Abstract
Fish oil, enriched in bioactive n-3 polyunsaturated fatty acids (PUFA), has therapeutic value for the treatment of inflammation-associated disorders. The effects of n-3 PUFAs are pleiotropic and complex; hence, an understanding of their cellular targets and molecular mechanisms of action remains incomplete. Here we focus on recent data indicating n-3 PUFAs exert immunosuppressive effects on the function of effector and regulatory CD4+ T cells. In addition, we also present emerging evidence that n-3 PUFAs have immunomodulatory effects on B cells. We then focus on one multifaceted mechanism of n-3 PUFAs, which is the alteration of the biophysical and biochemical organization of the plasma membrane. This mechanism is central for downstream signaling, eicosanoid production, transcriptional regulation and cytokine secretion. We highlight recent work demonstrating n-3 PUFA acyl chains in the plasma membrane target the lateral organization of membrane signaling assemblies (i.e. lipid rafts or signaling networks) and de novo phospholipid biosynthesis. We conclude by proposing new functional and mechanistic questions in this area of research that will aid in the development of fish oil as adjuvant therapy for treating unresolved chronic inflammation.
Keywords: Fish oil, T cells, B cells, plasma membrane domains
1. Introduction
A combination of clinical, animal, and in vitro studies suggest consumption of fish oil has potential therapeutic use for the treatment of inflammatory and autoimmune diseases (Galli and Calder, 2009). Some studies even suggest other naturally occurring n-3 PUFAs such as alpha-linolenic (ALA) or stearidonic (SDA) acid may also have clinical applications, although far less is known about these fatty acids (Mozaffarian, 2005; Whelan, 2009). The primary bioactive components of fish oil are the n-3 polyunsaturated fatty acids (PUFA) eicosapentaenoic (EPA, 20:5) and docosahexaenoic (DHA, 22:6) acids. Generally, n-3 PUFAs exert suppressive effects on innate and adaptive immunity (Galli and Calder, 2009). However, our understanding of how these complex fatty acids from fish oil impact inflammatory processes remains incomplete.
The best-accepted pharmacological use of n-3 PUFAs is for lowering elevated triglycerides (Davidson et al., 2007). Many clinical studies have evaluated the effects of dietary fish oil supplements on disorders with an inflammatory component such as cardiovascular disease, rheumatoid arthritis, and irritable bowel disease. Some of the data suggest fish oil supplementation has benefits for atherosclerosis, asthma, colitis, and other disease states (Galli and Calder, 2009). Current recommendations for the public are to increase dietary intake of these fatty acids, especially for those suffering from cardiovascular diseases. However, inconsistent results have prevented the widespread use and regulatory approval of EPA and/or DHA in the clinic as immunosuppressants. At the clinical level, discrepancies arise due to differences in sample demographics, dosages, length of treatment, and sources of fish oil (e.g. menhaden, cod-liver oil, tuna oil). From a basic science perspective, translation is limited due to an incomplete understanding of cellular targets and molecular mechanisms of action. In this review, we focus on the direct effects of EPA and DHA on T and B cell function through changes in one important mechanism.
2. n-3 PUFAs and CD4+ T cells
CD4+ helper T (Th) cells are essential regulators of the immune response. Upon activation, naïve Th cells differentiate into effector cells. For example, classical Th1 and Th2 lineages are characterized by their cytokine secretion and immunoregulatory function. Interleukin-2 (IL-2) and interferon-gamma (IFN-γ) are signature cytokines produced by Th1 cells, which regulate cell-mediated immune response, whereas Th2 cells regulate humoral immune response in part by secreting IL-4, IL-5, IL-10 and IL-13. Th1 cells are of pivotal importance for the pathogenesis of inflammatory bowel diseases (Maloy and Powrie, 2011). Furthermore, an IL-17 producing Th cell lineage activated by IL-23 was recently identified. These cells are classified as distinct pro-inflammatory Th17 cells (Maloy and Powrie, 2011). Hence, autoreactive Th1 cells, as well as Th17 cells, are major players in chronic inflammatory diseases (Sanchez-Munoz et al., 2008), and inhibition of the expansion of Th1 cells can be one of the mechanisms by which anti-inflammatory agents exert their effect.
In vitro and ex vivo studies from humans and rodents have established that EPA and/or DHA suppress the activation of CD4+ T cells in response to an activating stimulus and tip the balance from a pro-inflammatory Th1 to an anti-inflammatory Th2 phenotype (we acknowledge that Th1 and Th2 designation may represent some oversimplification) (Kim et al., 2010; Monk et al., 2010; Zhang et al., 2006). Specifically, n-3 PUFAs suppress T cell proliferation, lower activation markers (CD69, CD25), and subsequent secretion of cytokines (i.e. IL-2) and regulate essential lipid second messengers (Fowler et al., 1993a, b; Jolly et al., 1997; Pompos and Fritsche, 2002). The suppression of T cell activation is clearly dependent on the type of antigen. n-3 PUFAs suppress stimulation of naïve CD4+ T cells when activated with anti-CD3/CD28 antibodies or antigen presenting cells, but not with concanavalin A (Chapkin et al., 2002).
With respect to the suppression of T cell signaling, the Chapkin lab has recently demonstrated that n-3 PUFAs inhibit mitochondrial translocation to the immunological synapse, thereby reducing Ca2+ uptake by mitochondria (Yog et al., 2010). This in turn limits the cytosolic Ca2+ concentration, thereby blocking the phosphatase activity of calcineurin. Collectively, these events reduce the dephosphorylation and nuclear translocation of NFAT and suppress the transcription of genes involved in T-cell activation (Yog et al., 2010). Recent data show that n-3 PUFAs also exert immunosuppressive effects on regulatory Foxp3 CD4+CD25+ T cells (Yessoufou et al., 2009). DHA treatment dose-dependently lowered the ability of regulatory T cells to inhibit effector T cell proliferation in cell culture and ex vivo. This study provokes the question of what impact n-3 PUFAs may have on the function of other T cell subsets, such as Th17 cells.
3. n-3 PUFAs and B cells
B cells are generally thought of as regulators of humoral immunity. However, there is emerging evidence that B cells also have an important role in inflammation and autoimmunity (Hikada and Zouali, 2010; Winer et al., 2011). The effects of fish oil on B cell function (antigen presentation, antigen stimulated activation, proliferation, antibody production) are not well known. A few studies, which are not in agreement, demonstrate that fish oil can modify B cell function. For instance, one study reported that JY B cells, an immortal cell line, treated with an n-6 or an n-3 PUFA overnight decreased presentation of antigen to alloreactive human CD8+ T cells (Shaikh and Edidin, 2007). This was highly consistent with several in vitro studies with dendritic cells (DC) demonstrating that n-3 PUFAs inhibit stimulation of naïve CD4+ T cells (Kong et al., 2011; Zeyda et al., 2005). One open-ended question in this area is whether n-3 PUFAs also suppress the ability of DCs to stimulate naïve T cell in vivo.
The effects of n-3 PUFAs on B cell proliferation appear to be the opposite of those for effector CD4+ T cells. An in vitro study demonstrated that treatment of Raji B cells, an immortal cell type, enhanced the proliferation of this cell type (Verlengia et al., 2004). In agreement, a very recent study from the Shaikh lab also showed that EPA and DHA treatment, relative to controls, promoted the proliferation of EL4 cells (a murine lymphoma line often used as a surrogate antigen presenting cell) (Rockett et al., 2011). These data suggest the impact of n-3 PUFAs is uniquely cell type-dependent. It is likely that n-3 PUFAs exert differential effects between immortal cell types (e.g. Raji B cells) and primary cells that require a stimulus for growth.
The Shaikh lab reported that treatment of murine B220+ B cells with DHA, but not EPA, suppressed IL-6 secretion in response to lipopolysaccharide (LPS) stimulation (Rockett et al., 2010). This was consistent with aforementioned data on n-3 PUFAs and T cell activation as well as an in vitro study in macrophages and Ba/F3 B cells in which DHA treatment suppressed inflammatory gene expression (Wong et al., 2009). However, ex vivo experiments in the same study revealed a high dose of a mixed fish/flaxseed oil diet administered to C57BL/6 mice enhanced the activation of B cells in response to stimulation with LPS (Rockett et al., 2010). Specifically, B cells isolated from the n-3 PUFA fed animals secreted higher levels of IL-6 and IFNγ and upregulated CD69 surface expression, relative to those fed a purified control diet. The interpretation of these results is unclear. From one perspective, the data suggest n-3 PUFAs are promoting a pro-inflammatory response. This would be consistent with a few studies to show that ex vivo stimulation of select splenic macrophages with LPS enhanced TNFα secretion (Blok et al., 1996; Petursdottir and Hardardottir, 2007). A second interpretation is that the effects could be driven by the high dose of n-3 PUFAs used. In this study, ~5% of the total kcal came from EPA/DHA and ~11% from ALA. These levels are well above those used in most studies and therefore have limited physiological relevance. Finally, it is possible that n-3 PUFAs impact B cells in a way that would enhance their activation in vivo, which could have utility for increasing antibody production.
A few labs have tested the effects of n-3 PUFAs on antibody production with varying results, likely due to differences in model systems. Beli et al., reported that IgA antibody responses to respiratory enteric orphan virus was not modified by a fish oil diet relative to controls (Beli et al., 2008). A very recent set of experiments showed consumption of fish oil by Balb/c mothers lowered the antibody response for pups in response to ovalbumin (Lauritzen et al., 2011). Similarly, human B cells treated with DHA and activated with IL-4 and anti-CD40 suppressed IgE levels. In contrast, in broiler birds, n-3 PUFA diets (both fish and flaxseed oils) enhanced the antibody response compared to an n-6 PUFA diet (Selvaraj and Cherian, 2004).
4. Mechanisms by which n-3 PUFAs target plasma membrane molecular organization
The mechanisms by which n-3 PUFAs modulate lymphocyte function are pleiotropic (Shaikh and Edidin, 2006a). EPA and DHA can lower levels of arachidonic acid-derived eicosanoids, increase production of less inflammatory n-3 PUFA derived eicosanoids and specialized anti-inflammatory/pro-resolving mediators (e.g. resolvins, protectins), serve as ligands for PPARγ, and disrupt cellular signaling by targeting the molecular organization of the plasma membrane (Chapkin et al., 2009). This latter mechanism is highly relevant given that the plasma membrane accommodates the initial entry of lipid into the cell and thereby influences the aforementioned downstream mechanisms that converge on activation of inflammatory genes. In particular, n-3 PUFAs can alter numerous physical properties of membranes including compressibility, phospholipid flip-flop, acyl chain packing (i.e. microviscosity), elasticity, and lipid domain formation (Stillwell and Wassall, 2003). Recently, there has been an interest in elucidating how n-3 PUFAs disrupt membrane domain organization through changes in lipid rafts and/or by targeting specific signaling networks.
Membrane lipid rafts are defined as transient sphingolipid/cholesterol assemblies of high molecular order that coalescence under specific circumstances in order to compartmentalize signaling proteins (Lingwood and Simons, 2010). The raft hypothesis has remained contentious for several reasons. One, there are contradictions in the literature as to whether lipid rafts specifically are required to promote intracellular signals (Kenworthy, 2008). Second, biochemical detergent extraction can create artifacts and its overuse has simplified the concept (Heerklotz, 2002; Kenworthy, 2008; Lingwood and Simons, 2007). Third, until recently, direct visualization of rafts has remained elusive. However, the aforementioned issues are now being resolved using new methodologies in multiple model systems. For instance, lipidomics and microscopy approaches, that permit the study of domains below the diffraction limit of a microscope, are pointing to far more convincing evidence for the formation of sphingolipid/cholesterol clusters (Eggeling et al., 2009; Simons and Gerl, 2010). Furthermore, integrating multiple model systems including cells, plasma membrane blebs and artificial lipid vesicles, has shed light on how liquid-liquid immiscibility promotes formation of membrane assemblies that are highly dynamic and heterogeneous (Levental et al., 2011). Finally, the formation of sphingolipid/cholesterol domains is not limited to the plasma membrane, but also occurs in the Golgi where proteins are sorted (Lingwood and Simons, 2010).
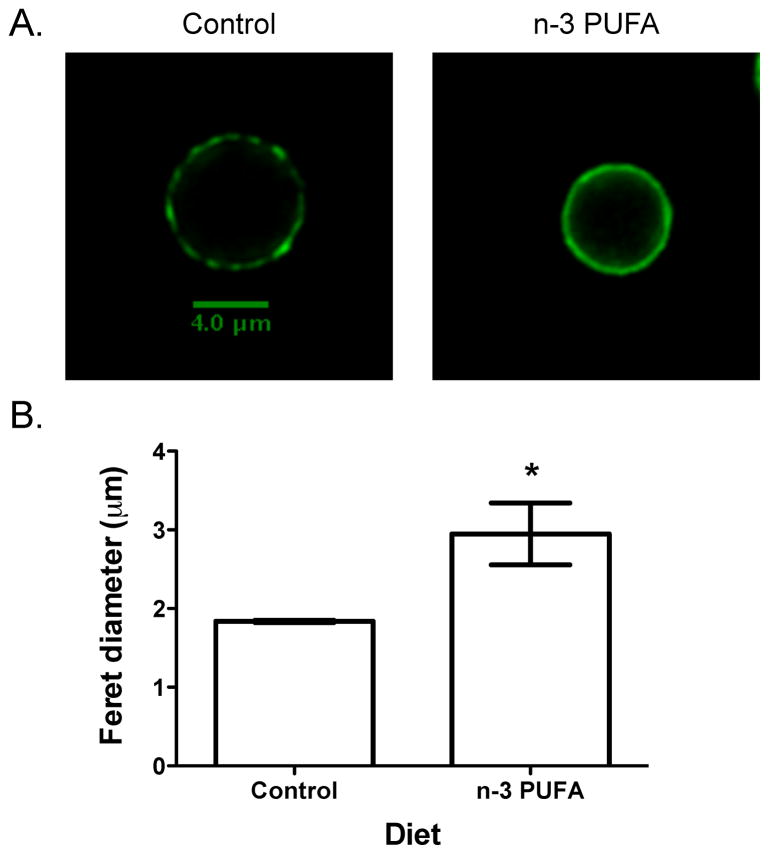
Initially, simplistic studies from detergent extraction showed EPA and DHA incorporated into detergent resistant membranes (a very crude fraction of rafts isolated from cells), which was associated with a disruption in the recruitment of specific CD4+ T cell proteins into rafts and ultimately a suppression in T cell activation (Stulnig et al., 2001). A major limitation of these experiments was the use of detergent extraction that did not provide molecular details (Heerklotz, 2002). Recently, imaging experiments have delivered more convincing data that n-3 PUFAs disrupt both outer and inner membrane leaflet lipid and protein lateral organization. The Shaikh lab demonstrated with confocal and acceptor photobleaching FRET microscopy that n-3 PUFAs, administered in vitro and in vivo, respectively disrupted the spatial distribution of outer leaflet GM1 clustering of EL4 and B cells, which was also associated with some changes in protein distribution (Rockett et al., 2011). Figure 1 shows an example of n-3 PUFAs, administered through the diet of C57BL/6 mice, dramatically disrupting lipid raft clustering and size of B cells. Similarly, the Chapkin lab used immuno-gold electron microscopy of plasma membrane sheets with spatial point analysis to show that DHA specifically disrupted the distribution of inner leaflet rafts of HeLa cells with no impact on non-raft markers (Chapkin et al., 2008a).
Figure 1. n-3 PUFAs disrupt cholera toxin induced clustering of GM1 molecules on the outer leaflet of the B cell plasma membrane.
(A) Sample images of naïve B220+ B cells isolated from a C57BL/6 mouse fed a control diet (CD) or a diet enriched in a high dose of n-3 PUFAs. (B) Quantification of cholera toxin induced clusters. Data are from a recent study on n-3 PUFAs and rafts by the Shaikh lab (Rockett et al., 2011).
Upon incorporation of n-3 PUFAs into rafts, these domains undergo a change in their molecular order. Incorporation of n-3 PUFAs into the immunological synapse of CD4+ T cells from the fat-1 transgenic mouse increased the molecular order of lipid rafts, as measured with polarization microscopy (Kim et al., 2008). Similarly, dietary n-3 PUFAs lowered the uptake of C16-Bodipy into splenic B cells, suggesting n-3 PUFAs may promote an increase in membrane order upon increasing raft size (Rockett et al., 2011). The potential effect on lipid raft size is noteworthy, because lipid rafts are small (6–14 nm in diameter) in order to promote nanoscale protein-protein interactions at the plasma membrane (Nicolau et al., 2006). In contrast, EPA treatment of Jurkat T cells in vitro decreased the molecular order of lipid rafts in the synapse (Zech et al, 2009). Chapkin and co-workers have also analyzed lipid raft formation in Laurdan labeled Jurkat T cells at the immunological synapse following co-culture with superantigen-pulsed human Raji B cells. General polarization values at the synapse were significantly suppressed in DHA treated T cells, as compared to control cells, indicating a diminution of membrane order/raft formation (Kim et al., 2010). These data suggest that malignant transformed Jurkat cell lines may be inherently different from primary T-cells with respect to specific plasma membrane properties, and therefore may not be a suitable model to probe fatty acid effects on lipid raft-dependent signaling. Clearly, more work is needed in this area since it is difficult to directly compare data from in vitro and in vivo studies in different cell types.
In parallel with a disruption in lipid molecular organization, protein distribution and thereby signaling is also modified with n-3 PUFAs. For instance, in the immunological synapse, n-3 PUFAs displace key signaling proteins. Fan et al from the Chapkin lab demonstrated that fish oil-derived n-3 PUFAs differentially modulate T-cell detergent resistant and soluble membrane phospholipid fatty acyl composition, resulting in the suppression of PKCθ colocalization into GM-1 specific lipid rafts in mitogen-stimulated murine CD4+ T-cells (Fan et al., 2003, 2004). Kim et al further demonstrated that n-3 PUFAs down-modulated the migration and activation status of PKCθ and PLCγ-1, as assessed by immunostaining of pan- or phospho-proteins in fixed cells, respectively (Kim et al., 2008) (model depicting raft induced changes in cell signaling are shown in Figure 2). These data suggest that following the formation of an immunological synapse between CD4+ T cells and antigen presenting cells, dietary n-3 PUFAs alter the temporal location of critical signaling proteins at the immunological synapse by affecting the formation of lipid rafts (Figure 2).
Figure 2. n-3 PUFAs increase lipid raft molecular order and suppress the recruitment and activation status of signaling proteins in the CD4+ T cell synapse.
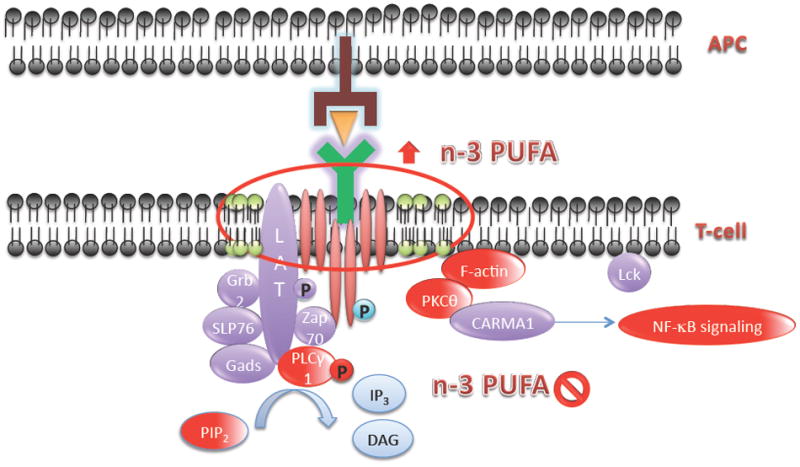
T cell activation results in an increase in lipid raft molecular order following the formation of the T cell – antigen presenting cell dependent immunological synapse. Green circles indicate cholesterol-enriched liquid ordered domains, which stabilize at the immunological synapse. Red circles indicate n-3 PUFA-dependent suppression of lipid second messengers (PIP2, DAG) and proteins (PLCγ-1, PKCθ, F-actin, NF-kB) required for T cell activation. APC, antigen presenting cell.
Additional mechanistic details on how n-3 PUFAs manipulate membrane domain organization are emerging from atomic level measurements using model membranes. Studies in model membranes show the DHA acyl chain (EPA has not been studied) to have unique physical properties (Mihailescu et al., 2011; Rosetti and Pastorino, 2011; Stillwell and Wassall, 2003). Solid-state NMR and molecular dynamic simulation studies demonstrated DHA avoids saturated acyl chains and most importantly has a very poor affinity for cholesterol (Mihailescu et al., 2011; Shaikh et al., 2004; Soni et al., 2008). Therefore, this fatty acid does not interact favorably with lipid rafts. The working model, based largely on in vitro work, is that when DHA acyl chains incorporate into ordered domains, the domains become de-clustered, driven by the poor affinity between DHA and cholesterol/saturated acyl chains (Shaikh et al., 2006b). As a consequence, cholesterol should be pushed away from rafts into non-raft domains (Grimm et al., 2011). Specifically, movement of cholesterol from rafts to non-rafts would have an effect on the ability of proteins (i.e. TLR4, MHC I and II, TCR complex) to optimally diffuse and cluster. The relationship between the n-3 PUFA driven de-clustering of rafts and associated proteins and changes in raft molecular order remains unclear.
In addition to n-3 PUFAs regulating membrane structure and function based on their biophysical properties, these fatty acids can also regulate membrane phospholipid mass (Collison et al., 2005a). For instance, the Jolly lab has shown that changes in membrane phospholipid mass may be a mechanism that also contributes toward the ability of n-3 PUFAs to regulate TCR signaling. Jolly and co-workers reported that glycerol-3-phosphate acyltransferase-1 (GPAT-1) activity, within 30 min of stimulation, dramatically increased in T cells (Collison et al., 2005b). This was significant because GPAT-1 is the initial and rate limiting enzyme in de novo glycerophospholipid biosynthesis. The increase in GPAT-1 activity was due to direct phosphorylation and subsequent activation by PKCθ and casein kinase II (Collison and Jolly, 2006). These observations were further supported by data showing that T cells from GPAT-1 knockout mice had reduced IL-2 production and subsequent proliferation which was associated with a significant reduction in glycerophophospholipid mass while sphingomyelin mass remained unchanged (Collison et al., 2008). This is highly relevant for two reasons. First, aged T cells fail to activate GPAT-1 (Collison et al., 2005b) and also exhibit reduced PKCθ activation. Second, DHA suppresses PKCθ activation in young adult mouse T cells (Kim et al., 2008). These data suggest a novel mechanism whereby n-3 PUFA can suppress TCR signaling which reduces the activation of GPAT-1. Taken together, a decrease in de novo membrane glycerophospholipid biosynthesis, i.e. decreased GPAT-1 activity, would reduce the supply of phospholipids for proper membrane integrity and subsequent plasma membrane receptor function. Limited data are available in this area but may be a common mechanism by which antigen receptors like T and B cell receptors can be modulated.
5. Future Directions
The study of n-3 PUFAs and membrane organization is rapidly evolving. Currently, the field needs an integrative approach that relies on multiple model systems, more sophisticated imaging, and employment of lipidomics to address critical questions on how these fatty acids disrupt membrane molecular organization. We present new questions below that are of importance toward moving the field forward.
What are the structural and thereby functional differences between EPA and DHA? There are very few studies that address this question. In measurements from the Shaikh lab with EL4 cells, EPA and DHA exerted differential effects on cholera-toxin induced raft clustering (Shaikh et al, 2009). The Wassall lab showed cholesterol had a poor affinity for DHA compared to fatty acids with four double bonds or less (Shaikh et al, 2006b); however, a thorough investigation with EPA was not conducted. A combination of atomic and molecular level studies will address how the dynamics of these fatty acids influence their interaction with surrounding cholesterol molecules and with neighboring saturated acyl chains. We propose integrating experiments using model membranes in parallel with in vitro and ex vivo studies. We also propose that it would be of high relevance to compare both EPA and DHA from fish oil with other n-3 PUFAs such as ALA and SDA.
How do n-3 PUFAs impact lipid-lipid and lipid-protein clustering between cell types? The majority of work on n-3 PUFAs and membrane domains has focused on immune cells (primarily T cells and very recently with B cells, macrophages, etc). However, it is unclear if the effects of n-3 PUFAs on raft organization are generalizable to other cell types. Along these lines, Chapkin and co-workers have demonstrated that n-3 PUFAs alter intestinal caveolae/raft lipid composition and resident protein localization in vivo (Ma et al., 2004a, b). Therefore, it is essential to compare the effects of differing n-3 PUFAs on lipid-lipid and lipid-protein clustering on a combination of both immune and non-immune cell types. For instance, how do n-3 fatty acids impact protein clusters on myotubes and what are the downstream functional consequences?
What impact do n-3 PUFAs have on the plasma membrane cytoskeleton and membrane models outside of lipid rafts? As discussed above, the mechanistic effects of n-3 PUFAs are pleiotropic. Thus, it is essential to determine how the bioactive components of fish oil alter the underlying actin cytoskeleton. The cytoskeleton regulates protein lateral diffusion and formation of clusters and it is conceivable that n-3 PUFAs may target signaling molecules by disrupting the cytoskeleton (Suzuki, et al., 2007). Furthermore, there are emerging models to consider with n-3 PUFA studies that are alternatives to the lipid raft model. For instance, diffusional trapping through protein-protein interactions may be a means by which membrane domains form (Douglass and Vale, 2005). Therefore, studying protein diffusion in response to n-3 PUFAs is important.
On what timescale(s) do n-3 PUFAs disrupt membrane lipid/protein clusters and signaling networks? To date, most studies on n-3 PUFAs and membrane organization have not addressed the issue of timescale. Membrane clusters are coalescing on a very rapid timescale and how this is modified is critical toward a complete understanding of the impact of n-3 PUFAs on membrane domain formation and stability.
How is endomembrane physical organization and function influenced by n-3 PUFAs? n-3 PUFA acyl chains incorporate into endomembranes (Shaikh and Edidin, 2006c). For example, one area of importance is the impact of EPA and DHA acyl chains in the mitochondrial membrane. Several labs have shown that EPA and DHA can incorporate into cardiolipin; furthermore, Jolly and co-workers have shown that n-3 PUFAs can modify cardiolipin mass (Collison et al., 2005a). Therefore, it is plausible that remodeling of cardiolipin has an effect on mitochondrial bioenergetics. This could explain potential benefits of n-3 PUFAs on mitochondrial function in several model systems (Fan et al., 2011). Also, incorporation of EPA/DHA into the Golgi membrane may have an influence on lipid shell formation and protein trafficking. Consistent with this viewpoint, the Chapkin lab have demonstrated that DHA selectively alters the plasma membrane targeting of lipidated cytosolic proteins, including Ras isoforms, by modifying membrane lipid composition (Seo et al., 2004; Chapkin et al., 2008b). Shaikh and Edidin have also observed that DHA can suppress the forward traffic of MHC class I molecules (Shaikh and Edidin, 2007).
To what extent do n-3 PUFAs modify the lipidome? A comprehensive analysis of the lipidome (totality of lipids in the cell) in response to fish oil feeding or treatment in vitro with specific cell types (perhaps CD4+ T cells or B cells as a starting point) would provide a global picture of the impact of these fatty acids. This would advance the entire field of n-3 PUFAs and this approach has the potential to open many new doors. For instance, analysis of the lipidome with n-3 PUFA treatment could also provide a potential link between changes in membrane organization to changes in cellular metabolism.
What is the relationship between phospholipid mass and membrane domain organization in response to n-3 PUFAs? As discussed above, the Jolly lab found that n-3 PUFAs can increase the mass of select phospholipids in T cells (Collison et al., 2005a). It is conceivable that changes in the delivery of select phospholipids to the plasma membrane, or other membranes like the mitochondria, would have an influence on lipid-lipid and lipid-protein interactions. In addition, this could impact the generation intracellular lipid second messengers. For example, the decrease Jolly and co-workers report in phosphatidylcholine and phosphatidylinositol in GPAT-1 KO mice could decrease the amount of diacylglycerol produced (Collison et. al. 2008). Furthermore, sphingomyelin mass, and ultimately ceramide generation, remains unchanged in these mice, which could tip the balance of pro-and anti-proliferative signaling molecules in the T cells.
6. Conclusion
We have reviewed recent evidence demonstrating that fish oil has differential effects on the function of CD4+ T cells and B cells. Mechanistically, we focused on advances addressing how n-3 PUFAs disrupt membrane domain organization and phospholipid metabolism in these cell types and have emphasized new areas of investigation. Taken together, fish oil has tremendous potential for the treatment of inflammatory diseases, perhaps by specifically targeting the molecular organization of the plasma membrane. This mechanism is of high importance since the plasma membrane regulates downstream signaling, eicosanoid generation, and gene transcription.
Acknowledgments
This work was supported in part by NIH Grants R15AT006122 (to S.R.S), RO1 AG/AR20651 (to C.A.J.) and NIH grants DK071707, P30ES09106, and USDA 2008-34402-19195, “Designing Foods for Health” (to R.S.C).
Biographies
Dr. Saame Raza Shaikh is a biochemist in the Department of Biochemistry & Molecular Biology and a member of the East Carolina Diabetes and Obesity Institute at East Carolina University. He received his B.S. in Biology/Psychology from Purdue University and his Ph.D. in Biophysics from Indiana University. He then completed postdoctoral training in immunology and cell biology at Johns Hopkins University in the lab of Dr. Michael Edidin. He is currently an Assistant Professor at East Carolina University.
Dr. Robert S. Chapkin is a biochemist in the Program in Integrative Nutrition & Complex Diseases, Center for Environmental & Rural Health at Texas A&M University. He received his B.S. in nutrition and biochemistry from the University of Guelph, Ontario, Canada, an M.S. in nutrition from the University of Guelph, and a Ph.D. in nutrition and physiology chemistry from the University of California-Davis. After completing a postdoctoral fellowship in cancer biology in the School of Medicine at the University of California-Davis, he joined the faculty at Texas A&M University. Dr. Chapkin is currently a Regents Professor and University Faculty Fellow.
Dr. Christopher A. Jolly is a biochemist in the Department of Nutritional Sciences at The University of Texas at Austin. He received his B.S. in Nutritional Sciences and a Ph.D. in Nutrition from Texas A&M University. He then completed a postdoc in lipid biochemistry/biophysics in the Texas A&M Veterinary School followed by being a postdoctoral fellow in nutrition and immunology at The University of Texas Health Science Center at San Antonio. Dr. Jolly is currently an Associate Professor of Nutritional Sciences at The University of Texas at Austin.
Footnotes
There are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beli E, Li M, Cuff C, Pestka JJ. Docosahexaenoic acid–enriched fish oil consumption modulates immunoglobulin responses to and clearance of enteric reovirus infection in mice. J Nutr. 2008;138:813–819. doi: 10.1093/jn/138.4.813. [DOI] [PubMed] [Google Scholar]
- Blok WL, de Bruijn MF, Leenen PJ, Eling WM, van Rooijen N, Stanley ER, Buurman WA, van der Meer JW. Dietary n-3 fatty acids increase spleen size and postendotoxin circulating TNF in mice; role of macrophages, macrophage precursors, and colony-stimulating factor-1. J Immunol. 1996;157:5569–5573. [PubMed] [Google Scholar]
- Chapkin RS, Arrington JL, Apanasovich TV, Carroll RJ, McMurray DN. Dietary n-3 PUFA affect TcR-mediated activation of purified murine T cells and accessory cell function in co-cultures. Clin Exp Immunol. 2002;130:12–18. doi: 10.1046/j.1365-2249.2002.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008a;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaeonic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem Phys Lipids. 2008b;153:14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leuk Essent Fatty Acid. 2009;81:187–91. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Collison RE, Murphy EJ, Jolly CA. Dietary n-3 polyunsaturaetd fatty acids increase T-lymphocyte phospholipid mass and acyl-CoA binding protein expression. Lipids. 2005a;40:81–87. doi: 10.1007/s11745-005-1362-8. [DOI] [PubMed] [Google Scholar]
- Collison LW, Kannan L, Onorato TM, Knudsen J, Haldar D, Jolly CA. Aging reduces glycerol-3 phosphate acyltransferase activity in activated rat splenic T cells. Biochim Biophys Acta. 2005b;1687:164–172. doi: 10.1016/j.bbalip.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Collison LW, Jolly CA. Phosphorylation regulates mitochondrial glycerol-3-phosphate-1 acyltransferase activity in T-lymphocytes. Biochim Biophys Acta. 2006;1761:129–139. doi: 10.1016/j.bbalip.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Collison LW, Murphy EJ, Jolly CA. Glycerol-3-phosphate acyltransferase-1 regulates murine T-lymphocyte proliferation and cytokine production. Am J Physiol Cell Physiol. 2008;295:C1543–C1549. doi: 10.1152/ajpcell.00371.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, Ballantyne CM, Ginsberg HN. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–U1121. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T-cell protein kinase C-theta lipid raft recruitment and interleukin-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- Fan YY, Ran Q, Toyokuni S, Okazaki Y, Callaway ES, Lupton JR, Chapkin RS. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res. 2011 Apr 13; doi: 10.1158/1940-6207.CAPR-10-0368. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler KH, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T-lymphocyte function. J Immunol. 1993a;151:5186–5197. [PubMed] [Google Scholar]
- Fowler KH, McMurray DN, Fan YY, Aukema HM, Chapkin RS. Purified dietary n-3 polyunsaturated fatty acids alter diacylglycerol mass and molecular species composition in concanavalin A stimulated murine splenocytes. Biochim Biophys Acta. 1993b;1210:89–96. doi: 10.1016/0005-2760(93)90053-c. [DOI] [PubMed] [Google Scholar]
- Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann Nutr Metab. 2009;55:123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- Grimm MOW, Kuchenbecker J, Groesgen S, Burg VK, Hundsdoerfer B, Rothhaar TL, Friess P, et al. Docosahexaenoic acid reduces amyloid β production via multiple, pleiotropic mechanism. J Biol Chem. 2011;286:14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures, Biophys. J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikada M, Zouali M. Multistoried roles for B lymphocytes in autoimmunity. Nat Immunol. 2010;11:1065–1068. doi: 10.1038/ni1210-1065. [DOI] [PubMed] [Google Scholar]
- Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK. Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010;49:250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen L, Kjær TM, Porsgaard T, Fruekilde MB, Mu H, Frøkiær H. Maternal intake of fish oil but not of linseed oil reduces the antibody response in neonatal mice. Lipids. 2011;46:171–178. doi: 10.1007/s11745-010-3519-8. [DOI] [PubMed] [Google Scholar]
- Levental I, Grzybek M, Simons K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc Natl Acad Sci USA. 2011;108:11411–11416. doi: 10.1073/pnas.1105996108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protocols. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Ma DWL, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. Faseb J. 2004a;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- Ma DWL, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004b;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Mihailescu M, Soubias O, Worcester D, White SH, Gawrisch K. Structure and dynamics of cholesterol-containing polyunsaturated lipid membranes studied by neutron diffraction and NMR. J Membr Biol. 2011;239:63–71. doi: 10.1007/s00232-010-9326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, McMurray DN, Chapkin RS. Clinical effects of n-3 PUFA supplementation in health and inflammatory diseases. In: Hernandez E, Hoskawa M, editors. Omega 3 oils: Applications in Functional Foods. American Oil Chemists’ Society; 2011. pp. 31–60. [Google Scholar]
- Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11:24–30. [PubMed] [Google Scholar]
- Nicolau DV, Burrage K, Parton RG, Hancock JF. Identifying optimal raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petursdottir DH, Hardardottir I. Dietary fish oil increases the number of splenic macrophages secreting TNF-α and IL-10 but decreases the secretion of these cytokines by splenic T cells from mice. J Nutr. 2007;137:665–670. doi: 10.1093/jn/137.3.665. [DOI] [PubMed] [Google Scholar]
- Pompos LJ, Fritsche KL. Antigen-driven murine CD4+ T lymphocyte proliferation and interleukin-2 production are diminished by dietary (n-3) polyunsaturated fatty acids. J Nutr. 2002;132:3293–3300. doi: 10.1093/jn/132.11.3293. [DOI] [PubMed] [Google Scholar]
- Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. N-3 PUFAs improve fatty acid composition, prevent palmitate induced apoptosis, and differentially modify B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1294–1297. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than non-rafts of EL4 and B cells. J Nutr. 2011;141:1041–1048. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosetti C, Pastorino C. Polyunsaturated and saturated phospholipids in mixed bilayers: A study from the molecular scale to the lateral lipid organization. J Phys Chem B. 2011;115:1002–1013. doi: 10.1021/jp1082888. [DOI] [PubMed] [Google Scholar]
- Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj RK, Cherian G. Dietary n-3 fatty acids reduce the delayed hypersensitivity reaction and antibody production more than n-6 fatty acids in broiler birds. Eur J Lipid Sci Technol. 2004;106:3–10. [Google Scholar]
- Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Dumaual AC, Castillo A, LoCascio D, Siddiqui RA, Stillwell W, Wassall SR. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: A comparative NMR, DSC, AFM, and detergent extraction study. Biophys J. 2004;87:1752–1766. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am J Clin Nutr. 2006a;84:1277–1289. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Cherezov V, Caffrey M, Soni SP, LoCascio D, Stillwell W, Wassall SR. Molecular organization of cholesterol in unsaturated phosphatidylethanolamines: X-ray diffraction and solid state 2H NMR reveal differences with phosphatidylcholines. J Am Chem Soc. 2006b;128:5375–5383. doi: 10.1021/ja057949b. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Membranes are not just rafts. Chem Phys Lipids. 2006c;144:1–3. doi: 10.1016/j.chemphyslip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J Lipid Res. 2007;48:127–138. doi: 10.1194/jlr.M600365-JLR200. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- Soni SP, LoCascio DS, Liu Y, Williams JA, Bittman R, Stillwell W, Wassall SR. Docosahexaenoic acid enhances segregation of lipids between raft and nonraft domains: 2H-NMR study. Biophys J. 2008;95:203–314. doi: 10.1529/biophysj.107.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177(4):717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlengia R, Gorjao R, Kanunfre CC, Bordin S, de Lima TM, Martins EF, Newsholme P, Curi R. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in raji cells. Lipids. 2004;39:857–864. doi: 10.1007/s11745-004-1307-2. [DOI] [PubMed] [Google Scholar]
- Weise C, Hilt K, Milovanovic M, Ernst D, Rühl R, Worm M. Inhibition of IgE production by docosahexaenoic acid is mediated by direct interference with STAT6 and NFκB pathway in human B cells. J Nutr Biochem. 2011;22:269–275. doi: 10.1016/j.jnutbio.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Whelan J. Dietary stearidonic acid is a long chain (n-3) polyunsaturated fatty acid with potential health benefits. J Nutr. 2009;139:5–10. doi: 10.3945/jn.108.094268. [DOI] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Kwon M, Choi AMK, Kim H, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yessoufou A, Plé A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009;50(12):2377–2388. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunological synapse and modulate calcium signaling in T cells. J Immunol. 2010;184:5864–5873. doi: 10.4049/jimmunol.0904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Hader T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Säemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhäusl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB. J Biol Chem. 2005;280(14):14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kim W, Zhou RL, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–2398. doi: 10.1093/jn/136.9.2391. [DOI] [PubMed] [Google Scholar]