Abstract
The use of regular yeast (RY) and selenium-enriched yeast (SEY) as dietary supplement is of interest because the Nutritional Prevention of Cancer (NPC) trial revealed that SEY but not RY decreased the incidence of prostate cancer (PC). Using two-dimensional difference in gel electrophoresis (2D-DIGE) – tandem mass spectrometry (MS/MS) approach, we performed proteomic analysis of RY and SEY to identify proteins that are differentially expressed as a result of selenium enrichment. 2D-DIGE revealed 96 candidate protein spots that were differentially expressed (p≤0.05) between SEY and RY. The 96 spots were selected, sequenced by LC/MS/MS and 37 proteins were unequivocally identified. The 37 identified proteins were verified with ProteinProphet software and mapped to existing Gene Ontology categories. Furthermore, the expression profile of 5 of the proteins with validated or putative roles in the carcinogenesis process, and for which antibodies against human forms of the proteins are available commercially were verified by western analysis. This study provides evidence for the first time that SEY contains higher levels of Pyruvate Kinase, HSP70, and Elongation factor 2 and lower levels of Eukaryotic Translation Initiation Factor 5A-2 and Triosephosphate Isomerase than those found in RY.
Keywords: Baker s yeast, Cancer prevention, Proteomics, Selenium, Pyruvate kinase
Introduction
Several agents including selenium compounds have been shown to have chemopreventive potential against several experimental cancers including prostate cancer [1, 2]. In preclinical model systems, we and others have clearly demonstrated that both dose and form (structure) are critical factors that determine the chemopreventive efficacy of selenium compounds [3–5]. The results of previous clinical chemoprevention trials [6–8] suggest that the effect of selenium may depend on several factors including the form of selenium, levels of selenium at baseline, population and genetic makeup, the target organ and the disease under examination. A recent clinical investigation was performed to determine the role of SEY (200 μg selenium daily for a mean of 4 years) against lung cancer recurrence in patients with resected stage I non-small cell lung cancer; the results indicate that SEY had no apparent benefit [6]. However, the Nutritional Prevention of Cancer (NPC) trial showed a 63% reduction in prostate cancer incidence, as a secondary endpoint, in individuals that received a daily supplement of SEY (200 μg selenium daily for a mean of 4.6 years) but not RY [7]. The Selenium and Vitamin E Cancer Prevention Trial (SELECT), a phase III randomized placebo-controlled study and the largest cancer chemoprevention trial ever conducted [8], demonstrated that the oral supplementation of selenium in the form of selenomethionine, the major component of selenium in SEY (200 μg daily for a mean of about 5 years), vitamin E (α-tochopherol acetate, 400 IU) or selenium + vitamin E did not prevent prostate cancer in the generally healthy, heterogeneous population of 35,000 men over age 50, including 79% white, 12.4% African Americans, and 8.6% other (Hispanics and Asians).
Considering the many potential limitations and pitfalls inherent in long-term placebo-controlled chemoprevention trials [8, 9], it may be difficult to predict whether SEY - the form that has been shown to be protective in the NPC trial [7] - would have been more effective than selenomethionine [10, 11]. However, the results of previous reports [12, 13] indicate that levels of selenium that can reach the prostate tissue vary following supplementation with different forms of selenium (SEY vs. selenomethionine). Since some yeast proteins including Enolase 1 and Enolase 2, superoxide dismutase and Thioredoxin II are reported to be selenium-containing proteins [14], it is important to determine the extent to which selenium impacts differential protein expression in both SEY and RY.
In the present study we tested the hypothesis that SEY effectively alters the levels of dietary-proteins that may play a critical role in cancer prevention. We were motivated to test this hypothesis because majority of studies related to selenium-rich yeast focus on selenomethionine and the identification of selenium containing proteins [14–16] and not on the effect of selenium on global protein expression. Elucidating the effect of selenium on protein expression, and which selenium-containing proteins show altered expression is important because it is known that certain proteins generated in diets or added to diets can be transferred intact into the circulatory system from the gastrointestinal tract. In fact, the 53 kDa Urokinase protein used to treat patients with several thromboembolic diseases can be detected in blood after oral administration[17, 18]. To test our hypothesis, we have examined the global protein expression of SEY and RY using the two dimensional difference in gel electrophoresis (2D-DIGE) – tandem mass spectrometry (MS/MS) approach and validated the expression profile of selected proteins by western analysis. We validated the protein identities with ProteinProphet and performed Gene Ontology classification [19] to define the potential role of the identified proteins and hence their potential relevance to prostate cancer prevention.
Materials and Methods
Sample Procurement
Selenium Yeast (SEY, Lot No. SE-84) and spray dried nutritional yeast (RY, Lot No. 07078) were obtained from Cypress Systems, Inc. (Fresno, CA); both forms were grown from a single culture and strain of Saccharomyces cerevisiae. Briefly, the standardized production process for SelenoExcell® High Selenium Yeast utilizes the commonly known standard baker s yeast strain (S. cerevisiae). These strains are recognized for food applications and are listed as “Generally Regarded As Safe” (GRAS) on the FDA list. In addition this specific high selenium yeast has received a GRAS “Letter of No Objection” from FDA. The product is produced from the introduction of selenium salt during active, aseptic, aerobic fermentation. During fermentation the temperature, pH, and percentage growth are closely regulated to assure proper uptake of selenium. This process produces a primary grown high protein yeast, which is fortified with a biologically bound mineral composition. The resulting product is washed, separated from its growth media and held in refrigerated storage to assure cell viability and the absence of any free minerals. Prior to spray drying the chilled yeast cream is pasteurized through a high temperature sterilization system to assure that it meets or exceeds established USDA food grade microbial requirements. Composite samples are collected during spray drying and packaging. The collected sample is analyzed for nutrient and microbial composition by an outside, independent, USDA approved food and pharmaceutical grade laboratory. Once product has met all QA/QC requirements it is released for sale with a supporting Certificate of Analysis. This closely controlled process produces a product which provides a 100% organically-bound natural food form of selenium. The level of selenium is 1200 μg/g of yeast and the major form of selenium is selenomethionine which accounts for about 70%.
Sample Preparation for Proteomics
Approximately 150 mg dry weight of SEY and RY were transferred to separate tubes and suspended in 1ml ToPI-DIGE™ Buffer – 2 (ITSI – Biosciences, Johnstown, PA) consisting of 7 M urea, 2 M thiourea, 4% CHAPS, 0.5% NP-40, 5 mM magnesium acetate, 30 mM Tris-HCl, pH 8.5, and disrupted using a polytron homogenizer as previously described [20]. After homogenization, the samples were incubated on ice for 40 minutes, while vortexing every 10 minutes, and then centrifuged at 13,000xg for 10 minutes at 4°C. The supernatant was transferred to a new tube and the total protein concentration was determined with the ToPA™ total protein assay kit (ITSI – Biosciences, Johnstown, PA) as previously described [20]. Proteins isolated were used for both comparative proteomics and Western blot analysis.
Comparative Proteomics by 2D-DIGE
The SEY and RY samples were split into 3 portions each and analyzed by 2D-DIGE as previously described by our group [21]. Specifically, 50 μg of total protein from each aliquot was labeled with 200 pmoles Cy dyes (Cy3 or Cy5). Additionally, 50 μg of total protein from all the SEY and RY samples were pooled to form a universal internal standard and 50 μg was labeled with Cy2. The Cy2 labeled sample, is added to all gels to allow comparison across all gels in the batch. Dye swapping between the SEY and RY samples were performed to compensate for slight differences in intensity that may exist between Cy3 and Cy5 fluorescent dyes. The Cy3 (e.g. SEY1), Cy5 (e.g. RY1) and Cy2 (pooled standard) labeled samples were mixed and subjected to 1st and 2nd dimension electrophoresis separations [21]. The 1st dimension separation (Iso Electric Focusing, IEF) was performed on an IPGphor electrophoresis unit (GE Healthcare) using immobiline dry strips (24 cm, IPG pH 3–10, GE Healthcare) for a total of 65,000 volt hours. The focused strips were equilibrated for 15 minutes in equilibration buffer containing 0.5% dithiothreitol and then for 15 minutes in equilibration buffer containing 4.5% iodoacetamide. The strips were transferred to large format (20 cm x 24 cm) 12.5% SDS-PAGE gels and electrophoresed for about 4.0 hours.
Image Capture and Analysis
After the 2nd dimension, the 3 gels were scanned to capture the Cy3, Cy5, and Cy2 signals using a DIGE-enabled Typhoon variable mode imager (GE Healthcare) using the following excitation/emission wavelengths: Cy2; 488/520 nm, Cy3; 532/580 nm and Cy5; 633/670 nm. The captured images from the three gels were imported into DeCyder (Version 6.5) and analyzed with False Discovery Rate mode enabled. Specifically, the signals obtained from SEY samples were compared to the same signals from the RY samples to identify spots that show true differences as a function of selenium enrichment based on the Mean Fold Difference of triplicate samples [20]. Candidate protein spots were considered differentially expressed ONLY if: a) the spot demonstrated ≥ 1.2-fold difference in abundance between SEY and RY, b) the protein spot occurred in all the gels and c) the difference in abundance was statistically significant at the 95% confidence level. Finally, all the spots selected automatically by DeCyder software were independently confirmed by manual inspection of the simulated 3D spot image generated by DeCyder. Spots that were considered artifacts because of the absence of a smooth curved surface were excluded.
Protein identification by LC/MS/MS
To identify the proteins-of-interest, the spots were picked from the gels and in-gel digested with trypsin using Ettan robotic workstations (GE Healthcare) and sequenced by LC/MS/MS as previously reported by our group [20]. All peptide digests were sequenced using the LCQ DECA XP Plus mass spectrometer (ThermoElectron) operating downstream of a Surveyor LC system (ThermoElectron). The LC system was configured for nanoflow, and controlled with the Xcalibur 2.0 SR2 software (ThermoElectron). To sequence the peptides, all the tryptic digested samples were reconstituted with ultra pure water and loaded onto a PicoFrit column (New Objective ProteoPep II C18, 100 mm length x .075mm internal diameter) using a helium pressure cell operated at 500 psi. A linear acetonitrile gradient was used from 2 to 30% acetonitrile over 30 minutes to flush the peptides into the mass spectrometer nanospray ion source. The flow rate of the Surveyor LC was 250 μL/min and the flow was split upstream of the column to achieve a flow rate of 500 nL/min at the spray tip. The mass spectrometer was operated in the positive ion mode with spray voltage set at 1.8 kV. Masses were measured from m/z 400–1500, and MS/MS data were collected using a “Top Three” method, in which the instrument was programmed to automatically perform MS/MS on the three most abundant ions, to generate fragmentation ions. Trypsin was used for digestion and for assignment of protein identity to the acquired mass spectra and precursor mass tolerance was set to 1.4 Da and fragment mass tolerance was 1 Da. The MS/MS data obtained were searched against the yeast database (version 12.2) using Bioworks-SEQUEST version 3.31 (Thermo Electron) with maximum of two missed cleavage and carbamidomethyl as the fixed modification. Additionally, search results were subjected to statistical filtering and validation using PeptideProphet [22] and ProteinProphet [23] (version 3.0) both under default settings for peptide and protein identification scoring, respectively. Supplementary Table 1 includes results from both ProteinProphet and Proteome Discoverer 1.2.
Gene Ontology Classification
All identified proteins were assigned molecular functions, biological processes, and cellular components, based on the unified Gene Ontology (GO) Consortium classification [19], to determine their relevance and potential role in the carcinogenesis process. Five representative proteins that have validated or putative roles in the carcinogenesis process and for which suitable antibodies were commercially available were selected for independent validation by western blot analysis. All bioinformatics were performed with caGEDA (http://bioinformatics2.pitt.edu/GE2/GEDA.html). Briefly, the CY3 and CY5 data were normalized to CY2 (Standard) data by ratio (CY3/CY2 and CY5/CY2). To determine spot distribution on the gels, Box plots were plotted using caGEDA (by input of previously normalized data, without additional transformation/normalization within caGEDA
Western Blot Analysis
Western blot analysis was performed to independently validate the expression profile of 5 proteins that a) were determined by 2D-DIGE to be differentially expressed between SEY and RY, b) have a validated or putative role in the carcinogenesis process and c) antibodies suitable for western analysis were commercially available. Three different protein aliquots (30 μg/lane) from RY and SEY were denatured and resolved in a 10% SDS-PAGE gel and probed with antibodies against five differentially expressed proteins selected on the basis of their putative/validated role in the carcinogenesis process. The antibodies for Pyruvate Kinase (PK), HSP70, Elongation factor 2 (eEF2), Eukaryotic Translation Initiation Factor 5A (IF5A2), Triosephosphate Isomerase (TSP isomerase) were obtained from Abcam, Cambridge, MA. Bands were detected using enhanced chemiluminescence reagents (ECL, GE Healthcare) and developed with autoradiography film (Imaging Resources, Inc.), images captured with Bio-Rad s GS800 Calibrated Densitometer and quantified with the Quantity One v4.5.0 1D Analysis Software (Bio-Rad Laboratories). Measurements were based on equal amount of protein loading and the average was taken from triplicate analysis. Bar diagram was constructed by normalizing RY average value to one for each protein. Statistical significance (p < 0.05) was determined using Student s t-test.
Results
2D-DIGE Identified Differences in Protein Expression in SEY and RY
The amount of extractable proteins from SEY and RY varied significantly (p≤0.012), averaging 47.69 μg/mg and 36.72 μg/mg of dry weight respectively. For 2D-DIGE 50 μg of total protein isolated from SEY and RY were labeled with cyanine dyes as described in the “Materials and Methods” section and separated in the first and second dimensions. After image capture, quantitative analysis of individual spot volumes was assessed, followed by statistical analysis (student t-test) with the Biological Variation Analysis (BVA) module of DeCyder software [20].
The distribution of the spot intensities (expression values) of the selected candidate proteins on the 2D-DIGE gels (SEY, n=3; RY, n=3) were obtained and plotted as Box plots. The Box plots showed that the spot intensities ranged from 0.55 (G2-HS) to 1.54 (G1-HS, G3-HS) for SEY and from 0.50 (G1-N) to 1.54 (G2-N) for RY (Figure 1) indicating that the SEY gels and RY gels were comparable, and the differences in protein expression observed is likely due to the effect of selenium enrichment on the proteome in SEY.
Figure 1.
Box plots demonstrating the spot intensity (expression values) distribution of the candidate proteins. The “red” plots show the distribution in SEY ranging from 0.55 to 1.54 and the blue plots show the distribution in RY ranging from 0.50 to 1.54;
A total of 184 candidate protein spots were automatically detected by DeCyder, as differentially expressed between SEY and RY. Out of the 184 spots, 75 (40.8%) were less abundant in SEY whereas 109 (59.2%) were more abundant in SEY compared to RY. The simulated 3D images of the 184 spots were manually inspected, after which 96 spots were selected for mass spectrometry based on the spots characteristics.
Identification of Protein Spots
The 96 candidate protein spots were excised from a representative 2D-DIGE gel and identified by MS/MS analysis. A total of 37 proteins were identified after LC/MS/MS analysis, use of the MS/MS data to search the yeast database and validation of the data with the PeptideProphet program. The PeptideProphet uses a range of SEQUEST scores and other parameters including peptide length, number of missed cleavages to calculate a probability score for each identified peptide. Additionally, the identified peptides were assigned a protein identity using the ProteinProphet software, which allowed the filtering of large data sets with predictable sensitivity and false-positive identification error rates. In this study, we used ProteinProphet probability score of ≥ 0.9 as the cutoff value for protein identification. This suggests that the false-positive rate (error rate) for protein identification in this study is ≤ 1%.
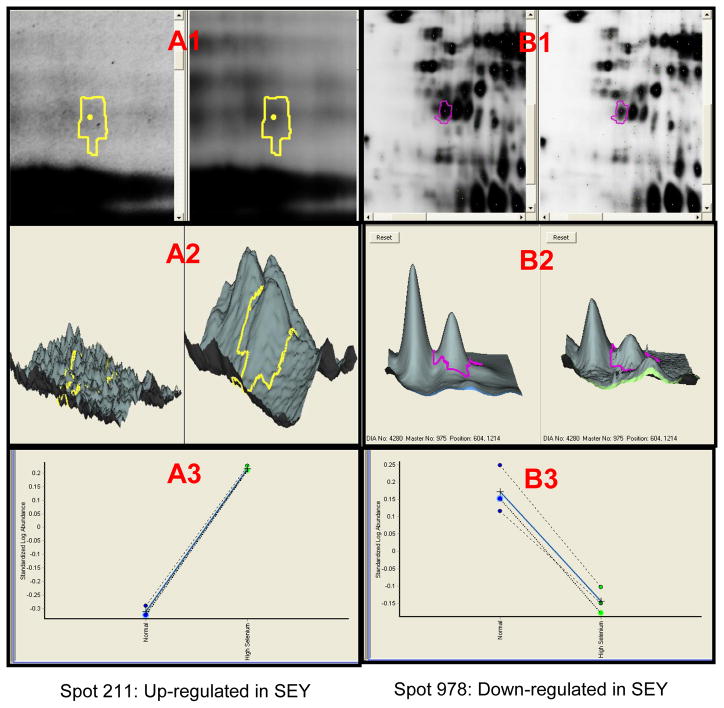
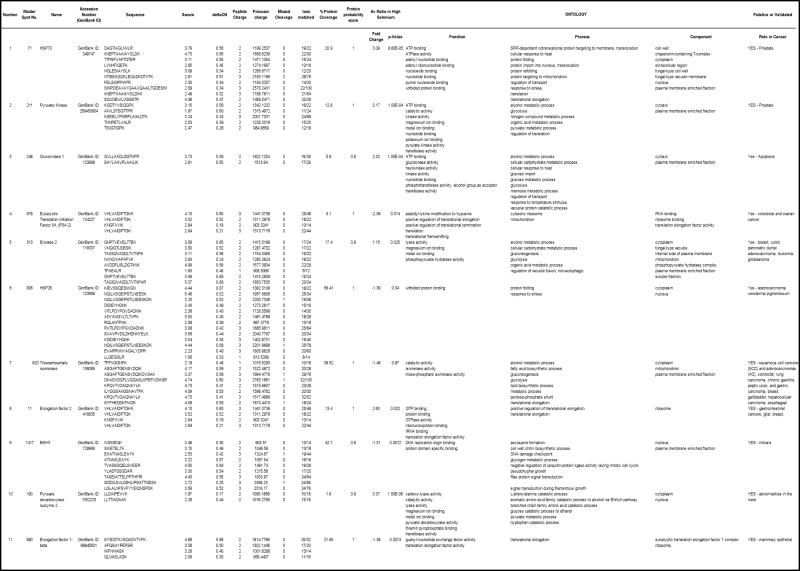
The 37 proteins differentially expressed are presented as supplemental data (Table 1S). GO classification indicates that 11 out of the 37 proteins have a validated or putative role in the carcinogenesis process (Table 1). Because of the GO classification, the cancer associated mechanism they are involved in and the availability of antibodies, five of the proteins differentially expressed between RY and SEY were selected and their expression were independently validated by western analysis. The selected proteins are HSP 70, Elongation factor 2, Pyruvate kinase, triosphosphate isomerase, Eukaryotic translation initiation factor 5A (Table 1). Figure 2 shows a representative image of candidate spots (2A1, 2B1), simulated 3D images (2A2, 2B2) and graphical illustrations (2A3, 2B3) of candidate protein spot 211 identified as PK (up-regulated in SEY compared to RY), and candidate spot 978 identified as eIF5A2 (down-regulated in SEY compared to RY). Figure 3 shows a ProteinProphet spectrum, scores and protein identity probability of a representative peptide (KGDTYVSIQGFK) used for identification of Pyruvate Kinase (spot #211).
Table 1.
Identities, fold difference and Gene Ontology of 11 proteins differentially expressed between SEY and RY
|
Figure 2.
2D-DIGE images for candidate protein spots 211 (A1, Pyruvate Kinase) up-regulated in SEY and 978 (B1, Eukaryotic Translation initiation Factor 5A-2; IF5A2) down-regulated in SEY. Images A2, B2 and A3, B3 are simulated 3-D and graphical representations of the spots illustrating differences in expression between SEY and RY.
Figure 3.
ProteinProphet spectrum, scores and protein identity probability of one of the five peptides (KGDTYVSIQGFK) used for identification of Pyruvate Kinase (PK).
Western Blotting Verified Modulation of Protein Levels Found in 2D-DIGE
The signal intensities generated after western blotting (Figure 4A) were quantified. As shown in Figure 4B, the antibodies against human forms of HSP70, PK and eEF2 were expressed at significantly higher levels in SEY compared to RY, whereas the expression of eIF5A2 and TSP isomerase were at lower levels in SEY compared to RY (p < 0.05).
Figure 4.
Western blot analysis of proteins expressed in selenium-enriched yeast (SEY) and regular yeast (RY). [A] Western blots of HSP70, Pyruvate Kinase (PK), Elongation Factor 2 (eEF2), Eukaryotic Translation Initiation Factor A-2 (eIF5A2) and Triosephosphate isomerase (TSP isomerase); [B] quantification of protein bond intensity (*, p < 0.05).
Discussion
In the present investigation we showed that selenium enrichment of baker s yeast results in up-regulation of significantly more proteins than those which are down-regulated. We established the identity of the differentially expressed proteins of interest by nano-LC/MS/MS, qualified the mass spectrometry data with the PeptideProphet and ProteinProphet programs and performed GO classification of the differentially expressed proteins to identify those that may be relevant in cancer. Out of the 37 proteins selected 11 have validated or putative roles in the carcinogenesis process. The protein probability score for the 5 proteins selected for mass spectrometry, including, pyruvate kinase was ≥0.9 (Figure 3).
The proteins were identified based on a search of a “yeast” database. Among the proteins identified (Table 1), a variety of biological functions were noted, including metabolic processes, glycolysis, ATP binding, metal binding, nucleoside and nucleotide binding, protein folding/unfolding, stress and signal transduction to cite a few. Interestingly, 11 of the proteins sequenced by mass spectrometry have a validated or putative role in the development of a variety of cancers including, prostate, colorectal, ovarian, breast, pancreatic, leukemia and lung. We selected three proteins that were up-regulated (fold difference +2.63, +3.09, +3.17) and two proteins that were down-regulated (fold difference −1.49, −2.06) in SEY compared to RY for independent validation by western blot analysis. The main criteria for selecting the 5 proteins out of the 11 were a) their GO classification b) validated or putative role in the carcinogenesis process and commercial availability of antibodies suitable for western analysis.
The antibodies for PK, HSP70, eEF2, eIF5A2 and TSP isomerase were used for semi-quantitative analysis of triplicate samples of SEY and RY to test the validity of the 2D-DIGE data and access the concordance between 2D-DIGE and western analysis. The western analysis displayed 100% concordance with the 2D-DIGE data.
The presence of genes encoding PK in yeast has long been reported [24–28]. The gene encoding of PK in yeast has been cloned [29]. We identified four Pyruvate proteins including Pyruvate decarboxylase isozyme 1 (Acc No: 30923172); Pyruvate decarboxylase isozyme 2 (Acc No.: 1352225); Pyruvate decarboxylase isozyme 3 (ACC No.: 118389) and Pyruvate Kinase (Acc No: 259450804). All the pyruvate proteins displayed approximately 3 fold higher expression in SEY compared to RY suggesting that the pathway associated with Pyruvate is indeed impacted by selenium supplementation. Not to overstate our finding, however, it is conceivable that the cancer preventive effect of SEY[7] may, in part, be due to the presence of high amounts of favorable proteins like Pyruvate Kinases (or their corresponding peptides) that impact the cancer prevention mechanism.
Amongst the other identified proteins, HSP70 and eEF2 were up-regulated whereas the other two were down-regulated. HSPs have strong cytoprotective effects and behave as molecular chaperones for other cellular proteins. Several HSPs directly interact with critical components of the cell signaling pathway [30]. HSPs 27 and 70 are the most strongly induced after stresses such as anticancer drugs, or irradiation and other damaging conditions. It is also known that HSPs bind to and inactivate androgen receptor (AR) [31]. Thus, on the basis of our results, which show an up-regulation of HSP70, it is clear that selenium fortification of baker s yeast leads to an increase in the amount of HSP70 in the yeast, and this increased levels may be effective in inhibiting the binding of androgens to the receptor. Obviously, the changes in HSP may be the consequence of stress response to cope with high selenium during the growth of yeast cells, a scenario similar to high-temperature tolerance. In fact it has also been reported that CDC19 encoding PK is important for high-temperature tolerance in yeast [29]. It is worth pointing out that the survival of the yeast cells and rate of biomass increase was not impacted by the selenium concentration used for producing the selenium-rich yeast.
Elongation factor 2 (eEF2) is a member of GTP-binding translation elongation factor family. This protein is an essential factor for protein synthesis and is negatively regulated by its phosphorylation mediated by EF-2 kinase [32]. It has been shown that the activation of eEF2 kinase-mediated degradation of proteins and organelles plays a protective role for cancer cells that are under metabolic stress conditions [32]. A recent study showed that activation of eEF2 kinase-mediated autophagy plays a protective role for cancer cells under metabolic stress conditions [33]. Our results clearly showed that eEF2 is up-regulated in SEY, and therefore its increased amounts in SEY compared to RY may contribute to increased protein synthesis in subjects receiving SEY. It will be interesting to determine if the levels of eEF2-kinase increases in human plasma as a result of selenium supplementation, and if the induced up-regulation of eEF2 following supplementation of SEY translates to a change of eEF2 levels in humans.
The phylogenetically conserved Eukaryotic translation initiation factor 5A-2 (eIF5A-2) is the only known cellular protein that contains the post-translationally derived amino acid hypusine [Nepsilon-(4-amino-2-hydroxybutyl)lysine]. Both eIF5A and its hypusine modification are essential for sustained cell proliferation. Normally only one eIF5A protein is expressed in human cells. However, further studies by Clement et al [34] identified a second human eIF5A gene that would encode an isoform (eIF5A2) of 84% sequence identity and the results suggest that it is a potential oncogene. Remarkably, Tastet et al recently reported the use of ICP-MS assisted proteomics to identify eIF5A-2 isolated from selenium-rich as a selenium-containing protein [14]. Based on this result and those of others, it will be interesting to determine the extent to which selenium fortification of baker s yeast can impact the oncogenic potential of proteins like eIF5A2, particularly in human subjects receiving selenium in the form of selenium-rich yeast.
TSP isomerase catalyzes the reversible interconversion of G3P and DHAP. Only G3P can be used in glycolysis and thus TSP isomerase is essential for energy production, allowing two molecules of G3P to be produced for every glucose molecule, thereby doubling the energy yield. In the present study we showed that selenium fortification of baker s yeast reduced the levels of TSP isomerase in yeast. If this scenario occurs in humans, then the deficiency of TSP isomerase may lead to disorder of glycolysis. Even though it is known that proteins work in concert and the elevation/or reduction in the levels of a single protein may not be responsible for cancer chemoprevention by SEY [7] it will be important to establish if TSP levels are altered in humans receiving selenium supplementation.
Although this manuscript reports on HSP70, eEF2, PK, eIF5A-2 and TSP isomerase for which validation by western analysis has been performed, it is worth mentioning that 32 other proteins identified by mass spectrometry also showed detectable differences in expression (see supplemental data). Amongst these, GO classification indicate that Pyruvate Decaboxylase Isozyme 1, Pyruvate Decarboxylase Isozyme 2, Enolase 2 and Pyruvate Kinase are metal binding proteins whereas, HSPSSC3, HSP70, HSP26 and HSP77 are stress response proteins. Dysregulation of carrier and stress response proteins would be expected since higher than normal levels of selenium are used to grow the yeast. HSP70, HSP77 and HSPSSC3 were up-regulated by an average of 2.6 folds in SEY compared to RY, whereas HSP26 was down-regulated (−1.39 fold) in SEY. This suggests that high selenium, though not toxic to the yeast, may stress the yeast to some extent.
Selenium is incorporated into proteins by the replacement of sulfur in methionine residues [35] and some HSP s identified in selenium-rich yeast; including HSP10, HSP12 and HSPSSA1 are classified as selenium-containing proteins [14]. Recently, Tastet et al [14] report the identification of seventeen selenium-containing proteins in selenium-rich yeast [14]. Remarkably, six of the differentially expressed proteins that we identified including, Enolase 2, eIF5A-2, GAPDH, Phosphoglycerate mutase 1, Elongation factor 1-beta and Malate dehydrogenase are part of the seventeen proteins reported by Tastet et al [14] and McSheehy et al [36] as selenium-containing proteins. Elongation factor is a critical protein involved in deciding when to add a selenocysteine at UGA codons instead of stopping [37]. Five of the 37 proteins, including Peroxiredoxin type-2, ATP synthase subunit beta, Malate dehydrogenase, Dihydrolipoyllysine-residue succinyltransferase, Glyceraldehyde-3-phosphate dehydrogenase are involved in oxidation reduction pathway and are probably differentially expressed in SEY due to the stress imposed by the selenium.
In summary, we showed for the first time, that selenium enrichment of baker s yeast simultaneously up-regulates certain proteins and down-regulates others. A number of the proteins are metal-binding, selenium-containing, stress response and reported to be relevant in the carcinogenesis process. While the results obtained here are noteworthy because we showed differential protein expression in selenium-rich yeast, the extent to which the active forms of the yeast proteins identified can be transported intact across the intestinal barrier and the biologic effect of such proteins (or the corresponding peptides) must be determined in preclinical animal models and pilot human clinical trials before any conclusions can be made on the potential beneficial effect. It is expected that this study will stimulate future research aimed at determining how the levels of proteins differentially expressed in SEY change in the blood of human subjects during selenium supplementation. Hopefully, such studies will eventually lead to the elucidation of other ways in which selenium and/or selenium supplementation of baker s yeast may impact prostate cancer prevention.
Supplementary Material
Acknowledgments
This work was supported in part by seed funds from the Penn State Hershey Cancer Institute and by the NCI Grant R01 CA127729. We thank Dr. Judith Bond for her constructive comments and Cypress Company for providing both SEY and RY.
Appendix A. Supplementary data
Supplementary information for the list of the 37 proteins and their GO classification are presented as supplemental data (Table 1S). Supplementary data associated with this article can be found, in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–36. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Nadiminty N, Gao AC. Mechanisms of selenium chemoprevention and therapy in prostate cancer. Mol Nutr Food Res. 2008;52:1247–60. doi: 10.1002/mnfr.200700369. [DOI] [PubMed] [Google Scholar]
- 5.Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–26. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 6.Karp DD, Lee SJ, Shaw Wright GL, Johnson DH, Johnston MR, Goodman GE. A phase III, intergroup, randomized, double-blind, chemoprevention trial of selenium (Se) supplementation in resected stage I non small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:7s. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–12. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 8.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristal AR. Are clinical trials the "gold standard" for cancer prevention research? Cancer Epidemiol Biomarkers Prev. 2008;17:3289–91. doi: 10.1158/1055-9965.EPI-08-1066. [DOI] [PubMed] [Google Scholar]
- 10.El-Bayoumy K. The negative results of the SELECT study do not necessarily discredit the selenium-cancer prevention hypothesis. Nutr Cancer. 2009;61:285–6. doi: 10.1080/01635580902892829. [DOI] [PubMed] [Google Scholar]
- 11.Facompre N, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: implications for cancer survivors. Cancer Res. 2009;69:2699–703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algotar AM, Stratton MS, Xu MJ, Dalkin BL, Nagle RB, Hsu CH, et al. Dose-dependent effects of selenized yeast on total selenium levels in prostatic tissue of men with prostate cancer. Nutr Cancer. 2011;63:1–5. doi: 10.1080/01635581.2010.516476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabichi AL, Lee JJ, Taylor RJ, Thompson IM, Miles BJ, Tangen CM, et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–84. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- 14.Tastet L, Schaumloffel D, Lobinski R. ICP-MS-assisted proteomics approach to the identification of selenium-containing proteins in selenium-rich yeast. J Anal Atom Spectrom. 2008;23:309–17. doi: 10.1016/j.talanta.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Díaz Huerta V, Hinojosa Reyes L, Marchante-Gayón JM, Fernández Sánchez ML, Sanz-Medel A. Total determination and quantitative speciation analysis of selenium in yeast and wheat flour by isotope dilution analysis ICP-MS. J Anal At Spectrom. 2003;18:1243–7. [Google Scholar]
- 16.Dumont E, De Cremer K, Van Hulle M, Chery CC, Vanhaecke F, Cornelis R. Identification of the major selenium compound, Se-Methionine, in three yeast (Saccharomyces cerevisiae) dietary supplements by on-line narrowbore liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2005;1071:191–6. doi: 10.1016/j.chroma.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Toki N, Sumi H, Sasaki K, Boreisha I, Robbins KC. Transport of urokinase across the intestinal tract of normal human subjects with stimulation of synthesis and/or release of urokinase-type proteins. J Clin Invest. 1985;75:1212–22. doi: 10.1172/JCI111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry S. Therapeutic Fibinolysis: past, present and future. In: Paoletti R, Sherry S, editors. Thrombosis and Urokinase. London, UK: Academic Press; 1977. pp. 1–9. [Google Scholar]
- 19.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyiri T, Somiari RI, Russell S, Aliaga C, El-Bayoumy K. Proteomics of rat prostate lobes treated with 2-N-hydroxylamino-1-methyl-6-phenylimidazo[4,5-b]pyridine, 5alpha-dihydrotestosterone, individually and in combination. Int J Oncol. 2009;35:559–67. doi: 10.3892/ijo_00000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somiari RI, Sullivan A, Russell S, Somiari S, Hu H, Jordan R, et al. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3:1863–73. doi: 10.1002/pmic.200300560. [DOI] [PubMed] [Google Scholar]
- 22.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 23.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 24.Sprague GF., Jr Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol. 1977;130:232–41. doi: 10.1128/jb.130.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boles E, Schulte F, Miosga T, Freidel K, Schluter E, Zimmermann FK, et al. Characterization of a glucose-repressed pyruvate kinase (Pyk2p) in Saccharomyces cerevisiae that is catalytically insensitive to fructose-1,6-bisphosphate. J Bacteriol. 1997;179:2987–93. doi: 10.1128/jb.179.9.2987-2993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portela P, Howell S, Moreno S, Rossi S. In vivo and in vitro phosphorylation of two isoforms of yeast pyruvate kinase by protein kinase A. J Biol Chem. 2002;277:30477–87. doi: 10.1074/jbc.M201094200. [DOI] [PubMed] [Google Scholar]
- 27.Fraenkel DG. The top genes: on the distance from transcript to function in yeast glycolysis. Curr Opin Microbiol. 2003;6:198–201. doi: 10.1016/s1369-5274(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 28.Williams JM, Chen GC, Zhu L, Rest RF. Using the yeast two-hybrid system to identify human epithelial cell proteins that bind gonococcal Opa proteins: intracellular gonococci bind pyruvate kinase via their Opa proteins and require host pyruvate for growth. Mol Microbiol. 1998;27:171–86. doi: 10.1046/j.1365-2958.1998.00670.x. [DOI] [PubMed] [Google Scholar]
- 29.Benjaphokee S, Koedrith P, Auesukaree C, Asvarak T, Sugiyama M, Kaneko Y, et al. CDC19 encoding pyruvate kinase is important for high-temperature tolerance in Saccharomyces cerevisiae. N Biotechnol. 2011 doi: 10.1016/j.nbt.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, et al. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Marquez M, Nilsson S, Holmberg AR. Comparison of protein expression in two prostate cancer cell-lines, LNCaP and DU145, after treatment with somatostatin. Oncol Rep. 2009;22:1451–8. doi: 10.3892/or_00000587. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura J, Aoyagi S, Nanchi I, Nakatsuka S, Hirata E, Shibata S, et al. Overexpression of eukaryotic elongation factor eEF2 in gastrointestinal cancers and its involvement in G2/M progression in the cell cycle. Int J Oncol. 2009;34:1181–9. [PubMed] [Google Scholar]
- 33.Cheng Y, Li H, Ren X, Niu T, Hait WN, Yang J. Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition. PLoS One. 2010;5:e9715. doi: 10.1371/journal.pone.0009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, et al. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254–63. doi: 10.1046/j.1432-1033.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- 35.Schrauzer GN. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. The Journal of nutrition. 2000;130:1653–6. doi: 10.1093/jn/130.7.1653. [DOI] [PubMed] [Google Scholar]
- 36.McSheehy S, Kelly J, Tessier L, Mester Z. Identification of selenomethionine in selenized yeast using two-dimensional liquid chromatography-mass spectrometry based proteomic analysis. Analyst. 2005;130:35–7. doi: 10.1039/b414246b. [DOI] [PubMed] [Google Scholar]
- 37.Leibundgut M, Frick C, Thanbichler M, Bock A, Ban N. Selenocysteine tRNA-specific elongation factor SelB is a structural chimaera of elongation and initiation factors. The EMBO journal. 2005;24:11–22. doi: 10.1038/sj.emboj.7600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.