Abstract
Th17 cells have been described as short-lived but this view is at odds with their capacity to trigger protracted damage to normal and transformed tissues. We report that Th17 cells, despite displaying low expression of CD27 and other phenotypic markers of terminal differentiation, efficiently eradicated tumors and caused autoimmunity, were long-lived and maintained a core molecular signature resembling early memory CD8+ cells with stem cell-like properties. In addition, we found that Th17 cells had high expression of Tcf7, a direct target of the Wnt and β-catenin signaling axis, and accumulated β-catenin, a feature observed in stem cells. In vivo, Th17 cells gave rise to Th1-like effector cell progeny and also self-renewed and persisted as IL-17A-secreting cells. Multipotency was required for Th17 cell-mediated tumor eradication because effector cells deficient in IFN-γ or IL-17A had impaired activity. Thus, Th17 cells are not always short-lived and are a less-differentiated subset capable of superior persistence and functionality.
Introduction
A key feature of adaptive immunity is the ability to generate long-lived populations of self-renewing memory cells; however, the evolutionary benefits of having robust anamnestic responses are balanced against the burden and hazard of maintaining large numbers of antigen-specific lymphocytes. Upon antigen stimulation, both CD8+ and CD4+ T cells experience a stereotypical clonal expansion followed by a contraction phase and the formation of memory (Kaech et al., 2002). While CD8+ memory can be retained almost indefinitely, the ability of CD4+ cells to persist is less understood and appears dependent upon the conditions of initial antigenic exposure (Homann et al., 2001; McKinstry et al., 2010; Taylor and Jenkins, 2011; Williams et al., 2008). The relative efficiency with which different CD4+ T cell subsets enter into the memory pool is the matter of discussion (MacLeod et al., 2009) and the analysis of memory formation is complicated because some polarized T helper (Th) cell subsets are meta-stable and experience plasticity (Zygmunt and Veldhoen, 2011).
In a recent report, Th17 cells were characterized as short-lived effector cells with a limited capacity to persist that was attributed to extinction of IL-17A secretion and low expression of CD27, when compared with Th1 cells (Pepper et al., 2010). In this elegant study, the authors analyzed endogenous Th1 and Th17 cells induced upon infection, thus allowing for in situ glimpses at the “real” T cell response in a more naturalistic setting than reports based on cells generated ex vivo (Surh and Sprent, 2010). However, the assertion that Th17 cells have a limited survival potential seems at odds with their protective role in antimicrobial immunity and the protracted tissue damage associated with Th17 responses in autoimmune disorders such as arthritis, multiple sclerosis, Crohn’s disease, uveitis, psoriasis and graft-versus-host disease (Carlson et al., 2009; Maynard and Weaver, 2009; Sallusto and Lanzavecchia, 2009; Shi et al., 2009). The view that Th17 cells are short-lived also seems contrary to the superior anti-tumor activity of adoptively transferred Th17 cells (Martin-Orozco et al., 2009b; Muranski et al., 2008; Muranski and Restifo, 2009), where persistence is critical to achieving complete tumor eradication (Shen et al., 2007; Zhou et al., 2005).
We therefore sought to study the phenotype, functional maturation and survival of Th17 cells in vivo using a T cell receptor (TCR) transgenic model where CD4+ cells are specific for the TRP-1 tissue differentiation antigen expressed by normal and transformed melanocytes and are capable of eradicating large established tumors (Muranski et al., 2008). Although Th17 cells can become “Th1-like” (Bending et al., 2009; Lee et al., 2009; Palmer and Weaver, 2010; Wei et al., 2009), it remains unclear why anti-tumor Th17-derived cells are more potent than their Th1 cell counterparts. In addition, the specific roles of IL-17A and other type 17-related pro-inflammatory cytokines remain controversial as they might either inhibit or promote early tumor progression (Murugaiyan and Saha, 2009; Zou and Restifo, 2010).
We confirmed observations that Th17 cells resembled a terminally-differentiated CD8+ T cell population defined by low expression of CD62L and CD27. We observed, however, that those Th17-derived cells critically required Th1-like features for the eradication of tumor, implying that the transferred Th17 cells were not terminally differentiated and functioned – at least in part – as precursors to Th1-like cells. Therefore, we hypothesized that a static immunophenotypic description may not be sufficient to explain the functionality of Th17 cells in vivo, as late plasticity of Th17 cells might introduce an additional layer of complexity to Th cell-mediated responses as they mature. Th17-derived cells maintained a molecular profile distinct from their Th1 cell-derived counterparts and were enriched with genes associated with a less differentiated CD8+ memory subset (Wirth et al., 2010). We discovered that Th17 cells expressed a signature closely resembling the pattern observed in stem cell-like memory cells (SCM) originally generated pharmacologically by activation of the Wnt-β-catenin pathway in a CD8-based model (Gattinoni et al., 2009) and recently identified in normal human peripheral blood (Gattinoni et al., 2011). Functionally, these characteristics manifested themselves not only as a superior ability to treat tumor and cause autoimmune self-tissue destruction, but also by stem cell-like properties such as an enhanced capability to survive, self-renew, generate effector progeny and enter the memory pool with efficiency dramatically superior to that of Th1 cells.
Results
Th17-polarized TRP-1 cells efficiently reject established tumors [Au: Shortened OK?]
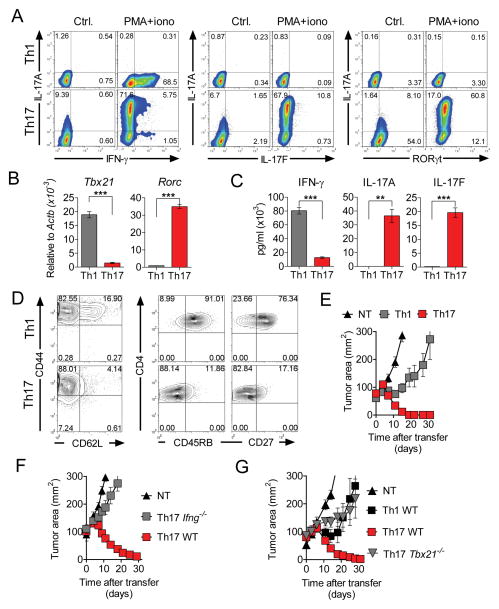
The conditions required for the induction of Th17 cells from naïve cells are becoming better understood (Ghoreschi et al., 2010; Yang et al., 2011). Based on those advances we generated highly-polarized populations of Th1 and Th17 TRP-1 cells. Intracellular staining revealed that the majority (>90%) of cells found in the Th17 cell-polarized cultures expressed IL-17A or canonical Th17 cell-defining transcription factor RORγt (Figure 1A). A high degree of polarization was further confirmed by quantitative RT-PCR measurement of subset-specific transcription factors Rorc (RORγt) and Tbx21 (T-bet) (Figure 1B) and by ELISA detection of IFN-γ, IL-17A and IL-17F following overnight peptide restimulation (Figure 1C).
Figure 1. Th17-polarized cells effectively reject large tumors despite phenotypic features suggesting terminal differentiation, but must acquire type 1-like features in vivo.
(A) Representative intracellular staining demonstrating production of IFN-γ IL-17A, IL-17F and Rorγt by Th1 and Th17-polarized TRP-1 cells generated in vitro following 4 hour re-stimulation with phorbol myrystate acetate (PMA) and ionomycin in the presence of brefeldin A. Resting polarized cells were used as negative control.
(B) Expression of Tbx21 and Rorc in in vitro polarized Th1 and Th17 cells was measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and expressed relative to β-actin (Actb).
(C) Secretion of indicated cytokines upon peptide stimulation was measured by ELISA. Error bars for (B) and (C) represent SEM (n=3).
(D) Contour plots show expression of CD44 vs. CD62L and CD4 vs. CD45RB and CD27 on the surface of Th1 and Th17-polarized TRP-1 cells at day 7 following stimulation.
(E) A total of 1×106 TRP-1 cells polarized into Th1 and Th17 subsets were injected into sublethally (5 Gy) irradiated C57/B6 mice bearing B16.10 tumors. Tumor growth was measured serially and is represented as tumor area. Error bars represent SEM (n=5–7).
(F)(G) Relative contribution of IFN-γ and T-bet to the antitumor activity of TCR-engineered Th17-polarized cells. CD4+ cells from WT, Ifng−/− and Tbx21−/− mice were polarized as indicated and transduced with a retroviral vector encoding the TRP-1 TCR. A total of 1.2×106 transduced cells were transferred as in (E). Recipients were treated with exogenous IL-2 and rVV TRP-1 vaccine. Error bars represent SEM (n=4–7). All tumor treatment experiments were reproduced at least twice. See also Figure S1.
Judging by their phenotype, Th17 polarization directed CD4+ cells towards terminal differentiation that could be expected to impair in vivo functionality, as we found that the vast majority of Th17 cells were CD44hi, CD62Llo, CD45RBlo and CD27lo, whereas Th1 cells retained less differentiated characteristics, as they were mostly CD45RBhi and CD27hi and retained a higher percentage of cells expressing CD62L (Figure 1D). These phenotypic differences could not be simply explained by variations in proliferative history as indicated by similar rapid CFSE dilution following the initial stimulation of naïve cells under type 1 and type 17 polarizing conditions (Figure S1A). When TRP-1 TCR transgenic Th17 cells were transferred into mice bearing established subcutaneous melanomas, they rapidly eradicated tumors whereas Th1-polarized cells were less effective (p<0.05, Figure 1E). Thus, in our model, the differentiation state estimated by phenotype of the cells did not correlate with responses observed in a functional assay of tumor elimination. Moreover, the low expression of some other phenotypic markers of senescence, such as CD25, KLRG1 and PD-1, were not consistent with the view that Th17 cells are more terminally differentiated (Figure S1B).
Th17-polarized cells must acquire Th1 cell features in vivo to eradicate tumor
The ability of Th17-polarized cells to acquire Th1 cell properties is increasingly recognized, but the contribution of such plasticity to the anti-tumor functionality of Th17 cells in vivo remains poorly defined. In order to assess the function of Th17 cells in a variety of genetically deficient mouse strains, we cloned the TRP-1 TCR into a retroviral vector (Figure S1C)(Kerkar et al., 2011). Comparable transduction efficiency was achieved in Th1 and Th17 cells derived from wild-type (WT) mice and Th17-polarized cells derived from Tbx21−/− and Ifng−/− mice (Figure S1D). TCR gene-modified cells specifically recognized cognate TRP-1 peptide and secreted Th1 and Th17-defining cytokines in a pattern consistent with polarization conditions (Figure S1E). Notably, we observed that Th17-polarized cells secreted high concentrations of IL-2, a feature typically associated with the early maturation stage in CD8+ memory cells (Klebanoff et al., 2006).
Upon adoptive transfer, we observed a profound impairment of anti-tumor functionality in Ifng−/− Th17 polarized cells (p<0.05, Figure 1F). Similarly, the activity of Tbx21−/− Th17 cells was significantly diminished (p<0.05) in comparison to WT Th17 TCR-transduced cells (Figure 1G). In the same experiment, WT TCR-transduced Th1-polarized cells were less efficient (p<0.05) than their Th17-polarized counterparts, recapitulating the results observed in the TCR transgenic TRP-1 model. In conclusion, the ability to differentiate into Th1-like effectors is required for effective eradication of tumor by Th17-polarized cells, underscoring the plasticity of this subset and suggesting its multipotent precursor status. However, it remains unclear why T cells already polarized to be Th1 cells were less effective in treating tumors.
Th17-polarized cells evolve in vivo into a distinct Th1 cell-like subset
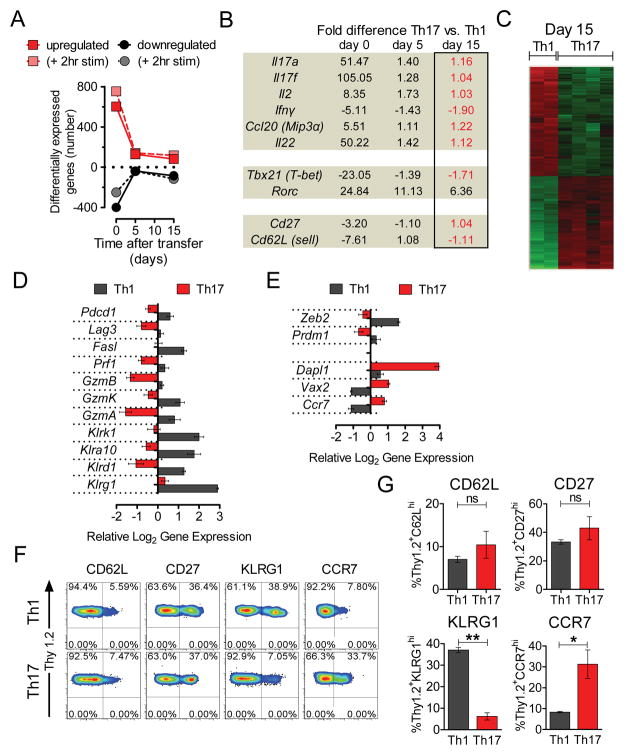
To understand the underlying differences between Th17-derived and Th1-derived cells in vivo, we compared in vitro differentiated Th1 and Th17 cells prior to transfer (day 0) and 5 and 15 days after adoptive cell transfer by global gene expression analysis. Analysis of in vitro generated cells showed 1001 differentially expressed transcripts: 602 were up and 399 were down with a minimum fold change (FC) >2 comparing Th17 vs. Th1 (File S1). Following transfer, the number of differentially expressed transcripts rapidly declined (Figure 2A). Notably, comparing Th17 derived cells with Th1 cells in vivo, the mRNA expression of genes encoding Th17-related cytokines (Il17a, Il17f, Il22, and Ccl20) decreased with time following transfer (Figure 2B), while the abundance of transcripts encoding Th1 cell-defining molecules Ifng and Tbx21 increased to amounts comparable to Th1-derived cells. The expression of Th17-related transcription factors Rora and Rorc (encoding for Rorγt) also declined, but the latter remained overexpressed even at day 15 (6.36 fold vs. Th1). The initial in vitro differences in expression of Sell (Cd62l) and Cd27 also disappeared (Figure 2B).
Figure 2. Adoptively transferred Th17 cells evolve in vivo into a distinct Th1-like population.
TRP-1 CD4+ T cells (Thy1.2+) were cultured under Th1 and Th17 conditions for 7 days. Serial gene expression profiling was performed on cells preserved on the day of adoptive transfer (day 0) and on highly purified cells recovered on day 5 and 15 from spleens and lymph nodes 5Gy irradiated B6.PL (Thy1.1+) animals (2–4 independent replicates/condition for days 5 and 15)
(A) Number of genes 2-fold up- or down-regulated (FDR<0.05 for days 5 and 15) between Th17 and Th1-polarized populations on indicated days is shown in their resting state and upon 2 hour re-stimulation in vitro.
(B) Fold differences in expression of indicated genes as measured by microarray on indicated days, red font indicates a fold difference <2 on day 15.
(C) Heat map of the genes differentially expressed by Th17 and Th1-derived cells recovered ex vivo on day 15 (FC>2, FDR<0.05).
(D) Relative log2 expression of selected differentially expressed genes encoding phenotypic markers of terminal differentiation and end-effector function in Th17 and Th1-derived cells recovered on day 15 after adoptive transfer.
(E) Relative log2 expression of selected differentially-expressed genes correlating with in vivo functionality in Th17 and Th1-derived cells recovered at day 15. Error bars for (D) and (E) represent SEM (n=2–4). List of differentially expressed genes at day 0, 5 and 15 is shown in File S1. See also Figure S2.
(F)(G) Expression of CD62L, CD27, KLRG1 and CCR7 on surviving Th17 or Th1-derived cells. Persisting Thy1.2+ cells in spleens of B6.PL (Thy1.1+) mice treated with Th17 or Th1 polarized cells were analyzed by flow cytometry on day 18 after adoptive transfer. Representative dot plots for indicated phenotypic markers are shown. Bar graphs depict frequency of positive cells in Th1 and Th17 populations. Error bars represent SEM (n=3), (*=p<0.05, **=p<0.01, ***=p<0.001).
Despite the acquisition of many Th1-related features by the transferred Th17 cells, the gene expression profiles of Th1 and Th17-derived cells recovered ex vivo remained different. There remained 167 differentially expressed transcripts on day 5 (FC>2, false discovery rate (FDR) <0.05, n=3) and 166 on day 15 (FC>2, FDR<0.05, n=2–4) and these numbers increased to 188 and 237 respectively when cells were re-stimulated in vitro for 2 hours (Figures 2A and C; File S1). The highest differentially expressed transcript found in Th1 cells on day 15 was Klrg1, which encodes a well-known marker of T cell senescence (Beyersdorf et al., 2007; Reiley et al., 2010) (Figure 2D).
Th1-derived cells also overexpressed inhibitory molecules and other markers of end-effector function, including granzymes, multiple killer lectin-like subfamily receptors, Pdcd1, Lag3 and Fasl in a pattern previously associated with terminal differentiation and less effective in vivo anti-tumor activity (Gattinoni et al., 2005; Grosso et al., 2007; Zhou et al., 2010) (Figure 2D). Th1-derived cells also overexpressed Prdm1, which encodes BLIMP1, a hallmark transcriptional repressor indicating terminal differentiation (Rutishauser et al., 2009), and Zeb2, a gene progressively up-regulated during late differentiation in CD8+ cells that encodes for zinc finger E-box binding homeobox 2 (Wirth et al., 2010) (Figure 2E).
Th17-polarized cells markedly up-regulated the gene encoding CCR7 that is closely associated with less differentiated memory subsets and higher anti-tumor activity in CD8+ models. Interestingly, Th17 cells highly overexpressed (10.38 fold on day 15) the gene encoding death associated protein-like 1 (Dapl1). Dapl1 was the most overexpressed gene in the early memory cells lost during repeat stimulations of CD8+ cells recovered ex vivo (Wirth et al., 2010). Dapl1 is active during early epithelial differentiation (Sun et al., 2006), but its function in T cells is unknown. Similarly, ventral anterior homebox 2 (Vax2), a gene important in early ocular development that is a target of the stem cell-associated sonic hedgehog signaling pathway, was prominently overexpressed throughout the timecourse in Th17-derived cells (Figure 2E) (Kim and Lemke, 2006). Examples of other genes differentially-regulated ex vivo on day 5 or 15 are shown in Figure S2
We verified our microarray results by flow cytometry (Figure 2F–2G). We observed higher expression of KLRG1 on the surface of surviving congenically marked Th1-derived TRP-1 cells and a higher frequency of CCR7hi cells in the Th17-derived population. In addition, both populations had low expression of CD62L and equal frequency of cells expressing CD27 that was initially suppressed in Th17 cells, consistent with the microarray data. Overall, serial measurements of gene expression indicated that despite a dramatic transcriptional convergence with the Th1 subset, Th17-derived cells existed as a transcriptionally and phenotypically distinct population. Th17-derived cells retained expression of a core set of genes previously associated with enhanced anti-tumor functionality in CD8+ T cells and suggesting they are less terminally differentiated than Th1-derived cells.
Th17-derived cells maintain a less mature molecular signature with stem-cell-like features
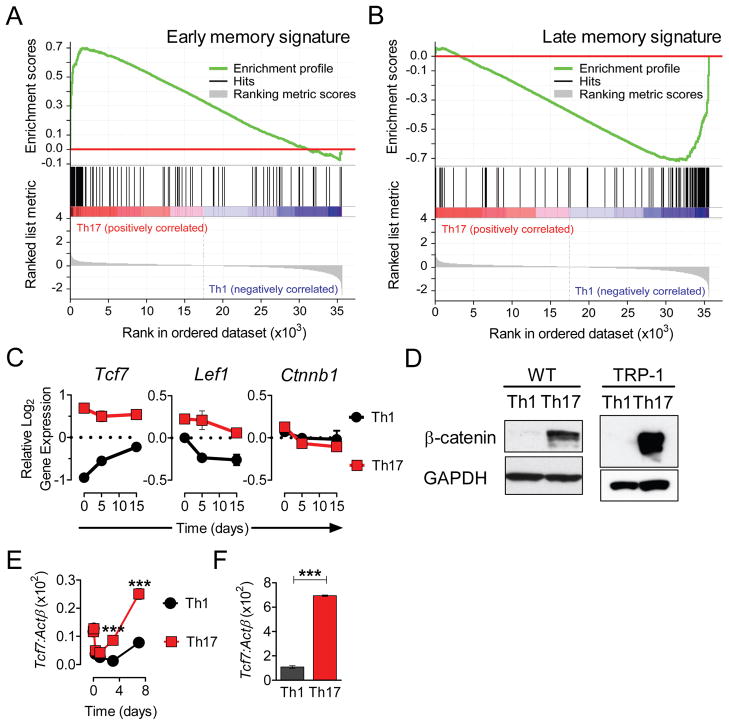
To further assess the maturation stage of the surviving Th1 and Th17 cell-derived populations in an unbiased manner we performed gene set enrichment analysis (GSEA) of our microarray data (Subramanian et al., 2005). This method examines the distribution of a reference gene set within the ranked list of genes generated from microarray data. From a recently published analysis of CD8+ T cells stimulated once vs. multiple times in vivo (Wirth et al., 2010), we created two gene sets. The first consisted of genes that were greater than 50% downregulated between the first and the last round of stimulation (lost with maturation), thus representing a signature of early (1°) memory. The second set included genes that were greater than 2-fold upregulated as early (1°) memory cells matured into late (4°) memory cells (induced with maturation) and consistent with a signature of late (4°) memory. Upon GSEA we observed a highly significant, non-random distribution of genes associated with less maturity (early memory signature) at the top of the ranked gene list from the Th17 vs. Th1 cell comparison on day 15 (NES=1.5557741, p<0.0001, FDR q=0.07, Figure 3A, Figure S3A). Out of 117 genes representing a less differentiated effector gene set, 53 (45%) were significantly enriched in the Th17 vs. Th1 list for day 15. Conversely, out of 103 genes contained in the late memory signature set, 60 (58%) were overrepresented at the opposite end (bottom) of the ranked gene expression list and were therefore relatively enriched in the Th1-derived population (NES=1.346455 p<0.0001, FDR q=0.15, Figure 3B, Figure S3B). Thus, GSEA indicates that even after the Th17-derived cells acquired Th1-like properties, they maintained a molecular signature of a less mature population, while surviving Th1-derived cells carried a molecular signature suggesting terminal differentiation.
Figure 3. Th17-polarized cells maintain the core molecular signature of a less differentiated T cell subset with stem-cell-like features.
Gene sets representing molecular signatures of early memory cells or late memory cells were generated from data set obtained following multiple rounds of in vivo stimulation of CD8+ T cells (Wirth et al., 2010).
(A)(B) Gene Set Enrichment Analysis (GSEA) enrichment plot of early memory signature set (NES=1.5557741, p<0.0001, FDR q=0.07) and late memory signature set (NES=1.346455 p<0.0001, FDR q=0.15) of the running enrichment score (ES) and positions of gene set members on the rank ordered list based on fold-change of Th17 versus Th1 gene expression profiles obtained on day 15 after adoptive transfer are shown. Full list of genes contained in the early and late memory signature sets with GSEA core enrichment heat maps for each analysis are shown in Figure S3A and S3B.
(C) Relative log2 expression of Tcf7, Lef1 and β-catenin (Ctnnb1) on indicated days following adoptive cell transfer.
(D) Presence of stable β-catenin was assessed by Western blot on day 7 in purified CD4+ T cells from WT (C57BL/6) donors (left panel) stimulated with aCD3/aCD28 and in fresh TRP-1 cells (right panel) peptide stimulated under Th1 and Th17-polarizing conditions.
(E)(F) WT CD4+ cells were cultured as in (D) and harvested at indicated times, while TRP-1 CD4+ T cells were grown for 7 days. Expression of Tcf7 was measured by qRT-PCR and shown as ratio vs. β-actin (Actb). Error bars represent SEM (n=3); (*=p<0.05, **=p<0.01, ***=p<0.001). See also Figure S3C-S3D.
One of the genes at the top of the enrichment list for the molecular signature of early memory cells was Tcf7, the gene encoding T cell factor 1 (TCF-1) (Figures S4 and 3C). Tcf7 is a direct target of the Wnt-β-catenin signaling axis, a pathway associated with self-renewal of stem cells and survival of thymocytes (Gattinoni et al., 2010; Staal and Sen, 2008). Tcf7 is expressed by undifferentiated CD8+ T cells and is rapidly lost when T cells acquire an effector phenotype (Gattinoni et al., 2009; Willinger et al., 2006). Tcf7, and to a lesser extent the β-catenin binding partner encoded by Lef1, were up-regulated in Th17 cells in vitro on day 0 and they remained relatively overexpressed ex vivo (Figure 3C). Differential expression of Axin2, Dab2, Igbf4, Jun, Myc, Satb1 and Tcf4 (Figure S2 and S3) also may reflect the activity of the Wnt-β-catenin pathway (Notani et al., 2010; Railo et al., 2009). The expression of β-catenin (Ctnnb1) itself was not affected by polarizing conditions (Figure 3C). However, β-catenin is regulated at the protein level by the activity of the Axin2-GSK3β-APC complex that mediates its degradation (Gattinoni et al., 2010). Consistent with this post-translational regulatory mechanism, we detected a massive accumulation of β-catenin protein in Th17-polarized WT and TRP-1 transgenic cells harvested at day 6, while there was very little protein detected in Th1 cells (Figure 3D). Additionally, we analyzed the kinetics of Tcf7 expression in wild-type cells polarized in vitro under Th1 and Th17 conditions (Figure 3E). Notably, Tcf7 expression was higher at the end of the culture in Th17 polarized cells than in naïve cells and Th1-polarized controls. Parallel observations were made in transgenic TRP-1 Th17 cells (Figure 3F) and in type 17 polarized CD8+ cells (Figure S3C-S3E).
Thus, Th17-derived cells had a molecular signature enriched with genes present in a well-defined early memory CD8+ population. Th17 polarization induced Tcf7 expression and an accumulation of β-catenin, a pattern associated with self-renewal of long-lived, stem cell-like memory CD8+ T cells that in the setting of adoptive cell transfer display superior anti-tumor activity (Gattinoni et al., 2009).
Th17 cells are long-lived in vivo
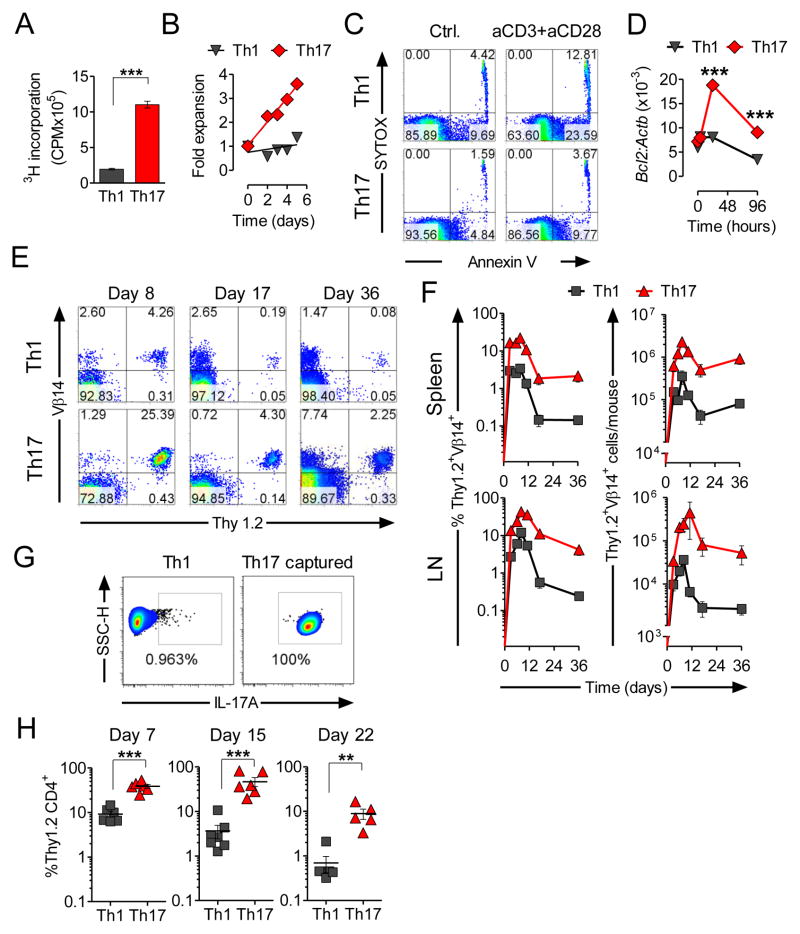
Analysis of the transcriptome indicated that Th17 cells resembled self-renewing, long-lived memory CD8+ T cells with stem cell-like properties. We therefore sought to assess the functional properties of these Th17 cells by analyzing their resistance to apoptosis and their ability to survive, expand, self-renew and generate more differentiated progeny. We used a 3H-thymidine incorporation assay to measure proliferation in Th1 and Th17-polarized cells (Figure 4A). We observed significantly lower thymidine incorporation in the Th1 cultures following restimulation with anti-CD3 and anti-CD28 mAbs. Accordingly, we enumerated more viable Th17-derived cells than Th1 control cells (Figure 4B). These two measures represent a complex mixture of proliferation and activation-induced cell death (AICD). Because CFSE-dilution experiments indicated that levels of proliferation were similar (data not shown), we sought to assess the relative contribution of apoptosis in the increased accumulation of cells in the Th17-derived cell cultures. We found greater induction of apoptosis in Th1-derived cultures as assessed by co-staining with annexin V and a nucleic acid viability stain (Figure 4C). We also found that Th1 cells rapidly down-regulated the anti-apoptotic factor Bcl2, while in Th17 cells the expression of Bcl2 increased following re-activation (Figure 4D). Thus, Th17-polarized cells are resistant to AICD in vitro. These data are consistent with the hypothesis that Th17 cells are less senescent.
Figure 4. Th17-polarized TRP-1 cells expand upon secondary stimulation in vitro and are long-lived in vivo.
(A) 3H-Thymidine incorporation in re-stimulated Th1 and Th17 cells was measured upon overnight incubation using the equal numbers of viable cells. Error bars represent SEM (n=3).
(B) Absolute number of viable cells in Th1 and Th17 cultures re-stimulated with anti-CD3/anti-CD28 was calculated serially at indicated days and represented as fold expansion.
(C) Annexin V expression was measured in Th1 and Th17 cells following overnight in vitro stimulation with anti-CD3 and anti-CD28 and compared to resting cells.
(D) Expression of mRNA encoding Bcl2 was measured serially by qRT-PCR following in vitro re-stimulation of Th1 and Th17-polarized cells. Expression relative to β-actin (Actb) is shown. Error bars represent SEM (n=3). Experiments (A, B and C) are representative and were replicated at least twice. (*=p<0.05, **=p<0.01, ***=p<0.001).
(E) A total of 1×106 Th1 and Th17 TRP-1 cells (Thy1.2+) were adoptively transferred into sublethally (5Gy) irradiated B6.PL mice (Thy1.1+). Spleens and inguinal lymph nodes were harvested and analyzed by flow cytometry at indicated timepoints for the presence of Thy1.2+Vβ14+ cells. Representative contour plots illustrate the frequency of Thy1.2+ Vβ14+ cells in spleens of animals injected with Th1 and Th17 polarized TRP-1 cells at the indicated days post transfer.
(F) Mean frequency (left panels) and total number (right panels) of persisting Thy1.2+ Vβ14+ cells from animals treated with Th1 or Th17 cells recovered at indicated days from spleens and inguinal lymph nodes are shown. Error bars represent SEM (n=3–6).
(G) (H) Purification of in vitro polarized Th17 TRP-1 cells was performed using IL-17A capture reagent and flow cytometric sorting of labeled cells. Dot plots show result of FACS-sorting, as compared with similarly labeled Th1 cells used as a negative control. A total of 4×105 Th1 and purified Th17 cells were adoptively transferred into B6.PL mice as described in (E). Frequency of Thy1.2+CD4+Vβ14 T cells was measured in blood of recipient animals at indicated time point. Error bars represent SEM (n=5–7, *=p<0.05, **=p<0.01, ***=p<0.001). See also Figure S4.
We sought to apply a very stringent in vivo assessment of T cell “fitness” to TRP-1 TCR transgenic T cells polarized using Th1 or Th17 conditions. We have previously observed that the ability of T cells to engraft and expand after transfer into lymphopenic hosts is severely impaired upon terminal differentiation (Gattinoni et al., 2005; Klebanoff et al., 2005). We therefore compared the persistence of congenically marked Th1- and Th17-polarized cells in vivo after transfer of cells in mice that had received 5 Gy of total body irradiation. A representative example for cells recovered from a spleen is shown in Figure 4E. We found that transferred Th17 cells engrafted and expanded more efficiently than their Th1-derived counterparts in both spleen and lymph nodes (Figure 4F). Differences in persistence were noted as late as 5 weeks following transfer. Addition of vaccine and exogenous IL-2 following transfer (Muranski et al., 2008; Overwijk et al., 2003) did not abrogate the survival advantage of Th17 cells (Figure S4A-S4B). We also compared the persistence of adoptively transferred Th1 and Th17 cells in non-irradiated recipients and we observed significantly higher numbers of Th17-derived cells in recipient animals (Figure S6C-S6D).
Because in vitro induced Th17 population is not homogenous and contains some cells not able to produce IL-17, it seemed plausible that this small uncommitted fraction was responsible for the profound survival difference that we observed upon adoptive transfer. To investigate this possibility we used a cytokine capture technique and flow cytometry to sort a highly purified preparation of IL-17A-secreting TRP-1 cells (Figure 4G). We observed superior persistence after adoptive transfer of sorted, congenically-labeled Th17 cells, while similarly treated Th1-polarized cells were detectable at significantly lower frequencies (Figure 4H). Analogous results were obtained using an IL-17F reporter system (Supplementary Figure S4E). These results led to a conclusion that observed survival of Th17 polarized cells was not due to a mere preferential expansion of contaminating uncommitted cells found in heterogenous Th17-skewed in vitro population.
Taken together, our data indicate that some Th1-derived cells survived under highly activating lymphopenic conditions; however, Th17 cells underwent less AICD in vitro and exhibited superior ability to engraft, enter memory pool and persist in vivo. These observations provide functional substantiation of the observed molecular program of survival and self-renewal displayed by Th17-polarized cells.
Expression of CD27 does not correlate with capacity of Th17 cells to survive in vivo
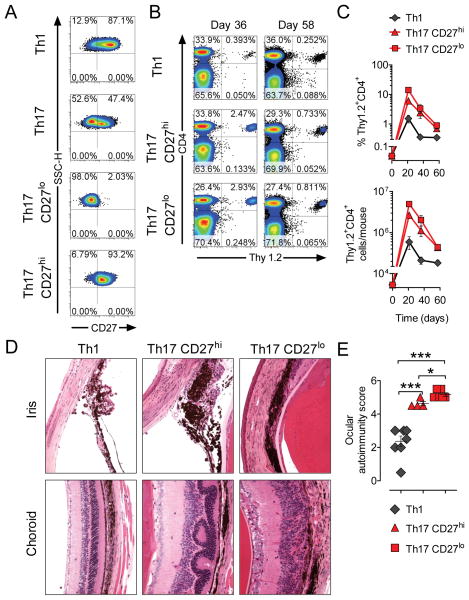
Diminished persistence of Th17 cells has been previously attributed to the low CD27 expression in this population (Hendriks et al., 2000; Pepper et al., 2010). Consistent with those reports, TRP-1 cells polarized under Th17 conditions demonstrated downregulation of CD27, but a certain degree of heterogeneity in expression of CD27 can be observed in flow cytometric analysis (Figure 1E), raising the possibility that only less-differentiated cells with higher CD27 expression survive and expand in vivo. To address this question we performed flow cytometric sorting of Th17-polarized cells into CD27hi and CD27lo subsets (Figure 5A). We subsequently evaluated the persistence of those cells in comparison to Th1 counterparts in an adoptive cell transfer experiment (Figure 5B–5C). Three weeks after transfer we found the highest frequency and total number of transferred cells in mice treated with Th17 CD27lo cells, while mice treated with Th17 cells purified for the high expression of CD27 persisted significantly less. This difference disappeared at 5 and 8 weeks after adoptive cell transfer. At every time point analyzed, Th1 polarized cells were found at significantly lower numbers (p<0.05 vs. Th17 CD27lo subset).
Figure 5. Expression level of CD27 on Th17 cells does not affect their ability to survive and function in vivo.
In vitro cultured Th1 and Th17 TRP-1 cells were labeled for the expression of Thy1.2 and CD27. CD27hi and CD27lo Th17 subsets sorted using Th1 population as a positive control. 1×106 cells were adoptively transferred into 5Gy irradiated B6.PL (Thy1.1) recipient animals.
(A) Expression of CD27 is shown on Th17 cells before and after sorting into CD27hi and CD27lo subsets.
(B) Dot plots demonstrate frequency of surviving CD4+Thy1.2+ TRP-1 cells in spleens of recipient animals treated with indicated cell subset at day 36 and 58 after adoptive cell transfer.
(C) Mean frequency (upper panel) and total number (lower panel) of CD4+Thy1.2+ cells recovered at indicated time points from the spleens of recipient animals (n=3–4 for Th1 and Th17 CD27lo cells, n=2–3 for Th17 CD27hi cells).
(D) Eyes from animals analyzed on day 58 in (B) and (C) were H&E stained and examined for evidence of autoimmunity in cornea, iris, photoreceptors and choroid. Representative examples for indicated experimental groups are shown.
(E) Degree of self-tissue destruction in eyes harvested from indicated experimental groups on day 58 after adoptive cell transfer was evaluated using masked autoimmunity score as described in materials and methods. Error bars represent SEM (n=4–8); (*=p<0.05, **=p<0.01, ***=p<0.001)
Persistence of CD27hi and CD27lo subsets of Th17 polarized TRP-1 cells was associated with an autoimmune-like syndrome manifested by progressive damage of melanocytes. Recipient mice treated with Th17 cells developed extensive diffuse vitiligo (data not shown). We have previously demonstrated a close correlation between the degree of ocular autoimmunity and efficacy of anti-tumor responses mediated by CD8+ Pmel1 cells (Palmer et al., 2008). Therefore we systematically analyzed the degree of eye damage in recipient animals on day 58 after transfer (Figure 5D–5E). We observed only evidence of mild, resolved irydocyclitis and chondroititis in mice treated with Th1 polarized cells. Animals treated with Th17 CD27hi cells developed much more pronounced damage of pigmented epithelium in the eyes, as manifested by moderate irydocyclitis, significant edema, inflammatory infiltration of the choroid, retinal folding and cataract formation. Strikingly, the changes observed in the group treated with Th17 CD27lo cells were the most severe, resulting in complete disruption of eye structure, large areas of retinal degeneration, corneal neovascularization and formation of synechiae (adhesions between iris and cornea) with obliteration of the anterior chamber of the eyes (Figure 5D–5E).
Our findings thus prompt a re-evaluation of the view that Th17 cells are short-lived and destined to die because of the low expression of CD27. In contrast, it appears that the level of CD27 expression observed in the initial Th17 polarized population does not affect the long term viability of Th17-derived cells following adoptive cell transfer in vivo. Moreover, even the Th17-derived cells with the lowest levels of this costimulatory receptor are capable of expansion and survival in significant numbers in vivo. In addition, CD27 expression, Th17 cells were capable of mediating dramatic autoimmune self-tissue destruction.
IL-17A-secreting cells are capable of self-renewal in vivo and contribute to tumor rejection
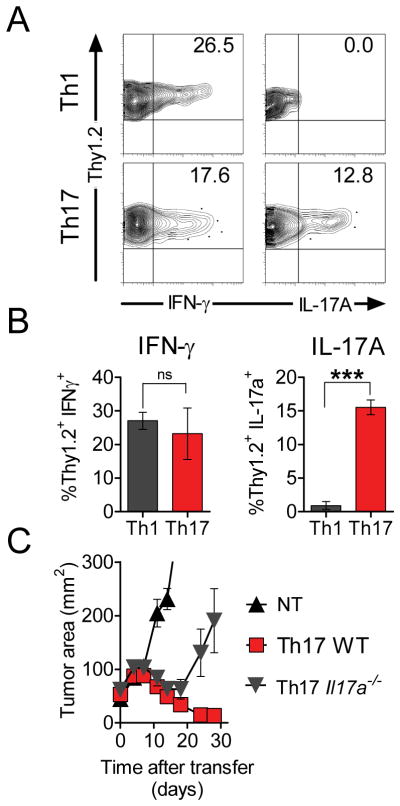
The development of Th1-like progeny from Th17-polarized precursors was evidenced by the critical importance of Th1-related factors for the in vivo anti-tumor effect of TRP-1 cells (Figure 1F–1G). However, the importance of continued capacity to produce IL-17A remained unclear. We found that Th1-derived cells did not give rise to IL-17A-producing cells when measured by intracellular cytokine analysis 5 weeks following adoptive transfer (Figure 6A-B). By sharp contrast, highly polarized Th17 cells acquired the ability to secrete IFN-γ. However, some cells also retained the ability to produce the Th17-defining cytokine IL-17A (Figure 6A-B). We therefore ascertained the role of IL17A in tumor rejection by adoptively transferring TCR-transduced Il17a−/− Th17-polarized CD4+ cells into tumor bearing mice. We found that they were significantly less effective in mediating elimination of the tumor than wild-type derived Th17-polarized effectors (p<0.05, Figure 6C) suggesting that IL-17A secretion by Th17 derived cells was also contributed to tumor rejection. These observations indicated that Th17 cells were both self-renewing (i.e. capable of IL-17A secretion) and had the multipotent capacity to generate progeny producing IFN-γ and that both qualities contributed to tumor treatment.
Figure 6. IL-17A-secreting cells maintain the ability to self-renew upon transfer into a highly activating environment and contribute to the effective rejection of tumor.
A total of 1×106 in vitro polarized Th17 and Th1 TRP-1 cells (Thy1.2+) were adoptively transferred into sublethally irradiated (5Gy) B6.PL (Thy1.1+) mice. On day 36 following transfer spleens from recipient mice were harvested and stimulated for 6 hours with TRP-1 peptide in presence of brefeldin A.
(A) Representative dot plots show intracellular IFN-γ and IL-17A in Thy1.2+ cells in the indicated experimental groups.
(B) Bar graphs show mean frequency of IFN-γ- and IL-17A-secreting cells among surviving Thy1.2+ Th1- and Th17-derived cells 36 days after adoptive transfer.
(C) Purified CD4+ cells from C57/B6 and IL-17A−/− donors were stimulated under Th17 conditions and transduced with retroviral vector encoding for TRP-1 TCR. A total of 1.2×106 cells were adoptively transferred into 5Gy irradiated C57BL/6 mice. Tumor growth was measured serially. Error bars represent SEM (n=5–6); (*=p<0.05, **=p<0.01, ***=p<0.001).
Discussion
In this report we investigated the anti-cancer responses mediated by Th17-polarized cells that effectively eradicated established tumors. The acquisition of type 1 effector properties, including T-bet expression and the secretion of IFN-γ in vivo, was required for the anti-tumor activity of highly-polarized Th17 cells. Plasticity of Th17 cells in vivo has been previously described (Lee et al., 2009; Martin-Orozco et al., 2009a; Wei et al., 2009; Yang et al., 2009), but plasticity alone seemed insufficient to explain why Th17-polarized CD4+ T cells demonstrated enhanced eradication of established tumors when compared with their Th1 counterparts that already express large amounts of T-bet and IFN-γ. Although IL-17A contributes to the anti-tumor function of Th17-derived cells in our model, possibly via recruitment of other arms of the immune system (Martin-Orozco et al., 2009b), the expression of IL-17A does not explain this conundrum, because unlike the case of genetic disruption of IFN-γ expression, elimination of IL17A only partially diminished treatment.
The superior functionality of Th17 cells in mediating tumor rejection and autoimmunity was associated with enhanced survival of Th17-derived cells upon adoptive transfer. IFN-γ-producing Th1 cells have been previously reported as terminal effectors prone to apoptosis (Wu et al., 2002), while Th17 cells engrafted better and were more resistant to apoptosis (McKinstry et al., 2010; Shi et al., 2009). A hypothetical explanation for this survival advantage was provided by Waddington more than 50 years ago who theorized that cellular differentiation was due to epigenetic changes which he analogized to the ball rolling downhill (Waddington, 1957). More recently, the epigenetic control of Th cell subset plasticity was analogized to a dissipation of biochemical or physical energy and the accumulation of entropy, a process that in a closed system efficiently proceeds only in one direction (Murphy and Stockinger, 2010; Wei et al., 2009). This metaphor can also be applied to the progressive maturation of CD8+ T cells, as their anti-tumor efficacy inversely correlates with advanced maturational state through limitation of their capacity to self-renew and survive in vivo (Gattinoni et al., 2005; Zhou et al., 2005). Terminally differentiated, end-effector CD8+ cells had the greatest ability to release IFN-γ and to lyse targets in vitro, but paradoxically exhibited limited or no anti-tumor function in vivo (Gattinoni et al., 2006). Conversely, less differentiated CD8+ populations, including naïve, central memory (CM) and more recently stem cell-like memory cells (SCM) were able to efficiently survive and eradicate tumor upon adoptive transfer (Berger et al., 2008; Gattinoni et al., 2009; Klebanoff et al., 2005; Xie et al., 2010).
One perplexing observation is that Th17 polarization induces a phenotype that resembles end effector memory cells (CD62LloCD27lo). CD27 is known to play a crucial role in the survival of memory CD8+ T cells (Hendriks et al., 2000). Pepper et al. explored the fate of endogenously generated Th cell subsets during an infection in normal hosts (Pepper et al., 2010). However, the generation of Th1 or Th17 cells in vivo required incommensurate methods of immunization that varied by route (intravenous vs. intranasal respectively) and magnitude of response, relying on pMHC tetramers to track the antigen specific cells, interpreting the extinction of IL-17A production as evidence for a limited lifespan of Th17 cells. The possibility that some Th17 cells simply experienced plasticity and lost the capacity to produce IL-17A was discounted by the authors because the IL-17A-secreting cells expressed low CD27, and thus were thought to not have been able to enter into the memory T cell pool. To support this claim, they showed that 80% of non-polarized, open-repertoire, CD27lo CD4+ cells disappeared within 14 days after the adoptive transfer, but Th17-polarized cells were not explicitly evaluated in this context (Pepper et al., 2010). Thus, Pepper, et al. assumed the equivalency between CD27lo cells and Th17 polarized cells and their conclusions were further limited by the expectation of a static regulation of CD27 expression in every Th cell subset. In our system, we observed excellent persistence of the congenically-marked Th17 subset purified for the expression of even the lowest levels of CD27, thus excluding the possibility that CD27hi cells phenotypically heterogeneous in vitro cultures enjoyed a selective survival advantage. Moreover, purified CD27lo Th17 polarized TRP-1 cells induced the most aggressive autoimmune-like syndrome resulting in almost complete elimination of ocular melanocytes and severe disruption of eye architecture, while CD27hi Th17 cells were slightly less effective.
The apparently discrepant conclusions from data presented in our manuscript and findings observed by Pepper, et al. mainly represent differences of interpretation. Nevertheless, there are important dissimilarities in experimental design. In vitro cultured cells used in our work likely differ from cells arising in vivo following the resolution of acute infection (Stockinger et al., 2011; Wahren-Herlenius and Kuchroo, 2011). Endogenous Th17 cells and their offspring are difficult to trace, but the recently published fate-mapping system linked to the IL-17A promoter could potentially advance this knowledge (Hirota et al., 2011). We transferred cells into transiently lymphopenic, sublethaly irradiated but otherwise immunocompetent animals. This host state was designed to mimic clinical conditions of host lymphopenia, including those observed in cancer patients treated with chemotherapy and radiotherapy (Muranski et al., 2006), but this lymphopenia was likely to speed the differentiation of Th17-polarized TRP-1 cells into the Th1 phenotype. At its essence, our findings are that Th17 cells represent an “earlier,” less-differentiated, plastic and more stem cell-like state than Th1 cells. These findings have been confirmed recently by a description of pro-survival molecular features in human Th17 cells and associated with expression of hypoxia-inducible factor 1 (HIF1α)(Kryczek et al., 2011), a molecule regulating Th17 differentiation and linked to stem cell-like behavior (Dang et al., 2011; Shi et al., 2011). Taken together, these data indicate that Th17 cells are long-lived cells capable of maturational plasticity.
The fairly simple associations between phenotypic features (CM vs. EM) and in vivo efficacy is applicable in many situations, but the core maturational program responsible for the fate of short- or long-lived effector cells might be more important (Haining et al., 2008). Ex vivo measurements of gene expression profiles demonstrated that Th17-derived cells remained clearly distinct from their Th1 counterparts, despite their evolution into a Th1-like cellular population, albeit with lower expression of Tbx21. We used a systems biology approach to characterize this persistent difference (Benoist et al., 2006; Haining and Wherry, 2010). GSEA showed that Th17-derived cells retained a molecular signature enriched in genes expressed at an earlier stage of CD8+ memory development, while the Th1-derived population displayed features associated with late effectors. GSEA was performed in an unsupervised manner taking into consideration the global pattern of gene expression rather than merely relying on the pre-defined expression level selection criteria (Subramanian et al., 2005). We used a well-characterized gene set that reflected the signatures of more or less differentiated memory CD8+ cells generated in vivo (Wirth et al., 2010). Recent work by Pepper, et al. and other groups indicated that high expression of T-bet in Th1 cells has been associated with terminal differentiation and limited survival, whereas T-betlo CD4+ T cells formed stable long-lived CM population, consistently with our findings (Ballesteros-Tato and Randall, 2011; Joshi et al., 2007; Marshall et al., 2011; Pepper et al., 2011). Thus, a core transcriptional program might be shared between polarized Th subsets and defined subsets of CD8+ T memory cells in progressive maturational states.
Because of the self-renewal capabilities displayed by long-lived memory T cells, analogies between stem cells and T cells have been postulated; this notion has been recently reinforced by descriptions of stemness-associated biological pathways including Wnt-β-catenin in peripheral lymphocytes (Luckey et al., 2006; Stemberger et al., 2009), We found that Th17 polarization was associated with accumulation of β-catenin and expression of Tcf7, factors important in the maintenance of self-renewal in stem cells and cancer cells (Roose et al., 1999; Staal et al., 2008). Tcf-1-β-catenin signaling is active in earlier T cell subsets (naïve, SCM and CM cells) with high self-renewal potential and anti-tumor activity (Gattinoni et al., 2009; Willinger et al., 2006) and is associated with preservation of long-term CD8+ and CD4+ T cell memory (Gattinoni et al., 2010; Williams et al., 2008). (Ding et al., 2008). Tcf7 inhibits production of effector cytokines (Yu et al., 2011; Yu et al., 2009). T-bet directly represses Tcf7 gene in Th1 cells (Oestreich et al., 2011), whereas STAT3 signaling, critical for Th17-polarization, also influences survival of T cells as well as self-renewal capacity of stem cells and cancer growth and directly regulates Tcf7 (Durant et al., 2010; Hirano et al., 2000). The physiological relevance of this conserved and dynamically-regulated self-renewal pathway in Th17 cells remains unclear, but multiple lines of evidence suggest that the activity of Tcf7 can be used as an indicator of early maturational stage and a predictor of T cell survival capability (Gattinoni et al., 2010).
In conclusion, cytokine signals received by CD4+ T cells during priming can launch them toward a fate of programmed senescence or long-term self-renewal, sustained survival and enhanced activity during the secondary response. Th17 polarization diverted cells from premature terminal differentiation and generated a population with stem cell-like features of self-renewal and enhanced ability to enter the memory pool. In addition, Th17 cells gave rise to Th1 effector progeny in vivo, a process that was functionally prerequisite to effectively eliminate tumors, indicating multipotency. Our findings provide potential insight into the role of these cells in autoimmune disorders (Carlson et al., 2009; Palmer and Weaver, 2010) and chronic infections, including HIV, where depletion of mucosal IL-17A secreting cells has been reported (Brenchley et al., 2008). Finally, our observations offer a mechanistic basis for the anti-tumor efficacy of Th17 cells in a highly realistic and clinically-relevant animal model of advanced cancer, and thus they have implications for the design of future T cell based immunotherapy.
Experimental Procedures
Mice and cell lines
BwRag1−/−TRP-1 and Pmel1 TCR transgenic mice were previously described and bred at the National Cancer Institute, NIH animal facility (Muranski et al., 2008; Overwijk et al., 2003). For description of all mice and cell lines used refer to Supplementary Experimental Procedures.
In vitro polarization and stimulation, flow cytometry, antibodies, cytokine release assays, proliferation assays, Immunoblot analysis and quantitative gene expression analysis by RT-PCR and statistical methods
For description see Supplementary Experimental Procedures
Adoptive cell transfer protocol
Tumor treatment experiments with TRP-1 cells were described previously (Muranski et al., 2008). For detailed description see Supplementary Experimental Procedures.
Retroviral Production and Transduction
Polarized CD4+ T cells from indicated donors were transduced with retroviral vector encoding TRP-1 TCR. For detailed description refer to Supplementary Experimental Procedures.
Microarray methods
Gene expression levels were determined using GeneChip Mouse Gene 1.0 ST arrays according to manufacturer’s protocols (Affymetrix). Description of methods and statistical analysis is included in Supplementary Experimental Procedures. GEO accession number: GSE26030. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rtujjwaumosuilm&acc=GSE26030
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Enrico Lugli and Dr. Christian S. Hinrichs for sharing their technical expertise, Megan Bachinski for editorial assistance and Ethan Tyler and Alan Hoofring for creating the visual abstract.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballesteros-Tato A, Randall Troy D. Memory: The Incomplete Unhappening of Differentiation. Immunity. 2011;35:496–498. doi: 10.1016/j.immuni.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. The Journal of clinical investigation. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C, Germain RN, Mathis D. A plaidoyer for ‘systems immunology’. Immunological reviews. 2006;210:229–234. doi: 10.1111/j.0105-2896.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8(+) T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersdorf N, Ding X, Tietze JK, Hanke T. Characterization of mouse CD4 T cell subsets defined by expression of KLRG1. European journal of immunology. 2007;37:3445–3454. doi: 10.1002/eji.200737126. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Eric V, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nature medicine. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Ji Y, Restifo NP. Wnt/{beta}-Catenin Signaling in T-Cell Immunity and Cancer Immunotherapy. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. The Journal of clinical investigation. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu ZY, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–U1325. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature reviews. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature medicine. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–U144. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. The Journal of clinical investigation. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, et al. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. J Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hendriks J, Gravestein LA, Tesselaar K, van Lier RAW, Schumacher TNM, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nature immunology. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MBA. Differential regulation of antiviral T-cell immunity results in stable CD8(+) but declining CD4(+) T-cell memory. Nature medicine. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature reviews. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kerkar SP, Sanchez-Perez L, Yang SC, Borman ZA, Muranski P, Ji Y, Chinnasamy D, Kaiser ADM, Hinrichs CS, Klebanoff CA, et al. Genetic Engineering of Murine CD8(+) and CD4(+) T Cells for Preclinical Adoptive Immunotherapy Studies. J Immunother. 2011;34:343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lemke G. Hedgehog-regulated localization of Vax2 controls eye development. Genes & development. 2006;20:2833–2847. doi: 10.1101/gad.1462706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunological reviews. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Zhao ED, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou WP. Human T(H)17 Cells Are Long-Lived Effector Memory Cells. Science translational medicine. 2011;3 doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod MKL, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do? Semin Immunol. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng HL, Poholek AC, Parish IA, Rutishauser R, Cui WG, Kleinstein SH, Craft J, et al. Differential Expression of Ly6C and T-bet Distinguish Effector and Memory Th1 CD4(+) Cell Properties during Viral Infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. European journal of immunology. 2009a;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009b;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunological reviews. 2010;236:110–124. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nature clinical practice. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Current opinion in immunology. 2009 doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nature immunology. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. Global Regulator SATB1 Recruits β-Catenin and Regulates TH2 Differentiation in Wnt-Dependent Manner. PLoS Biol. 2010;8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. The Journal of experimental medicine. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. The Journal of experimental medicine. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, Powell DJ, Jr, Klebanoff CA, Finkelstein SE, Fariss RN, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4(+) T cell lineup. Nature immunology. 2010;11:36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing Signals from the Bcl6 Transcription Factor and the Interleukin-2 Receptor Generate T Helper 1 Central and Effector Memory Cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railo A, Pajunen A, Itaranta P, Naillat F, Vuoristo J, Kilpelainen P, Vainio S. Genomic response to Wnt signalling is highly context-dependent - Evidence from DNA microarray and chromatin immunoprecipitation screens of Wnt/TCF targets. Exp Cell Res. 2009;315:2690–2704. doi: 10.1016/j.yexcr.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard Gs, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proceedings of the National Academy of Sciences. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Huls G, Beest Mv, Moerer P, Horn Kvd, Goldschmeding R, Logtenberg T, Clevers H. Synergy Between Tumor Suppressor APC and the -Catenin-Tcf4 Target Tcf1. Science (New York, NY. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Shen XL, Zhou JH, Hathcock KS, Robbins P, Powell DJ, Rosenberg SA, Hodes RJ. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, Siegel RM, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1α– dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nature reviews. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. European journal of immunology. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stem cell-like plasticity of naive and distinct memory CD8(+) T cell subsets. Semin Immunol. 2009;21:62–68. doi: 10.1016/j.smim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Ryan DG, Zhou MY, Sun TT, Lavker RM. EEDA: A protein associated with an early stage of stratified epithelial differentiation. J Cell Physiol. 2006;206:103–111. doi: 10.1002/jcp.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Glimpsing the real CD4+ T cell response. Nature immunology. 2010;11:47–49. doi: 10.1038/ni0110-47. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Jenkins MK. CD4(+) memory T cell survival. Current opinion in immunology. 2011;23:319–323. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The Strategy of the Genes. London: George Allen & Unwin; 1957. [Google Scholar]
- Wahren-Herlenius M, Kuchroo VK. Gene-environment interaction in induction of autoimmunity. Semin Immunol. 2011;23:65–66. doi: 10.1016/j.smim.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive Antigen Stimulation Induces Stepwise Transcriptome Diversification but Preserves a Core Signature of Memory CD8(+) T Cell Differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nature immunology. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. The Journal of experimental medicine. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. The Journal of experimental medicine. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Ghosh A, Sen JM. T Cell Factor-1 Negatively Regulates Expression of IL-17 Family of Cytokines and Protects Mice from Experimental Autoimmune Encephalomyelitis. J Immunol. 2011;186:3946–3952. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nature immunology. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere Length of Transferred Lymphocytes Correlates with In Vivo Persistence and Tumor Regression in Melanoma Patients Receiving Cell Transfer Therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nature reviews. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt B, Veldhoen M. T Helper Cell Differentiation: More than Just Cytokines. In: Frederick WA, editor. Advances in immunology. Academic Press; 2011. pp. 159–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.