular, intracranial, or epidural) 43. However, VTEPS data has shown that enoxaparin chemoprophylaxis, when initiated 6–8 hours after surgery and continued for the duration of inpatient stay, does not significantly increase rates of re-operative hematoma. Our results, which demonstrate an absolute difference of 0.73% in re-operative hematoma rates, are similar to results from Leonardi and colleagues’ meta-analysis in general surgery patients 42.
Januszyk and Gurtner note that “clinically trivial results may exhibit statistical significance and, likewise, results that fail to achieve statistically significance may nonetheless be clinically relevant” 44. For VTEPS, the observed difference in re-operative hematoma when patients did or did not receive post-operative enoxaparin was small (0.73%). A post-hoc power analysis indicates that VTEPS was underpowered to detect if this difference was statistically significant. Such a trial would require 8,885 patients per cohort, assuming alpha equal to 0.05, beta equal to 0.80, and n1:n2 of 1:1. Regardless of whether the observed difference was statistically significant, VTEPS data demonstrates that absolute differences in re-operative hematoma rates when stratified by receipt of post-operative enoxaparin are small and likely irrelevant to everyday clinical practice.
The consequences of VTE and hematoma are quite different. Although small amounts of bleeding into critical spaces (e.g. intracranial bleeding or an expanding neck hematoma) can be life-threatening, post-operative bleeding can generally be addressed by transfusion and/or operative drainage without long term sequelae. In contrast, a pulmonary embolus can be immediately fatal or can have long-term morbidity among survivors. We agree with Dr. David Green, a board certified hematologist interviewed in 2006 by Drs. Felmont Eaves and V. Leroy Young for the Aesthetic Surgery Journal. Dr. Green noted that “pulmonary embolism is potentially a lethal factor. Nobody likes the idea of transfusion, [but]…you can better tolerate some bleeding than you could tolerate a massive PE” 45. We also agree with Drs. Davison and Massoumi’s 2007 editorial in Plastic and Reconstructive Surgery. This piece, entitled “Our Complication, Your Problem”, notes that “a hematoma is a medical stress, an inconvenience, an embarrassment, or an additional procedure, but [unlike PE] rarely does it kill a patient” 46.
CONCLUSION
Risk of re-operative hematoma has previously been identified as the major driver of plastic surgeons’ decisions not to use VTE chemoprophylaxis. Analysis of the VTEPS database shows that receipt of post-operative enoxaparin does not create a statistically or clinically significant increase in observed rates of re-operative hematoma. Independent predictors for re-operative hematoma include breast surgery, post-bariatric body contouring, and microsurgical procedure.
Acknowledgments
The VTEPS study was funded by the Plastic Surgery Foundation.
Dr. Pannucci receives salary support from the NIH T32 grant program (T32 GM-08616).
Footnotes
Meeting disclosure: Portions of this work were presented at the 2011 American Association of Plastic Surgeons meeting (Boca Raton, Florida) and the 2011 Plastic Surgery Research Council (Louisville, Kentucky).
FINANCIAL DISCLOSURE AND PRODUCTS PAGE
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Contributor Information
Christopher J. Pannucci, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
Christine Fisher Wachtman, Division of Plastic and Reconstructive Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
George Dreszer, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Steven H. Bailey, Department of Plastic Surgery, University of Texas-Southwestern, Dallas, Texas.
Pamela R. Portschy, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
Jennifer B. Hamill, JBH Consulting, Shohola, Pennsylvania.
Keith M. Hume, American Society of Plastic Surgeons, Arlington Heights, Illinois.
Ronald E. Hoxworth, Department of Plastic Surgery, University of Texas-Southwestern, Dallas, Texas.
Loree K. Kalliainen, Department of Plastic and Hand Surgery, Regions Hospital, St. Paul, Minnesota.
J. Peter Rubin, Division of Plastic and Reconstructive Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Andrea L. Pusic, Plastic and Reconstructive Surgery Service, Memorial Sloan Kettering Cancer Center, New York, New York.
Edwin G. Wilkins, Section of Plastic Surgery, University of Michigan, Ann Arbor, Michigan.
References
- 1.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 4.Arnold DM, Kahn SR, Shrier I. Missed opportunities for prevention of venous thromboembolism: An evaluation of the use of thromboprophylaxis guidelines. Chest. 2001;120:1964–1971. doi: 10.1378/chest.120.6.1964. [DOI] [PubMed] [Google Scholar]
- 5.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–10. doi: 10.1016/j.amjsurg.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Henke PK, Pannucci CJ. Venous thromboembolism risk factor assessment and prophylaxis. Phlebology. 2010;25:219–223. doi: 10.1258/phleb.2010.010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy RX, Jr, Peterson EA, Adkinson JM, Reed JF., 3rd Plastic surgeon compliance with national safety initiatives: Clinical outcomes and “never events”. Plast Reconstr Surg. 2010;126:653–656. doi: 10.1097/PRS.0b013e3181de1929. [DOI] [PubMed] [Google Scholar]
- 10.Lembitz A, Clarke TJ. Clarifying “never events and introducing “always events”. Patient Saf Surg. 2009;3:26. doi: 10.1186/1754-9493-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [Accessed May 9, 2011.];National Quality Forum press release. 2008 May 15; Available at www.qualityforum.org.
- 12. [Accessed May 9, 2011.];Centers for Medicare and Medicaid Services press release. 2008 April 14; www.cms.hhs.gov.
- 13.Wakefield TW, McLafferty RB, Lohr JM, et al. Call to action to prevent venous thromboembolism. J Vasc Surg. 2009;49:1620–1623. doi: 10.1016/j.jvs.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed May 9, 2011.];The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. http://www.surgeongeneral.gov/library/calls/index.html.
- 15.Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: A double-blind randomized multicentre trial with venographic assessment. ENOXACAN study group. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]
- 16.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 19.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen MS. Preventing thromboembolic complications in cancer patients after surgery: A role for prolonged thromboprophylaxis. Cancer Treat Rev. 2002;28:141–144. doi: 10.1016/s0305-7372(02)00043-9. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: A multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 22.Turpie AG, Bauer KA, Caprini JA, et al. Fondaparinux combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: A randomized, double-blind comparison. J Thromb Haemost. 2007;5:1854–1861. doi: 10.1111/j.1538-7836.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 23.Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: Adherence to the 1995 american college of chest physicians consensus guidelines for surgical patients. Arch Intern Med. 2000;160:334–340. doi: 10.1001/archinte.160.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 25.Clavijo-Alvarez JA, Pannucci CJ, Oppenheimer AJ, Wilkins EG, Rubin JP. Prevention of venous thromboembolism in body contouring surgery: A national survey of 596 ASPS surgeons. Ann Plast Surg. 2011;66:228–232. doi: 10.1097/SAP.0b013e3181e35c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannucci CJ, Oppenheimer AJ, Wilkins EG. Practice patterns in venous thromboembolism prophylaxis: A survey of 606 reconstructive breast surgeons. Ann Plast Surg. 2010;64:732–737. doi: 10.1097/SAP.0b013e3181ba57a0. [DOI] [PubMed] [Google Scholar]
- 27.Chen CM, Disa JJ, Cordeiro PG, Pusic AL, McCarthy CM, Mehrara BJ. The incidence of venous thromboembolism after oncologic head and neck reconstruction. Ann Plast Surg. 2008;60:476–479. doi: 10.1097/SAP.0b013e31816fd7e7. [DOI] [PubMed] [Google Scholar]
- 28.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 29.Keyes GR, Singer R, Iverson RE, et al. Mortality in outpatient surgery. Plast Reconstr Surg. 2008;122:245–50. doi: 10.1097/PRS.0b013e31817747fd. discussion 251–3. [DOI] [PubMed] [Google Scholar]
- 30.Keyes GR, Singer R, Iverson RE, et al. Analysis of outpatient surgery center safety using an internet-based quality improvement and peer review program. Plast Reconstr Surg. 2004;113:1760–1770. doi: 10.1097/01.prs.0000124743.75839.11. [DOI] [PubMed] [Google Scholar]
- 31.Liao EC, Taghinia AH, Nguyen LP, Yueh JH, May JW, Jr, Orgill DP. Incidence of hematoma complication with heparin venous thrombosis prophylaxis after TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121:1101–1107. doi: 10.1097/01.prs.0000302454.43201.83. [DOI] [PubMed] [Google Scholar]
- 32.Murray DJ, Neligan PC, Novak CB, Howley B, Wunder JS, Lipa JE. Free tissue transfer and deep vein thrombosis. J Plast Reconstr Aesthet Surg. 2008;61:687–692. doi: 10.1016/j.bjps.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Pannucci CJ, Chang EY, Wilkins EG. Venous thromboembolic disease in autogenous breast reconstruction. Ann Plast Surg. 2009;63:34–38. doi: 10.1097/SAP.0b013e318188bedf. [DOI] [PubMed] [Google Scholar]
- 34.Davison SP, Venturi ML, Attinger CE, Baker SB, Spear SL. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg. 2004;114:43E–51E. doi: 10.1097/01.prs.0000131276.48992.ee. [DOI] [PubMed] [Google Scholar]
- 35.Seruya M, Venturi ML, Iorio ML, Davison SP. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122:1701–1708. doi: 10.1097/PRS.0b013e31818dbffd. [DOI] [PubMed] [Google Scholar]
- 36.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 37.Lemaine V, McCarthy C, Kaplan K, et al. Venous thromboembolism following microsurgical breast reconstruction: An objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2010 doi: 10.1097/PRS.0b013e318208d025. [DOI] [PubMed] [Google Scholar]
- 38.Kroll SS, Miller MJ, Reece GP, et al. Anticoagulants and hematomas in free flap surgery. Plast Reconstr Surg. 1995;96:643–647. doi: 10.1097/00006534-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Farkas JP, Kenkel JM, Hatef DA, et al. The effect of blood pressure on hematoma formation with perioperative lovenox in excisional body contouring surgery. Aesthet Surg J. 2007;27:589–593. doi: 10.1016/j.asj.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Deleyiannis FW, Clavijo-Alvarez JA, Pullikkotil B, et al. Development of consensus guidelines for venous thromboembolism prophylaxis in patients undergoing microvascular reconstruction of the mandible. Head Neck. 2010 doi: 10.1002/hed.21571. [DOI] [PubMed] [Google Scholar]
- 41.Durnig P, Jungwirth W. Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg. 2006;118:502–7. doi: 10.1097/01.prs.0000228180.78071.44. discussion 508–9. [DOI] [PubMed] [Google Scholar]
- 42.Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: A systematic review of 33 randomized controlled trials. Arch Surg. 2006;141:790–7. doi: 10.1001/archsurg.141.8.790. discussion 797–9. [DOI] [PubMed] [Google Scholar]
- 43.Muntz JE, Michota FA. Prevention and management of venous thromboembolism in the surgical patient: Options by surgery type and individual patient risk factors. Am J Surg. 2010;199:S11–20. doi: 10.1016/j.amjsurg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Januszyk M, Gurtner GC. Statistics in medicine. Plast Reconstr Surg. 2011;127:437–444. doi: 10.1097/PRS.0b013e3181f95dd2. [DOI] [PubMed] [Google Scholar]
- 45.Green D. VTE prophylaxis in aesthetic surgery patients. Aesthet Surg J. 2006;26:317–324. doi: 10.1016/j.asj.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Davison SP, Massoumi W. Our complication, your problem. Plast Reconstr Surg. 2007;120:1428–1429. doi: 10.1097/01.prs.0000279376.12476.b4. [DOI] [PubMed] [Google Scholar]