Abstract
Objectives
Near-infrared (NIR) fluorescence imaging has the potential to improve sentinel lymph node (SLN) mapping in vulvar cancer, which was assessed in the current study. Furthermore, dose optimization of indocyanine green adsorbed to human serum albumin (ICG:HSA) was performed.
Study Design
Nine vulvar cancer patients underwent the standard SLN procedure using 99mtechnetium-nancolloid and patent blue. In addition, intraoperative imaging was performed after peritumoral injection of 1.6 mL of 500, 750 or 1000 µM of ICG:HSA.
Results
NIR fluorescence SLN mapping was successful in all patients. A total of 14 SLNs (average 1.6, range 1–4) were detected: 14 radioactive (100%), 11 blue (79%), and 14 NIR fluorescent (100%).
Conclusions
This study demonstrates feasibility and accuracy of SLN mapping using ICG:HSA. Considering safety, cost, and pharmacy preferences, an ICG:HSA concentration of 500 µM appears optimal for SLN mapping in vulvar cancer.
Keywords: Image-Guided Surgery, Indocyanine Green, Near-Infrared Fluorescence Imaging, Sentinel Lymph Node Mapping, Vulvar Cancer
INTRODUCTION
Vulvar cancer is a relatively rare disease with an annual incidence of approximately 4000 cases in the United States, resulting in 900 deaths per year.1 Tumor size and invasion into adjacent tissues are important factors for staging vulvar cancers, but nodal status remains the single most important prognosticator.2 Radical vulvectomy with en bloc inguinofemoral lymphadenectomy has been replaced in the surgical treatment of vulvar cancer by radical wide local excision or radical vulvectomy with inguinofemoral lymphadenectomy using separate groin incisions.3 The latter modification has significantly decreased surgery-related morbidity.3 However, 30% to 70% of patients treated with full inguinofemoral lymphadenectomy still suffer from lymphedema.4–6 Only 27% of patients with clinically stage I or II vulvar cancer have tumor positive lymph nodes; therefore, approximately 70% of patients undergo unnecessary lymphadenectomy.6
The sentinel lymph node (SLN) biopsy, as introduced in the management of cutaneous melanoma by Morton,7 was first described in vulvar cancer by Levenback in 1994.8 The SLN procedure in vulvar cancer patients has been validated in multicenter trials and its introduction in regular clinical practice has marked a significant reduction in lymphedema, wound infection, and wound dehiscence.9, 10 Currently, the procedure usually involves a combination of a radioactive colloid and a blue dye. However, the use of radiotracers requires complex logistics including the involvement of a nuclear medicine physician and the transport of radioactivity, and is therefore not available in all clinics.. Moreover, blue dyes cannot be visualized when the lymph nodes and lymphatic channels are covered by tissue, such as skin or fat.
The use of invisible near-infrared (NIR) light (700–900 nm) has several characteristics that can be advantageous in the SLN procedure, which include relatively high penetration into living tissue (millimeters to centimeters), when compared to blue dyes, and the lack of ionizing radiation.11 Indocyanine green (ICG) is one of only 2 clinically available NIR fluorescent agents and is currently the most optimal agent for SLN mapping.12 In several studies, intraoperative imaging systems in combination with ICG have been used for the SLN procedure for various types of cancer.13–18 The lymphatic channels and SLNs in vulvar cancer are often located in a relatively superficial location in the groin when compared to other tumors; therefore, NIR fluorescence imaging could be particularly useful for this indication. Indeed, Crane et al. reported the successful use of ICG alone at a concentration of 645 µM, in conjunction with an intraoperative imaging system for the SLN procedure in vulvar cancer.19 In that study involving 10 patients, 26 of 29 SLNs (90%) were detected in vivo by NIR fluorescence. Furthermore, lymphatic channels could be visualized in 5 of 16 groins (31%) containing SLNs.
Preclinical evidence demonstrated that premixing of ICG with human serum albumin (HSA, complex is ICG:HSA) increases the fluorescence intensity and hydrodynamic diameter of ICG, resulting in better retention in the SLN.20 The aims of the current study were to assess the use of NIR fluorescence imaging using ICG:HSA and the Mini-FLARE intraoperative imaging system for the SLN procedure in vulvar cancer and to optimize ICG:HSA dose.
MATERIALS AND METHODS
Preparation of Indocyanine Green Adsorbed to Human Serum Albumin
ICG (25 mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) and resuspended in 10 mL of sterile water for injection for the 500 µM group, or in 5 mL of sterile water for injection for the 750 µM and 1000 µM groups, to yield stock solutions of 3.2 mM and 6.4 mM, respectively. Various amounts of this stock solution were transferred to a 50 cc vial of Cealb (20% human serum albumin [HSA] solution; Sanquin, Amsterdam, The Netherlands) to yield ICG in HSA (ICG:HSA) at a final concentration of 500 µM, 750 µM, or 1000 µM.
Intraoperative NIR Fluorescence Imaging
SLN mapping was performed using the Mini-FLARE image-guided surgery system as described in detail previously.14 Briefly, the system consists of 2 wavelength separated light sources: a “white” LED light source, generating 26,600 l× of 400 to 650 nm light to illuminate the surgical field and an NIR LED light source, generating 7.7 mW / cm2 of fluorescence excitation light. White light and NIR fluorescence images are acquired simultaneously and displayed in real time, using custom designed optics and software. A pseudo-colored (lime green) image of NIR fluorescence superimposed over the white light image is also displayed, to provide the NIR fluorescence signal in proper anatomical context.
Clinical Trial
The current dose escalation clinical trial was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in concordance with the ethical standards of the Helsinki Declaration of 1975. Nine consecutive patients that planned to undergo a SLN procedure for squamous cell vulvar carcinoma were included in this study between June 2010 and January 2011. All patients had clinically FIGO stage I vulvar cancer with a unifocal carcinoma measuring less than 4 cm in diameter, not encroaching the vagina, anus or urethra and with negative inguinofemoral nodes as determined by palpation and ultrasonography. Exclusion criteria were pregnancy, lactation or an allergy to iodine, shellfish, or indocyanine green.
All patients gave informed consent and were anonymized. Patients received the standard-of-care SLN procedure.9 For our institution, this implies peritumoral injections of 60–100 MBq 99mtechnetium-nanocolloid on the afternoon of the day before, or the morning prior to surgery. Before the start of the operation, 1 mL total of patent blue V (Guerbet, France) was injected at 4 sites peritumorally. Immediately after injection of patent blue, 1.6 mL total of ICG:HSA was injected as 4 injections at the same location as the patent blue injections. After surgical scrub, the Mini-FLARE imaging head was positioned at approximately 30 cm above the surgical field. The NIR fluorescence signal was measured percutaneously, prior to skin incision, and continuously during the surgical procedure. Throughout the procedure, the surgeon was continuously provided with real-time NIR fluorescence image guidance. When the SLN could not be found easily by NIR fluorescence, the handheld gamma probe could be used for the localization of SLNs. Relative brightness of the SLNs was determined by measuring signal-to-background ratios (SBR), that is the NIR fluorescence signal of the SLN divided by a directly adjacent region. Excised sentinel lymph nodes were analyzed ex vivo for NIR fluorescence and radioactivity and were routinely analyzed by histopathological frozen section analysis. SLNs were fixed in formalin and embedded in paraffin for hematoxylin, eosin, and immunohistopathological staining for AE1/AE3 at multiple levels, with an interval of 250 µm, according to the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) study protocol.9
Statistical Analysis
For statistical analysis, SPSS statistical software package (Version 17.0, Chicago, IL) was used. Graphs were generated using GraphPad Prism Software (Version 5.01, La Jolla, CA). To compare the SBR between concentration groups, a one-way analysis of variance (ANOVA) was performed with pairwise comparison with least square difference (LSD) adjustment for multiple comparisons. Assumption of homogeneity of variances was assessed using Levene’s test. All statistical tests were two-tailed and P < 0.05 was considered significant.
RESULTS
Patient and Tumor Characteristics
Nine consecutive patients with vulvar cancer undergoing SLN mapping were included in this study. Patients and tumor characteristics are described in Table 1. Median body mass index (BMI) was 27 (range 23–45), median age was 50 years (range 30–72 years), and median tumor size was 13 mm (range 4–22 mm). In 6 patients, the tumor was laterally located and in 3 patients the tumor was located on, or near, the midline.
Table 1.
Patient and Tumor Characteristics
| Patient | Dose (µM) |
Age (Years) |
BMI | Localization | FIGO stage | Tumor Type |
Tumor Size (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 500 | 72 | 33 | Right | 2 | Squamous | 22 |
| 2 | 500 | 30 | 22 | Right | 1 | Squamous | 10 |
| 3 | 500 | 67 | 26 | Left | 1 | Squamous | 10 |
| 4 | 750 | 37 | 29 | Midline | 1 | Squamous | 17 |
| 5 | 750 | 42 | 45 | Left | 2 | Squamous | 22 |
| 6 | 750 | 37 | 23 | Right | 1 | Squamous | 20 |
| 7 | 1000 | 72 | 27 | Midline | 1 | VIN III | 5 |
| 8 | 1000 | 50 | 26 | Midline | 1 | Squamous | 4 |
| 9 | 1000 | 74 | 32 | Left | 1 | Squamous | 13 |
Abbreviations: BMI = body mass index, VIN III = vulvar intraepithelial neoplasia grade III
Intraoperative NIR Fluorescence Imaging
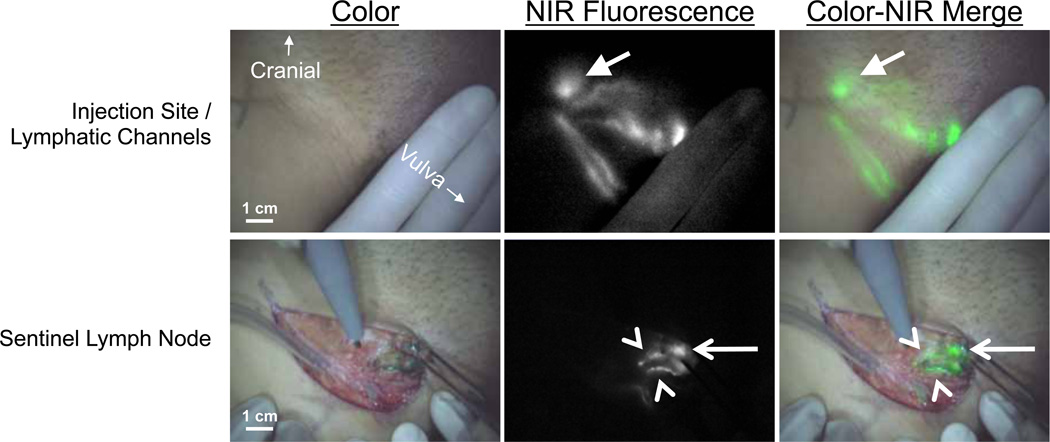
Average time between ICG:HSA injection and skin incision was 19 ± 4 minutes. In all patients (N = 9), NIR fluorescence imaging using the Mini-FLARE system enabled visualization of one or more SLNs (Figure 1 and Supplementary Video 1). Average time between skin incision and resection of the first SLN was 13 ± 5 minutes. A total of 14 SLNs were detected, all of which were radioactive and fluorescent (Table 2). Four SLNs from 3 patients did not have blue staining from patent blue. After all NIR fluorescent nodes were resected, the surgical field was systematically inspected for remaining radioactivity or blue nodes. No additional nodes were found that were not detected by NIR fluorescence. No adverse reactions associated with the use of ICG:HSA or the Mini-FLARE imaging system were observed.
Figure 1. Sentinel lymph node mapping using NIR fluorescence imaging in vulvar cancer.
Peritumoral injection of 1.6 mL of 500 µM ICG:HSA (injection site covered by hand) identifies lymphatic channels, which converge in a SLN (arrow) that can be seen percutaneously (top row). Identification of the SLN (arrow) and 2 afferent lymphatic channels (arrowheads) is demonstrated using NIR fluorescence imaging 17 min after injection of ICG:HSA (bottom row). Camera exposure times were 100 msec (top row) and 45 msec (bottom row). Scale bars represent 1 cm.
Table 2.
SLN Identification Results
| Patien t |
Number of SLN detected |
Radiocolloi d |
Blue | NIR Fluorescence |
SLN+ | Percutaneou s |
|---|---|---|---|---|---|---|
| 1 | 2 (1 left, 1 right) | 2 | 2 | 2 | 0 | Unilateral |
| 2 | 1 (right) | 1 | 1 | 1 | 0 | Yes |
| 3 | 1 (left) | 1 | 1 | 1 | 1 | Yes |
| 4 | 2 (1 right, 1 left) | 2 | 1 | 2 | 0 | Unilateral |
| 5 | 1 (left) | 1 | 1 | 1 | 0 | No |
| 6 | 1 (right) | 1 | 1 | 1 | 0 | Yes |
| 7 | 1 (left) | 1 | 0 | 1 | 0 | Yes |
| 8 | 4 (2 left, 2 right) | 4 | 3 | 4 | 0 | Yes |
| 9 | 1 (left) | 1 | 1 | 1 | 1 | No |
| Total | 14 | 14 (100%) | 11 (79%) | 14 (100%) | 2 | 8 of 12 groins |
The Effect of Lymphatic Tracer Dose on SLN Brightness
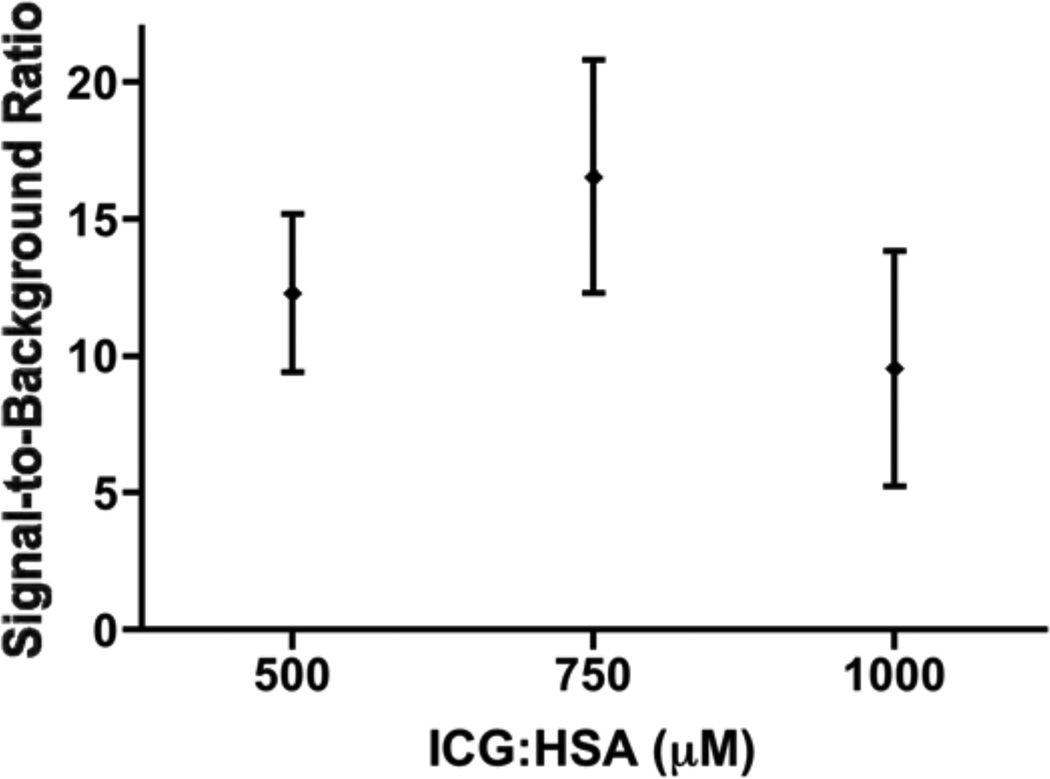
The effect of injected lymphatic tracer dose on fluorescence brightness was determined by comparing SBRs between concentration groups. Mean SBRs of the SLNs were 12.3 ± 2.9, 16.6 ± 4.3, and 9.5 ± 4.3 for the 500, 750 and 1000 µM concentration groups, respectively. A one-way ANOVA showed no significant effect of concentration on SBR (P = 0.16), and pairwise comparison with LSD adjustment for multiple comparison showed no difference between the individual concentration and SBR, although a trend was found for a decreased SBR of the 1000 µM group when compared to the 750 µM group (P = 0.07). However, due to the small sample size, the current pilot study may not have sufficient power to detect significant differences in dose groups.
Percutaneous Visualization of Lymphatic Channels
The mean BMI of patients in which lymphatic channels were visualized percutaneously to all SLN containing groins (N = 5 patients) was 24.8 ± 2.2. In patients with bilateral SLNs in which percutaneous visualization was possible for the groin (N = 2 patients), the mean BMI was 31.0 ± 2.8, and in patients where percutaneous visualization of lymphatic channels was not possible (N = 2 patients), the mean BMI was 38.5 ± 9.2. A one-way ANOVA showed a significant difference in the BMIs between these 3 groups (P = 0.024). A pairwise comparison with LSD adjustment for multiple comparison showed a significantly higher BMI for the group in which percutaneous visualization of lymphatic channels was not possible, when compared to the group in which percutaneous visualization of lymphatic channels was possible (P = 0.009).
COMMENT
The current study showed feasibility and accuracy of SLN mapping in vulvar cancer using ICG:HSA and the Mini-FLARE imaging system. In all 9 cases, the Mini-FLARE permitted the gynecologist to perform SLN mapping under direct image-guidance after skin incision. The flexible gooseneck of the Mini-FLARE could be used to position the system at any required location over the surgical field, which ensured no interference with the procedure. Indeed, no additional time was needed to complete the procedure, with an average time between skin incision and resection of the first SLN of 13 ± 5 minutes.
All radioactive SLNs could also be detected by NIR fluorescence. This higher detectability compared to the study by Crane et al. that utilized ICG alone19 may be due to improved brightness of ICG:HSA over ICG, better retention in the SLN, better imaging system performance, an optimized tradeoff between injection concentration and tracer dilution within lymphatic channels, or a combination thereof.14 In contrast, 4 out of 14 SLNs could not be detected by patent blue, which is comparable to previous findings.10, 19, 21 Lymphatic channels could be visualized in the majority of patients prior to skin incision. This aided the gynecologist in determining the location of the incision and facilitated a more efficient identification of SLNs. In the patients where percutaneous visualization of lymphatic channels was not possible, a higher BMI was observed when compared to patients where the lymphatic channels could be visualized percutaneously. Crane et al. observed similar findings.19 Future studies will have to determine whether NIR fluorescence imaging can replace radiocolloids in the SLN procedure in vulvar cancer, which could potentially be feasible only in non-obese patients. This could be particularly beneficial in clinics where radiotracers are not available. Furthermore, as the patient population in Western societies tends to suffer from increasingly higher BMIs, the penetration depth has to be increased for NIR fluorescence imaging to be a generally usable imaging modality. To accomplish this, current research is focusing on improved fluorophores (some of which are currently in the process of clinical approval22) and improved camera systems using optimized detection techniques to maximize the depth at which a fluorophore can be detected.23, 24
In the current study, no significant differences in NIR fluorescence signal were observed between the different concentrations that were administered. However, a trend was observed showing a decline of signal in the 1000 µM group, which is in line with previously reported results in breast cancer.14 A decrease of fluorescence signal with an increase in concentration can be explained by an effect in fluorescence quenching.12 Fluorescence quenching occurs when the concentration of a fluorophore is too high, causing molecules to absorb the emitted light of other nearby molecules, thereby effectively attenuating the fluorescence signal. In the current study, ICG was adsorbed to HSA prior to injection. Preclinical work has shown premixing to increase the fluorescence brightness of ICG and improve retention in the SLN.20 Flow to higher tier nodes seemed to have been avoided, as no additional NIR fluorescent nodes were identified that were not radioactive. An ongoing study is investigating whether the albumin is actually needed for optimal SLN mapping in vulvar cancer. When the optimal imaging parameters have been determined, larger trials can be performed to assess patient benefit.
In conclusion, the current study demonstrates the feasibility of SLN mapping in vulvar cancer patients using ICG:HSA and the Mini-FLARE image-guided surgery system. The preferred dose can be determined by local preparation preferences because no differences between tested doses were observed. In general, a dose of 500 µM seemed to be optimal, as it requires minimal manipulation of ICG and albumin volumes.
Supplementary Material
In this supplementary video, images as displayed in real time to the gynecologist, are shown. Patient was injected peritumorally with 1.6 mL of 500 µM of ICG:HSA.
Figure 2. Optimization of ICG:HSA dose.
Signal-to-background ratio (mean ± S.D.) of vulvar SLNs (ordinate) is plotted as a function of injected dose of ICG:HSA (abscissa). The SBRs of the 500, 750, and 100 µM concentration groups were not significantly different, although a trend was found favoring 750 µM over 1000 µM (P = 0.07).
ACKNOWLEDGEMENTS
The authors like to thank Dorien M.A. Berends-van der Meer and Margriet J.G. Löwik for their assistance with inclusion of patients, and Lindsey Gendall for editing. This work was supported in part by the Dutch Cancer Society grant UL2010-4732 and National Institutes of Health grant R01-CA-115296.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hacker NF. Revised FIGO staging for carcinoma of the vulva. Int J Gynaecol Obstet. 2009;105:105–106. doi: 10.1016/j.ijgo.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Ansink A, Van Der Velden J. Surgical interventions for early squamous cell carcinoma of the vulva. Cochrane database of systematic reviews (Online) 2000 doi: 10.1002/14651858.CD002036. CD002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaarenstroom KN, Kenter GG, Trimbos JB, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer. 2003;13:522–527. doi: 10.1046/j.1525-1438.2003.13304.x. [DOI] [PubMed] [Google Scholar]
- 5.Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg. 2003;196:442–450. doi: 10.1016/S1072-7515(02)01895-1. [DOI] [PubMed] [Google Scholar]
- 6.De Hullu JA, Van Der Zee AG. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006;60:38–58. doi: 10.1016/j.critrevonc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 8.Levenback C, Burke TW, Gershenson DM, Morris M, Malpica A, Ross MI. Intraoperative lymphatic mapping for vulvar cancer. Obstet Gynecol. 1994;84:163–167. [PubMed] [Google Scholar]
- 9.Van Der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 10.Hampl M, Hantschmann P, Michels W, Hillemanns P German Multicenter Study G. Validation of the accuracy of the sentinel lymph node procedure in patients with vulvar cancer: results of a multicenter study in Germany. Gynecol Oncol. 2008;111:282–288. doi: 10.1016/j.ygyno.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 13.Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010 doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Mieog JS, Troyan SL, Hutteman M, et al. Towards Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 17.Crane LM, Themelis G, Pleijhuis RG, et al. Intraoperative Multispectral Fluorescence Imaging for the Detection of the Sentinel Lymph Node in Cervical Cancer: A Novel Concept. Mol Imaging Biol. 2010 doi: 10.1007/s11307-010-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara M, Mizukami T, Suzuki A, Fukamizu H. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2009;62:e373–e378. doi: 10.1016/j.bjps.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 19.Crane LM, Themelis G, Arts HJ, et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: First clinical results. Gynecol Oncol. 2010 doi: 10.1016/j.ygyno.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 21.Rob L, Robova H, Pluta M, et al. Further data on sentinel lymph node mapping in vulvar cancer by blue dye and radiocolloid Tc99. Int J Gynecol Cancer. 2007;17:147–153. doi: 10.1111/j.1525-1438.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 22.Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Mol Imaging Biol. 2010;12:583–594. doi: 10.1007/s11307-010-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gioux S, Mazhar A, Cuccia DJ, Durkin AJ, Tromberg BJ, Frangioni JV. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009;14:034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar AT, Raymond SB, Bacskai BJ, Boas DA. Comparison of frequency-domain and time-domain fluorescence lifetime tomography. Opt Lett. 2008;33:470–472. doi: 10.1364/ol.33.000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this supplementary video, images as displayed in real time to the gynecologist, are shown. Patient was injected peritumorally with 1.6 mL of 500 µM of ICG:HSA.