Abstract
Objective
To conduct a pilot study for feasibility of planning a definitive clinical trial comparing traditional acupuncture (TA) to sham acupuncture (SA) and waiting control (WC) on menopause related vasomotor symptoms (VMS), quality of life (QOL), and the hypothalamic-pituitary-adrenal (HPA) axis in peri and post-menopausal women.
Methods
Thirty-three peri and post-menopausal women with at least 7 VMS daily were randomized to TA, SA or WC. The TA and SA groups were given three treatments per week for 12 weeks. Outcomes included the number and severity of VMS, MENQOL questionnaire, Beck Depression Inventory, Spielberg State-Trait Anxiety Instrument, Pittsburgh Quality Sleep Index, 24 hour urine cortisol and metabolites, and ACTH stimulation testing.
Results
Both TA and SA groups demonstrated improved VMS trends compared to WC (Δ −3.5±3.00 vs. −4.1±3.79 vs. −1.2±2.4, respectively, p=.20), and significantly improved MENQOL vasomotor scores (Δ − 1.5±2.02 vs. −1.8±1.52 vs. 0.3±0.64, respectively, p=.04). There were no psychosocial group differences. Exit 24-hour urinary measures were lower in the TA vs the SA or WC in total cortisol metabolites (4,658.9±1,670.9 vs 7,735.8±3,747.9 vs 5,166.0±2,234.5, p=0.03, respectively) and DHEA (41.4±27.46, 161.2±222.77, 252.4±385.40, respectively, p=0.05). The ACTH stimulation cortisol response data also trended in the hypothesized direction (p=0.17).
Conclusion
Both TA and SA reduce VMS frequency and severity and improve VMS-related quality of life compared to WC; however, TA alone may impact the HPA axis. This association is viewed as preliminary and hypothesis-generating and should be explored in a large clinical trial.
Keywords: Menopause, VMS, acupuncture, cortisol, mechanisms, randomized control trial
INTRODUCTION
Vasomotor symptoms (VMS) are experienced by 68-82% of women transitioning through menopause and is the primary reason that women use hormone therapy (HT)1-2. Although HT is effective for the reduction of VMS, concern regarding the findings of the Women’s Health Initiative (WHI)3-9 specific to the greater risks compared to health benefits of HT has fueled interest in alternative non-hormonal treatments. Other pharmaceutical agents such as venoflaximen and gabapentine have been used with moderate success but can also produce undesirable side-effects10, and longer term safety is relatively unknown. A non-pharmacologic intervention that can be used to reduce VMS with little to no side-effects is acupuncture. Prior study of acupuncture on VMS, has suggested that acupuncture is no better than placebo, however these studies have been limited by relatively small sample sizes, relatively short duration, and absent or inadequate controls. Furthermore, there has been limited investigation of the impact of acupuncture on mechanistic pathways of VMS.
Dysfunction of the central thermoregulatory center associated with declining ovarian function is the leading hypothesized etiology of VMS11. An understanding of the hypothalamic-pituitary-ovarian (HPO) axis provides a framework from which to investigate additional/alternative hypothesized mechanistic pathways of VMS. It is known that the hypothalamic-pituitary-adrenal (HPA) axis under stressful conditions can negatively impact the HPO axis12-13, however there is limited knowledge regarding the HPA axis, specifically cortisol (F) production, and VMS. Recent data suggests that women with severe VMS have increased urinary F secretion (>10 ng/mg creatinine) during the late menopausal transition stage compared to women with less severe VMS, despite the fact that the groups did not differ in terms of age, body mass index, follicle stimulating hormone (FSH) or estrone glucuronide levels, health practices, exercise, mood, sleep, cognition, or stress.14
Prior study suggests that acupuncture reduces hot flashes better than usual care alone, and as well as anti-depressant medication, but not significantly better than sham acupuncture, suggestive of a placebo effect15-18. Other study suggests that acupuncture may decrease VMS relatively longer term compared to other interventions17, 19-22 suggesting a mechanistic pathway more complex than a psychological placebo pathway. We conducted a pilot study for feasibility, outcome determination, and sample size estimation purposes in order to plan a definitive clinical trial comparing traditional acupuncture (TA) to sham acupuncture (SA) and waiting control (WC) on vasomotor symptoms (VMS), menopause-related quality of life (QOL), and the potential VMS mechanistic pathway of the hypothalamic-pituitary-adrenal (HPA) axis in peri and postmenopausal women.
METHODS
The study was approved by the Cedars-Sinai Institutional Review Board.
Study overview
We designed a randomized, single blind, placebo controlled trial of equal randomization into TA, SA, or WC. The intervention period was three months (12 weeks), with the TA and SA participants receiving treatment three times per week for a maximum total of 36 treatments. Data and specimen collections was carried out at week 0 (entry), week 5 (TA and SA: hot flash diary only) and week 12 (exit).
Participants
We recruited 33 women through mailing advertisements to women within a 5-mile radius as well as advertisements placed on the Cedars-Sinai Medical Center intranet. We recruited women age over 40 years with menopause-related VMS. Eligibility criteria included at least 7 hot flashes per day, and at least one missed menstrual cycle or spontaneous or medically-induced menopause. Exclusion criteria included concomitant illness with reasonable likelihood of limiting survival to less than one year, current substance abuse, known, suspected or planned pregnancy in next year, other concomitant menopause treatment, participating in acupuncture treatment or formal psychological stress management program within the last year, participating in another treatment for VMS, unless willing to stop it 4 weeks in advance of participation, HIV infection, chronic or active hepatitis or other blood-borne illness.
Randomization
To eliminate bias in treatment assignment, participants were allocated to one of three study arms with equal probability using a randomized block design after signing the consent form. Appropriate statistical analyses that took the blocking into account were employed.
Blinding
The TA and SA participants were blinded as to randomization status however the WC group necessarily knew their randomization status. The treating acupuncturists were unblinded, in order to know whether to deliver TA or SA. Both TA and SA groups were required to wear eye covers throughout the treatment. We have previously validated our SA technique and delivery protocol23.
Intervention
Participants in the TA and SA groups alternated between two groups of points. The TA group included eleven “front” points (DU 20, PC 6, HT 7, LIV 3, LI 4, LI 11, KD 3, SP 6, ST 36, REN 17, REN 6,) which were placed with the patient lying supine and seven “back” points (DU 14, UB 15, UB 18, UB 20, UB 23, GB 34 and KD 3) which were accessed in the prone position. The TA needles were inserted 0.5 to 1.5 inches and then manually stimulated to reach “de-qi” and then retained for 30 minutes. The SA points were selected by our team to be proximate to the TA site (to enhance the blind), but not considered active (Figure 1). In the SA group, the disposable acupuncture needle and plastic needle tube were placed on the sham points, manipulated without skin penetration and secured with adhesive tape. The TA group had similar use of adhesive tape holding the plastic tubing in place. The WC group received no treatment for 3 months, underwent exit testing and subsequently had the option of 1 month (12 sessions) of complimentary TA.
Figure 1. Protocol Traditional Acupuncture (TA) and Sham Acupuncture (SA) Points.
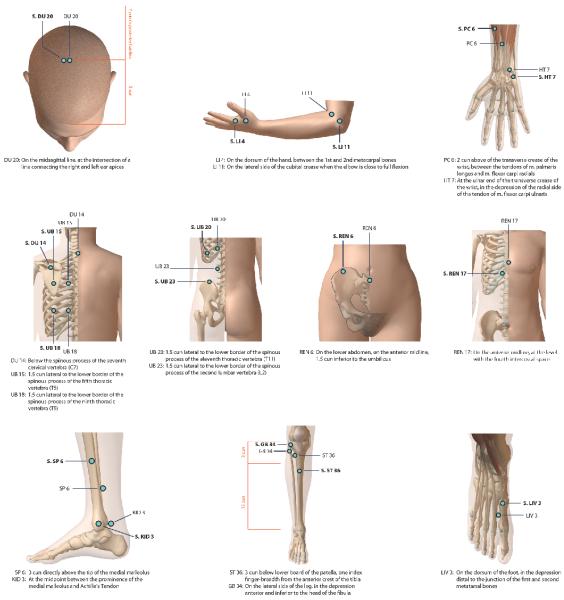
Diagram illustrating Traditional and Sham acupuncture points used during the treatments. SA sites are signified by S prior to site code. Front and back points were used on alternate days. DU=Governing meridian, GB=Gallbladder meridian, HT=Heart meridian, KID=Kidney meridian, LI=Large Intestine meridian, LIV=Liver meridian, PC=Pericardium meridian, Ren=Ren meridian, SP=Spleen meridian, ST=Stomach meridian, UB=Urinary Bladder meridian.
Outcome Measures
The 7-day hot flash diary was filled out at week 0 (entry) before starting treatment, week 5 and week 12 (exit). Participants recorded the number of mild, moderate, severe and very severe VMS for 7 consecutive days. The VMS frequency was an average number of hot flashes recorded per day for 7 days. The severity score was computed as the average severity-weighted number of VMS per day. The MENQOL questionnaire was also collected as a condition-specific instrument that measures the impact of menopause symptoms on quality of life (QOL)24. The MENQOL questionnaire provided a summary score along with 4 domain scores, vasomotor, psychological, physical, and sexual, and was collected to study entry and exit.
Additional psychosocial measures included: 1) the Pittsburgh Sleep Quality Index (PSI)25 which is a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month time interval. Nineteen individual items generate seven component scores: subjective sleep, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medications and daytime dysfunction. The sum of component scores yields one global score between 0 and 21, with higher scores indicating worse sleep quality; 2) the Beck Depression Inventory II (BDI)26 which is a 21 item questionnaire used to assess the intensity of depression in clinical and normal patients. Each item is a list of four statements arranged in increasing severity about a particular symptom of depression; 3) the State-Trait Anxiety Inventory (STAI)27 which is the definitive instrument for measuring anxiety in adults. It differentiates between the temporary condition of state anxiety and the more general and long-standing quality of trait anxiety.
To evaluate the HPA axis and specifically F production and metabolism, 24-hour urine samples were collected and an adrenocorticotropic hormone (ACTH) stimulation test was performed. Twenty-four hour urine samples were assayed for urinary free F and free cortisone, measured by a dichloromethane extraction RIA with inter-assay CVs of less than 16% over the range of 153–798 nmol/liter. In addition, a urinary steroid metabolite profile was obtained using gas chromatography/mass spectrometry, as previously reported28. These included the F metabolites THF, 5α-THF, THE, cortols and cortolones; and the adrenal androgen metabolites etiocholanolone and androsterone. The sum of the concentrations of the principal cortisol metabolites (tetrahydrocortisone [THE], tetrahydrocortisol [THF], 5α-THF, cortolones, and cortols), along with the concentrations of free F and free E, were used as an assessment of the total daily cortisol production, as previously validated29. Finally, the production of adrenal androgens was estimated from the content of etiocholanolone + androsterone.
Participants underwent the ACTH stimulation test on the morning of completing the 24-hour urine collection. Because of the effect of the circadian rhythm on adrenocortical function all stimulations started between 7:00 and 9:00 am. After a 30 minute rest period following the placement of an IV angiocath and heparin lock, three blood samples were drawn at −30 min, −15 min and 0 min, and pooled to form the baseline sample (0 min). Following, 0.25 mg (1 vial) of ACTH-1-24 (Cortrosyn®, Organon Co., New Orange, NJ) was injected IV over 60 seconds and a final blood sample obtained 60 minutes later. Samples were assayed for F, DHEA, androstenedione (A4). During the initial blood draw at −30 min, two other samples were drawn for the measurement of follicle stimulating hormone (FSH), estradiol (E2), and estrone (E1). Samples were frozen at −70°C. until assayed.
Statistical Analysis
Data are presented in tables as means and SD or SE for all continuous variables. Analyses were performed by applying non-parametric statistics. Comparing the demographic and symptom variables at baseline, we employed the Kruskal-Wallis test. Kruskal-Wallis test was applied for comparing the median in the three groups or the Wilcoxon rank sum test for comparing two related groups. All tests of hypotheses were two-sided with Type I error rate of 0.05. A p < 0.05 was considered statistically significant. All statistical analyses were done using SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Participants
Of the 60 participants enrolled, 27 dropped out and 33 completed the study. The TA and SA group both had 8 drop outs with all but two participants occurring after consenting but before treatment began because the participants felt they could not commit to the 3 month treatment protocol. The majority of the 11 WC participants dropped due to being randomized to a no treatment group despite being offered free acupuncture after the three month waiting period. Compliance to the 36 scheduled treatments in the TA and SA groups was 29 (80%) and 30 (83%) respectively. Demographic and clinical variables at entry are shown in Table 1. No significant entry differences existed between groups.
Table 1.
Study Population Demographic and Symptoms (Entry)
| TA (n=12) | SA (n=12) | WC (n=9) | P value* | |
|---|---|---|---|---|
| Hot flash diary | ||||
| VMS frequency | 8.3 ± 4.4 | 9.0 ± 3.8 | 9.9 ± 4.6 | 0.48 |
| VMS severity | 14.9 ± 9.1 | 15.2 ± 8.4 | 16.7 ± 8.1 | 0.76 |
| Anxiety | 36.1 ± 11.2 | 36.0 ± 10.4 | 35.6 ± 11.5 | 0.94 |
| Depression | 9.7 ± 8.7 | 11.5 ± 10.2 | 8.1 ± 5.8 | 0.89 |
| Pitts sleep | 8.5 ± 3.6 | 7.4 ± 3.9 | 9.4 ± 5.0 | 0.58 |
| MENQOL | ||||
| Vasomotor | 5.4 ± 1.4 | 6.1 ± 1.0 | 5.4 ± 1.6 | 0.22 |
| Psychosocial | 2.8 ± 1.6 | 3.5 ± 1.8 | 3.2 ± 1.8 | 0.68 |
| Physical | 3.4 ± 1.3 | 3.7 ± 1.3 | 3.9 ± 1.1 | 0.58 |
| Sexual | 3.8 ± 2.1 | 3.5 ± 2.6 | 3.3 ± 1.8 | 0.89 |
| Overall | 3.8 ± 1.1 | 4.2 ± 1.1 | 3.9 ± 1.4 | 0.73 |
| Age, mean ± SD | 57.2 ± 5.2 | 56.8 ± 6.5 | 54.9 ± 6.4 | 0.43 |
| BMI, mean ± SD | 26.9 ± 3.6 | 31.4 ± 4.5 | 31.2 ± 9.8 | 0.13 |
|
Alcohol intake (drinks/week),
mean ± SD |
2.1 ± 4.5 | 3.6 ± 3.8 | 2.3 ± 2.5 | 0.15 |
|
Time from menopause
(years), mean ± SD |
6.1 ± 4.5 | 8.4 ± 5.5 | 5.1 ± 9.9 | 0.20 |
All data presented as mean ± SD (standard deviation)
BMI-body mass index
MENQOL=menopause quality of life
VMS=vasomotor symptoms
P value is from Kruskal-Wallis test
7-Day Hot Flash Diary and MENQOL Results
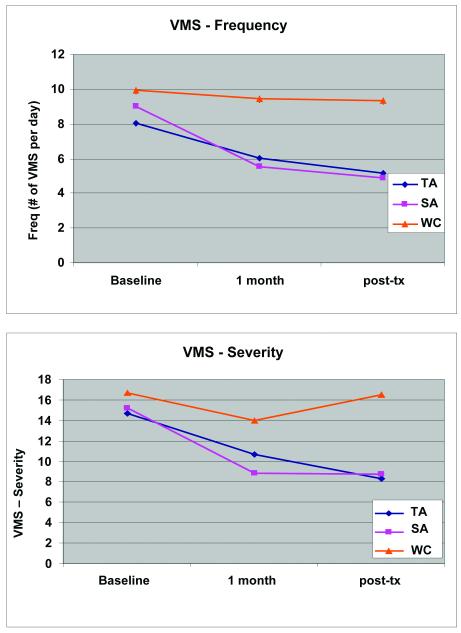
There was no significant difference in reduction of VMS frequency and severity between the TA and SA groups (exit-entry), however both groups improved compared to the WC group (Figure 2). The majority of the reduction of VMS frequency (≥ 86%) and severity (≥78%) occurred by week five of treatment, however, the SA severity scores remained static after the initial reduction while the TA scores continued to reduce throughout the three month period. The MENQOL results demonstrated a significant improvement in the vasomotor domain, and a trend towards improvement in the overall MENQOL in the TA and SA compared to the WC group (Table 2).
Figure 2. 7-Day Hot Flash Diary Results (mean ±SE).
Graph representing the effect of TA and SA used three times per week for 30 minutes for 12 weeks compared to WC on VMS frequency (p=.24) and severity (p=.20). Comparisons made at baseline, week 5 and week 12 (exit) compared to WC. Numbers represent means ± SE.
Table 2.
MENQOL Results (Exit - Entry)
| TA (n=12) | SA (n=12) | WC (n=9) | P value* | P value** | |
|---|---|---|---|---|---|
| MENQOL | |||||
| Vasomotor | −1.5 ± 2.1 | −1.8 ± 1.5 | 0.3 ± 0.6 | 0.04 | 0.01 |
| Psychosocial | −0.5 ± 1.4 | −0.9 ± 1.7 | 1.0 ± 1.6 | 0.16 | 0.07 |
| Physical | −0.5 ± 1.6 | −1.1 ± 1.4 | 0.3 ± 0.9 | 0.17 | 0.12 |
| Sexual | −0.3 ± 2.1 | −0.3 ± 2.2 | 0.3 ± 0.9 | 0.72 | 0.44 |
| Overall | −0.7 ± 1.3 | −1.0 ± 1.2 | 0.4 ± 0.7 | 0.07 | 0.03 |
All data is presented as mean ± SD
Abbreviations as previous
p value is from Kruskal-Wallis test for TA vs. SA vs. WC
p value from Wilcoxon-rank sum test for TA + SA vs. WC
Psychosocial Results
There were no group differences between entry and exit in the Pittsburgh Sleep Quality Index (PSQI), Beck Depression Inventory (BDI) and the State-Trait Anxiety Inventory (STAI). As seen in Table 3, there are trends for VMS improvement among the subgroup where anxiety, depression and sleep improved in both the TA and SA groups. This relationship became significant when the TA and SA groups were combined compared to WC.
Table 3.
VMS Change in the Subgroups With Improved Clinical Psychosocial and Sleep Variables (Exit-Entry)
| TA | SA | WC | P value* | P value** | |
|---|---|---|---|---|---|
| Anxiety ≤0† (n=33) | |||||
| VMS frequency | −3.4 ± 3.1 | −4.9 ± 3.8 | −1.5 ± 2.3 | 0.20 | 0.11 |
| VMS severity | −7.0 ± 7.5 | −10.4 ± 5.9 | −1.5 ± 3.4 | 0.06 | 0.03 |
| Depression ≤0† (n=33) | |||||
| VMS frequency | −3.5 ± 3.0 | −5.1 ± 3.3 | −1.2 ± 2.1 | 0.09 | 0.055 |
| VMS severity | −7.1 ± 7.2 | −8.7 ± 7.5 | −1.0 ± 3.3 | 0.07 | 0.02 |
| Pitts sleep ≤0† (n=33) | |||||
| VMS frequency | −5.5 ± 3.5 | −4.6 ± 2.3 | −1.3 ± 2.6 | 0.15 | 0.06 |
| VMS severity | −9.1 ± 8.1 | −9.1 ± 6.7 | −1.4 ± 3.9 | 0.14 | 0.05 |
All data is presented as mean ± SD
Abbreviations as previous
p value from Kruskal-Wallis test for TA vs. SA vs. WC
p value from Wilcoxon-rank sum test for TA + SA vs. WC
Scores ≤0 indicate improvement in variable (Exit-Entry)
HPA Results
There was no correlation between 24 hour urinary F and metabolites and frequency or severity of VMS at entry in cross-sectional analysis of all women. Exit 24-hour urinary cortisol measures demonstrated results in the hypothesized direction, where the TA group had the lowest levels of DHEA, cortisol metabolites and adrenal androgens (etiocholanolone + androsterone) (Table 4). Additionally, we observed a correlation between improved urinary F levels (exit-entry) and improved VMS frequency (exit-entry) in the TA group (r=0.62, P=0.043) compared to no correlations in the SA (r=0.18, P=0.586) and WC (r=0.8, P=0.20). The ACTH stimulation testing results were also in a hypothesized direction, however these differences appeared to be confounded by baseline differences (Table 5).
Table 4.
24 hour Urinary Cortisol and Metabolites
| TA (n=12) | SA (n=12) | WC (n=9) | P valuea | |
|---|---|---|---|---|
| Study Entry | ||||
| DHEA | 47.7 ± 38.7 | 104.0 ± 127.1 | 265.7 ± 551.8 | 0.41 |
| F (Cortisol) | 40.8 ± 17.4 | 50.4 ± 32.5 | 39.7 ± 16.0 | 0.90 |
| Total F metabolites | 4,907.3 ± 2128.1 | 6,923.5 ± 3687.1 | 6,507.4 ± 4952.9 | 0.35 |
| Adrenal androgens | 972.5 ± 626.0 | 1,543.7 ± 1,508.4 | 1,524.4 ± 1,120.1 | 0.41 |
| Study Exit | ||||
| DHEA | 41.4 ± 27.4 | 161.2 ± 222.7 | 252.4 ± 385.4 | 0.04 |
| F (Cortisol) | 37.3 ± 16.7 | 66.2 ± 56.3 | 30.4 ± 11.3 | 0.05 |
| Total F metabolites | 4,658.9 ± 1,670.9 | 7,735.8 ± 3,747.9 | 5,166.0 ± 2,234.5 | 0.03 |
| Adrenal androgens | 888.1 ± 614.6 | 1,506.1 ± 766.6 | 1,219.0 ± 684.3 | 0.09 |
All data is presented as mean ± SD
DHEA= Dehydroepiandrosterone sulfate
Adrenal Androgens = etiocholanolone + androsterone
P value for Kruskal-Wallis test
Table 5.
ACTH Stimulation Test Measures*
| TA (n=7) | SA (n=10) | WC (n=9) | P value** | |
|---|---|---|---|---|
| Study entry | ||||
| Cortisol (mcg/dl) | 16.9 ± 2.1 | 24.2 ± 5.2 | 20.4 ± 2.7 | 0.01 |
| Androstenedione (ng/mL) | 0.4 ± 0.1 | 0.6 ± 0.5 | 0.9 ± 0.6 | 0.43 |
| DHEA (ng/mL) | 3.0 ± 2.8 | 4.2 ± 3.2 | 6.0 ± 4.5 | 0.42 |
| Study exit | ||||
| Cortisol (mcg/dl) | 21.1 ± 3.8 | 23.0 ± 3.7 | 19.8 ± 2.5 | 0.17 |
| Androstenedione (ng/mL) | 0.4 ± 0.07 | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.67 |
| DHEA (ng/mL) | 3.1 ± 2.1 | 3.8 ± 1.5 | 2.4 ± 2.8 | 0.43 |
All data shown as mean ± SD
Abbreviations as previous, ACTH= Adrenocorticotropic hormone
60 minutes post-ACTH stimulation
P value is for Kruskal-Wallis test
In our pilot study, mean MENQOL vasomotor domain core was 5.68 with a standard deviation 1.3 among all study participants. With a sample size of 72 patients in each group, we would have adequate power (more than 95%) to detect a minimum 15% difference between SA (or TA) and WC groups at the significant level of 0.025. For the 24-hour urinary measures, our observed baseline mean of 24-hour F level of 45.2 ug/dL (SD 16.42 ug/dL), in order to detect a clinically relevant 40% reduction in F in women treated with TA (exit level=27.1) and 15% in SA group (exit level=38.4), a sample size of 72 women per group would provide a 96% power with a α-value of 0.025, for detecting the estimated differences between TA and SA groups.
DISCUSSION
Our pilot study results demonstrate the feasibility of an appropriately controlled and adequate duration acupuncture intervention trial to more definitively test the impact of TA on VMS. Both the TA and SA interventions improved subjective self-report VMS and MENQOL compared to WC, yet the urinary F and metabolites data reflective of the HPA axis suggests that TA alone may impact this putative mechanistic pathway. These pilot results further suggest correlations between improved depression, anxiety and sleep scores with improved VMS, supporting further study into the psychological and physiological role of stress in VMS mechanistic pathways and treatment. Determination of clinically relevant differences between TA and SA and WC are important – if TA is not superior to SA, this suggests that TA operates predominantly via a placebo effect and that a simplified approach to acupuncture therapy could be undertaken, reducing expense and need for qualified acupuncturists. If SA is not superior to WC, this would suggest that SA does not provide an effect via psychosocial stress reduction, and that alternative mechanistic pathways should be explored.
Our results additionally support further testing of the impact of TA on the HPA axis as a mechanistic and therapeutic pathway. From a physiologic perspective, TA appears to have an impact on F, F metabolites and DHEA levels when compared to SA as well as correlate to a reduction in VMS. These results are novel and intriguing; should the HPA axis be identified as a mechanistic pathway and treatment target for VMS, a variety of new treatment options should be further explored, including selective serotonin inhibitors (SSRIs), specifically scitalopram which has been demonstrated to reduce cortisol levels in older adults with generalized anxiety disorder30. Additional pharmacological manipulation of F pathways include octreotide, which is a long-acting analogue of somatostatin that rapidly reduces ectopic ACTH secretion, as well as the adrenal enzyme inhibitors, metyrapone or ketoconazole which reduce cortisol secretion. The current study results also suggest that 24 hour urinary F and F metabolites should be measured as an outcome to reflect the HPA axis as a mechanistic pathway in the definitive clinical trial.
Our current pilot study results add to the existing literature. One prior study which used similar group comparisons (TA, SA, WC) also found significant improvement compared to WC, but no difference between the TA and SA groups for hot flash frequency and index scores16, supportive of the concept that TA and SA both likely improve self-report VMS via a placebo effect via psychosocial stress reduction. Their findings of a lack of difference in psychosocial stress and sleep measures between the TA and SA groups further supports this concept and mirrors our pilot study findings. The current results are also consistent with the majority of studies which exclusively compared TA and SA reporting improved VMS but no group differences15, 22, 31-32, suggesting that further work with objective VMS measures should be pursued33. Two of the three studies using individualized treatments rather then predetermined control protocols did report that TA significantly reduced hot flash severity but not frequency compared to SA15, 16, 23. These findings may suggest that using adaptable point protocols, as is done in traditional acupuncture clinic settings may produce a greater reduction in VMS severity. Of the studies examining variables other than VMS, no direct group differences were seen in QOL, sleep dysfunction or psychological well being14, 29 similar to our findings for these variables.
Prior work in acupuncture VMS mechanistic pathways has been not particularly fruitful. One study demonstrated that acupuncture decreases levels of calcitonin gene-related peptide (CGRP), a powerful vasodilator released into circulation during VMS while another study did not17, 32. When examining change in reproductive hormone levels, acupuncture does not appear to exert an effect34. In our pilot study, we did not observe a correlation between either urinary F or ACTH stimulation results and VMS, likely due to small sample size and restricted range of values in our study population selected for a high frequency of VMS. Higher F levels have been previously linked to menopause, includingVMS14, depressed mood35, decrease in bone density36, arousal and sleeplessness37, as well as greater body fat, especially centripetal obesity36, 38-40 and age41-42. Both acute and chronic F elevations have been demonstrated to be associated with cognitive deficits in humans43-44. In the one study that directly examined the association of VMS and serum cortisol levels, no direct correlation was found14. The studies investigating the effects of acupuncture on serum F levels demonstrated mixed results45-49, potentially due to methodological limitations. Methods which accurately represent F production and metabolism over longer periods of time, such as 24 hour urinary are likely superior in this regard50-51. Our pilot results suggest that 24 hour urinary cortisol collection was, but the ACTH testing was not, sufficiently sensitive and accurate for this purpose.
We increased the duration of treatment in this pilot study compared to prior studies15, 22, 31 in response to thought that a longer treatment period could result in distinct clinical outcomes between active and sham acupuncture groups. Although we observed similar VMS and MENQOL outcomes in this pilot study, we also noted that there appeared to be a stabilization of SA effect after one month, compared the TA group which continued to decrease throughout the treatment. We hypothesize that we will see a significant TA vs SA group difference with a larger sample size in our longer duration trial design. We also realize, however that a longer duration of treatment appears to be associated with a higher drop out rate. Given that the majority of VMS improvements occur within one month (12 treatments), one solution may be to reduce treatment demand (one to two treatments per week) during months two and three, thus maintaining a longer duration with less burden to participants.
Limitations
The sample size of this pilot study was, by design, small and therefore limited conclusions regarding the intervention effect sizes can be drawn. Our eligibility criteria included at least 7 hot flashes per day, and at least one missed menstrual cycle or spontaneous or medically-induced menopause which could allow for a heterogeneous group. Future studies may want to further delineate between peri, post and medically induced menopause to control for possible pathophysiologic differences. The lack of a consistent method of determining the ideal acupuncture point prescription remains an inherent limitation of acupuncture research, however the current results are suggestive that our standardized point and delivery protocol may have had the desired mechanistic pathway effect. A further limitation of our study is the reliance on self-reported VMS rather than an objective measure such as the skin conductance ambulatory Biolog monitor 23, 52. Placebo response seen in SA along with the use of subjective measures enhances the chance of type II error in any study. A recent summary report of the National Institutes of Health workshop on measuring VMS called for studies with both objective biologically measured and subjective self-reported measures of VMS to provide an adequate understanding of efficacy53. Given our pilot study findings suggesting a possible physiological mechanistic pathway effect of TA, incorporation of the objective ambulatory VMS monitor which is now available and validated for VMS measurement33 into trial designs is important for objective outcome assessment. Although a previous acupuncture study suggested that increased number of treatments may result in greater between group differences (TA vs. SA) 22, this can also increase placebo response54, likely explaining our and prior study results which failed to find a difference between TA and SA55. Because F levels follow diurnal patterns and differ day to day, their use as an outcome variable can be controversial. However, since the principal metabolites of F account for > 95% of F excretion, using their measurements offers a more sensitive means of detecting changes in rates of F secretion in 24 hour urines and lessens the possibility of confusion resulting form circadian rhythm or metabolism56.
Implications
The current pilot study results support conductance of a definitive clinical trial employing objective measures and investigating mechanistic pathways to determine the efficacy of TA for VMS in peri- and postmenopausal women. The current study results provide feasibility for trial design and recruitment, identification of primary outcome selection, and variance estimate for the VMS and F outcome measures useful for designing a rigorous clinical trial to guide the application of acupuncture in clinical practice. The use of objective VMS measures and exploration of the role and modulation of the HPA axis are novel additions that should provide clarity and insight into this time-honored non-hormonal therapy.
Conclusions
In this pilot, randomized, single-blinded, placebo-controlled study conducted for trial feasibility, outcome determination, and sample size estimation purposes, both the TA and SA interventions improved VMS and menopause-related QOL compared to WC. Improvement in VMS appeared related to improvement in depression, anxiety and sleep in both TA and SA, while TA only appeared to have an effect on the HPA axis as a mechanistic and therapeutic pathway. Given the small sample size, these findings reported should be viewed as preliminary, hypothesis generating and useful for the planning of a well-designed study including objective measures to determine the efficacy of acupuncture for VMS in peri- and postmenopausal women.
Acknowledgments
This work was supported by grants from the National Institutes of Health-National Center for Alternative and Complementary Medicine, nos. R01 5 R01 AT 1482, and a GCRC grant MO1-RR00425 from the National Center for Research Resources. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Koster A, Davidsen M. Climacteric complaints and their relation to menopausal development--a retrospective analysis. Maturitas. 1993;17:155–66. doi: 10.1016/0378-5122(93)90043-h. [DOI] [PubMed] [Google Scholar]
- 2.Rabin DS, Cipparrone N, Linn ES, Moen M. Why menopausal women do not want to take hormone replacement therapy. Menopause. 1999;6:61–7. [PubMed] [Google Scholar]
- 3.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 6.Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292:1573–80. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 10.Nachtigall LE. Therapy: nonhormonal treatment of hot flashes-a viable alternative? Nat Rev Endocrinol. 2010;6:66–7. doi: 10.1038/nrendo.2009.269. [DOI] [PubMed] [Google Scholar]
- 11.Shanafelt TBD, Adjei A, Loprinzi C. Pathophysiology and Treatment of Hot Flashes. Mayo Clin Proc. 2002;77:1207–18. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 12.Berga SLLT. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 1092:114–9. doi: 10.1196/annals.1365.010. 206. [DOI] [PubMed] [Google Scholar]
- 13.Tolis GDE. Distress Amenorrhea. Ann N Y Acad Sci. 2006;771:660–4. doi: 10.1111/j.1749-6632.1995.tb44718.x. [DOI] [PubMed] [Google Scholar]
- 14.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13:212–21. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 15.Nir Y, Huang MI, Schnyer R, Chen B, Manber R. Acupuncture for postmenopausal hot flashes. Maturitas. 2007;56:383–95. doi: 10.1016/j.maturitas.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Avis NE, Legault C, Coeytaux RR, et al. A randomized, controlled pilot study of acupuncture treatment for menopausal hot flashes. Menopause. 2008;15:1070–8. doi: 10.1097/gme.0b013e31816d5b03. [DOI] [PubMed] [Google Scholar]
- 17.Borud EK, Alraek T, White A, et al. The Acupuncture on Hot Flushes Among Menopausal Women (ACUFLASH) study, a randomized controlled trial. Menopause. 2009;16:484–93. doi: 10.1097/gme.0b013e31818c02ad. [DOI] [PubMed] [Google Scholar]
- 18.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture Versus Venlafaxine for the Management of Vasomotor Symptoms in Patients With Hormone Receptor-Positive Breast Cancer: A Randomized Controlled Trial. J Clin Oncol. 2010;28:634–40. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 19.Wyon YAM, Spetz ACE, Theodorsson GE, Hammar ML. Concentrations of calcitonin gene-related peptide and neuropeptide Y in plasma increase during flushes in postmenopausal women. Menopause. 2000;7:25–30. doi: 10.1097/00042192-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Zaborowska E, Brynhildsen J, Damberg S, et al. Effects of acupuncture, applied relaxation, estrogens and placebo on hot flushes in postmenopausal women: an analysis of two prospective, parallel, randomized studies. Climacteric. 2007;10:38–45. doi: 10.1080/13697130601165059. [DOI] [PubMed] [Google Scholar]
- 21.Filshie J, Bolton T, Browne D, Ashley S. Acupuncture and self acupuncture for long-term treatment of vasomotor symptoms in cancer patients--audit and treatment algorithm. Acupunct Med. 2005;23:171–80. doi: 10.1136/aim.23.4.171. [DOI] [PubMed] [Google Scholar]
- 22.Deng G, Vickers AJ, Yeung KS, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clinical Oncol. 2007;25:5584–90. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 23.Kirschner JCA, Williams J, Paul-Labrador M, et al. Successful recruitment and retention strategies for the study, “The Effects of Traditional Acupuncture on Coronary Heart Disease. Altern Ther Health M. 2009;15:S166. [Google Scholar]
- 24.Lewis JEHJ, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005;50:209–21. doi: 10.1016/j.maturitas.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJRC, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. 1989. 193-213. [DOI] [PubMed] [Google Scholar]
- 26.Beck ATWC, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger CGR, Lushene R. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- 28.Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–7. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 29.Palermo M, Shackleton CH, Mantero F, Stewart PM. Urinary free cortisone and the assessment of 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf) 1996;45:605–11. doi: 10.1046/j.1365-2265.1996.00853.x. [DOI] [PubMed] [Google Scholar]
- 30.Lenze EJMR, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL. Elevated Cortisol in Older Adults With Generalized Anxiety Disorder Is Reduced by Treatment: A Placebo-Controlled Evaluation of Escitalopram. Am J Geriatr Psychiatr. 2010 doi: 10.1097/JGP.0b013e3181ec806c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent A, Barton DL, Mandrekar JN, et al. Acupuncture for hot flashes: a randomized, sham-controlled clinical study. Menopause. 2007;14:45–52. doi: 10.1097/01.gme.0000227854.27603.7d. [DOI] [PubMed] [Google Scholar]
- 32.Wyon Y, Lindgren R, Lundeberg T, Hammar M. Effects of Acupuncture on Climacteric Vasomotor Symptoms, Quality-of-Life, and Urinary-Excretion of Neuropeptides among Postmenopausal Women. Menopause. 1995;2:3–12. [Google Scholar]
- 33.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–15. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Ludicke F, Comte I, Campana A, Graff P, Bischof P. An exploratory pilot study of acupuncture on the quality of life and reproductive hormone secretion in menopausal women. J Altern Complement Med. 2001;7:651–8. doi: 10.1089/10755530152755207. [DOI] [PubMed] [Google Scholar]
- 35.Ballinger S. Stress as a factor in lowered estrogen levels in the early postmenopause. Ann N Y Acad Sci. 1990;592:95–113. doi: 10.1111/j.1749-6632.1990.tb30318.x. discussion 23-33. [DOI] [PubMed] [Google Scholar]
- 36.Greendale GA, Unger JB, Rowe JW, Seeman TE. The relation between cortisol excretion and fractures in healthy older people: results from the MacArthur studies-Mac. J Am Geriatr Soc. 1999;47:799–803. doi: 10.1111/j.1532-5415.1999.tb03835.x. [DOI] [PubMed] [Google Scholar]
- 37.Shaver JL, Johnston SK, Lentz MJ, Landis CA. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med. 2002;64:793–802. doi: 10.1097/01.psy.0000024235.11538.9a. [DOI] [PubMed] [Google Scholar]
- 38.Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 39.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997;82:912–9. doi: 10.1210/jcem.82.3.3834. [DOI] [PubMed] [Google Scholar]
- 40.Meldrum DR, Defazio JD, Erlik Y, et al. Pituitary hormones during the menopausal hot flash. Obstet Gynecol. 1984;64:752–6. [PubMed] [Google Scholar]
- 41.Yen SS, Laughlin GA. Aging and the adrenal cortex. Exp Gerontol. 1998;33:897–910. doi: 10.1016/s0531-5565(98)00046-1. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:3561–8. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- 43.Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 44.Wolkowitz OM, Reus VI, Canick J, Levin B, Lupien S. Glucocorticoid medication, memory and steroid psychosis in medical illness. Ann N Y Acad Sci. 1997;823:81–96. doi: 10.1111/j.1749-6632.1997.tb48381.x. [DOI] [PubMed] [Google Scholar]
- 45.Ahsin S, Saleem S, Bhatti AM, Iles RK, Aslam M. Clinical and endocrinological changes after electro-acupuncture treatment in patients with osteoarthritis of the knee. Pain. 2009;147:60–6. doi: 10.1016/j.pain.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Lee SC, Yin SJ, Lee ML, Tsai WJ, Sim CB. Effects of Acupuncture on Serum Cortisol Level and Dopamine Beta-Hydroxylase Activity in Normal Chinese. Am J of Chin Med. 1982;10:62–9. doi: 10.1142/S0192415X82000117. [DOI] [PubMed] [Google Scholar]
- 47.Magarelli PC, Cridennda DK, Cohen M. Changes in serum cortisol and prolactin associated with acupuncture during controlled ovarian hyperstimulation in women undergoing in vitro fertilization-embryo transfer treatment. Fertil Steril. 2009;92:1870–9. doi: 10.1016/j.fertnstert.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 48.Kotani N, Hashimoto H, Sato Y, et al. Preoperative intradermal acupuncture reduces postoperative pain, nausea and vomiting, analgesic requirement, and sympathoadrenal responses. Anesthesiology. 2001;95:349–56. doi: 10.1097/00000542-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 49.So EWS, Ng EHY, Wong YY, Lau EYL, Yeung WSB, Ho PC. A randomized double blind comparison of real and placebo acupuncture in IVF treatment. Human Reproduction. 2009;24:341–8. doi: 10.1093/humrep/den380. [DOI] [PubMed] [Google Scholar]
- 50.Villanueva ALSC, Hopper B. Increased cortisol production in women runners. J Clin Endocr Metab. 1986;63:133–6. doi: 10.1210/jcem-63-1-133. [DOI] [PubMed] [Google Scholar]
- 51.Neary JP, Malbon L, McKenzie DC. Relationship between serum, saliva and urinary cortisol and its implication during recovery from training. J Sci Med Sport. 2002;5:108–14. doi: 10.1016/s1440-2440(02)80031-7. [DOI] [PubMed] [Google Scholar]
- 52.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–6. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 53.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 54.Kaptchuk TJ. The placebo effect in alternative medicine: Can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002;136:817–25. doi: 10.7326/0003-4819-136-11-200206040-00011. [DOI] [PubMed] [Google Scholar]
- 55.Painovich JSC, Azziz R, Yang Y, et al. A Randomized, Single Blind Controlled Pilot Trial Examining the Effects of Traditional Acupuncture on the Menopause Symptoms and Pathophysiology. 2010. (in press)
- 56.Jerjes WTN, Peters T, Wessely S, Cleare A. Urinary Cortisol and Cortisol Metabolite Excretion in Chronic Fatigue Syndrome. Psychosom Med. 2006;68:578–82. doi: 10.1097/01.psy.0000222358.01096.54. [DOI] [PubMed] [Google Scholar]