Abstract
Efficient antigen extraction from vaccines formulated on aluminum hydroxide gels is a critical step for the evaluation of the quality of vaccines following formulation. It has been shown in our laboratory that the efficiency of antigen extraction from vaccines formulated on Alhydrogel decreased significantly with increased storage time. To increase antigen extraction efficiency, the present study determined the effect of surfactants on antigen recovery from vaccine formulations. The Plasmodium falciparum apical membrane antigen 1 (AMA1) formulated on Alhydrogel and stored at 2-8 °C for three years was used as a model in this study. The AMA1 on Alhydrogel was extracted in the presence or absence of 30 mM sodium dodecyl sulfate (SDS) or 20 mM cetylpyridinium chloride in the extraction buffer (0.60 M citrate, 0.55 M phosphate, pH 8.5) using our standard antigen extraction protocols. Extracted AMA1 antigen was analyzed by 4-20% Tris-glycine SDS-PAGE followed by silver staining or western blotting. The results showed that inclusion of SDS or cetylpyridinium chloride in extraction buffer increased the antigen recovery dramatically and can be used for efficient characterization of Alhydrogel vaccines.
Keywords: Alhydrogel, AMA1, extraction, SDS, cetylpyridinium chloride
1. Introduction
Adjuvant is one of the critical components of vaccine formulations. Aluminum compounds, including aluminum phosphate (AlPO4) and aluminum hydroxide (Al(OH)3 , Alhydrogel) have been used as adjuvants in vaccines for more than 80 years1, and are the most established adjuvants approved by the United States Food and Drug Administration as components of human vaccines. Because of the good track record of safety, low cost and adjuvanticity with a variety of antigens, aluminum compounds will continue to be used as adjuvants for many years. To ensure the vaccine quality, regulatory authorities require that antigens have to be adsorbed at certain percentage by aluminum-containing adjuvants, for example, at least 80% for tetanus toxoid vaccine by the World Health Organization2. On the other hand, due to the difficulties of analyzing the identity and integrity of the antigen in the formulated vaccine, it needs to be eluted from aluminum-containing adjuvants in order to perform the quality evaluation prior to vaccine administration to humans.
There are many factors that affect the antigen adsorption to and dissociation from aluminum compounds. The adsorption and dissociation of antigens on aluminum compounds heavily depends on electrostatic forces between antigen and adjuvant. Other interactive forces such as hydrophobic interactions, hydrogen bond and Van der Waals forces are contributed as well. The factors such as ligand exchange, pH, temperature, size of gel particles, ionic strength of reaction mixture will affect the interactive forces between antigen and adjuvant, and therefore will also affect the adsorption and dissociation3-14. A number of in vitro studies have shown that the antigen can be rapidly eluted from aluminum-containing adjuvants after exposure of the vaccine to interstitial fluid, which normally contains phosphate anion and citrate anion 15-20. Our previously study also found that phosphate inhibits CPG 7909, a 24-mer oligonucleotide that contains three CpG motifs (5′-GTCGTT-3′), binding to Alhydrogel21, indicating that phosphate reduces the binding capacity of Alhydrogel. In addition to selection of extraction buffer and ionic strength for extraction purpose, a numbers of publications have reviewed and reported on the effects of surfactants on the adsorption and dissociation of proteins from solid surfaces or aluminum compounds. Horbett and others reported studies of removal of fibrinogen and other proteins by surfactant from polymers22-26. They found that the elution efficiency decreased as the time of protein adhered to the surface of polymers increased22-25, 27-28. Rinella and colleague tested a numbers of surfactants including Triton X-100, lauryl maltoside, lauryl sulfobetaine, sodium dodecyl sulfate (SDS), cetylpyridinium chloride (CPC) and dodecyltrimethylammonium chloride, and found the best elutability was achieved with the concentration greater than 20 mM for SDS, or 15 mM for CPC, when freshly prepared ovalbumin and lysozyme were used as model antigens29. However, they only used total proteins to assess the extraction efficacy, and it was unclear if the proteins were degraded or the functional epitopes of the proteins were altered or destroyed during the extraction process. The method may not be useful in the vaccine quality control if the process changed antigen integrity or function. Further evaluation to demonstrate the proteins to retain the same integrity after extraction is needed in order to adopt this method for general vaccine quality control applications. Furthermore, extraction, in the presence of surfactants, of vaccines stored for prolonged period of time, has not been reported and merits vigorous investigation.
In this paper, we report the study of factors which may affect the elution of two Plasmodium falciparum apical membrane antigen 1 (AMA1) allelic forms - AMA1-FVO and AMA1-3D7 - when combined referred to as AMA1-C1, from Alhydrogel. The effects of surfactants including sodium dodecyl sulfate (SDS) and cetylpyridinnium chloride (CPC) in the elution of AMA1-C1 from Alhydrogel for the vaccine stored at 2-8°C for up to 3 years was evaluated. We also describe the methods used to assess the identity and integrity of vaccine over time.
2. Material and method
2.1. Preparation of AMA1-C1/Alhydrogel formulations
The AMA1-FVO and AMA1-3D7 proteins were manufactured according to current good manufacturing practice at the Walter Reed Army Institute of Research Pilot Bioproduction Facility (Silver Spring, MD) with methods developed at the Laboratory of Malaria Immunology and Vaccinology (LMIV) (formerly known as Malaria Vaccine Development Branch (MVDB)), National Institute of Allergy and Infectious Diseases, National Institutes of Health30. Purified AMA1-FVO and AMA1-3D7 were mixed at 1:1 ratio and prepared at concentrations of 10, 40, or 160 μg/ml in 1,600 μg/ml Alhydrogel® (Aluminum Hydroxide Gel Adjuvant, Brenntag Biosector, Denmark) by Pharmaceutical Development Service, National Institutes of Health as previously described31. The formulations were aliquoted and kept at 2-8°C until use.
AMA1-C1/Alhydrogel reference standard were prepared freshly at final concentrations of 10, 40, or 160 μg/ml in 1,600 μg/ml Alhydrogel® by rotating the mixture at 16 - 24 rpm on a rotary spinner (Appropriate Technical Resources, Laurel, Maryland) for 60 minutes at room temperature, and then aliquoted and kept at 2-8°C until use.
2.2. Antigen extraction
AMA1-C1 on Alhydrogel was extracted in the presence or absence of surfactants including 30 mM of sodium dodecyl sulfate or 20 mM of cetylpyridinium chloride in the extraction buffer (0.60 M citrate, 0.55 M phosphate, pH 8.5) using our standard antigen extraction protocol. Briefly, after vortexing the vaccine for 1 minute at 5.5 rpm on a Daigger vortex genie 2 (Daigger & Co. Inc.,), 0.3 mL of vaccine was immediately transferred to an eppendorf microcentrifuge tube and 0.6 mL of extraction buffer (0.60 M sodium citrate dihydrate/0.55 M sodium phosphate dibasic, with or without 30 mM SDS or 20 mM CPC, pH 8.5) was added. The tube was mixed by inversion 10 times and incubated for 2.5 hours at 60°C with a gently mixing every 20 minutes during the incubation. The tube was then centrifuged at 425 g for 2 minutes at room temperature. The supernatant was transferred to a new microcentrifuge tube and used for analysis by SDS-PAGE or western blot analysis, and the remaining volume was then stored at –80°C.
Vaccine samples were extracted at year 1, 2 and 3 after formulation. Freshly prepared formulations were also exacted with the standard extraction method described above as references. Due to the un-availability of extraction method at the time the vaccine was prepared, the extracts for T=0 samples were not accessible.
2.3. SDS-PAGE and Western blot
Approximately 43 ng (calculation based on 100% recovery) of extracted AMA1-C1 were resolved on 4-20% gradient Tris-glycine SDS-PAGE gels (Invitrogen Corp) under non-reducing conditions using an XCell SureLock electrophoresis Mini-Cell apparatus (Invitrogen Corp). Extractions of reference formulations or stored AMA1-C1/Alhydrogel formulations, at 2-8°C for 1, 2 or 3 years, were analyzed on the SDS-PAGE and visualized by silver staining. Silver stained gels were scanned with a Laser Densitometer (Molecular Dynamics) and the intensity of all visible bands was analyzed by ImageQuant software (GE Health Care). The extraction efficiency of the extracted proteins at each time point was calculated as [Extraction efficiency = (intensity of all bands of test sample/ intensity of all bands of reference bulk antigen sample) × 100].
Similarly prepared SDS-PAGE gels were transferred to nitrocellulose membranes (Invitrogen Corp.) and used to perform Western blots (43 ng per lane, calculation based on 100% recovery) by probing with 3 monoclonal antibodies including 1G4 that primarily recognizes AMA1 domains I/II of the FVO allele, 1E9 that predominantly recognizes an unknown epitope of AMA1 of the 3D7 allele, and a Penta-His mAb which recognizes the C-terminal His-tag of all recombinant AMA1 proteins. The Western blot was performed with alkaline phosphatase system (KPL Labs, Gaithersburg, MD) and 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT, KPL Labs) was used as substrate.
2.4. Potency studies
Animal studies were performed in compliance with National Institutes of Health guidelines and under the auspices of an Animal Care and Use Committee approved protocol. The vaccine potency assays were carried out using 24 groups (N = 10) of 6-week old female BALB/c mice immunized intraperitoneally (i.p.) as previously described32. Sera were collected 14 days after the secondary immunization and serum antibodies to AMA1-FVO or AMA1-3D7 were assayed by ELISA as described previously32. One antibody unit was defined as the reciprocal of the sera dilution that gives an optical density (O.D.) at 405 nm of 1.033.
2.5 Zeta potential
Zeta potential is the electric potential across the ion layer around a charged colloidal particle and causes colloidal particles to repel each other and stay in suspension, which measures the magnitude of the repulsion or attraction between particles. The zeta potentials of unformulated AMA1-C1 in saline, placebo (1600 μg/mL of Alhydrogel alone diluted with saline), AMA1-C1/Alhydrogel vaccine were measured by Zetasizer Nano ZS (Malvern Instrument, Westborough, MA). The Zeta potential of a mixture of AMA1-C1/Alhydrogel incubated with extraction buffer (with or without addition of surfactants) for 2.0 or 2.5 hr at 60 °C was also measured. The supernatants of AMA1-C1/Alhydrogel extractions were collected in separate eppendorf tubes, and their Zeta potential was measured as well.
2.6 Statistical analysis
Extraction efficiency and Zeta potential data were analyzed by Student's t-test and one-way ANOVA with one-way analysis of variance test for multiple comparisons (GraphPad Prism 5 Software). Potency data were analyzed by one tailed Mann-Whitney U test.
3. Results
3.1 The effect of storage time
The samples of AMA1-C1/Alhydrogel formulations at concentrations of 10 μg/ml, 40 μg/ml and 160 μg/ml and stored for 1, 2 and 3 years at 2-8°C were extracted by a standard extraction method (0.60 M citrate, 0.55 M phosphate, pH 8.5, 60°C, 2 hrs). The efficiency of antigen extraction decreased significantly (P<0.05) with increased age of the vaccine. For the year one extraction samples, the 10 μg/ml, 40 μg/ml and 160 μg/ml formulations showed about 60% recovery of AMA1-C1 compared to the same amount of AMA1-C1 reference antigen when detected by SDS-PAGE with the estimation by ImageQuant software (Table 1). For year two extraction samples, the recovery of AMA1-C1 was further reduced for all three concentrations with the significant reduction in recovery of 22.4 % for the lower concentration (10μg/mL) when compare to AMA1-C1 reference antigen. After the vaccines were stored at 2-8°C for three years, the extraction of 40 μg/ml and 160 μg/ml showed the lowest recovery of the 3 time points when compared to the reference antigen, and the 10μg/ml formulation gave only a very weak signal on the SDS-PAGE. The year one extraction samples appeared to be a slightly higher recovery when compared to the freshly made vaccine. This may be caused by the use of different lot of Alhydrogel® for preparation of these two sets of vaccines. However, there is no significant difference in extraction efficacy between these two sets of samples (P>0.05). In addition, some high molecular weight bands were also observed in the extraction samples, indicating the long-term storage may cause slight aggregation of AMA1 protein. Fig. 1 and Table 1 show the analysis by SDS-PAGE and subsequent quantification over the 3 years of analysis.
Table 1.
Comparison of extraction efficiency with or without the use of surfactant in extraction buffer
| Years in storage1 | Surfactant2 added to standard extraction buffer | % Extraction efficiency3 |
||
|---|---|---|---|---|
| 160μg/ml4 | 40μg/ml4 | 10μg/ml4 | ||
| 0 (Reference)5 | None | 72.9 | 45.9 | 53.9 |
| 1 | None | 58.4 | 62.1 | 67.4 |
| 2 | None | 48.0 | 34.1 | 22.4 |
| 3 | None | 24.1 | 15.7 | 4.5 |
| 3 | SDS | 100 | 100 | 100 |
| 3 | CPC | 100 | 100 | ND6 |
Number of years vaccine formulations stored at 2-8°C;
30 mM SDS (sodium dodecyl sulfate) or 20 mM CPC (cetylpyridinium chloride) in extraction buffer (0.60 M citrate, 0.55 M phosphate, pH 8.5);
Extraction efficiency = (intensity of all bands of test sample/ intensity of all bands of reference bulk antigen sample) × 100;
Formulation from which protein was extracted;
Reference was prepared freshly
Not determined.
Fig.1.
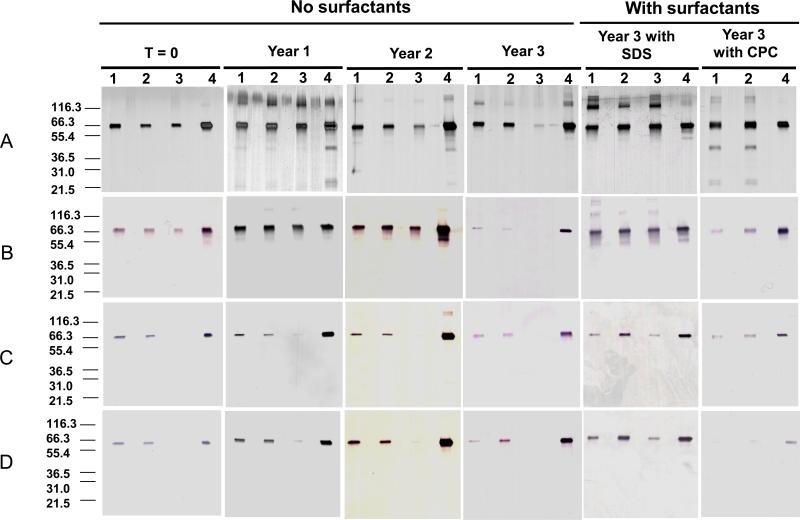
Characterization of AMA1 extracted from vaccines following 3 years of storage at 2-8°C. A. SDS-PAGE visualized by silver staining; B. Western blot results probed with monoclonal antibody 1E9; C. Western blot results probed with monoclonal antibody 1G4; D. Western blot results probed with Penta-His. Lane 1, 160 μg/mL AMA1-C1/Alhydrogel extraction; Lane 2, 40 μg/mL AMA1-C1/Alhydrogel extraction; Lane 3, 10 μg/mL AMA1-C1/Alhydrogel extraction, Lane 4, AMA1-C1 reference standard (without extraction). All samples were loaded at 43 ng protein (calculation based on 100% recovery) per lane. Molecular weight markers are indicated in kDa.
3.2 Extraction efficiency increased with addition of SDS or cetylpyridinium chloride
To evaluate the effect of SDS on the extraction efficiency, 30 mM SDS was added to the standard extraction buffer and incubated at 60°C for 2.5 hr. The results from all three vaccine formulations stored at 2-8°C for 3 years showed that the greatest antigen recovery was achieved using this condition, with an extraction efficiency of 100% when compared to the same amount of AMA1-C1 reference antigen loaded on SDS-PAGE (Fig.1A and Table 1). The analysis by Western blot with mAb 1E9, 1G4 or Penta-His showed similar results (Fig.1B-D). Higher molecular weight bands were also observed in the presence of SDS visualized by silver staining despite that these protein bands were barely recognized by monoclonal antibodies 1E9, 1G4 and Penta-His in Western blot. This may be due to the fact that the amount of protein (calculated as 43ng per lane) analyzed was not sufficient to generate detectable aggregates, although the loading amount was maximized for the 10 μg/mL dose vaccine. The condition using standard extraction buffer with addition of 30 mM SDS at pH 4.8 at 60°C for 2.5 hr was also tested, but resulted in poor recovery of antigen from vaccines (data not shown).
Due to the limited availability of vaccine, only the 40 μg/ml and 160μg/ml formulations of AMA1-C1/Alhydrogel stored at 2-8°C for 3 years were tested with extraction buffer containing 20 mM cetylpyridinium chloride (CPC). The formulations stored at 2-8°C for 3 years were incubated at 60 °C for 2.0 hr. The results showed that the antigen recoveries increased dramatically to 100% when compared to the same amount of AMA1-C1 reference antigen detected by SDS-PAGE (Fig.1 and Table 1). In contrast to extraction in the presence of SDS, bands with lower molecular weight were observed, and the extracted proteins were not recognized by Penta-His mAb. Conditions of standard extraction buffer with 20 mM of CPC for 0.5 hr or 1.0 hr were also tested, but recovery of antigen from vaccines was poor (data not shown).
The conditions using standard extraction buffer with 3.0 hr incubation time at 60°C and at pH 4.8 at 60°C for 2.5 hr were also tested, but resulted in poor recovery of antigen from vaccines (data not shown).
3.3 Potency of AMA1 vaccines stored at 2-8°C
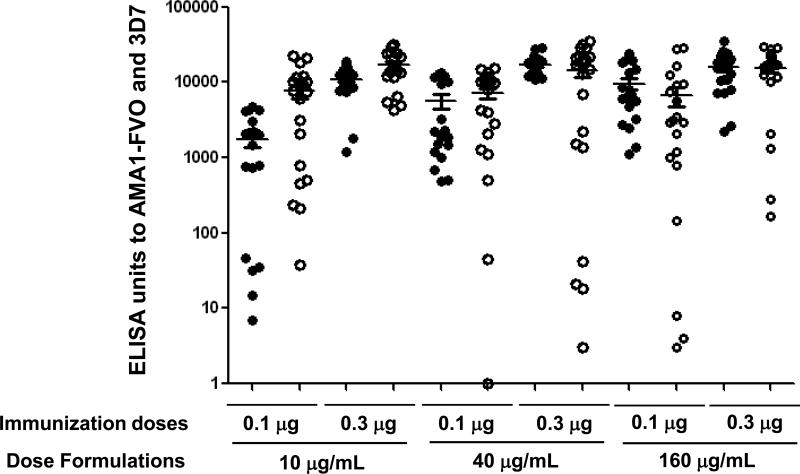
Potency of different formulations of AMA1-C1/Alhydrogel after storage at 2-8°C for 0.5, 1.0, 1.5, 2.0, 2.5 and 3 years was determined by serum antibody responses in mice compared to a freshly formulated reference vaccine, considered to be fully potent. Mouse anti-AMA1-FVO and anti-AMA1-3D7 IgG titers were measured by ELISA. The vaccines were fully potent at 0.5, 1.0, 1.5, 2.0 and 2.5 years (data not shown). Figure 2 shows results of the T=3 years potency assay, and there was no significantly statistical difference (P>0.05) in antibody titers between the AMA1-C1/Alhydrogel vaccines and the freshly formulated reference vaccine, indicating the vaccines remained immunogenic following prolonged storage under recommended conditions.
Fig. 2.
Mouse potency study of vaccines stored for 3 years at 2-8 °C. Each group of 10 mice was immunized intraperitoneally (i.p.) with 0.1 μg or 0.3 μg of AMA1-C1/Alhydrogel per mouse, respectively. This graph shows the average ELISA unit ± SEM ( ) of anti-AMA1-FVO and AMA1-3D7 alleles (P>0.05).
) of anti-AMA1-FVO and AMA1-3D7 alleles (P>0.05).  Reference formulations,
Reference formulations,  Clinical formulations. Shown are antibody levels of mice receiving 0.1 μg or 0.3 μg per immunization, diluted from the Reference or Clinical formulations of 10 μg/mL, 40 μg/mL and 160 μg/mL, respectively.
Clinical formulations. Shown are antibody levels of mice receiving 0.1 μg or 0.3 μg per immunization, diluted from the Reference or Clinical formulations of 10 μg/mL, 40 μg/mL and 160 μg/mL, respectively.
3.4 Zeta potential
The Zeta potential results of unadjuvanted AMA1-C1 antigen alone, placebo, AMA1-C1/Alhydrogel vaccine and AMA1-C1/Alhydrogel vaccine with extraction solution (with or without addition of surfactants) are summarized in Table 2. AMA1-C1 antigen alone has a negative Zeta potential as expected, AMA1-C1/Alhydrogel formulation has a positive Zeta potential, and the Zeta potential of vaccine formulations remained the same (P>0.05) after 3 years of storage at 2-8°C. However, the Zeta potential of AMA1-C1/Alhydrogel suspensions and supernatants of extracted formulations changed significantly (P<0.05) from positive to negative when mixed with standard extraction buffer or standard extraction buffer with addition of SDS. A significant decrease (P<0.05) in Zeta potential for samples in standard extraction buffer with SDS as compared to that for samples in standard extraction buffer alone was also apparent. In addition, the Zeta potentials of the vaccine suspensions in extraction buffer with CPC and the extraction supernatants decreased significantly (P<0.05) when compared to those of the vaccine formulations without any treatment.
Table 2.
Zeta Potential measurement of reference and 3 years samples
| Years in storage1 | Sample Form | Sample ID | Zeta Potential (mV) |
|---|---|---|---|
| 0 (Reference2) | Aqueous Solution | AMA1-C1 Antigen in saline | -23.22 ± 1.48 |
| Suspension | Placebo3 | 26.15 ± 1.51 | |
| AMA1-C1/Alhydrogel Formulation | 26.41 ± 3.09 | ||
| 3 | Suspension | AMA1-C1/Alhydrogel, prior to Extraction | 29.15 ± 4.06 |
| AMA1-C1/Alhydrogel, Standard Extraction Method4 | -7.94 ± 1.65 | ||
| AMA1-C1/Alhydrogel, Standard Extraction Method with SDS5 | -17.7 ± 3.45 | ||
| AMA1-C1/Alhydrogel, Standard Extraction Method with CPC6 | 15.95 ± 1.97 | ||
| 3 | Supernatant of Vaccine Extractions | AMA1-C1/Alhydrogel supernatant, Standard Extraction Method4 | -7.99 ± 1.61 |
| AMA1-C1/Alhydrogel supernatant, Standard Extraction Method with SDS5 | -21.88 ± 1.75 | ||
| AMA1-C1 supernatant, Standard Extraction Method with CPC6 | 10.67 ± 8.39 | ||
Number of years vaccine formulations stored at 2-8°C;
Reference was prepared freshly
Placebo =1600μg/mL Alhydrogel in pH 7.0 saline;
Standard extraction solution = 0.6 M sodium citrate dihydrate /0.55 M sodium phosphate dibasic, pH 8.5
30 mM SDS (sodium dodecyl sulfate)
20 mM CPC (cetylpyridinium chloride)
4. Discussion
At neutral pH, aluminum hydroxide (Alhydrogel), having an isoelectric point (pI) of 11.4, is positively charged whereas aluminum phosphate, with pI of between 4.5 and 6.0, is negatively charged. Therefore, Alhydrogel has a higher adsorption capacity and better adsorption properties for proteins with pI below neutral pH 4, 13. Because the pI of AMA1-FVO and AMA1-3D7 proteins are 5.54 and 5.55, respectively, and they will be negatively charged at neutral pH, Alhydrogel was chosen as the adjuvant for our malaria vaccine development.
Previous studies by Hem and colleagues indicated that ligand exchange and electrostatic attraction are the predominant mechanisms contributing to the adsorption and desorption of antigen to aluminum-containing adjuvants. At neutral pH, positively charged Alhydrogel supplied a good binding environment for the antigens with pIs below neutral pH through electrostatic attractions. However, the presence of phosphate in the extraction buffer will displace the surface hydroxyls of Alhydrogel through ligand exchange mechanism5, 14-16 as a result of its stronger binding to aluminum than hydroxyl groups, resulting in the surface charge of Alhydrogel changing from positive to negative; at that point the negatively charged antigen will dissociate from negatively charged Alhydrogel due to electrostatic repulsion and hydrophobic interaction. This hypothesis was supported by the present study, the AMA1-C1 antigen alone and placebo have Zeta potentials of negative 23 mV and positive 26 mV, respectively, and it indicates that AMA1-C1 antigen should bind to Alhydrogel efficiently through electrostatic attraction. However, after the formulation is exposed to phosphate anions (ie, extraction solution) for 2.5 hours, the zeta potential of Alhydrogel suspension shifted dramatically from positive (26 mV) to negative (-8 mV). This may explain why vaccines made fresh or less aged could be eluted by the current extraction buffer (phosphate and citrate buffer). This finding was also reported by Rinella9.
However, aged vaccine was difficult to elute with extraction buffers containing only phosphate and citrate. This suggested that the properties of AMA1-C1/Alhydrogel formulations may be changed or modified over the time. As reviewed by Hem5, as storage time increased, aluminum ions were linked together by forming double hydroxide bridges through the deprotonation and dehydration reactions. It is feasible that some AMA1-C1absorbed on Alhydrogel may have become trapped in the void spaces within the aluminum aggregates. These aggregates can be disrupted by surfactants.
SDS, an anionic surfactant and cetylpyridinium chloride, a cationic quaternary ammonium compound, could supply strong ionic charges to the surface of Alhydrogel, as well to the surface of AMA1-C1. These surfactants could reduce or even eliminate the electrostatic attraction or hydrogen bonds between the target protein and Alhydrogel, particularly with the help of heat providing the energy for reversing the equilibrium of deprotonation. In the present study, the Zeta potentials of AMA1-C1/Alhydrogel changed from positive 29 mV to negative 18 mV after addition of SDS, and AMA1-C1 in aqueous solution was negative 22 mV, indicating that the SDS caused both proteins and the surface of Alhydrogel to shift to strong negative charge. On the other hand, the addition of cetylpyridinium chloride caused both AMA1-C1 and Alhydrogel to shift to a strong positive charge, 11 mV and 16 mV respectively. These observations suggested that the electrostatic repulsion of protein with Alhydrogel and hydrophobic interaction of surfactant with the protein probably played an important role for releasing the antigen from Alhydrogel.
Differences of extraction efficiency of SDS or cetylpyridinium chloride compared to standard extraction buffer were observed in this study. Both SDS and cetylpyridinium chloride increased the extraction efficiency dramatically, but it seems that addition of SDS was better than cetylpyridinium chloride although AMA1-C1 on Alhydrogel extracted in the presence of SDS gave a considerable amount of aggregation when compared to the AMA1 antigen standard. The aggregation of AMA1-C1 may be caused by hydrophobic interaction during the extraction process.
AMA1-C1/Alhydrogel extracted in the presence of cetylpyridinium chloride gave less aggregation but considerable degradation products, exhibiting a similar protein banding under reducing conditions. This suggested that CPC disrupting the AMA1 in a similar manner with reducing reagent and liberating the lower molecular weight populations of AMA1-C1. In addition, the main band of protein was not recognized by the Penta-His tag antibody, indicating that the His-tag in the C-terminal was lost during the extraction procedure. Further studies need to be carried out to determine if the aggregation/degradation were caused by the addition of surfactants during the extraction process or formed before the extraction.
In summary, the strength of protein adhered to Alhydrogel increased with residence time, i.e., the vaccine will bind tighter to Alhydrogel as storage time increased. Therefore, more “harsh” conditions were needed to elute antigen from aged vaccines. The addition of ionic surfactants, SDS or cetylpyridinium chloride, significantly (by SDS method, P<0.05) increased the extraction efficiency and may be used for efficient antigen extraction of AMA1-C1 from Alhydrogel in aged vaccines. The potency results indicated that the vaccines maintained the same biological activity when compared to the fresh made formulations, suggesting that the AMA1-C1/Alhydrogel vaccine was biochemically and biologically stable after three years storage at 2-8°C.
Highlight.
Efficient antigen extraction from Alhydrogel is a critical step for the evaluation of the quality of vaccines.
The efficiency of antigen extraction from vaccines decreased with increased storage time.
Antigen recovery was dramatically increased by inclusion of surfactants in extraction buffer.
Acknowledgment
We thank Aaron Miles for coordination and production of AMA1-C1/Alhydrogel, Lynn Lambert for animal immunization and Xia Liu for antibody characterization.
This research was supported by the Intramural Research Program of the NIAID, NIH
Abbreviations
- AMA1
apical membrane antigen 1
- SDS
sodium dodecyl sulfate
- CPC
cetylpyridinium chloride
- pI
isoelectric point
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glenny AT, Pope CG, Waddington H, Wallace U. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;(29):38–45. [Google Scholar]
- 2.World Health Organization . WHO. Geneva: 1977. Manual for the Production and Control of Vaccines—Tetanus Toxoid. [Google Scholar]
- 3.Gupta RK. Aluminum compounds as vaccine adjuvants. Advanced Drug Delivery Reviews. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RK, Rost BE. Aluminum compounds as vaccine adjuvants, Vaccine Adjuvant. Humana Press; 2000. pp. 65–89. [Google Scholar]
- 5.Hem SL, HogenEsch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Review of Vaccines. 2007;6(5):685–698. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 6.Lindblad EB. Aluminum compounds for use in vaccines. Immunology and Cell Biology. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Iyer S, Robinett RSR, HogenEsch H, Hem SL. Mechanism of adsorption of hepatitis B surface antigen by aluminium hydroxide adjuvant. Vaccine. 2004;22:1475–9. doi: 10.1016/j.vaccine.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shakhshir RH, Regnier FE, White JL, Hem SL. Contribution of electrostatic and hydrophobic interactions to the adsorption of proteins by aluminium-containing adjuvants. Vaccine. 1995;13:41–4. doi: 10.1016/0264-410x(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 9.Rinella JV, White JL, Hem SL. Effect of anions on model aluminium-adjuvant-containing vaccines. J Colloid Interface Sci. 1995;172:121–30. [Google Scholar]
- 10.Rinella JV, White JL, Hem SL. Effect of pH on the elution of model antigens from aluminium-containing adjuvants. J Colloid Interface Sci. 1998;205:161–5. doi: 10.1006/jcis.1998.5648. [DOI] [PubMed] [Google Scholar]
- 11.Rinella JV, Jr, White JL, Hem SL. Treatment of aluminium hydroxide adjuvant to optimize the adsorption of basic proteins. Vaccine. 1996;14(4):298–300. doi: 10.1016/0264-410x(95)00194-6. [DOI] [PubMed] [Google Scholar]
- 12.Chang MF, White JL, Nail SL, Hem SL. Role of the electrostatic attractive force in the adsorption of proteins by aluminum hydroxide adjuvant. PDA J Pharm Sci Technol. 1997;51:25–29. [PubMed] [Google Scholar]
- 13.Seeber SJ, White JL, Hem SL. Predicting the adsorption of proteins by aluminium-containing adjuvants. Vaccine. 1991;9(3):201–3. doi: 10.1016/0264-410x(91)90154-x. [DOI] [PubMed] [Google Scholar]
- 14.Iyer S, HogenEsch H, Hem SL. Effect of the degree of phosphate substitution in aluminum hydroxide adjuvant on the adsorption of phosphorylated proteins. Pharm Dev Technol. 2003;8(1):81–6. doi: 10.1081/pdt-120017526. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, HogenEsch H, Hem SL. Change in the degree of adsorption of proteins by aluminum-containing adjuvants following exposure to interstitial fluid: freshly prepared and aged model vaccines. Vaccine. 2001;20(1-2):80–5. doi: 10.1016/s0264-410x(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 16.Heimlich JM, Regnier FE, White JL, Hem SL. The in vitro displacement of adsorbed model antigens from aluminium-containing adjuvants by interstitial fluid. Vaccine. 1999;17:2873–2881. doi: 10.1016/s0264-410x(99)00126-7. [DOI] [PubMed] [Google Scholar]
- 17.Chang MF, Shi Y, Nail SL, HogenEsch H, Adams SB, White JL, et al. Degree of antigen adsorption in the vaccine or interstitial fluid and its effect on the antibody response in rabbits. Vaccine. 2001;19:2884–2889. doi: 10.1016/s0264-410x(00)00559-4. [DOI] [PubMed] [Google Scholar]
- 18.Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC, et al. In vivo absorption of aluminium-containing vaccine adjuvants using 26Al. Vaccine. 1997;15:1314–1318. doi: 10.1016/s0264-410x(97)00041-8. [DOI] [PubMed] [Google Scholar]
- 19.Iyera S, HogenEschb H, Hem SL. Relationship between the degree of antigen adsorption to aluminum hydroxide adjuvant in interstitial fluid and antibody production. Vaccine. 2003;21:1219–1223. doi: 10.1016/s0264-410x(02)00556-x. [DOI] [PubMed] [Google Scholar]
- 20.Seeber SJ, White JL, Hem SL. Solubilization of aluminum-containing adjuvants by constituents of interstitial fluid. J Parenter Sci Technol. 1991;45:156–160. [PubMed] [Google Scholar]
- 21.Aebig JA, Mullen GE, Dobrescu G, Rausch K, Miles AP, et al. Formulation of vaccines containing CpG oligonucleotides and alum. J Immunol Methods. 2007;323(2):139–46. doi: 10.1016/j.jim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapoza RJ, Horbett TA. Changes in the SDS elutability of fibrinogen adsorbed from plasma to polymers. J Biomater Sci Polym Ed. 1989;1(2):99–110. doi: 10.1163/156856289x00091. [DOI] [PubMed] [Google Scholar]
- 23.Rapoza RJ, Horbett TA. Postadsorptive transitions in fibrinogen: influence of polymer properties. J Biomed Mater Res. 1990;24(10):1263–87. doi: 10.1002/jbm.820241002. [DOI] [PubMed] [Google Scholar]
- 24.Bohnert JL, Horbett TA. Changes in adsorbed fibrinogen and albumin interactions with polymers indicated by decreases in detergent elutability. J Colloid Interface Sci. 1986;111:363–377. [Google Scholar]
- 25.Rapoza RJ, Horbett TA. The effects of concentration and adsorption time on the elutability of adsorbed proteins in surfactant solutions of varying structures and concentrations. J. Colloid Interface Sci. 1990;136:480–493. [Google Scholar]
- 26.Krisdhasima V, Vinaraphong P, McGuire J. Adsorption Kinetics and Elutability of α-Lactalbumin, β-Casein, β-Lactoglobulin, and Bovine Serum Albumin at Hydrophobic and Hydrophilic Interfaces. J Colloid Interface Sci. 1993;161:325–334. [Google Scholar]
- 27.Elwing H, Golander C. Protein and detergent interaction phenomena on solid surfaces with gradients in chemical composition. Adv Colloid Interface Sci. 1990;32:317–339. [Google Scholar]
- 28.Chinn J, Posso SE, Horbett TA, Ratner BD. Postadsorptive transitions in fibrinogen adsorbed to polyurethanes: changes in antibody binding and sodium dodecyl sulfate elutability. J Biomed Mater Res. 1992;26(6):757–78. doi: 10.1002/jbm.820260606. [DOI] [PubMed] [Google Scholar]
- 29.Rinella JV, Workman RF, Hermodson MA, White JL, Hem SL. Elutability of proteins from aluminium-containing vaccine adjuvants by treatment with surfactants. J Colloid Interface Sci. 1998;197:48–56. doi: 10.1006/jcis.1997.5230. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy MC, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–60. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu D, McClellan H, Dai W, Gebregeorgis E, Wu Y, et al. Long term stability of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Montanide® ISA 720 and stabilized with glycine. Vaccine. 2011;29(20):3640–5. doi: 10.1016/j.vaccine.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]