Abstract
CD8+ T cell synthesis of IFN-γ is an important component of the CD8+ T cell immune response. In short-term cultures of murine pan-T cells, we found that IL-4 was the principal cytokine responsible for driving IFN-γ synthesis by CD3/CD28-activated CD8+ T cells. IL-4 was able to induce low levels of IFN-γ mRNA in CD8+ T cells even in the absence of CD3/CD28 engagement, although concomitant CD3/CD28 stimulation was necessary for IFN-γ secretion. IL-4 induction of IFN-γ was explained by its ability to induce Eomesodermin and T-bet transcription factors whose expression was further increased by CD3/CD28. Expression of Eomesodermin, T-bet and IFN-γ induced by IL-4 was partially dependent upon activation of MAPK and PI3K but independent of the canonical IL-4-activated transcription factor, STAT6. In contrast, expression of IFN-γ induced by IL-4/CD3/CD28 stimulation showed additional dependency upon STAT6 which functions to increase expression of Eomesodermin specifically. These novel findings point to a function for IL-4 as a direct regulator of IFN-γ expression in CD8+ T cells and reveal the molecular mechanisms involved.
Keywords: Cytokines, CD8 T cell, Signal transduction, Knock out mice
1. Introduction
CD8+ T cells are the principal effector cells of the adaptive immune response directed toward intracellular pathogens. Initial activation of CD8+ T cells results from TCR and costimulatory receptor recognition of MHC class I-antigenic peptide complexes and costimulatory ligands respectively that are both displayed upon the surface of professional APC. Once activated, effector CD8+ T cells then target infected cells through TCR recognition of the same MHC class I-peptide complexes displayed on the target cell surface. Another important mechanism by which CD8+ T cells control intracellular infections is through synthesis of IFN-γ that has antiviral and macrophage-activating properties.
In many, although not all types of infection, activation of CD8+ T cells and development of memory CD8+ T cells is additionally dependent upon signals provided by CD4+ T cells or other immune cell types [1–5]. One important CD4+ T cell-derived factor that promotes CD8+ T cell activation is the IL-2 cytokine [6]. In addition, IL-4, classically studied as a regulator of CD4+ Th2 cell differentiation and B cell Ig class switching and differentiation [7, 8], has also been implicated as a helper factor for CD8+ T cell responses. In lymphochoriomeningitis virus and vaccinia virus infection models, mice that are double-deficient for IL-2 and IL-4 show a more severe impairment in an ability to generate specific CTL upon restimulation ex vivo compared to mice singly-deficient for IL-2 [9]. In addition, in an influenza virus infection model, mice deficient in the IL-4R alpha receptor showed reduced CTL responses ex vivo [10]. IL-4 has also been shown to regulate CD8+ T cell responses in parasitic infection models. In Plasmodium yoelii mouse malaria, Leishmania Donovani and Schistosoma mansoni infection models, generation of IFN-γ expressing CD8+ T cells following ex vivo restimulation was profoundly reduced in IL-4-deficient animals [11–13]. However, in each of these studies, it is unclear if IL-4 acts directly upon CD8+ T cells to regulate IFN-γ synthesis or cytotoxic activity or indirectly through action upon another cell type. Moreover, IL-4 has been shown to be required for the generation of memory CD8+ T cells which compared to naïve T cells synthesize much greater quantities of IFN-γ and cytotoxic effector molecules [14]. Therefore, an apparent role for IL-4 in the induction of IFN-γ and cytoxicity as revealed upon ex vivo stimulation could reflect a role for this cytokine in induction of memory cell formation in vivo rather than its ability to directly regulate these responses per se.
In the current studies we show that IL-4 acts as a direct regulator of CD8+ T cell function independent of its role as an inducer of CD8+ T cell memory. The mechanism by which IL-4 induces expression of IFN-γ in CD8+ T cells was investigated in detail. IL-4 induces expression of Eomesodermin (Eomes) and T-bet transcription factors that activate transcription of the ifng gene. Coupling of the IL-4R to both transcription factors in part depends upon IL-4-mediated activation of the intracellular signaling enzymes, MAPK and PI3K. In addition, in the presence of concomitant TCR and costimulatory receptor stimulation, an important role for IL-4-mediated STAT6 transcription factor activation in IFN-γ induction, specifically through induction of Eomes, was demonstrated.
2. Materials and methods
2.1. Mice
C57BL/6 × 129 Sv mice were bred in the animal facility at the University of Michigan Medical School. C57BL/6, IL-4-deficient and STAT6-deficient (both on a C57BL/6 background) and BALB/c mice were purchased from the Jackson Laboratory. All mice were 2–3 mo old at the time of experiments. All experiments were performed in compliance with University of Michigan guidelines and were approved by the University Committee on the Use and Care of Animals.
2.2. Isolation and stimulation of peripheral T cells
Pan-T cells or CD8+ T cell populations were prepared from pooled spleen and lymph node cell suspensions using MACS pan-T cell or CD8+ T cell negative selection kits (Miltenyi) respectively according to manufacturer’s instructions. For isolation of NKT cell-depleted pan-T cells and CD44lo CD8+ T cell populations, NK1.1 and CD44 mAb (eBioScience) were used in conjunction with pan-T cell and CD8+ T cell isolation kits respectively. Purity of negatively selected T cell populations was routinely determined by flow cytometric analysis.
T cells were stimulated in wells of 96 well U-bottomed plates in RPMI 1640 containing 10% FBS and antibiotics (2 × 105 cells/200 ml/well). For CD3/CD28 mAb stimulation, wells were pre-coated with CD3 mAb (1 μg/ml; eBioScience) and soluble CD28 mAb was included in the culture medium (1 μg/ml; eBioScience). Neutralizing anti-IL-2 and anti-IL-4 mAb (BD Pharmingen) were added to wells at 1 μg/ml. Recombinant murine IL-2 and IL-4 (R&D Systems) were added to wells at 100 and 10 ng/ml respectively. PD98059 and wortmannin inhibitors (Calbiochem) were added to wells at 50 and 1 nM respectively.
2.3. Flow cytometry
Cells were harvested from wells and analyzed for cell surface expression of CD4, CD8, CD44, CD49b (DX5) and intracellular expression of IFN-γ, Eomes and T-bet by flow cytometry using respective fluorochrome-labeled mAb (BD Pharmingen except CD49b, Eomes and T-bet mAb which were purchased from eBioScience). CD1d-α-GalCer tetramer used for detection of NKT cells was purchased from the NIH Tetramer Core Facility. For intracellular staining, harvested cells were treated with PMA and ionomycin (50 ng/ml and 1.5 μM respectively; Sigma Aldrich) for 5 h with addition of brefeldin A (1:1000 dilution of stock; BD Biosciences) for the last 4 h of culture. Cells were then surface stained prior to fixation and permeabilization and intracellular staining. Cellular data was collected on a FACSCanto flow cytometer equipped with FACSDiva software (BD Biosciences).
2.4. ELISA
Concentrations of IFN-γ in well supernatants were determined with the use of a Duo-set ELISA kit (R&D Systems). To assay Granzyme B secretion, stimulated T cells were harvested and restimulated in CD3 mAb coated wells as above. Granzyme B concentrations in well supernatants were then determined with the use of a Granzyme B Duo-set ELISA kit (R&D Systems).
2.5. Real time RT-PCR
RNA was isolated from stimulated CD8+ T cells using Trizol (Invitrogen) and reverse transcribed using SuperScript reverse transcriptase (Invitrogen). Real time PCR was performed using established IFN-γ and β-actin Taqman primer/probe sets in a 7500 Fast Lightcycler (Applied Biosystems). Fold change in IFN-γ mRNA expression relative to unstimulated CD8+ T cells was determined as described [15].
2.6. Statistical analysis
Statistical significance was determined using two-tailed Student’s two sample or paired sample t-tests as indicated. *P<0.05; **P<0.01; ***P<0.001.
3. Results
3.1. A major role for IL-4 in induction of IFN-γ expression in CD8+ T cells
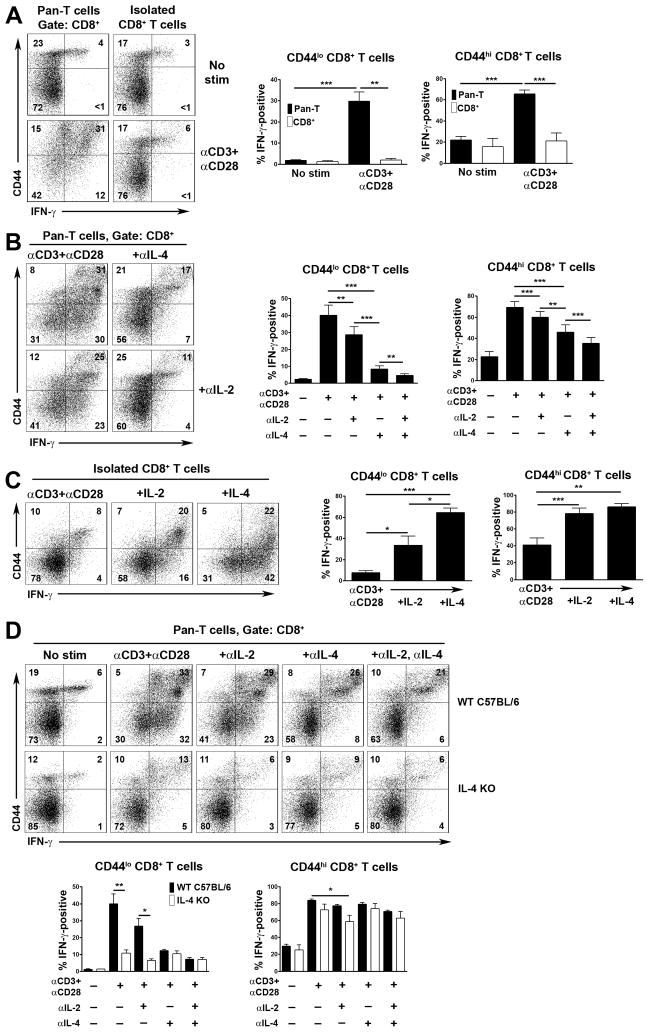
As shown by intracellular staining, stimulation of pan-T cells with CD3 mAb (directed to the TCR complex) and mAb against the CD28 costimulatory receptor for 48 h in vitro results in CD8+ T cell expression of IFN-γ (Fig. 1A). Substantial induction of IFN-γ is observed in both CD44lo naïve and CD44hi memory phenotype CD8+ T cells in these cultures. In contrast, purified CD8+ T cells synthesize little IFN-γ in response to the same stimulus, at least as detected by intracellular staining (Fig. 1A). Thus, other T cells provide help to CD8+ T cells in order for them to synthesize IFN-γ.
Fig. 1.
Role of IL-2 and IL-4 in induction of IFN-γ expression in CD8+ T cells. Experiments were performed with T cells from littermate C57BL/6 × 129 Sv (A–C) or C57BL/6 (D) mice. (A) Pan-T cells or purified CD8+ T cells were stimulated or not with CD3 and CD28 mAb. After 48 h, expression of IFN-γ upon CD44lo and CD44hi CD8+ T cells was determined by flow cytometry. At left are shown representative two color flow cytometry plots of CD44 versus IFN-γ staining. Numbers indicate percentage of CD8+ T cells in each quadrant. Bar charts at right show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells determined in repeat experiments (n=5 mice). (B) Pan-T cells were stimulated as in (A) in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 antibodies. Representative flow cytometry plots of CD44 versus IFN-γ staining are shown at left. Bar charts show mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells under each stimulation condition determined in repeat experiments (n=5 mice). (C) Purified CD8+ T cells were stimulated as in (A) in the presence or absence of recombinant IL-2 or IL-4. Representative flow cytometry plots of CD44 versus IFN-γ staining are shown at left. Bar graphs at right show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells under the indicated stimulation conditions determined in repeat experiments (n=4 mice). (D) Pan-T cells from C57BL/6 IL-4-deficient (IL-4 KO) and wild type control C57BL/6 mice were stimulated as in (A) in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 mAb. Expression of IFN-γ upon CD44lo and CD44hi CD8+ T cells was determined by flow cytometry after 48 h of culture. At top are shown representative flow cytometry plots of CD44 versus IFN-γ staining. Bar graphs show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells for each mouse genotype and condition of stimulation as determined in repeat experiments (n=5 mice each genotype). Statistical significance was determined using the paired Student’s t-test.
IL-2 is considered an important helper factor for CD8+ T cell responses [6]. To address this, we included a neutralizing anti-IL-2 antibody in pan-T cell cultures. However, blockade of IL-2 only partially inhibited the ability of CD8+ T cells to synthesize IFN-γ in pan-T cell cultures (Fig. 1B). Therefore, we examined the effect of blocking antibodies against other cytokines whose receptors share the same common gamma chain subunit with the IL-2R [16, 17]. Of these, antibodies against IL-7, IL-9, IL-15 and IL-21 had no effect upon CD8+ T cell IFN-γ synthesis (data not shown). In contrast, a neutralizing antibody against IL-4 profoundly blocked CD8+ T cell IFN-γ expression, particularly in CD44lo CD8+ T cells (Fig. 1B). Furthermore, combined blockade of IL-2 and IL-4 essentially abrogated the ability of both CD44lo and CD44hi CD8+ T cells to synthesize IFN-γ (Fig. 1B).
Based on these findings, we next examined if addition of IL-4 to cultures of purified CD8+ T cells could restore IFN-γ expression in response to CD3/CD28 mAb stimulation. In comparison with IL-2, IL-4 was found to be a potent inducer of IFN-γ expression in CD3/CD28 mAb-stimulated CD8+ T cells, particularly in the CD44lo subset (Fig. 1C).
To confirm a predominant role for IL-4 in induction of CD8+ T cell synthesis of IFN-γ, we examined IFN-γ expression in CD8+ T cells from IL-4-deficient mice. Consistent with results of antibody blocking studies, CD8+ T cells in CD3/CD28-stimulated pan-T cell cultures from IL-4-deficient mice synthesized reduced levels of IFN-γ in comparison to CD8+ T cells from C57BL/6 control mice (Fig. 1D). Again, the dependency upon IL-4 was most apparent in CD44lo CD8+ T cells. A major role for IL-4 in the induction of IFN-γ synthesis in CD44lo CD8+ T cells was also demonstrated in mouse strains other than C57BL/6 or 129 Sv, e.g. BALB/c (Supporting Fig. 1).
NKT cells are known to rapidly synthesize IL-4 in response to TCR engagement [18]. Therefore, since NKT cells would be present in pan-T cell cultures, we asked if they were required for CD8+ T cell synthesis of IFN-γ through provision of IL-4. For this purpose, we performed additional negative selection for the NK1.1 marker that is expressed upon the majority of mature NKT cells in the peripheral immune system of mice (Supporting Fig. 2) [19]. As shown by staining with CD1d-α-GalCer tetramers and antibodies against the DX5 marker that is also expressed by NKT cells [19], NK1.1 negative selection was only partially effective at depleting NKT cells in C57BL/6 × 129 Sv mice. Furthermore, NK1.1 depletion did not affect CD8+ T cell synthesis of IFN-γ in response to CD3/CD28 mAb stimulation in this strain. By contrast, the same NK1.1 negative selection procedure resulted in depletion of the vast majority of NKT cells in C57BL/6 mice, which could potentially be explained by differences in the level of expression of NK1.1 between the two strains. More importantly, in C57BL/6 mice, NKT cell depletion resulted in much reduced IFN-γ synthesis by CD44lo CD8+ T cells following stimulation with CD3/CD28 mAb. In addition, the residual IFN-γ response of these cells was not inhibited with an anti-IL-4 mAb. These results, therefore, indicate that NKT cells are the principal source of IL-4 that drives CD8+ T cell synthesis of IFN-γ at least in the CD44lo subset.
3.2. IL-4 induces the expression of intracellular IFN-γ in CD8+ T cells in the absence of TCR and costimulatory receptor engagement
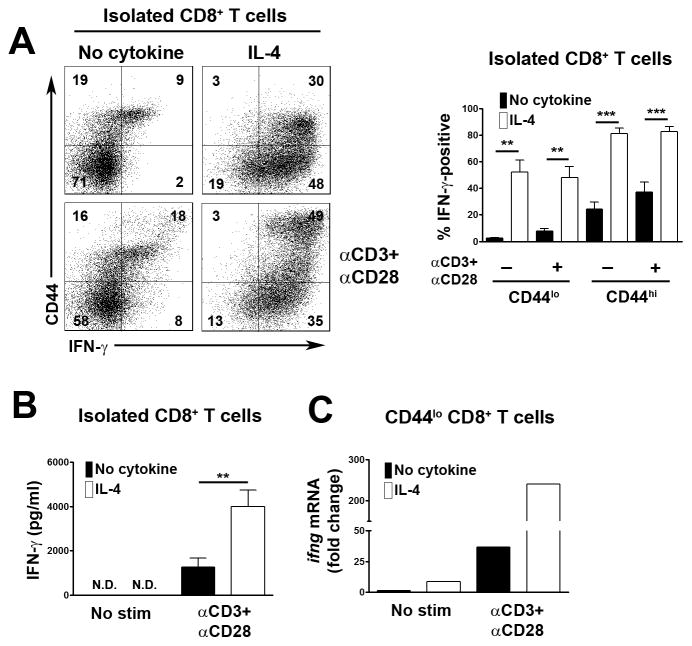
As a negative control in experiments, we examined the influence of IL-4 alone upon CD8+ T cell expression of IFN-γ. Surprisingly, as detected by intracellular staining, IL-4 alone induced similar levels of IFN-γ expression in CD44lo and CD44hi CD8+ T cells to that observed when T cells were stimulated with IL-4 plus CD3 and CD28 mAb (Fig. 2A). However, as determined by ELISA of well supernatants, none of the IFN-γ induced by IL-4 in the absence of CD3/CD28 engagement was secreted from cells but instead remained intracellular (Fig. 2B). Thus, CD3/CD28 engagement is necessary for the secretion of IL-4-induced IFN-γ in CD8+ T cells. With this knowledge, we considered that IL-4 plus CD3/CD28 engagement might in fact induce much higher levels of IFN-γ expression in CD8+ T cells compared to IL-4 stimulation alone. However, the difference would not be detected by intracellular staining since concurrent CD3/CD28 stimulation would result in continual release of de novo synthesized IFN-γ whereas in IL-4 alone stimulated CD8+ T cells the de novo synthesized IFN-γ would accumulate. To confirm this possibility, we examined IFN-γ mRNA levels in CD8+ T cells by real time RT-PCR. As predicted, stimulation of CD44lo CD8+ T cells with the combination of IL-4 plus CD3/CD28 mAb resulted in much higher levels of IFN-γ mRNA expression than stimulation with IL-4 alone (Fig. 2C). Thus, although IL-4 alone is able to induce IFN-γ expression in CD8+ T cells, IL-4 is in fact a relatively weak inducer of IFN-γ in the absence of concurrent CD3/CD28 stimulation. In addition, stimulation of CD8+ T cells with IL-4 alone does not result in IFN-γ secretion.
Fig. 2.
IL-4 induced synthesis of IFN-γ in CD8+ T cells with and without concurrent CD3/CD28 stimulation. All experiments were performed with T cells from C57BL/6 × 129 Sv mice. (A) Purified CD8+ T cells were stimulated or not with IL-4 and/or CD3 and CD28 mAb for 48 h. Expression of IFN-γ in CD44lo and CD44hi CD8+ T cells was determined by flow cytometry. At left are shown representative flow cytometric plots of CD44 vs IFN-γ staining. Bar graph at right shows the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells under different stimulation conditions as determined in repeat experiments (n=7 mice). (B) Purified CD8+ T cells were stimulated as in (A). Concentrations of IFN-γ in well supernatants were determined by ELISA. Shown is the mean concentration of IFN-γ + 1 SEM for each stimulation condition as determined repeat experiments (n=4 mice). N.D., not detectable. (C) Purified CD8+ CD44lo T cells were stimulated or not with IL-4 and/or CD3 and CD28 mAb for 6 h. Relative expression levels of IFN-γ mRNA were determined by real time RT-PCR. Results are expressed as fold change in IFN-γ expression compared to non-stimulated T cells. Similar results were obtained in a repeat experiment. In (A) and (B) statistical significance was determined using the paired Student’s t-test.
3.3. IL-4 promotes the cytotoxic function of CD8+ T cells but is not required for CD8+ T cell proliferation
We further asked if IL-4 was necessary for the acquisition of CD8+ T cell cytotoxic function in vitro. Efficiency of CTL priming was measured by expression of the lysosomal marker CD107a that is induced in CD8+ T cells during the acquisition of cytotoxic function [20]. In wild type mice, CD3/CD28 mAb stimulation of pan-T cells resulted in expression of CD107a in CD44hi CD8+ T cells that was blocked by approximately 50 percent by anti-IL-2 or anti-IL-4 mAb and completely by both types of mAb when included in priming cultures (Supporting Fig. 3A). Consistent with these results, in IL-4-deficient mice, induction of CD107a surface expression in CD8+ T cells was substantially reduced (Supporting Fig. 3A). IL-4 also induced expression of CD107a in purified CD44lo and CD44hi CD8+ T cells, albeit not to the levels observed with concurrent CD3 and CD28 stimulation (Supporting Fig. 3B). We also examined secretion of the cytotoxic effector molecule, Granzyme B, by ELISA. Blockade of either IL-2 or IL-4 during CD3/CD28 mAb-induced priming of wild type CD8+ T cells, or intrinsic IL-4 deficiency, was sufficient to almost completely block the synthesis and secretion of Granzyme B by CD8+ T cells (Supporting Fig. 3C).
We also asked if IL-4 played a role in the induction of CD8+ T cell proliferation in CD3/CD28-stimulated pan-T cell cultures by monitoring CD8+ T cell dilution of CFSE label by flow cytometry. In these experiments, blocking IL-2 and IL-4 mAb, when used either alone or together, did not affect the CD8+ T cell proliferative response (Supporting Fig. 4).
3.4. IL-4 induces the expression of Eomes and T-bet transcription factors in CD8+ T cells
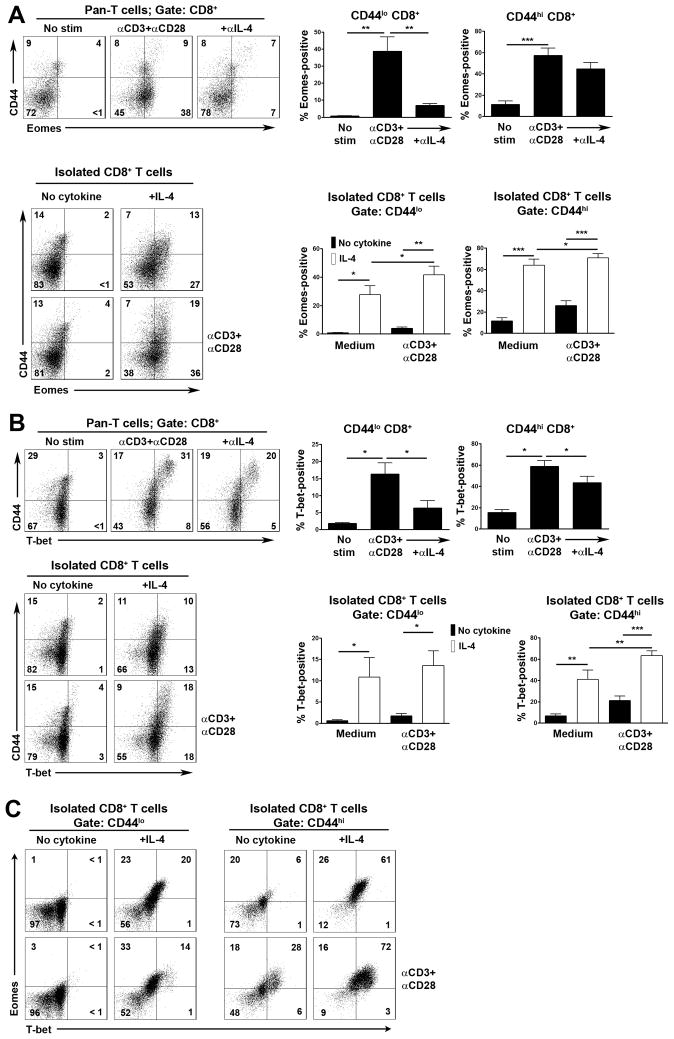
Expression of IFN-γ in CD8+ T cells is controlled by Eomes and T-bet transcription factors [21, 22]. Therefore, we asked if IL-4 induced the expression of either transcription factor in CD8+ T cells that would account for IFN-γ gene transcription. First, we examined if expression of Eomes and T-bet in CD8+ T cells in CD3/CD28 mAb-stimulated pan-T cell cultures was dependent upon IL-4. As shown, CD3/CD28 mAb stimulation of pan-T cells resulted in an induction of Eomes and T-bet expression in CD8+ T cells (Fig. 3A and B). In CD44lo T cells, stronger induction of Eomes compared to T-bet was noted, whereas in CD44hi subpopulations these transcription factors were induced to a similar degree. Significantly, expression of both of these transcription factors in both CD8+ T cell subpopulations was inhibited by a blocking anti-IL-4 mAb (Fig. 3A and B).
Fig. 3.
IL-4 induction of Eomes and T-bet transcription factors in CD8+ T cells. Pan-T cells or purified CD8+ T cells from C57BL/6 × 129 Sv mice were stimulated or not with CD3 and CD28 mAb in the presence or absence of a neutralizing IL-4 mAb or with or without IL-4 or with IL-4 alone as indicated. After 48 h, expression of CD44 and Eomes (A) or T-bet (B) upon CD8+ T cells was determined by flow cytometry. At left are shown representative flow cytometry plots of CD44 versus transcription factor staining. Bar graphs at right show the mean percentage of transcription factor positive cells + 1 SEM within CD44lo and CD44hi CD8+ T cell populations under the different stimulation conditions as determined in repeat experiments (A, n= at least 5 mice; B, n= at least 4 mice). (C) Shown are two-color flow cytometry plots of Eomes versus T-bet expression on CD44lo and CD44hi populations from A and B.
We next examined if IL-4 could induce expression of either transcription factor in purified CD8+ T cells. IL-4 alone induced expression of Eomes and T-bet in CD44lo and CD44hi CD8+ T cell subpopulations, although in CD44lo cells, Eomes was more strongly induced, consistent with findings with pan-T cells (Fig. 3A–C). Furthermore, the combination of IL-4 plus CD3/CD28 mAb resulted in increased expression of both transcription factors in CD8+ T cells (Eomes in CD44lo and CD44hi cells and T-bet in CD44hi cells only), consistent with increased levels of IFN-γ mRNA (Fig. 2C; Fig. 3A and B). We conclude that IL-4 drives IFN-γ expression in CD8+ T cells through induction of the expression of Eomes and T-bet.
3.5. STAT6 promotes IFN-γ expression in CD8+ T cells induced by the combination of IL-4 and CD3 plus CD28 mAb stimulation
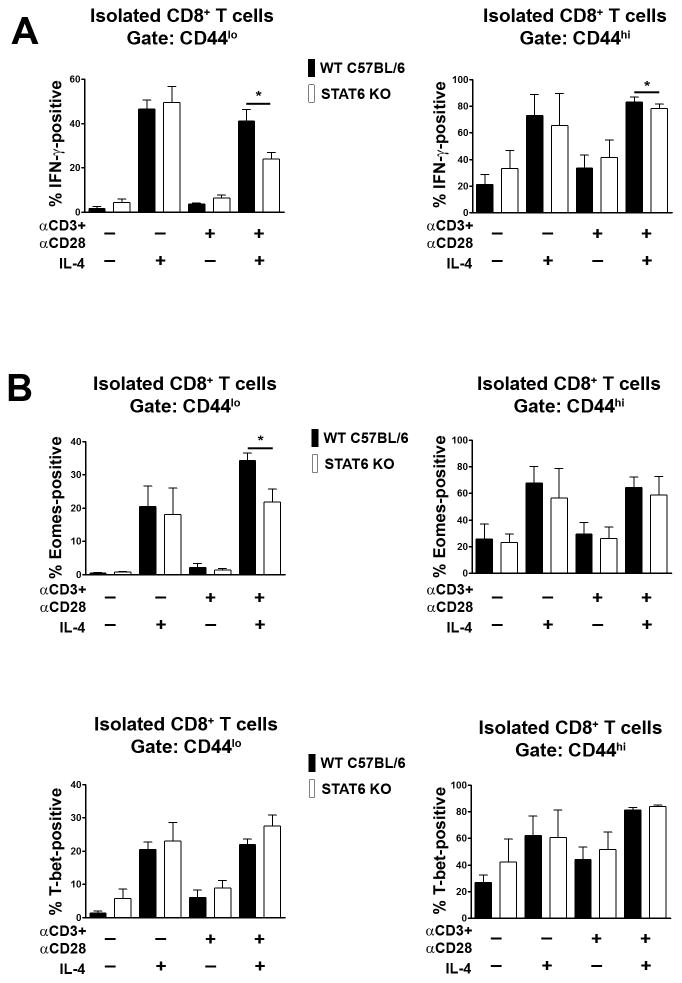
IL-4 signal transduction proceeds through the JAK-STAT pathway [23, 24]. Binding of IL-4 to the IL-4R results in activation of receptor-associated JAK protein tyrosine kinases, which phosphorylate the IL-4R leading to recruitment of the STAT6 transcription factor. JAK-mediated phosphorylation of STAT6 then promotes STAT6 dimerization and nuclear translocation and activation of gene transcription programs. Given the established role of STAT6 in the IL-4 signal transduction pathway, we examined if this transcription factor was necessary for IL-4 induced expression of IFN-γ. To this end, we examined the ability of IL-4 to induce expression of IFN-γ in purified CD8+ T cells from STAT6-deficient mice. Surprisingly, when stimulated with IL-4 alone, loss of STAT6 did not impact upon the ability of CD8+ T cells to synthesize IFN-γ (Fig. 4A). In contrast, loss of STAT6 did result in reduced levels of IFN-γ expression in CD44lo CD8+ and CD44hi CD8+ T cells following stimulation with the combination of IL-4 and CD3/CD28 mAb (Fig. 4A). Therefore, stimulation of CD8+ T cells with IL-4 plus CD3/CD28 mAb appears to license STAT6 involvement in a signaling pathway that is associated with much increased levels of IFN-γ gene transcription (Fig. 2C).
Fig. 4.
Role of STAT6 in IL-4-mediated induction of IFN-γ expression in CD8+ T cells. Purified CD8+ T cells from STAT6-deficient (STAT6 KO) or wild type C57BL/6 control mice were stimulated or not with CD3 and CD28 mAb and/or IL-4. After 48 h, expression of IFN-γ (A), Eomes and T-bet (B) within CD44lo and CD44hi CD8+ T cell populations was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ or transcription factor-positive cells + 1 SEM for each condition of stimulation and mouse strain as determined in repeat experiments (n= at least 3 mice each genotype). Statistical significance was determined using the paired Student’s t-test.
We further examined if STAT6 was necessary for IL-4 induced expression of Eomes and T-bet in CD8+ T cells. Loss of STAT6 did not affect expression of Eomes or T-bet in CD8+ T cells that were stimulated with IL-4 alone (Fig. 4B and C). However, as with IFN-γ induction, loss of STAT6 did result in reduced levels of expression of Eomes in CD44lo CD8+ T cells following stimulation with IL-4 plus CD3 and CD28 mAb (Fig. 4B). Expression of T-bet was not affected by loss of STAT6 in CD8+ T cells following stimulation with IL-4 plus CD3 and CD28 mAb (Fig. 4C). From these findings we conclude that STAT6 functions to increase IFN-γ expression in IL-4 plus CD3/CD28 mAb-stimulated CD8+ T cells primarily through increasing the expression of Eomes.
3.6. IL-4 induction of IFN-γ expression in CD8+ T cells is partially dependent upon activation of MAPK and PI3K
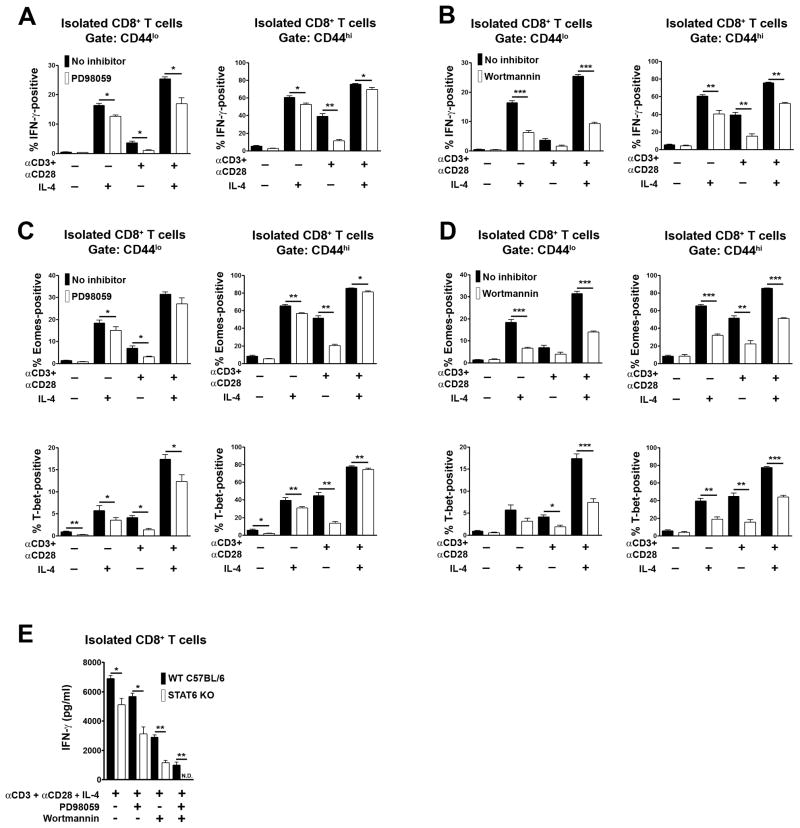
JAK-mediated phosphorylation of the IL-4R also leads to recruitment of the insulin receptor substrate adapter proteins that couple the IL-4R to activation of MAPK and PI3K [23–25]. Furthermore, MAPK and PI3K are activated upon TCR and CD28 engagement and are essential for a number of different types of T cell response [26, 27]. Therefore, we asked if MAPK and PI3K activation are necessary for IL-4-induced CD8+ T cell expression of IFN-γ. For this purpose, purified CD8+ T cells were stimulated with IL-4 and/or CD3 and CD28 mAb in the presence or absence of the MAPK inhibitor, PD98059, or the PI3K inhibitor, wortmannin. PD98059 and wortmannin both partially inhibited CD8+ T cell expression of IFN-γ induced by IL-4 alone and IL-4 plus CD3/CD28 mAb (Fig. 5A and B). Furthermore, this was associated with partial inhibition of expression of Eomes and T-bet for both types of induced response (Fig. 5C and D). Therefore, MAPK and PI3K promote IL-4 and IL-4 plus CD23/CD28-induced IFN-γ expression in CD8+ T cells by increasing the expression of Eomes and T-bet.
Fig. 5.
Roles of ERK and PI3K activation in IL-4-mediated induction of IFN-γ expression in CD8+ T cells. Purified CD8+ T cells from C57BL/6 mice were stimulated or not with CD3 and CD28 mAb and/or IL-4 in the presence or absence of the ERK inhibitor PD98059 (A, C) or the PI3K inhibitor wortmannin (B, D). After 48 h, expression of IFN-γ (A), Eomes (B) and T-bet (C) within CD44lo and CD44hi CD8+ T cell populations was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ or transcription factor-positive cells + 1 SEM for each condition of stimulation as determined in repeat experiments (n= at least 3 mice each genotype). Statistical significance was determined using the paired Student’s t-test. (E) Purified CD8+ T cells from STAT6-deficient or wild type C57BL/6 control mice were stimulated or not as in (A–D). After 48 h, the concentration of IFN-γ in well supernatants was determined by ELISA. Shown is the mean concentration of IFN-γ + 1 SEM for each condition of stimulation as determined in repeat experiments (n=3). Statistical significance was determined using the two sample Student’s t-test.
We additionally examined the effect of combined MAPK and PI3K inhibition and STAT6 loss upon IL-4 plus CD3/CD28-induced IFN-γ secretion in CD8+ T cells (Fig. 5E). Pair-wise combination of inhibitors and STAT6 loss resulted in further reduction of IFN-γ secretion compared to single inhibitors or STAT6 loss alone. Furthermore, combination of MAPK and PI3K inhibitors with STAT6 loss resulted in a complete blockade of the CD8+ T cell IFN-γ response.
4. Discussion
We show here that IL-4 acts as a potent stimulator of IFN-γ synthesis and cytotoxic activity in CD8+ T cells. These functions for IL-4 cannot be explained on the basis of any role for IL-4 in CD8+ T cell memory development or proliferation. Importantly, therefore, findings expand upon previous studies and show that IL-4 activates CD8+ T cell IFN-γ synthesis and cytotoxic effector function directly.
Regarding the mechanism by which IL-4 induces expression of IFN-γ in CD8+ T cells, interestingly, some induction of IFN-γ was observed when CD8+ T cells were stimulated with IL-4 alone in the absence of TCR and costimulatory receptor engagement. However, the IFN-γ synthesized upon IL-4 stimulation alone was not secreted from cells but was instead retained intracellularly. Therefore, IL-4 produced in the course of an immune response would not trigger IFN-γ secretion from bystander antigen non-specific CD8+ T cells unless their TCR and costimulatory receptors were also engaged. If IL-4 alone-induced IFN-γ synthesis in CD8+ T cells has a physiological purpose, one possibility is that it serves to amplify a TCR/CD28 plus IL-4 induced IFN-γ response of antigen specific CD8+ T cells.
The role of TCR/CD28 engagement upon CD8+ T cells in induction of IFN-γ secretion is not limited to an ability to stimulate release of IFN-γ. Thus, TCR and CD28 signals synergize with IL-4 signals to induce much higher levels of IFN-γ gene transcription. This is associated with increased expression of Eomes and T-bet transcription factors that are known activators of IFN-γ gene transcription in CD8+ T cells [22]. Eomes and T-bet also induce the expression of genes that mediate CTL lysis of target cells [22]. Therefore, an ability of IL-4 with and without TCR/CD28 engagement to induce both transcription factors in CD8+ T cells would also account for the role of this cytokine in the acquisition of cytotoxic effector function.
IL-4-alone and IL-4 plus TCR/CD28-induced CD8+ T cell IFN-γ synthesis was partially dependent upon activation of MAPK and PI3K. This could be accounted for on the basis that both signaling intermediates are necessary for full induction of Eomes and T-bet expression. Exactly how MAPK and PI3K promote Eomes and T-bet expression in CD8+ T cells is unknown at present. However, MAPK and PI3K participate in signaling pathways that culminate in the activation of transcription factors such as activator protein 1 and nuclear factor kappa-B that have the potential to activate transcription of the Eomes and T-bet genes directly.
STAT6 was not required for CD8+ T cell IFN-γ synthesis induced by IL-4 alone but did promote IFN-γ synthesis in response to IL-4 plus TCR/CD28 engagement. However, the magnitude and kinetics of STAT6 activation in CD8+ T cells as detected by phospho-STAT6 western blotting was comparable following IL-4 alone or IL-4 plus TCR/CD28 activation (data not shown). Therefore, participation of STAT6 specifically upon additional TCR/CD28 engagement cannot be explained by increased activation of this transcription factor. Instead, TCR/CD28 triggering must act to license STAT6 involvement in the IFN-γ response through an as yet undetermined mechanism. Whatever this mechanism, STAT6 appears to increase IFN-γ synthesis through increased expression of Eomes but not T-bet. Potentially, this specificity could be accounted for by an ability of STAT6 to activate transcription of the Eomes gene directly.
The discovery that IL-4 promotes the expression of Eomes in naive CD8+ T cells is exciting in light of recent findings that state the critical role of Eomes in the promotion and maintenance of memory CD8+ T cell populations. Eomes-deficient CD8+ T cells have been reported as defective in establishing central memory cell populations in vivo [21]. In addition, IL-4 is required for the development of a recently described population of innate CD8+ T cells in mice with a memory-like phenotype and constitutive expression of Eomes [28]. IL-4 has also been shown been shown to promote memory development in adaptive CD8+ T cells in vivo, as well as their survival and proliferation [14, 29, 30]. With regards to memory development, in the current studies we also found that blockade of IL-4 in CD3/28-mAb stimulated pan-T cell cultures or addition of IL-4 to CD3/CD28 mAb-stimulated purified CD8+ T cells inhibited or promoted the generation of CD44hi memory phenotype CD8+ T cells respectively (data not shown). Potentially, therefore, the role of IL-4 in CD8+ T cell IFN-γ expression might be explained by increased generation of memory phenotype CD8+ T cells and that memory CD8+ T cells intrinsically have an increased propensity to synthesize this cytokine in comparison with naïve CD8+ T cells. However, this possibility can be ruled out since IL-4 was shown to induce high levels of expression of IFN-γ in CD44lo naïve CD8+ T cells. In addition, IL-4 was generally shown to induce higher levels of IFN-γ expression in CD44hi memory CD8+ T cells compared to memory CD8+ T cells deprived of this cytokine.
In conclusion, we have identified IL-4 as a major direct inducer of IFN-γ expression in CD8+ T cells and have dissected the mechanisms involved. In concert with the TCR and costimulatory receptors, IL-4 induces expression of Eomes and T-bet and de novo transcription of the IFN-γ gene. Induction of both transcription factors is partially dependent upon activation of MAPK and PI3K enzymes in CD8+ T cells. However, only expression of Eomes is regulated by the STAT6 transcription factor. Altogether, these studies illuminate an important molecular mechanism involved in the control of CD8+ T cell immunity.
Supplementary Material
Pan-T cells from wild type C57BL/6 and BALB/c mice were stimulated or not with CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-4 mAb. After 48 h, expression of IFN-γ upon CD44lo and CD44hi CD8+ T cells was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells for each mouse genotype and condition of stimulation as determined in repeat experiments (n=5 mice each genotype). Statistical significance was determined using the paired Student’s t-test.
(A) Pan T cells were depleted or not of NKT cells by negative selection using an NK1.1 mAb. Shown are two-color plots of DX5 mAb (CD49b) and CD1d-α–GalCer tetramer staining from three mice of each genotype. (B) Pan-T cells or NKT cell-depleted pan-T cells from wild type C57BL/6 × 129 Sv mice or C57BL/6 mice (as in A) were stimulated with CD3 and CD28 mAb in the presence or absence of a neutralizing anti-IL-4 mAb. After 48 h, expression of IFN-γ upon CD44lo and CD44hi CD8+ T cell subsets was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells for each mouse genotype as determined in repeat experiments (n=5 mice each genotype). Statistical significance was determined using the two sample Student’s t-test.
(A) Pan-T cells from IL-4-deficient and wild type control C57BL/6 mice were stimulated or not with anti-CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 mAb. After 72 h, cells were restimulated in CD3 mAb coated (1 mg/well) wells (1 × 105 cells/well) in medium containing labeled CD107a mAb before staining for additional cell surface markers. Expression of cell surface CD107a upon CD8+ CD44lo and CD44hi T cell subsets was determined by flow cytometry. At left are shown representative flow cytometry plots of CD44 and CD107a staining. The bar graph at right shows the mean percentage of CD107a-positive cells + 1 SEM among CD8+ CD44hi cells under different conditions of stimulation in the two strains of mice as determined in repeat experiments (n=4 mice each genotype). (B) Purified CD8+ T cells from C57BL/6 mice were stimulated or not with CD3 and CD28 mAb and/or IL-4 as in (A) and expression of CD44 and CD107a was determined by flow cytometry. At left are shown representative flow cytometry plots of CD44 and CD107a staining. Bar graphs at right show the mean percentage of CD107a-positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells under different stimulation conditions as determined in repeat experiments (n=3 mice). (C) Pan-T cells from IL-4-deficient mice and wild type control C57BL/6 mice were stimulated as in (A). After 4 h restimulation, concentrations of Granzyme B in well supernatants were determined by ELISA. Shown is the mean concentration of Granzyme B +1 SEM for the different stimulation conditions and mouse genotypes as determined in repeat experiments (n=4 mice each genotype).
CFSE-labeled pan-T cells from C57BL/6 mice were stimulated or not with anti-CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 mAb. After 96 h, CFSE fluoresence of CD8+ T cells was determined by flow cytometry. At left are shown representative flow cytometry plots of CFSE fluoresence versus CD44 staining. The bar graph at right shows the mean percentage of live CD8+ T cells + 1 SEM that have undergone the indicated number of divisions under the different conditions of stimulation as determined in repeat experiments (n=4 mice). Statistical significance was determined using the paired Student’s t-test.
Highlights.
IL-4 induces CD8+ T cell IFN-γ expression via induction of T-bet and Eomes
IL-4 and TCR costimulation results in increased expression of T-bet, Eomes and IFN-γ
TCR costimulation is required for CD8+ T cell secretion of IFN-γ induced by IL4
IL-4 CD8+ T cell expression of IFN-γ is dependent upon MAPK and PI3K
IL-4 plus TCR induction of IFN-γ is also dependent upon STAT6 that augments Eomes
Acknowledgments
This work was supported by National Institutes of Health grants AI050699 to PDK.
Abbreviations
- Eomes
Eomesodermin
Footnotes
Disclosures
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–6. [PubMed] [Google Scholar]
- 2.Buller RM, Holmes KL, Hugin A, Frederickson TN, Morse HC., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–9. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- 3.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–71. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(−/−) mice. J Virol. 2000;74:9762–5. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 7.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 8.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann MF, Schorle H, Kuhn R, Muller W, Hengartner H, Zinkernagel RM, Horak I. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol. 1995;69:4842–6. doi: 10.1128/jvi.69.8.4842-4846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsland BJ, Schmitz N, Kopf M. IL-4Ralpha signaling is important for CD8+ T cell cytotoxicity in the absence of CD4+ T cell help. Eur J Immunol. 2005;35:1391–8. doi: 10.1002/eji.200425768. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–70. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 12.Pedras-Vasconcelos JA, Brunet LR, Pearce EJ. Profound effect of the absence of IL-4 on T cell responses during infection with Schistosoma mansoni. J Leukoc Biol. 2001;70:737–44. [PubMed] [Google Scholar]
- 13.Stager S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9:1287–92. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 14.Huang LR, Chen FL, Chen YT, Lin YM, Kung JT. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc Natl Acad Sci U S A. 2000;97:3406–11. doi: 10.1073/pnas.060026497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 16.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu Rev Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 19.Das R, Sant’Angelo DB, Nichols KE. Transcriptional control of invariant NKT cell development. Immunol Rev. 2010;238:195–215. doi: 10.1111/j.1600-065X.2010.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–11. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 23.Acacia de Sa Pinheiro A, Morrot A, Chakravarty S, Overstreet M, Bream JH, Irusta PM, Zavala F. IL-4 induces a wide-spectrum intracellular signaling cascade in CD8+ T cells. J Leukoc Biol. 2007;81:1102–10. doi: 10.1189/jlb.0906583. [DOI] [PubMed] [Google Scholar]
- 24.Wurster AL, Withers DJ, Uchida T, White MF, Grusby MJ. Stat6 and IRS-2 cooperate in interleukin 4 (IL-4)-induced proliferation and differentiation but are dispensable for IL-4-dependent rescue from apoptosis. Mol Cell Biol. 2002;22:117–26. doi: 10.1128/MCB.22.1.117-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang LM, Tai TY, Kahn RC, Wu HP, Lee SC, Lin BJ. Signal transduction pathways for interleukin 4 and insulin in human hepatoma cells. J Biochem. 1996;120:111–6. doi: 10.1093/oxfordjournals.jbchem.a021371. [DOI] [PubMed] [Google Scholar]
- 26.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 27.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–30. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 28.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–16. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris SC, Heidorn SM, Herbert DR, Perkins C, Hildeman DA, Khodoun MV, Finkelman FD. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J Immunol. 2009;182:1429–38. doi: 10.4049/jimmunol.182.3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202:551–60. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pan-T cells from wild type C57BL/6 and BALB/c mice were stimulated or not with CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-4 mAb. After 48 h, expression of IFN-γ upon CD44lo and CD44hi CD8+ T cells was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells for each mouse genotype and condition of stimulation as determined in repeat experiments (n=5 mice each genotype). Statistical significance was determined using the paired Student’s t-test.
(A) Pan T cells were depleted or not of NKT cells by negative selection using an NK1.1 mAb. Shown are two-color plots of DX5 mAb (CD49b) and CD1d-α–GalCer tetramer staining from three mice of each genotype. (B) Pan-T cells or NKT cell-depleted pan-T cells from wild type C57BL/6 × 129 Sv mice or C57BL/6 mice (as in A) were stimulated with CD3 and CD28 mAb in the presence or absence of a neutralizing anti-IL-4 mAb. After 48 h, expression of IFN-γ upon CD44lo and CD44hi CD8+ T cell subsets was determined by flow cytometry. Bar graphs show the mean percentage of IFN-γ positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells for each mouse genotype as determined in repeat experiments (n=5 mice each genotype). Statistical significance was determined using the two sample Student’s t-test.
(A) Pan-T cells from IL-4-deficient and wild type control C57BL/6 mice were stimulated or not with anti-CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 mAb. After 72 h, cells were restimulated in CD3 mAb coated (1 mg/well) wells (1 × 105 cells/well) in medium containing labeled CD107a mAb before staining for additional cell surface markers. Expression of cell surface CD107a upon CD8+ CD44lo and CD44hi T cell subsets was determined by flow cytometry. At left are shown representative flow cytometry plots of CD44 and CD107a staining. The bar graph at right shows the mean percentage of CD107a-positive cells + 1 SEM among CD8+ CD44hi cells under different conditions of stimulation in the two strains of mice as determined in repeat experiments (n=4 mice each genotype). (B) Purified CD8+ T cells from C57BL/6 mice were stimulated or not with CD3 and CD28 mAb and/or IL-4 as in (A) and expression of CD44 and CD107a was determined by flow cytometry. At left are shown representative flow cytometry plots of CD44 and CD107a staining. Bar graphs at right show the mean percentage of CD107a-positive cells + 1 SEM for CD44lo and CD44hi CD8+ T cells under different stimulation conditions as determined in repeat experiments (n=3 mice). (C) Pan-T cells from IL-4-deficient mice and wild type control C57BL/6 mice were stimulated as in (A). After 4 h restimulation, concentrations of Granzyme B in well supernatants were determined by ELISA. Shown is the mean concentration of Granzyme B +1 SEM for the different stimulation conditions and mouse genotypes as determined in repeat experiments (n=4 mice each genotype).
CFSE-labeled pan-T cells from C57BL/6 mice were stimulated or not with anti-CD3 and CD28 mAb in the presence or absence of neutralizing anti-IL-2 and/or anti-IL-4 mAb. After 96 h, CFSE fluoresence of CD8+ T cells was determined by flow cytometry. At left are shown representative flow cytometry plots of CFSE fluoresence versus CD44 staining. The bar graph at right shows the mean percentage of live CD8+ T cells + 1 SEM that have undergone the indicated number of divisions under the different conditions of stimulation as determined in repeat experiments (n=4 mice). Statistical significance was determined using the paired Student’s t-test.