Abstract
A sensitive, selective, and rapid ultra-high performance liquid chromatography-tandem mass spectrometry (uHPLC-MS/MS) was developed for the simultaneous quantification of clopidogrel (Plavix®) and its derivatized active metabolite (CAMD) in human plasma. Derivatization of the active metabolite in blood with 2-bromo-3’-methoxy acetophenone (MPB) immediately after collection ensured metabolite stability during sample handling and storage. Following addition of ticlopidine as an internal standard and simple protein precipitation, the analytes were separated on a Waters Acquity UPLC™ sub-2µm-C18 column via gradient elution before detection on a triple-quadrupole MS with multiple-reaction-monitoring via electrospray ionization. The method was validated across the clinically-relevant concentration range of 0.01–50 ng/mL for parent clopidogrel and 0.1–150 ng/mL (r2= 0.99) for CAMD, with a fast run time of 1.5 min to support pharmacokinetic studies using 75, 150, or 300 mg oral doses of clopidogrel. The analytical method measured concentrations of clopidogrel and CAMD with accuracy (%DEV) < ±12% and precision (%CV) of < ±6%. The method was successfully applied to measure the plasma concentrations of clopidogrel and CAMD in three subjects administered single oral doses of 75, 150, and 300 mg clopidogrel. It was further demonstrated that the derivatizing agent (MPB) does not affect clopidogrel levels, thus from one aliquot of blood drawn clinically, this method can simultaneously quantify both clopidogrel and CAMD with sensitivity in the picogram per mL range.
Keywords: Clopidogrel, Active Metabolite, Ultra HPLC-MS/MS
1. Introduction
Clopidogrel is a platelet aggregation inhibitor that is commonly prescribed to prevent cardiovascular events and death in patients with acute coronary syndromes or patients with recent ischemic stroke, myocardial infarction, or peripheral artery disease [1]. Clinical responses to clopidogrel-mediated platelet inhibition vary greatly between patients. Clopidogrel is an orally bioavailable prodrug where the majority (~85%) gets hydrolyzed by carboxyesterases to form inactive metabolites [2]. A portion of the remaining dose is transformed into the inactive intermediate, 2-oxo-clopidogrel, that is further oxidized to the active thiol metabolite, which belongs to a family of eight stereoisomers with the following primary chemical structure: 2-{1-[1-(2-chlorophenyl)-2-methoxy-2-oxoethyl]-4-sulfanyl-3-piperidinylidene} acetic acid [3]. These two activation steps are mediated by multiple cytochrome P450 (CYP) enzymes, including CYP2C19, CYP3A4, CYP1A2, CYP2C9, and CYP2B6 [4]. CYP2C19 appears to be involved in both steps of active metabolite formation. The importance of the CYP2C19 pathway was highlighted when Brandt et al demonstrated reduced active metabolite exposure and a subsequent attenuation of clopidogrel-mediated platelet aggregation due to loss of function CYP2C19 polymorphisms [5]. Numerous studies now suggest that the loss of function CYP2C19 polymorphisms as a way to explain some of the reduced efficacy, particularly the more common CYP2C19*2 and *3 alleles [6–9].
Though clopidogrel has been on the market since 1997, assay methods for the active metabolite were developed only recently. The active metabolite contains a free thiol, which is very reactive, thus making it difficult to obtain a reliable, accurate assessment of plasma concentration (Figure 1). Consequently, few studies such as these have quantified the active metabolite in order to determine differences in exposure levels between patients with genotypes [5,7]. Many previous studies have reported analytical methods quantifying either parent clopidogrel [10–13] or clopidogrel and its inactive metabolites (resulting from carboxyesterase activity) in an effort to measure clopidogrel active metabolite (CAM) indirectly [14–18]. Another group attempted semiquantitation of underivatized CAM, using clopidogrel to establish the calibration curve [19], but this provided only an estimate of active metabolite plasma concentrations. To date, only three groups have reported quantification of CAM using the 2-bromo-3’-methoxyphenone (MPB)-derivatized product (CAMD) as the reference standard for calibration [20–22]. Takahashi et al utilized LC-MS/MS with a 6 min run time over a calibration range of 0.5–250 ng/mL of CAMD while also demonstrating greater than 90% yield for the MPB derivatization of CAM at multiple concentrations in rat blood [21]. Delavenne et al, validated an ultra-high performance tandem mass spectrometric (uHPLC-MS/MS) assay, with a 1.5 min run time over a CAMD calibration range of 1–150 ng/mL, but was not applicable for clopidogrel [20]. Recently, Tuffal et al developed a uHPLC-MS/MS assay to separate and identify four stereoisomers of CAMD [22].
Figure 1. Formation of MPB-Derivatized Clopidogrel Active Metabolite.
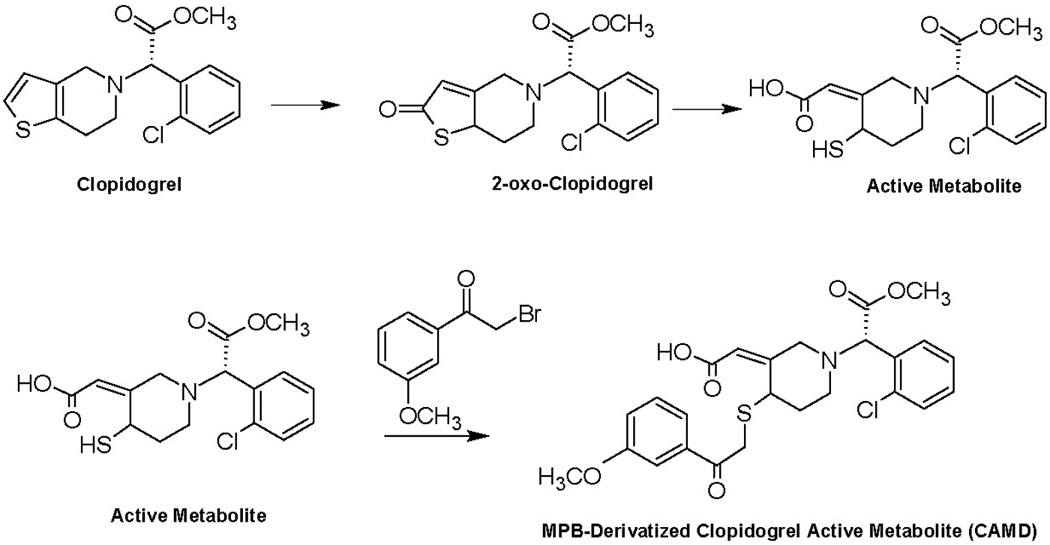
Clopidogrel is oxidized to the inactive intermediate 2-oxo-clopidogrel, before subsequent further oxidation to the free thiol-containing active metabolite. Due to its reactivity, the active metabolite is added to 2-bromo-3’-methoxyphenone (MPB) to derivatize the thiol to provide stability for more accurate quantification.
In this report, we validated a novel uHPLC-MS/MS assay for the simultaneous quantification of both parent clopidogrel (calibration range 0.01–50 ng/mL) and its MPB-derivatized active metabolite (CAMD; calibration range 0.1–150 ng/mL) with a rapid run time of 1.5 min. The novelty of our method resides in the rapid and simultaneously sensitive quantification of clinically-relevant plasma concentrations of both analytes. In addition, we administered clopidogrel tablets of 75, 150, and 300 mg to three volunteers and assessed their clopidogrel and CAMD plasma concentrations using this uHPLC-MS/MS method. Here, we report the fastest, most sensitive analytical assay for the simultaneous determination of both parent clopidogrel and its active metabolite in human plasma for application in pharmacokinetic studies.
2. Experimental
2.1. Materials
Clopidogrel hydrogen sulfate and ticlopidine hydrochloride were purchased from Sigma Aldrich (St. Louis, MO). The racemic (E)-2-bromo-3’-methoxyacetophenone (MPB)-derivatized clopidogrel active metabolite (CAMD) was synthesized by Alsachim (Illkirch, France). Optima-grade acetonitrile and methanol were obtained from Fisher Scientific (Fairlawn, NJ, USA) and de-ionized water was generated with a Hydro-Reverse Osmosis system (Durham, NC, USA) connected to a Milli-Q UV Plus purifying system (Billerica, MA, USA). Drug-free EDTA human plasma was obtained from the National Institutes of Health Clinical Center Blood Bank (Bethesda, MD, USA).
2.2. Preparation of Stock Solutions
Master stock solutions were prepared individually by dissolving clopidogrel, CAMD, and ticlopidine in methanol, acetonitrile, and acetonitrile, respectively, at free-base equivalent concentrations of 1 mg/mL. After vortex mixing and brief sonication, each of the three stock solutions were stored in glass vials at −80 °C. Serial dilutions (working stock cocktails) containing both clopidogrel and CAMD were prepared in acetonitrile from each individual master stock and stored in glass vials at −80 °C for the preparation of calibration and quality control (QC) samples. The chemical structures of clopidogrel and CAMD are pictured in Figure 1.
For each analytical run, calibration standards in drug-free human EDTA plasma were freshly prepared in duplicate at concentrations of 0.01, 0.05, 0.10, 0.50, 1.0, 5.0, 25, and 50 ng/ml for clopidogrel and 0.1, 0.5, 1.0, 5.0, 10, 50, 75, and 150 ng/mL for CAMD. QC samples were prepared in batch at concentrations of 0.04, 4.0, and 40 ng/mL for clopidogrel and 0.4, 12, and 120 ng/mL for CAMD by adding plasma to the required amount of working stock cocktail solution in a volumetric flask. QC samples were vortexed-mixed, then subdivided into aliquots and stored at −80 °C. Both calibration and quality control standards contained both clopidogrel and CAMD.
2.3. Sample Preparation
Frozen standards and samples were thawed on wet ice before homogenization by vortex-mixing. Fifty microliters of plasma calibrator aliquots, QC samples, and unknowns were each transferred into an Eppendorf mini-centrifuge tube. Protein precipitation was performed by adding 500 µL of ice-cold acetonitrile containing 15 ng/mL ticlopidine (internal standard) to each tube. This mixture was vortex-mixed for 30 sec and centrifuged for 10 min at 13,200 rpm (11,700 × g) before the supernatent was transferred to a Waters glass UPLC™ vial.
2.4. Instrument Conditions
The samples were chromatographically separated with a Waters Acquity UPLC™ system (Waters Corporation, Milford, MA, USA), which included a binary pump, a refrigerated autosampler, and a temperature-controlled column compartment. The injection volume was 5.0 µL, with the autosampler maintained at 4 °C, and the column compartment at 40 °C. Chromatographic separation was achieved on a Waters Acquity UPLC™ BEH C18 reverse-phase column (50 × 2.1 mm, internal diameter) and guard column packed with 1.7-µm packing material. The mobile phase consisted of A: 0.1% formic acid in water, and B: 0.1% formic acid in acetonitrile with a flow rate of 0.5 mL/min (the gradient scheme is provided in Table 1) and intended to elute the stereoisomers of the racemic CAMD together in one peak, as was previously performed for CAMD analysis alone [20]. This was coupled with an AB Sciex QTrap 5500 mass spectrometer (AB Sciex, Foster City, CA, USA). The mass spectrometer was set to monitor clopidogrel, CAMD, and ticlopidine (IS) using multiple reaction monitoring (MRM) in the positive ion mode. Table 2 provides the MRM settings for each compound. Universal mass spectrometric settings included ion spray voltage of 4500 V, source temperature of 400 °C, GS1 and GS2 at 50, entrance potential of 10, collision exit potential of 10, and dwell times of 50 msec. MRM peak integrations and data analyses were performed using the MultiQuant algorithm from MultiQuant 4.0 (Analyst®, AB Sciex).
Table 1.
Mobile Phase Composition
| Time | %A | %B | Flow (mL/min) |
|---|---|---|---|
| 0.00 | 60 | 40 | 0.5 |
| 0.10 | 60 | 40 | 0.5 |
| 0.20 | 10 | 90 | 0.5 |
| 1.20 | 10 | 90 | 0.5 |
| 1.30 | 60 | 40 | 0.5 |
Table 2.
Mass Spectrometric Settings
| Compound | Parent ion (m/z) |
Daughter ion (m/z) |
Collision Energy |
Declustering Potential |
|---|---|---|---|---|
| Clopidogrel | 322.0 | 212.1 | 23 | 44 |
| CAMD | 504.2 | 155.1 | 56 | 85 |
| Ticlopidine (IS) | 264.0 | 154.1 | 27 | 76 |
2.5. Validation
2.5.1. Linearity
Calibration curves for each analyte (clopidogrel and CAMD) were individually constructed by least-squares linear regression analysis of an eight-point calibration curve by plotting peak area of the analyte versus the peak area of the internal standard (ticlopidine), using 1/x2 as a weighting factor. Calibrator response functions and choice of regression analysis were investigated by calculating correlation coefficients (r) and the percent deviation (% DEV) for all calibrators.
2.5.2. Accuracy and Precision
Accuracy and precision were evaluated by determining clopidogrel and CAMD at three different concentrations of QC samples in five replicates analyzed over four different days. Each run consisted of blank plasma samples, internal standard only, and calibration standards in duplicate; and QC and lower limit of quantification (LLOQ) samples in replicates of five, with the LLOQ prepared independently in five different lots of plasma each of the four days (n=20). Accuracy (% DEV) was defined as the percent difference between the mean observed concentration and the nominal concentration. The repeatability of the assay was determined by the within-run precision (WRP) and between-run (BRP), as calculated below.
GM represents the grand mean over the four days, MSwit represents the within-group mean squared, MSbet represents the between-group mean squared, and n represents the number of repetitions (n=20, with QCs and LLOQs analyzed in quintuplet over four days). For the calculation of BRP, there are instances where MSwit > MSbet, thus making a negative number, of which a square root cannot be taken. In this case, it is assumed that no additional variation was observed as a result of performing the assay in different runs. FDA guidelines for bioanalytical accuracy and precision were followed, with ± 15% variability allowed except for the LLOQ, where ± 20% variability is acceptable [23].
2.5.3. Stability
Storage stability has been well characterized for both clopidogrel and CAMD in plasma at varying temperatures and lengths of time. Clopidogrel was previously shown to be stable in plasma for at least 2 months at −20 °C [10], and at least 6 months at −70 °C [13]. The stability of CAMD was previously demonstrated in plasma for at least 4 months at −20 °C and at least 8 months at −80 °C [22].
2.5.3.1. Freeze/Thaw Stability
Stability tests were performed to verify the stability of clopidogrel and CAMD during freeze/thaw cycles. Samples were assayed at two calibrator concentrations (0.1 and 25 ng/mL for clopidogrel; 1.0 and 75 ng/mL for CAMD). The samples were subjected to four freeze/thaw cycles at −80 °C, with each freeze cycle lasting at least 12 hr. The concentration of the drugs after each storage period was compared to the concentration of freshly prepared samples in the same analytical run.
2.5.3.2. Short-Term Autosampler Stability
The stability of clopidogrel and CAMD in the injection vials pending analysis in the autosampler (autosampler stability) was performed. Samples were re-injected and reanalyzed 24 hr after the initial analysis and compared to values obtained from those same samples prepared 24 hr prior.
2.5.4. Matrix Effects
Matrix effects from the plasma on the mass spectrometric signals for clopidogrel, CAMD, and the internal standard ticlopidine were assessed through direct comparison of samples spiked in plasma to samples spiked in water. Clopidogrel and CAMD peak areas were compared using a low (n=3) and a high (n=3) calibrator sample spiked into water with 500 µL of 15 ng/mL ticlopidine in ACN added, in addition to one low and one high calibrator spiked into each of 5 plasma lots. Matrix effects (ME) were calculated using analyte peak areas as follows:
2.6. Clinical Application
2.6.1. IRB Approval
The protocol was approved by the University of Maryland, Baltimore Institutional Review Board and the Food and Drug Administration Research Involving Human Subjects Committee.
2.6.2. Subject Treatment
Three subjects were each administered one tablet of 75, 150, or 300 mg of clopidogrel bisulfate (Plavix®), with all three subjects eventually receiving all three doses. A washout period of at least seven days was required between doses. Two separate aliquots of blood were drawn from the subjects at the following time points: pre-dose, 0.25, 0.50, 1, 2, and 4 hours post dose. The first aliquot of blood was drawn into an EDTA tube for analysis of parent clopidogrel. The second aliquot of blood was drawn into an EDTA tube pretreated with 30 µL of 500 mM of MPB to immediately derivatize the clopidogrel active metabolite for accurate analyses. Derivatization efficiency of the active metabolite (CAM) with MPB was previously assessed [21]. Based on this derivatization optimization, four previous clinical trials added at least 20 µL of 500 mM MPB in acetonitrile to human blood for the analysis of MPB-derivatized CAM [20– 22,24]. Therefore, 30 µL of 500 mM of MPB in acetonitrile was used to derivatize CAM without further optimization.
3. Results and Discussion
3.1. Limits of Quantification
Independent LLOQ experiments were performed by preparing the LLOQ in five different lots of plasma over four days (n=20), and back-calculating the concentration as a “quality control” sample. The LLOQ was 0.01 ng/mL for clopidogrel and 0.1 ng/mL for CAMD. The average back-calculated clopidogrel concentration was 0.01 ± 0.00 (mean ± SD). The precision (% CV) was 5.92% and the accuracy (% DEV from the nominal standard) was 2.05%. The average back-calculated CAMD concentration was 0.10 ± 0.00 (mean ± SD). The % CV was 3.71% and the % DEV from the nominal standard was 0.01%.
3.2. Selectivity
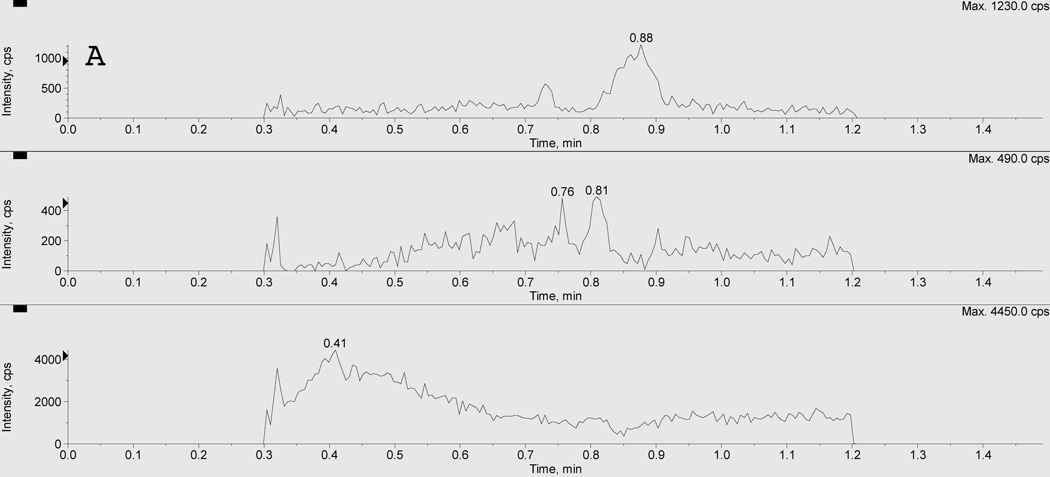
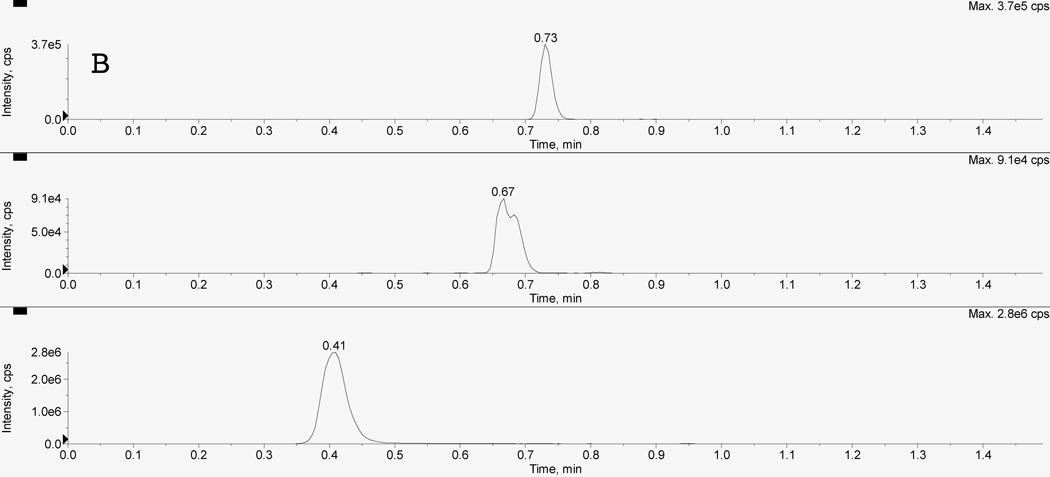
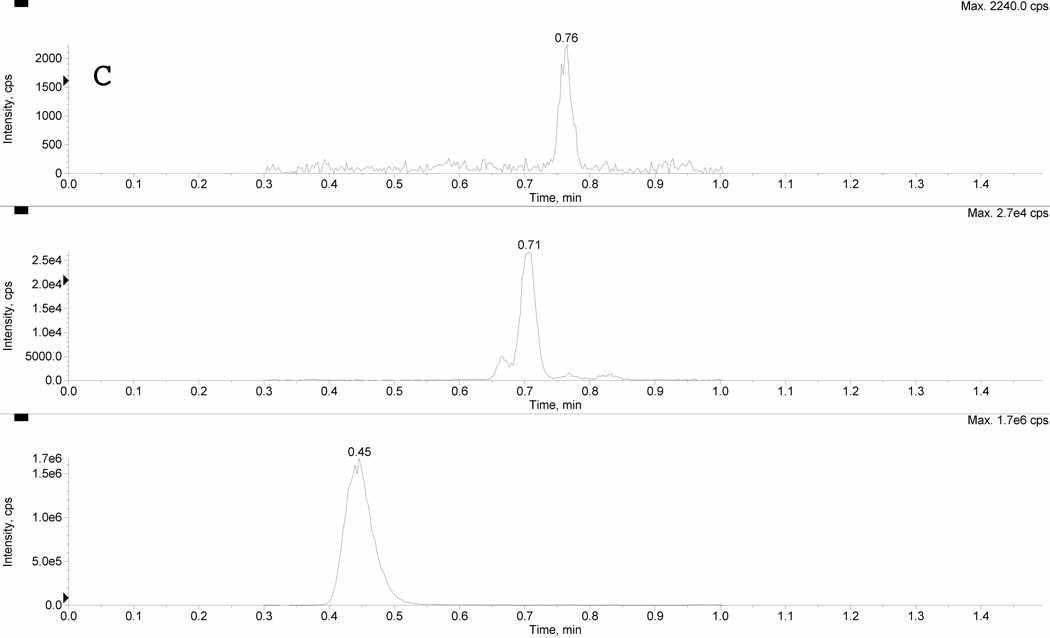
Figure 2 depicts typical chromatograms resulting from the uHPLC-MS/MS analysis of extracts of 50 µL plasma from a: (A) blank plasma sample, a (B) mid quality control (MQC), and (C) a clinical sample of clopidogrel and CAMD. The clopidogrel, CAMD, and internal standard peaks were sufficiently chromatographically separated under the optimized conditions, with retention times for ticlopidine (IS; bottom), CAMD (middle), and clopidogrel (top) of 0.41, 0.67, and 0.73 min, respectively. The total run time was 1.5 min. Clopidogrel peak shape, resolution, and signal to noise were acceptable to meet FDA criteria for LLOQ. The CAMD peak shape is relatively broad (compared to clopidogrel) for calibrators and QCs (Figure 2B) due to the presence of stereoisomers from the racemic synthesized reference standard. The CAMD stereoisomers were purposely eluted together, and our result was consistent with previous reports of broad CAMD peak shapes from synthetic racemates [20]. This was further supported by CAMD peak shape in clinical samples (Figure 2C), which demonstrated a clear stereoselective CAMD diastereomer, thus a sharper peak shape at a relatively low plasma concentration ([CAMD]=3.60 ng/mL). The excellent precision (%CV < 3.71) for CAMD at 0.1 ng/mL suggested sufficient selectivity and was acceptable for the LLOQ.
Figure 2. Chromatogram of A) blank plasma extract, B) mid quality control sample, and C) clinical sample.
Panel A represents a blank plasma extract, Panel B represents a mid quality control sample (MQC), and Panel C represents a clinical sample. There are three separate LC-MS/MS chromatographic tracings within each panel resulting from three simultaneous multiple reaction monitoring (MRM) transitions. The bottom pane represents the internal standard ticlopidine (m/z 264>154); the middle pane represents CAMD (m/z 504>155); and the top pane represents clopidogrel (m/z 322>212). The racemic CAMD reference standard produces a broad peak (B), due to the presence of both stereoisomers. Clinical samples demonstrate the stereoselective metabolism, preferentially forming one stereoisomer as evident by the relatively sharper peak shape.
Furthermore, no carryover was observed for either analyte or IS by running a blank sample following the upper limit of quantification (ULOQ; 50 ng/mL for clopidogrel, 150 ng/mL for CAMD) and not detecting any analyte or IS peaks at their respective retention times.
3.3. Linearity
For the clopidogrel standard curve, the calibrators were back-calculated from the peak area ratios of clopidogrel:IS and the intercept. The deviation for all concentrations from the nominal concentrations fell within acceptable limits, between −10.0 and 5.97%, whereas the (%CV) ranged from 0.49 to 5.74% (Table 3A). For each analytical run in plasma, an eight-point standard curve was constructed and shown to be linear over the tested range of 0.01–50 ng/mL. The mean (± standard deviation) correlation coefficient obtained on four separate days resulted in a mean r2 = 0.9945 ± 0.0016 (n = 4). The model with the least total bias across the concentration range investigated was obtained using 1/x2 as the weighting factor.
Table 3.
Linearity
| Table 3A. Clopidogrel concentrations from calibration curves | |||||
|---|---|---|---|---|---|
| Nominal (ng/ml) | GM (ng/ml) | S.D. (ng/ml) | DEV (%) | CV (%) | n |
| 0.01 | 0.01 | 0.00 | −2.38 | 0.49 | 8 |
| 0.05 | 0.05 | 0.00 | 5.97 | 5.74 | 8 |
| 0.1 | 0.10 | 0.01 | 4.81 | 4.85 | 8 |
| 0.5 | 0.55 | 0.01 | 9.37 | 1.95 | 8 |
| 1.0 | 1.05 | 0.03 | 5.16 | 2.78 | 8 |
| 5.0 | 4.72 | 0.06 | −5.64 | 1.37 | 8 |
| 25 | 22.5 | 0.47 | −10.0 | 2.09 | 8 |
| 50 | 45.9 | 1.25 | −8.05 | 2.73 | 8 |
| Table 3B. CAMD concentrations from calibration curves | |||||
|---|---|---|---|---|---|
| Nominal (ng/ml) | GM (ng/ml) | S.D. (ng/ml) | DEV (%) | CV (%) | n |
| 0.1 | 0.10 | 0.00 | −1.30 | 0.51 | 8 |
| 0.5 | 0.53 | 0.01 | 5.65 | 2.16 | 8 |
| 1.0 | 1.01 | 0.02 | 0.86 | 2.47 | 8 |
| 5.0 | 5.20 | 0.05 | 4.00 | 0.87 | 8 |
| 10 | 10.4 | 0.36 | 4.27 | 3.44 | 8 |
| 50 | 48.2 | 1.00 | −3.63 | 2.07 | 8 |
| 75 | 71.6 | 2.56 | −4.51 | 3.58 | 8 |
| 150 | 142 | 2.77 | −5.15 | 1.95 | 8 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV (%), relative deviation from nominal value; CV (%), coefficient of variation; n, number of replicate observations within each validation run, i.e. two samples at each concentration were run on four separate occasions, for a total (n) of eight samples at each concentration.
The calibrators for CAMD were back-calculated from the peak area ratios of CAMD:IS and the intercept. The deviations from the nominal concentrations were between −5.15 and 5.65%, whereas the precision (%CV) ranged from 0.51 to 3.58% (Table 3B). For each analytical run in plasma, an eight-point standard curve was constructed and was shown to be linear over the tested range of 0.1–150 ng/mL. The mean (± standard deviation) correlation coefficient obtained on four separate days resulted in a mean r2 = 0.9978 ± 0.0007 (n = 4) using 1/x2 as the weighting factor.
3.4. Accuracy and Precision
The assay performance data for the determination of independent QC samples of clopidogrel in plasma are presented in Table 4A. The deviation from nominal concentration (accuracy) ranged from −11.7 to −2.37% and within-run precision was all less than 1.1%.
Table 4.
Accuracy and Precision
| Nominal (ng/ml) |
GM (ng/ml) |
S.D. (ng/ml) |
DEV (%) |
WRP (%) |
n |
|---|---|---|---|---|---|
| 0.04 | 0.04 | 0.00 | −2.86 | 1.06 | 20 |
| 4.0 | 3.91 | 0.14 | −2.37 | 1.01 | 20 |
| 40 | 35.3 | 0.64 | −11.7 | 1.02 | 20 |
| Nominal (ng/ml) |
GM (ng/ml) |
S.D. (ng/ml) |
DEV (%) |
WRP (%) |
n |
|---|---|---|---|---|---|
| 0.4 | 0.38 | 0.03 | −5.69 | 1.11 | 20 |
| 12 | 11.7 | 0.63 | −2.23 | 1.04 | 20 |
| 120 | 111 | 5.16 | −6.78 | 1.02 | 20 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV (%) relative deviation from nominal value; WRP, within-run precision; n, number of replicate observations within each validation run.
For the active metabolite CAMD, deviation from nominal concentration (accuracy) ranged from −6.78 to −2.23% and within-run precision was all less than 1.2% (Table 4B). Between-run variation could not be calculated for any QCs for clopidogrel or CAMD due to the square of within-run means > square of between-run means, which results in taking the square root of a negative number. Thus, we concluded that no additional variation was observed as a result of performing the assay in different runs.
3.5. Stability
3.5.1. Freeze/Thaw Stability
No significant degradation was observed following four freeze/thaw cycles of plasma samples containing clopidogrel at concentrations of 0.1 or 25 ng/mL (Table 5A). Likewise, minimal degradation was observed following four freeze/thaw cycles of plasma samples containing CAMD at concentrations of 1.0 or 75 ng/mL (Table 5B).
Table 5.
Freeze/Thaw Stability
| Table 5A | 0.1 ng/mL | 25 ng/ML | ||
|---|---|---|---|---|
| Freeze/Thaw Cycles |
GM (ng/ml) |
DEV from fresh (%) |
GM (ng/ml) |
DEV from fresh (%) |
| 0 (Fresh) | 0.11 | - | 23.0 | - |
| 1 | 0.11 | −4.34 | 22.1 | −3.99 |
| 2 | 0.10 | −8.87 | 23.6 | 2.73 |
| 3 | 0.10 | −9.04 | 22.1 | −4.12 |
| 4 | 0.10 | −8.85 | 21.7 | −5.63 |
| Table 5B | 1.0 ng/mL | 75 ng/mL | ||
|---|---|---|---|---|
| Freeze/Thaw Cycles |
GM (ng/ml) |
DEV (%) |
GM (ng/ml) |
DEV (%) |
| 0 (Fresh) | 1.01 | - | 72.6 | - |
| 1 | 1.03 | 2.42 | 70.7 | −2.60 |
| 2 | 0.97 | −3.58 | 76.3 | 5.19 |
| 3 | 1.02 | 0.67 | 71.2 | −1.94 |
| 4 | 1.04 | 2.64 | 70.1 | −3.40 |
Abreviations: GM, grand mean; S.D., standard deviation; DEV (%) relative deviation from nominal value
3.5.2. Short-Term Autosampler Stability
Clopidogrel and CAMD short-term stability was assessed by re-running a validation set after sitting in the autosampler at 4 °C for 24 hr. Clopidogrel (Table 6A) and CAMD (Table 6B) both demonstrated good short-term stability in the autosampler, with mean deviations under ± 4.0% after 24 hr.
Table 6.
Short-Term Autosampler Stability
| Table 6A. Autosampler Stability for Clopidogrel | |||||
|---|---|---|---|---|---|
| Immediately after preparation | After 24 hours | Mean Change after 24h (%) |
|||
| Nominal (ng/ml) |
GM (ng/ml) | DEV (%) | GM (ng/ml) | DEV (%) | |
| 0.04 | 0.04 | −3.00 | 0.04 | −5.95 | −3.04 |
| 4 | 4.09 | 2.13 | 4.17 | 4.17 | 2.00 |
| 40 | 36.1 | −9.66 | 35.6 | −11.1 | −1.55 |
| Table 6B. Autosampler Stability for CAMD | |||||
|---|---|---|---|---|---|
| Immediately after preparation | After 24 hours | Mean Change after 24h (%) |
|||
| Nominal (ng/ml) |
GM (ng/ml) | DEV (%) | GM (ng/ml) | DEV (%) | |
| 0.4 | 0.39 | −2.78 | 0.39 | −2.94 | −0.16 |
| 12 | 12.0 | 0.30 | 12.4 | 3.70 | 3.39 |
| 120 | 111 | −7.27 | 112 | −5.99 | 1.38 |
Abbreviations: GM, grand mean; S.D., standard deviation; DEV (%) relative deviation from nominal value
3.6. Matrix Effects
Clopidogrel peak areas in plasma were 14% and 16% lower than in water, whereas CAMD peak areas in plasma were 1% higher and 2% lower than in water, for the low and high concentration calibrators, respectively. The internal standard, ticlopidine, demonstrated 8% higher peak areas in plasma compared to water.
3.7 Clinical Application
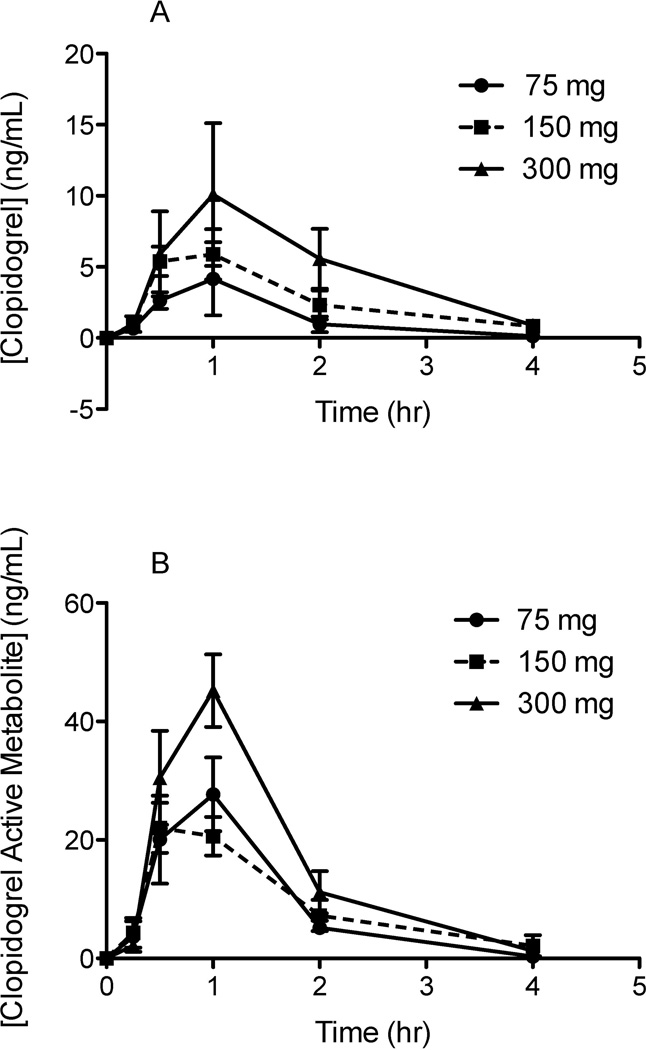
The method was subsequently applied to three subjects who were each administered clopidogrel tablets of 75 mg, 150 mg, or 300 mg with a washout period of at least seven days between doses. The first-dose concentration time curves (mean ± SEM; n=3) were plotted for both parent clopidogrel and its MPB-derivatized active metabolite (Figure 3). This uHPLC-MS/MS method demonstrated sufficient sensitivity and selectivity for both compounds. Inter-patient variability was observed in both parent clopidogrel and CAMD plasma concentrations, with differences in CYP2C19 genotype as a potential source of variability. Loss of function variants have been shown to demonstrate significantly decreased active metabolite exposure (P=0.004) and CMAX (P=0.020), which also affects parent drug levels [5]. Incurred sample re-analysis (24 hours in autosampler) demonstrated less than 20% differences in calculated clopidogrel and CAMD concentrations. However, the majority of the larger percent differences were due to changes in very small concentrations (e.g. 0.015 ng/mL vs 0.011 ng/mL provides a 33.3% difference).
Figure 3. Clinical Concentration-Time Curves over 3 dose levels for A) Clopidogrel, and B) Clopidogrel Active Metabolite-Derivatized (CAMD).
Three subjects were administered clopidogrel orally at three dose levels: 75 mg, 150 mg, and 300 mg. Two separate aliquots of blood were drawn from the subjects at the following time points: pre-dose, 0.25, 0.50, 1, 2, and 4 hours post dose. The first aliquot of blood was drawn into an EDTA tube for analysis of parent clopidogrel. The second aliquot of blood was drawn into an EDTA tube pretreated with 30 µL of 500 mM of MPB to immediately derivatize the clopidogrel active metabolite for accurate analyses.
The clopidogrel calibration range (0.01–50 ng/mL) was sufficient to measure plasma levels observed clinically, with one subject having a maximum plasma concentration (CMAX) of 20 ng/mL. As this was a relatively small subject population, the range was set with an upper limit of quantification (ULOQ) at 50 ng/mL to account for CYP2C19 poor metabolizers that would exhibit higher than normal clopidogrel plasma levels. The same is true for the CAMD calibration range (0.1–150 ng/mL), as one subject had a CMAX of 98 ng/mL. The calibration ranges of both compounds were maintained at relatively high ULOQs to account for both extensive and poor metabolizers of CYP2C19.
It was initially believed that MPB had a deleterious effect on clopidogrel signals in the mass spectrometer, thus this was controlled for clinically by drawing two separate aliquots of blood, one for clopidogrel without MPB, the other aliquot containing 30 µL of 500 mM MPB for derivatization of CAM. After the analysis of >1000 clinical samples, it was subsequently demonstrated that there was no statistically significant differences in clopidogrel plasma concentrations in clinical sample aliquots with and without MPB (Table 7). Retrospectively, there was no need to draw separate aliquots for analysis of parent and metabolite; hence this method can be applied for the simultaneous quantification of both clopidogrel and CAMD in a single clinical blood sample. This also suggests that MPB did not cause any significant matrix effects.
Table 7.
Effect of MPB on the quantification of clopidogrel
| Dose-Time Point | [Clop] (ng/mL) Without MPB (Mean ± SE; n=18) |
Mean [Clop] (ng/mL) With MPB (Mean ± SE; n=18) |
P-value |
|---|---|---|---|
| 75mg-0.25 hr | 0.3161 ± 0.0897 | 0.3144 ± 0.0849 | 0.9291 |
| 75mg-0.50 hr | 1.065 ± 0.3115 | 1.017 ± 0.3035 | 0.2287 |
| 75mg-1.0 hr | 1.189 ± 0.5259 | 1.123 ± 0.4693 | 0.3310 |
| 75mg-2.0 hr | 0.3381 ± 0.1110 | 0.3505 ± 0.1100 | 0.5405 |
| 75mg-4.0 hr | 0.0348 ± 0.0071 | 0.0315 ± 0.0069 | 0.1818 |
| 150mg-0.25 hr | 0.4487 ± 0.1687 | 0.4956 ± 0.1770 | 0.0554 |
| 150mg-0.50 hr | 1.612 ± 0.5310 | 1.569 ± 0.4856 | 0.5259 |
| 150mg-1.0 hr | 1.839 ± 0.5590 | 1.923 ± 0.5674 | 0.5867 |
| 150mg-2.0 hr | 1.097 ± 0.2681 | 1.059 ± 0.2583 | 0.0930 |
| 150mg-4.0 hr | 0.1663 ± 0.0467 | 0.1435 ± 0.0370 | 0.0914 |
| 300mg-0.25 hr | 0.6800 ± 0.3236 | 0.6556 ± 0.2937 | 0.4857 |
| 300mg-0.50 hr | 2.151 ± 0.7728 | 2.172 ± 0.8208 | 0.7937 |
| 300mg-1.0 hr | 3.191 ± 1.152 | 3.217 ± 1.200 | 0.6755 |
| 300mg-2.0 hr | 1.816 ± 0.6160 | 1.674 ± 0.5467 | 0.1053 |
| 300mg-4.0 hr | 0.3586 ± 0.1068 | 0.3381 ± 0.1000 | 0.1002 |
Conclusions
For the first time, both clopidogrel and its active metabolite were quantitatively assayed simultaneously over wide calibration ranges (clopidogrel: 0.01–50 ng/mL; CAMD: 0.1–150 ng/mL). The use of ultra HPLC allowed for efficient chromatographic separation with a short run time of 1.5 min, and the protein precipitation step allowed for short sample preparation time and the ability to run samples in a high-throughput manner.
The method proved sensitive, with a lower limit of quantification of 0.01 ng/mL for parent clopidogrel and 0.1 ng/mL for MPB-derivatized active metabolite. The assay was accurate, precise, and linear over the entire calibration range for both analytes. All validation criteria met with FDA bioanalytical guideline requirements. Both compounds demonstrated minimal degradation through four freeze/thaw cycles, which corresponds well with previous freeze/thaw stability studies for clopidogrel alone [13].
Overall, the method presented here allows for the rapid, selective, and sensitive quantitation of clopidogrel and its active metabolite, and is ideally suited towards analyzing pharmacokinetic samples in a high-throughput manner.
Highlight.
We developed and validated a novel uHPLC-MS/MS assay for the simultaneous quantification of clopidogrel and its derivatized active metabolite.> Clinically-relevant calibration ranges for clopidogrel (0.01–50 ng/mL) and active metabolite (0.1–150 ng/m) were used. > Successfully applied to clinical phase I study using 3 subjects.
Acknowledgements
This study was funded by the Bench to Bedside Program of the National Institutes of Health (128475) and the National Institute of General Medicine Science (U01GM074518-05S1).
Abbreviations
- uHPLC
Ultra-high performance liquid chromatography
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MPB
2-bromo-3’-methoxyphenone
- CAMD
MPB-derivatized clopidogrel active metabolite
- QC
Quality control
- LLOQ
Lower limit of quantification
- EDTA
Ethylenediaminetetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. The views in this manuscript are those of the authors and may not necessarily reflect FDA or NIH policy. No official endorsement is intended nor should be inferred.
References
- 1.Diener HC, Ringleb PA, Savi P. Expert Opin Pharmacother. 2005;6:755. doi: 10.1517/14656566.6.5.755. [DOI] [PubMed] [Google Scholar]
- 2.Heestermans AA, van Werkum JW, Schomig E, ten Berg JM, Taubert D. J Thromb Haemost. 2006;4:1143. doi: 10.1111/j.1538-7836.2006.01891.x. [DOI] [PubMed] [Google Scholar]
- 3.Pereillo JM, Maftouh M, Andrieu A, Uzabiaga MF, Fedeli O, Savi P, Pascal M, Herbert JM, Maffrand JP, Picard C. Drug Metab Dispos. 2002;30:1288. doi: 10.1124/dmd.30.11.1288. [DOI] [PubMed] [Google Scholar]
- 4.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Drug Metab Dispos. 2010;38:92. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 5.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, Lachno DR, Salazar D, Winters KJ. J Thromb Haemost. 2007;5:2429. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 6.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Lancet. 2009;373:309. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 7.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. N Engl J Med. 2009;360:354. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 8.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. N Engl J Med. 2009;360:363. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 9.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, Neumann FJ. J Am Coll Cardiol. 2008;51:1925. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 10.Shin BS, Yoo SD. Biomed Chromatogr. 2007;21:883. doi: 10.1002/bmc.850. [DOI] [PubMed] [Google Scholar]
- 11.Robinson A, Hillis J, Neal C, Leary AC. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:344. doi: 10.1016/j.jchromb.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 12.Nirogi RV, Kandikere VN, Shukla M, Mudigonda K, Maurya S, Boosi R. Rapid Commun Mass Spectrom. 2006;20:1695. doi: 10.1002/rcm.2497. [DOI] [PubMed] [Google Scholar]
- 13.Silvestro L, Gheorghe MC, Tarcomnicu I, Savu S, Savu SR, Iordachescu A, Dulea C. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3134. doi: 10.1016/j.jchromb.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Bahrami G, Mohammadi B, Sisakhtnezhad S. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;864:168. doi: 10.1016/j.jchromb.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Mani H, Toennes SW, Linnemann B, Urbanek DA, Schwonberg J, Kauert GF, Lindhoff-Last E. Ther Drug Monit. 2008;30:84. doi: 10.1097/FTD.0b013e31815c13fd. [DOI] [PubMed] [Google Scholar]
- 16.Souri E, Jalalizadeh H, Kebriaee-Zadeh A, Shekarchi M, Dalvandi A. Biomed Chromatogr. 2006;20:1309. doi: 10.1002/bmc.697. [DOI] [PubMed] [Google Scholar]
- 17.Ksycinska H, Rudzki P, Bukowska-Kiliszek M. J Pharm Biomed Anal. 2006;41:533. doi: 10.1016/j.jpba.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Patel NK, Subbaiah G, Shah H, Kundlik M, Shrivastav PS. J Chromatogr Sci. 2008;46:867. doi: 10.1093/chromsci/46.10.867. [DOI] [PubMed] [Google Scholar]
- 19.Taubert D, Kastrati A, Harlfinger S, Gorchakova O, Lazar A, von Beckerath N, Schomig A, Schomig E. Thromb Haemost. 2004;92:311. doi: 10.1160/TH04-02-0105. [DOI] [PubMed] [Google Scholar]
- 20.Delavenne X, Basset T, Zufferey P, Malouk N, Laporte S, Mismetti P. J Sep Sci. 2010;33:1968. doi: 10.1002/jssc.201000115. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Pang H, Kawabata K, Farid NA, Kurihara A. J Pharm Biomed Anal. 2008;48:1219. doi: 10.1016/j.jpba.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Tuffal G, Roy S, Lavisse M, Brasseur D, Schofield J, Delesque Touchard N, Savi P, Bremond N, Rouchon MC, Hurbin F, Sultan E. Thromb Haemost. 2011;105 doi: 10.1160/TH10-09-0582. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. Guidelines for Industry: Bioanalytical Methods Validation. 2001
- 24.Small DS, Payne CD, Kothare P, Yuen E, Natanegara F, Teng Loh M, Jakubowski JA, Richard Lachno D, Li YG, Winters KJ, Farid NA, Ni L, Salazar DE, Tomlin M, Kelly R. Clin Ther. 2010;32:365. doi: 10.1016/j.clinthera.2010.02.015. [DOI] [PubMed] [Google Scholar]